Abstract
The Drosophila esc-like gene (escl) encodes a protein very similar to ESC. Like ESC, ESCL binds directly to the E(Z) histone methyltransferase via its WD region. In contrast to ESC, which is present at highest levels during embryogenesis and low levels thereafter, ESCL is continuously present throughout development and in adults. ESC/E(Z) complexes are present at high levels mainly during embryogenesis but ESCL/E(Z) complexes are found throughout development. While depletion of either ESCL or ESC by RNAi in S2 and Kc cells has little effect on E(Z)-mediated methylation of histone H3 lysine 27 (H3K27), simultaneous depletion of ESCL and ESC results in loss of di- and trimethyl-H3K27, indicating that either ESC or ESCL is necessary and sufficient for di- and trimethylation of H3K27 in vivo. While E(Z) complexes in S2 cells contain predominantly ESC, in ESC-depleted S2 cells, ESCL levels rise dramatically and ESCL replaces ESC in E(Z) complexes. A mutation in escl that produces very little protein is viable and exhibits no phenotypes, but strongly enhances esc mutant phenotypes, suggesting they have similar functions. esc escl double homozygotes die at the end of the larval period, indicating that the well-known “maternal rescue” of esc homozygotes requires ESCL. Furthermore, maternal and zygotic over-expression of escl fully rescues the lethality of esc null mutant embryos that contain no ESC protein, indicating that ESCL can substitute fully for ESC in vivo. These data thus indicate that ESC and ESCL play similar if not identical functions in E(Z) complexes in vivo. Despite this, when esc is expressed normally, escl appears to be entirely dispensable, at least for development into morphologically normal fertile adults. Furthermore, the larval lethality of esc escl double mutants, together with the lack of phenotypes in the escl mutant further suggests that in wild type (esc+) animals it is the post-embryonic expression of esc, not escl, that is important for development of normal adults. Thus escl appears to function in a back-up capacity during development that becomes important only when normal esc expression is compromised.
Keywords: ESC-like, ESC, E(Z), histone methylation, Polycomb silencing
Introduction
The Drosophila Polycomb Group (PcG) proteins are required for heritable silencing of the homeotic genes and many others. PcG proteins form a number of distinct complexes. The ESC/E(Z) complex, also known as Polycomb Repressive Complex 2 (PRC2), methylates histone H3 on lysine 27 (H3K27) and contains the histone methyltransferase E(Z), the histone H3 binding protein ESC (Tie et al., 2007) and SU(Z)12. The PRC1 complex contains the PcG proteins PC, PH, PSC and RING, an E3 ubiquitin ligase that mono-ubiquitinates lysine 119 of histone H2A (Wang et al., 2004). Its PC subunit binds the trimethylated H3K27 (3mH3K27) sites created by E(Z). PcG complexes and their associated enzymatic activities are required continuously to maintain silencing.
ESC originally appeared to be unique among PcG proteins in being required predominantly during early embryogenesis. Temperature-shift experiments with a temperature-sensitive esc allele, suggested that esc is required only during early embryogenesis (Struhl and Brower, 1982) and similar experiments with a heat-inducible hsp70-esc transgene also suggested that early esc expression is sufficient to promote normal development (Simon et al., 1995). This early requirement for esc is reflected in its temporal expression profile: esc mRNA is most abundant in early embryos, peaking at 8 hours (Gutjahr et al., 1995; Sathe and Harte, 1995), and subsequently declines to almost undetectable levels by the end of embryogenesis. Similarly, the ESC protein is present at high levels during the first half of embryogenesis, peaking at mid-embryogenesis and declining to barely detectable levels by first instar (Simon et al., 1995). In contrast, the sole mammalian ESC ortholog, EED, appears to be expressed and required continuously (Schumacher et al., 1996).
