Abstract
Ongoing studies have developed strategies for identifying key bio-active compounds and chemical profiles in Echinacea with the goal of improving its human health benefits. Antiviral and antiinflammatory–antipain assays have targeted various classes of chemicals responsible for these activities. Analysis of polar fractions of E. purpurea extracts showed the presence of antiviral activity, with evidence suggesting that polyphenolic compounds other than the known HIV inhibitor, cichoric acid, may be involved. Antiinflammatory activity differed by species, with E. sanguinea having the greatest activity and E. angustifolia, E. pallida, and E. simulata having somewhat less. Fractionation and studies with pure compounds indicate that this activity is explained, at least in part, by the alkamide constituents. Ethanol extracts from Echinacea roots had potent activity as novel agonists of TRPV1, a mammalian pain receptor reported as an integrator of inflammatory pain and hyperalgesia and a prime therapeutic target for analgesic and antiinflammatory drugs. One fraction from E. purpurea ethanol extract was bioactive in this system. Interestingly, the antiinflammatory compounds identified to inhibit prostaglandin E2 production differed from those involved in TRPV1 receptor activation.
Keywords: Alkamide, antiinflammatory, antipain, antiviral, cichoric acid, Echinacea, Echinacea angustifolia, Echinacea pallida, Echinacea purpurea, Echinacea sanguinea, Echinacea simulate, HIV, TRPV1 receptor
CENTER ORGANIZATION
The primary goal of the Iowa Center for Research on Botanical Dietary Supplements is to improve our understanding of the characteristics of Echinacea, Hypericum, and Prunella that contribute to human health and thereby pave the way for optimizing these supplements for study in future clinical trials. Our center focuses on infection with an emphasis on antiviral, antiinflammatory, and antipain activities. This article summarizes some of our work on Echinacea.
A central strategy of the Iowa center has been to use biological diversity to help to identify active constituents and determine mechanisms of action. It is tempting to consider the diversity of these plant genera and the complexity of their constituents as barriers to understanding their potential health benefits. However, the range of variation in these plants, when systematically analyzed, provides a strong foundation on which to develop the strategies and tools needed to produce the most efficacious products for a growing body of consumers.
The center is organized into 3 cores: Germplasm and Phytochemical Profiling; Separations, Structure, Bioavailability; and Administration, Data Management, Statistics, and Bioinformatics. Three projects are supported: defining antiviral activities in Echinacea, Hypericum, and Prunella species; antiinflammatory activity of Echinacea, Hypericum, and Prunella species; and pain-receptor-mediated antiinflammatory activity of Echinacea and Hypericum species.
PRODUCTION OF WELL-CHARACTERIZED PLANT MATERIAL
A unique resource of the center is our collection of a genetically diverse set of well-documented plant populations of Echinacea and the conservation of these genetic stocks at the US Department of Agriculture-Agricultural Research Service North Central Regional Plant Introduction Station (NCRPIS). This resource gives us strength in controlling the production of plant materials and links with our expertise in genomic analysis and broad-based plant metabolic profiling and our ability to integrate complex datasets by using bioinformatics and other statistical tools. The NCRPIS, which is located at Iowa State University, is one of the main active gene banks in the US National Plant Germplasm System, and it conserves extensive collections of known-source medicinal plants, with an emphasis on Echinacea and Hypericum.
Since the late 1990s, the NCRPIS has acquired > 150 distinct wild populations (or accessions) of Echinacea, representing all recognized species and varieties from throughout their ranges, including 2 federally endangered taxa, E. laevigata and E. tennesseensis. The genetic integrity of these populations is preserved by regenerating seed samples under controlled conditions in screened field cages with insect pollinators, typically honeybees (1). To ensure unbiased sampling of Echinacea populations for regeneration, research has been directed toward understanding seed dormancy and methods to overcome it (2, 3).
During seed regeneration, taxonomic identities are verified and populations are characterized for phenotypic traits with a standardized descriptor list. Phenotypic descriptors along with images and detailed passport data describing each accession are available from the Germplasm Resources Information Network database (4). Seeds of all available accessions are distributed for research and educational purposes at no cost to the user.