The temporal profile of esc expression suggested that ESC might be specifically required only for the establishment but not the subsequent maintenance of Polycomb silencing. E(Z), however, is required continuously throughout development (Beuchle et al., 2001), and recent biochemical studies demonstrate that ESC is required for E(Z) HMTase activity both in vitro (Czermin et al., 2002; Nekrasov et al., 2005) and in vivo, at least during embryogenesis (Ketel et al., 2005). This suggested that this essential function of ESC may be carried out by another protein after ESC levels drop. One obvious possibility was that such a protein would be similar to ESC itself. When the complete sequence of the Drosophila melanogaster genome became available, we conducted a BLASTP search using the ESC protein sequence as a query and identified a single predicted protein with a high degree of sequence similarity to ESC. This protein is encoded by the CG5202 gene, which we have renamed extra sex combs-like (escl). While this work was in progress, Wang et al. demonstrated that recombinant ESCL can substitute for ESC in reconstituted PRC2 complexes in an in vitro histone H3K27 methylation assay, indicating that their biochemical functions are similar if not identical (Wang et al., 2006).
We report here that ESCL protein exhibits a temporal expression profile that is complementary to that of ESC, including substantially higher levels of expression during larval and adult stages than embryogenesis. We show that ESCL, like ESC, binds directly to E(Z) via its WD-repeats and is physically associated with E(Z), SU(Z)12, PCL and p55 in vivo. While RNAi-mediated knock-down of either ESC or ESCL in S2 cells has no appreciable effect on histone H3K27 methylation, simultaneous knock down of both strongly reduces tri-methyl and di-methyl (3m and 2m) H3K27 levels. Some ESCL is bound to the Ubx PRE in Drosophila Kc cells and this binding is increased when ESC is depleted by RNAi. A strong escl mutation is viable and fertile, but enhances the phenotypes of PcG mutants, consistent with a role in Polycomb silencing. Genetic analysis reveals that the well-known “maternal rescue” of esc− embryos to viable adults requires ESCL. GAL4-driven constitutive maternal and zygotic ESCL expression can fully substitute for ESC in vivo, indicating that their functions are qualitatively indistinguishable if not identical. We discuss why two apparently functionally equivalent proteins have been retained during Drosophila evolution.
Materials and methods
Constructs
A full-length escl cDNA, SD11903, was obtained from the Drosophila Genomics Resource Center. All escl constructs were generated from this cDNA by PCR using primers containing the appropriate restriction sites on either end for subcloning. PCR products were inserted into pGEM-T vector (Promega) and the sequences of all constructs were confirmed. Coding fragments cut from pGEM-ESCL were inserted into pGEX-2T and pET-15b to generate GST fusions and His-tagged constructs.
Antibodies
Guinea pig polyclonal anti-ESCL antibodies were raised against a GST-ESCL fusion protein containing the N-terminal 95 residues preceding the WD motifs (Tie et al. 2007), a region with limited identity to ESC. The specificities of anti-ESCL and anti-ESC antibodies (Tie et al., 2005) were confirmed by Western analyses of ESCL- or ESC-depleted cells (see Fig. 5 A and C). Other anti-PcG protein antibodies, including anti-E(Z), anti-SU(Z)12, anti-PCL were described previously (Tie et al., 2001; Tie et al., 2003). Anti-histone antibodies were described previously (Tie et al. 2007).
Fig. 5.

The requirement of ESC or ESCL in di- and trimethylation of H3K27. (A and B) Whole S2 cells were analyzed by Western after 7-day RNAi to knockdown E(Z) (lane 3), ESC (0.70-kb dsRNA in lanes 4 and 7, 0.49-kb dsRNA in lanes 5 and 8), ESCL (lane 6), both ESC and ESCL (lanes 7 and 8). RNAi knockdown of a non-PcG protein MTM1 (lane 2) as well as wild-type S2 cells (lane 1) served as control. Western blots were shown in A for target (E(Z), ESC, ESCL, SU(Z)12) proteins (2.5x105 cells/lane) and in B for methylated histone H3 (5x105 cells/lane). Whole histone H3 was detected with goat anti-H3 antibodies after stripping signals by 0.1 M NaOH solution from previous Western using rabbit antibodies. Tubulin at bottom panel of A and histone H3 at bottom panel of B severed as loading control. (C and D) Western analysis of whole Kc cells as same as A and B. A non-specific band appeared above E(Z) band in whole cell (top penal in A and C) was indicated by an asterisk.