Echinacea samples used by center researchers have typically been produced from NCRPIS accessions. By using these accessions produced under known conditions, we minimize both the genetic and the environmental components of biochemical variation in the resulting products, which increases the overall repeatability of bioassays. Long-term, replicated field plantings of the 3 primary medicinal species, E. angustifolia, E. pallida, and E. purpurea, were established in 2003 to optimize root production and determine the effects of disease and shading on plant survival and productivity. Dried roots from these plantings have been a major source of plant material for our research projects. Additional accessions have also been supplied to researchers as root samples from plants used for seed regeneration, after successful completion of the seed-production process, and as leaf and pollen samples. After production and processing, all plant samples for center use are inventoried with a standardized coding system, and unextracted dried plant materials are packaged in nitrogen and stored frozen at −20°C. In addition to the supply of plant materials for bioassays, a carefully selected, diverse array of ≈40 Echinacea accessions is being characterized biochemically for alkamides and caffeic-acid derivatives and genetically for both nuclear and chloroplast DNA variation. These characterization data should be valuable for describing the extent of chemical variation, elucidating taxonomic relations, and providing a framework for phylogenetic analysis and future bioassay.
ANTI-HIV ACTIVITIES OF ECHINACEA
The antiviral activities of Echinacea extracts and its metabolic constituents are surprisingly poorly studied. A recent, prominent study identifying a lack of efficacy of E. angustifolia extracts against rhinovirus infection has discouraged additional clinical antiviral studies on botanical extracts (5). However, recent in vitro studies within our center identifying antiviral activities in Echinacea against HIV are promising. Constituents responsible for the anti-HIV activity in our plant extracts were identified through bioactivity-driven fractionation studies. A example of our success with this approach is shown here for E. purpurea.
Initially, extracts from 6 Echinacea species were tested for inhibition of replication of the HIV molecular clone pNL4–3. All HIV infectivity assays were performed in the HeLa37 cell line that expresses the viral receptor and co-receptors (6). Known titers of HIV were added to cells in the presence of the botanical extract. Cells were fixed and immunostained for viral antigens at 40 h after infection. Cells immunopositive for HIV were represented as percentage of control values. Extracts from E. purpurea consistently provided the most robust inhibition of HIV with little or no cellular cytotoxicity. Increasing concentrations of E. purpurea extract had antiviral activity with a 50% inhibitory concentration (IC50) of 2.4 μg/mL (Figure 1). This species contains the anti-HIV compound cichoric acid and thus was anticipated to inhibit HIV replication (7). To determine whether constituents other than cichoric acid had antiviral activity, E. purpurea extracts were fractionated. Seven fractions were generated; no fraction had detectable cytotoxicity or endotoxin contamination (Figure 2A). Fraction 1, the most polar, had significant anti-HIV activity; the other fractions had no antiviral activity. HPLC analysis of fraction 1 documented that a series of caffeic acid derivatives and other polyphenolics including cichoric acid were present (Figure 2B). Subfractionation into 9 subfractions yielded 6 with anti-HIV activity (Figure 3); HPLC analysis of these subfractions showed that each fraction was composed of different constituents. Subfractions 1–6 all contained numerous constituents including cichoric acid; subfractions 1–3, 1–4, 1–7, and 1–8 exhibited some antiviral activity and contained multiple constituents that absorbed light at 330 nm and likely are polyphenolics. Interestingly, subfraction 5 absorbed light at 254 nm but not at 330 nm and may contain glycosylated flavanoids. We anticipate that we will successfully continue to use this bioactivity-driven fractionation approach to identify the botanical compounds responsible for the antiviral activity.
FIGURE 1.
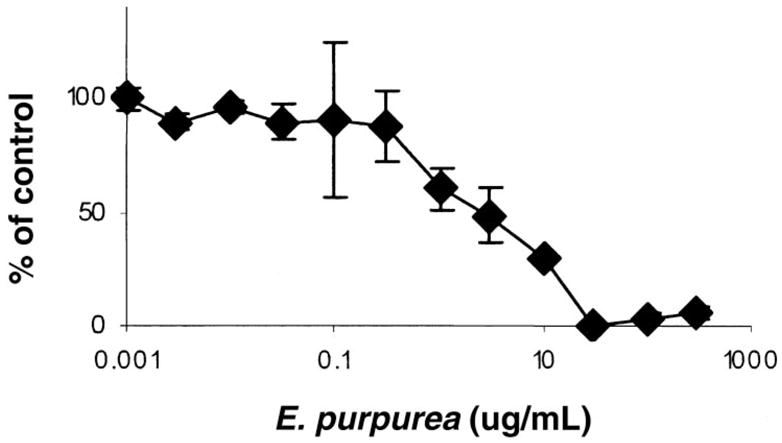
Ability of Echinacea purpurea ethanol extract (PI621307) to inhibit HIV replication. Data shown represent the mean ± SEM for each data point from 3 different experiments with triplicate samples in each experiment.