Extracts preparation and fractionation
Embryo nuclear extracts and whole embryo extracts were prepared as previously described (Tie et al., 2001). To prepare whole extracts from developmental stages of Drosophila, different stages of embryos were homogenized at 2.0 mL per gram by douncing in extraction buffer (40 mM HEPES, pH 7.4, 0.35 M NaCl, 10% glycerol, 0.1% Tween-20 plus protease inhibitor cocktail tablet (Roche) and phosphatase inhibitors: 5 mM KF, 1 mM sodium pyrophosphate, 1 mM glycerophosphate, 0.2 mM sodium molybdate, 0.2 mM sodium orthovanadate. Larvae and adult flies were pulverized under liquid nitrogen and added to the same buffer and centrifuged. Normal S2 cells and ESC- or ESCL-depleted S2 cells were homogenized at a ratio of 1x 108 cells/mL in extraction buffer (50 mM HEPES (pH7.6),110 mM KCl, 250 mM NaCl, 10% glycerol, 1mM DTT, 0.1 mM EDTA) containing protease inhibitors and phosphatase inhibitors (Tie et al, 2001). Cell extracts were centrifuged at 30,000xg for 1 hour at 4°C. Extracts were fractionated on a Superose 6 column as previous described (Tie et al., 2001; Tie et al., 2003).
Immunostaining
Embryos were stained with anti-ESCL antibodies as previously described (Furuyama et al., 2003; Tie et al., 1998). Imaginal discs were dissected from wandering third instar larvae raised in uncrowded bottles. Discs were fixed in 4% formaldehyde in PBS for 2 hours at 4°C, washed in PBS, and either mounted in aqueous mounting solution (Sigma) and visualized by light microscopy or used in antibody staining. For antibody staining fixed discs were blocked with 10% normal goat serum, incubated in the appropriate primary antibody in PBS overnight at 4°C, washed, then incubated with a 1:2,000 dilution of FITC-conjugated anti-guinea pig antibody (Vector, Burlingame, CA) and 100 μg/ml of RNase A overnight at 4°C. After extensive washing, a 1:200 dilution of propidium iodide (Oncor, Gaithersburg, MD) was added to the last wash to counterstain the chromosomes. Discs were mounted in antifade (Oncor) and images were scanned using a Bio-Rad (Hercules, CA) MRC-600 confocal imaging system.
GST pull-down assays and co-immunoprecipitation
GST pull-down assays, using extracts and in vitro translated proteins, and immunoprecipitation of proteins from extracts with anti-ESCL, anti-E(Z), and other antibodies were performed as described previously (Tie et al., 1998; Tie et al., 2001; Tie et al., 2003).
Cell culture and double-stranded RNA interference (RNAi)
Drosophila S2 cells (derived from late embryos) and Kc167 cells (derived from 6-12 hour embryos) were obtained from the Drosophila Genomics Resource Center and cultured at 27°C in Schneider’s medium (Invitrogen) supplemented with 10% (for S2) or 5% (for Kc) fetal bovine serum (FBS) (Invitrogen). Double-stranded RNAs (dsRNAs) were generated in vitro and RNAi was performed in Drosophila cells as previously described (Tie et al., 2007). For RNAi, Drosophila S2 or Kc cells were treated with 15 μg/ml of dsRNA for 7 days, with fresh dsRNA and media added every other day. To deplete ESC and ESCL, a 490 bp dsRNA extending from the start codon of esc and a 619 bp dsRNA extending from the start codon of escl were used for RNAi respectively. A 700-bp esc dsRNA (extending from the start codon), which contains a 20 bp stretch of sequence that is identical in escl (for secondary target or off-target), was used for complete knockdown of ESC and partial knockdown of ESCL (lane 4 of Fig. 5 A and C). A 700-bp E(z) dsRNA (extending from the start codon) was used as a positive control, and a dsRNA for the non-PcG gene mtm1 was used as a negative control. Whole cell extracts were prepared in 40 mM Tris (pH7.5), 8.0 M urea and analyzed by Western blot.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was conducted with the anti-ESCL antibody as described previously (Tie et al., 2007). PCR reactions amplified a 150-bp fragment of the Ubx core PRE. Primer sequences are available upon request.
escld01514 mutation and other genotypes used
The P element line P{XP}escld01514 contains an insertion of the 7.3 kb XP element 5’ of the second codon of escl (orientation: 3’ P{XP} 5’ – 5’ escl 3’) (Thibault et al., 2004). The precise location of the insertion was confirmed by sequencing PCR products from both ends of the insertion. As expected, an 8 bp duplication of escl coding sequence is present at both ends of the insertion site. The insertion lies 5’ of codon 2 of escl, separating the escl 5’ regulatory sequence and initiation codon from the rest of the escl coding sequence; however the last three bases of the XP element (ATG) restore an intact escl protein coding sequence and the sequence surrounding this ATG appear to be suitable for translation initiation (Cavener and Ray, 1991). Homozygotes are viable and fertile and exhibit no obvious morphological abnormalities but have greatly reduced escl RNA and protein levels (see Results). Hemizygotes (over three different deletions) are phenotypically indistinguishable from homozygotes, suggesting that it is at least a strong hypomorpic allele if not a null allele.
Other genotypes used in this study:
Df(2L)Prl, nub / CyO (32F1; 33F, removes both esc and escl genes)
Df(2L)esc-P3-0 / Cy, amosRoi (33A1; 33E, ~710 kb deletion that removes both esc and escl)
Df(2L)esc-P2-0 / Cy, amosRoi (33A1; 33B1-2, ~280 kb deletion that removes esc but not escl)
Df(2L)6031 / CyO (33B2; 33C2, 99 kb deletion (Exelixis) that removes escl but not esc)
y w ; P{αtub-GAL4}LL7 / TM3 Sb Ser.
w ; esc6 / CyO, esc2 ; αFe54-5 (FLAG-esc transgene under control of α tubulin promoter) (Furuyama et al., 2003)
y w ; esc4 / CyO
y w ; esc6 / CyO
y w; esc5 cn / CyO
esc9 pr / CyO
esc10 cn b / CyO
In(2LR)Gla / CyO, esc2
esc19 / CyO
y w ; P{w+ mat α-4 GAL-VP16}V37
w1118 ; Pc3 / TM3 Sb
E(z)60 / TM3 Sb.
Generation of the esc6 escld01514 recombinant chromosome
To examine the effects of removing both ESCL and ESC proteins we generated a recombinant chromosome containing the null esc6 mutation (Gutjahr et al., 1995; Struhl, 1981; Struhl, 1983) and escld01514. Females of the genotype w1118 ; escld01514 {w+} / CyO were mated to w1118 ; esc6 / CyO males and their w1118 ; esc6 + / + escld01514 {w+} female progeny were crossed to w1118 ; Sco / CyO males (the escld01514 XP insertion carries the dominant w+ marker). Potential recombinants were recovered as w+ Cy male progeny (w1118 ; (esc6 ?) escld01514 {w+} / CyO) and tested for the presence of esc6 by crossing them to Df(2L)escP2-0 / CyO, amosRoi females and scoring the non-Cy progeny for extra sex combs phenotypes, which were enhanced by the presence of escld01514. Recombinant chromosomes were recovered in the CyO, amosRoi sibs. esc6 escld01514 homozygotes are lethal, but this lethality can be rescued by an esc+ transgene, indicating that there are no other lethals present on the recombinant chromosomes. Consistent with this, esc6 escld01514 chromosomes are also viable over the original esc6 and escld01514 parental chromosomes used to generate them. Recombinant chromosomes were also maintained over a CyO, GFP balancer chromosome that allowed positive identification of esc6 escld01514 double homozygous embryos and larvae.