FIGURE 2.
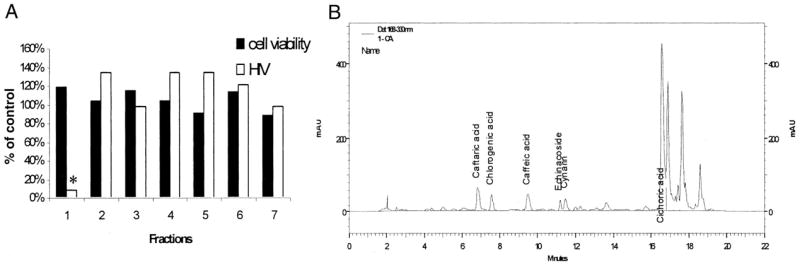
Inhibition of HIV infectivity by Echinacea purpurea fractions. (A) Ability of 7 HPLC-separated fractions (100 μg/mL) to inhibit HIV infection (white bars) or cytotoxicity (black bars). Each bar represents the mean of triplicates. The antiviral activity found in fraction 1 that is highlighted with an asterisk showed efficacy against HIV-1 as determined by a Student’s t test comparing the untreated and treated samples (P = 0.0001). (B) HPLC analysis of E. purpurea fraction 1. Phenolic acid standards were run to allow identification of compounds.
FIGURE 3.
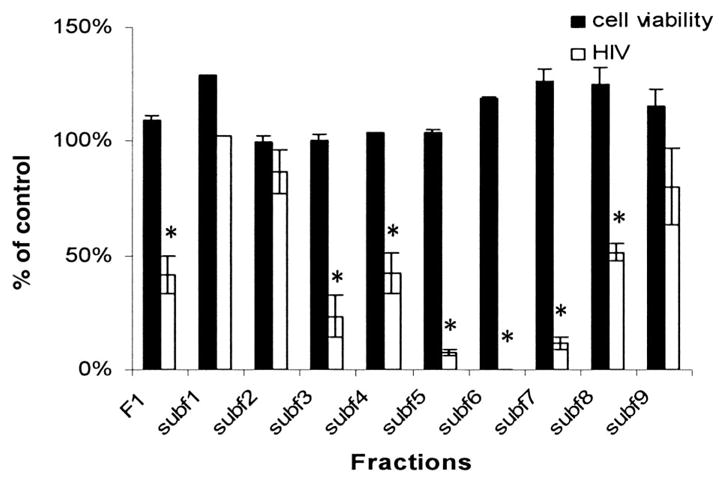
Antiviral activity and cytotoxicity associated with subfractions 1–9 from Echinacea purpurea fraction 1. All HIV infectivity (white bars) and cytotoxicity (black bars) assays were performed with 100 μg/mL of the fraction or subfraction. Values represent the mean ± SEM for 2 experiments performed in triplicate. Asterisks represent findings that are significantly different from control infections as determined by a Student’s t test (P = 0.0001).
ANTIINFLAMMATORY SCREENING USING RAW264.7 MACROPHAGES
The antiinflammatory activity of Echinacea extracts, fractions, and constituents was assessed with RAW264.7 cells treated with and without lipopolysaccharide, and prostaglandin E2 (PGE2) accumulation was measured. This widely used screen for antiinflammatory activity is based on PGE2 production arising from cyclooxygenase-1 and -2 activation, a key event in inflammation. Initial studies showed that soxhlet ethanol extracts of Echinacea provided the greatest antiinflammatory activity. Activity of soxhlet extracts of E. purpurea, E. angustifolia, E. pallida, E. tennesseensis, E. simulata, and E. sanguinea at 15 μg/mL, which were harvested during the fall of 2003, 2004, and 2005, did not differ significantly by repeat extraction or harvest. E. sanguinea inhibited PGE2 production to the greatest extent; E.angustifolia, E. pallida, and E. simulata inhibited less; and E. tennesseensis and E. purpurea extracts were not inhibitory at this concentration (Figure 4) but were at higher concentrations (8). Concentrations of alkamides and ketones in these extracts were determined, but these compounds did not simplistically explain bioactivity. For example, E. angustifolia and E. purpurea were rich in Bauer Amide 8, which was not abundant in E. pallida. There was relatively little cytotoxicity of Echinacea even at doses 10-fold higher than those assayed for antiinflammatory activity. All extracts reported here were negative for endotoxin contamination.
FIGURE 4.
Extracts of Echinacea species from different harvest dates were studied at 15 μg/mL for their effects on prostaglandin E2 (PGE2) production. Data (originally ng/mL PGE2) were normalized to the DMSO-lipopolysaccharide (LPS)–treated control and are presented as means ± 95% CIs. E. angustifolia, E. pallida, and E. sanguinea (P < 0.001) reduced PGE2 production compared with medium + DMSO controls. Data shown are with LPS; studies were conducted without LPS, but PGE2 values were not altered by Echinacea. *P < 0.05 compared with medium + LPS + DMSO in a Dunnett multiple-comparison test. Redrawn from reference (8).
HPLC fractions of E. pallida and E. angustifolia had similar patterns of effects on PGE2 production, with the strongest inhibition in fraction 3 for both species. Interestingly, the more polar fractions (fractions 1 and 2 containing polyphenols such as caffeic acid and Bauer alkamides 1–7) had less antiinflammatory activity at higher concentrations, but fraction 3, which contained abundant alkamides (unique in this fraction for E. purpurea and E. angustifolia were Bauer amides 8 and 9), significantly reduced LPS-induced PGE2 production (Figure 5). Our observations are consistent with observations of others showing antiinflammatory activity with alkamide extracts containing Bauer alkamides 8 or 9 (9). However, because E. pallida does not contain amides 8 or 9; other compounds, possibly ketones, must contribute the activity.
FIGURE 5.
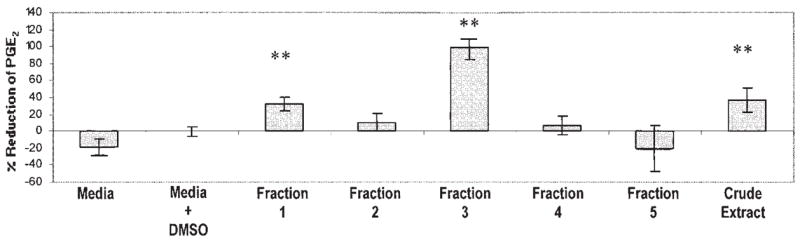
Preparative HPLC fractions of Echinacea angustifolia soxhlet ethanol extract were assessed for reduction of prostaglandin E2 (PGE2) production and are presented as means ± 95% CIs. All treatments are shown with lipopolysaccharide (LPS) because the fractions did not alter PGE2 production in the absence of LPS. Fractions 1 and 3 significantly reduced LPS-induced PGE2 production. n = 3. **P < 0.05, representative of a Dunnett multiple-comparison test.
Numerous alkamides synthesized by our center were tested for antiinflammatory activity, and all the synthesized alkamides (ie, Bauer 2, 8, 10, 11, 13, and 14) screened to date significantly inhibited the production of PGE2 (P < 0.001) at 50 μmol/L (8). Only Bauer amide 14 significantly inhibited PGE2 production at 10 μmol/L (P < 0.05), although amides 8 and 12 at 10 μmol/L inhibited at P < 0.08. From these data, amide 14 is the only synthesized alkamide that significantly reduced PGE2 at all concentrations screened. These data suggest that the alkamides in Echinacea are important for its antiinflammatory activity. Amide 8 may play a key role because of its abundance in E. angustifolia fraction 3, in which PGE2 production by LPS-treated RAW264.7 cells was potently reduced (Figure 5).
Subfractionation of E. angustifolia fraction 3 showed that the less polar subfractions have antiinflammatory activity, with subfractions 3D and 3E exhibiting the greatest reduction in PGE2 accumulation (data not shown). Alkamides are present in subfractions 3B–E: 3D contains alkamides 5, 8, 9, and 14; 3E contains 10 and 11; 3B contains alkamide 1; and 3C contains alkamides 1, 2, 3, 5, 12, 13, and 14.
This series of studies suggests that Echinacea alkamides may contribute to observed antiinflammatory activity because they are readily identified in the active extracts and exhibit appreciable activity. However, results with purified alkamides indicate that single compounds failed to completely account for antiinflammatory activity and, thus, may interact with each other or with other compounds to explain the observed antiinflammatory activity.
MEDIATION OF PAIN-RECEPTOR ACTIVITY BY ECHINACEA
TRPV1 in pain and inflammation
TRPV1 (transient receptor potential channel, vanilloid subfamily member 1, VR1) (10) is a ligand-gated cation channel pain receptor. It was initially cloned by using capsaicin, the potent compound of hot peppers, which is a strong ligand (11). TRP channels are extremely nonselective; TRPV1 is activated by factors including capsaicin, protons, noxious heat (>42 °C), the endocannabinoid anandamide, lipoxygenase product, and ethanol (12). It is considered to be a key integrator of external stimuli, both chemical and physical, into the common signal of inward ion currents (11). Receptors from the TRP channel family, as well as other classes of receptors such as CB, ASIC, and Trek-1, are predominantly expressed in sensory tissues, such as nociceptors and skin. These receptors are thought to provide feedback to sense, transmit, and integrate pain and associated inflammatory responses (11). Growing evidence indicates that TRPV1 acts as an integrator of inflammatory pain and hyperalgesia, making it an excellent potential target for analgesic or antiinflammatory agents. Because of its importance in pain and inflammatory responses, TRPV1 protein structure-activity relations are being analyzed extensively to determine which TRPV1 sites are important for interaction with each of its ligands. TRPV1 is also desensitized by its ligands. Desensitization of TRPV1 after its activation in these neurons is crucial in blocking pain transmission (11). Therefore, compounds that serve as agonists have potential use as analgesics or antiinflammatory agents. In addition, capsaicin can produce a hypotensive effect in spontaneously hypertensive rats, indicating that activation of TRPV1 may contribute to the treatment of hypertension (13).
Echinacea species comparison
Because of reported effects on inflammatory pain, we investigated the effects of Echinacea extracts on TRPV1-dependent inward ion currents by using transient expression of TRPV1 in frog oocytes (11). In this system, TRPV1 cRNA is injected into healthy oocytes and is expressed transiently; then the TRPV1-expressing oocytes are bathed in Echinacea extracts or capsaicin for 10 s. Whole-cell currents are recorded by a 2-electrode voltage clamp recording system. Extracts of roots of E. angustifolia evoked a current 10-fold greater than a saturating dose of capsaicin (Figure 6). Echinacea extracts rapidly desensitize the TRPV1 channel even in the absence of added calcium, which suggests that TRPV1 activation may occur through a mechanism different from that of capsaicin. Leaf and flower extracts also activated TRPV1.
FIGURE 6.

Relative potency of 70% ethanol Echinacea extracts (0.25 mg/mL) on TRPV1 (mean ± SEM, n ≥ 4 independent oocytes for each value). Current responses were normalized in each cell to responses obtained with capsaicin (10 μmol/L). Spinach extract (0.25 mg/mL) was used as the control. *P < 0.05, **P < 0.01, significantly different when compared with spinach control (Student’s t test). Extracts evoked no responses in water-injected cells.
Identification of bioactive constituents
Aqueous extracts of Echinacea contain unusual polysaccharides reported to be responsible for certain antiinflammatory activities (14). When Echinacea is extracted with solvent containing < 90% water, polysaccharides are absent; thus, our ethanol extracts would presumably not contain them. To directly test whether Echinacea polysaccharides activate the TVPR1 channel, we prepared water extracts of E. purpurea, E. angustifolia, and E. pallida and tested them with the frog-oocyte model. Echinacea aqueous extracts did not activate TRPV1, which indicates that the polysaccharides do not appear to be TRPV1 ligands. Because several bioactivities, including cannabinoid CB pain-receptor activation (15) and antiinflammatory effects (described here), can be attributed at least in part to the alkamide constituents of Echinacea, we also tested the effect of 6 purified Echinacea alkamides on TRPV1 with use of the frog oocyte model. Interestingly, no alkamide tested activated the TRPV1 channel. We are now using a combination of subfractionation of Echinacea extracts and bioassays to identify the bioactive constituents. CB and TRP receptors appear to be intimately related. CB1 stimulation modulates TRPV1 activities in cultured rat dorsal root ganglion cells, and CB1 and TRPV1 receptors are highly coexpressed in nociceptive primary sensory neurons (15).
FUTURE OBJECTIVES
Our center’s overarching objectives include identifying compounds contributing to the antiviral, antiinflammatory, and pain-control effects of Echinacea and to Echinacea toxicity. We further seek to assess the influence of plant species and population on bioactive constituents and to understand their mechanisms of action, in particular, the cellular signaling pathways and critical receptors and the effects of their interactions. We will assess the bioavailability of key constituents of Echinacea supplements to fill this important gap, because bioavailability likely plays a key role in translating the results of bioassays to potential human health effects.
Footnotes
Presented at the workshop “The Science of Botanical Supplements for Human Health,” held at Experimental Biology 2007, Washington, DC, 28 April 2007.
Supported by grant number P01 ES012020 from the National Institute of Environmental Health Sciences (NIEHS) and the Office of Dietary Supplements (ODS) and by grant 95P50AT004155 from the National Center of Complementary and Alternative Medicine (NCCAM) and ODS, NIH. The contents are the responsibility of the authors and do not necessarily represent the views of the funding agency.
The contributions of the authors were as follows—DFB: directs the Center and supervised the research of CAL; MPW: co-leads the Germplasm and Phytochemistry Core; CAL: conducted the studies reported in Figures 4 and 5; LW: conducted the studies reported in Figure 6; JB: synthesized alkamides; AKSS: conducted fractionation of Echinacea; GAK: supervised the research of JB; PAM: supervised the research of AKSS; ESW: supervised the research of LW; QL: instructed the Wurtele laboratory in the oocyte TRVP1 studies; SCH: consulted with the Wurtele laboratory in the interpretation of the oocyte TRVP1 studies; WJM: supervised the research of JPP; JPP: conducted the studies reported in Figures 1, 2, and 3.
DFB served on the National Toxicology Program (NIEHS) Board of Scientific Counselors during most of the time that this research was being conducted. The remaining authors had no financial or personal interests in any company or organization sponsoring the research, including advisory board affiliations.
References
- 1.Widrlechner MP, McKeown KA. Assembling and characterizing a comprehensive Echinacea germplasm collection. In: Janick J, Whipkey A, editors. Trends in new crops and new uses. Alexandria, VA: ASHS Press; 2002. pp. 506–8. [Google Scholar]
- 2.Qu L, Wang X, Chen Y, et al. Commercial seed lots exhibit reduced seed dormancy in comparison to wild seed lots of Echinacea purpurea. Hort-Science. 2005;40:1843–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Romero FR, Delate K, Hannapel DJ. The effect of seed source, light during germination, and cold-moist stratification on seed germination in three species of Echinacea for organic production. HortScience. 2005;40:1751–4. [PMC free article] [PubMed] [Google Scholar]
- 4.US Department of Agriculture. Germplasm Resources Information Network database. [accessed 3 May 2007]; Internet: http://www.arsgrin.gov/npgs.
- 5.Turner RB, Bauer R, Woelkart K, Hulsey TC, Gangemi JD. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. N Engl J Med. 2005;353:341–8. doi: 10.1056/NEJMoa044441. [DOI] [PubMed] [Google Scholar]
- 6.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–64. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson WE., Jr L-chicoric acid, an inhibitor of human immunodeficiency virus type 1 (HIV-1) integrase, improves on the in vitro anti-HIV-1 effect of Zidovudine plus a protease inhibitor (AG1350) Antiviral Res. 1998;39:101–11. doi: 10.1016/s0166-3542(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 8.LaLone CA, Hammer KDP, Wu L, et al. Echinacea species and alkamides inhibit prostaglandin E2, production in RAW264.7 mouse macrophage cells. J Agric Food Chem. 2007;55:7314–22. doi: 10.1021/jf063711a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merali S, Binns S, Paulin LM, et al. Antifungal and antiinflammatory activity of the genus Echinacea. Pharm Biol. 2003;41:412–20. [Google Scholar]
- 10.Zygmunt PM, Petersson J, Andersson DA, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–7. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 11.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 12.Trevisani M, Smart D, Gunthorpe MJ, et al. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–51. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Kaminski NE, Wang DH. Anandamide-induced depressor effect in spontaneously hypertensive rats: role of the vanilloid receptor. Hypertension. 2003;41:757–62. doi: 10.1161/01.HYP.0000051641.58674.F7. [DOI] [PubMed] [Google Scholar]
- 14.Bauer R. Echinacea: biological effects and active principles. In: Lawson LD, Bauer R, editors. Phytomedicines of Europe: chemistry and biological activity. American Chemical Society Symposium Series 691. Washington, DC: ACS; 1998. pp. 140–57. [Google Scholar]
- 15.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–24. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]