GAL4-induced expression of ESCL to rescue esc mutant phenotypes
Since the XP element inserted into escld01514 restores the wild type coding sequence, it can be used to induce escl expression/overexpression from its GAL4-regulated hsp70 minimal promoter when combined with a GAL4 driver. To test whether ESCL can completely replace ESC, the esc6 escld01514 recombinant chromosome was combined with maternal and zygotic GAL4 drivers to test whether constitutive expression of escl could rescue esc mutant phenotypes, including adult phenotypes of “maternally rescued” esc homozygotes and the embryonic lethality of esc6 embryos lacking both maternal and zygotic ESC (see below). A zygotically expressed α tubulin(84B)-GAL4 driver (“αtub-GAL4”) (stock: y w ; P{tubP-GAL4}LL7 / TM3, Sb) and a maternally expressed α tubulin(67C)-GAL4-VP16 driver (“αmat-GAL4”) (stock: w ; P{w+ matα4-GAL-VP16}V37) were used singly and in combination for this purpose. To test for rescue of embryos lacking both maternal and zygotic ESC, esc6 escld01514 / CyO; αtub-GAL4 / TM3 Sb females were crossed to esc6 escld01514 / CyO; αmat-GAL4 / TM3 Sb males to generate esc6 escld01514 / esc6 escld01514; αtub-GAL4 / αmat-GAL4 progeny, which were then crossed to each other. These females produce viable offspring. Of 50 esc6 escld01514 / esc6 escld01514; αtub-GAL4 / αmat-GAL4 progeny of this cross examined, all but two were indistinguishable from wild type. One had a partial wing to haltere transformation and one had spots of pigmentation in abdominal segment A4 similar to a mild Mcp phenotype.
Scoring scheme for evaluating enhancement of PcG phenotypes by escld01514
The escld01514 mutation was tested for its ability to enhance of low penetrance dominant Polycomb phenotypes in Pc3 and esc alleles. To do so, multiple phenotypes were scored for a total of 20 possible points for males. Each second thoracic (T2) and third thoracic (T3) leg was scored for severity of the extra sex comb phenotype: 1 = 1-4 bristles, 2 = 5-9 bristles, 3 = full sex comb. One point was given for each of the following transformations: presence of transverse row bristle(s) on T2 or T3, missing mesoplural bristles, reduced apical bristles on T2, reduction of sternoplural region (loss of bristles), transformations of T2 coxa to T1, A5 to A4, antenna to leg, and wing to haltere. The total points for all males scored was divided by the number of males scored to obtain a mean score for each genotype. Females were also scored for all but the extra sex comb and abdominal transformations (data not shown). All crosses were done reciprocally and no differences were observed in the severity of phenotypes scored in progeny of reciprocal crosses. In calculating the percent survival of a mutant genotype (observed/expected x 100) the expected number was the average of the number of adults in each of the two or three other viable sibling genotype classes produced from the same cross.
RT-PCR
To determine the effect of the escld01514 XP insertion on escl mRNA levels, RNA was extracted from wild type and homozygous escld01514 embryos using Trizol reagent according to the manufacturer’s protocol. Reverse Transcriptase reactions were carried out using total RNA and random primers (Promega) and the resulting cDNA was used as template for PCR with primers intron-spanning for escl, engrailed, and CG16969, the gene located adjacent to the escl 5’ end (and on the other side of the XP insertion present in escld01514).
Results
Identification of ESCL, a paralog of ESC
The escl gene is located on chromosome 2L, 160 kb proximal to esc. It contains two introns, located within the coding sequence, whose positions correspond precisely to the positions of the second and third introns of esc. The intron-exon structures of esc and escl are conserved in all other sequenced Drosophila species, strongly suggesting that the two genes arose from a common ancestor by gene duplication.
The ESC and ESCL proteins are highly similar. Both comprised of 7 WD motifs, predicted to fold into a β-propeller structure, preceded by a short N-terminal region. The regions containing their WD motifs are 63% identical (78% similar) and can be aligned with a single gap, an insertion of six residues between the first two WD motifs in ESCL. ESCL also contains an N-terminal extension of 33 residues not present in ESC. The rest of their N-termini (~60 residues), while less conserved than their WD motifs, share several regions of similar sequence composition, including an acidic region, a serine-/ threonine-rich region, and a basic region (see Supplemental Fig. S1). This region is conserved, to varying degrees, in the ESC and ESCL orthologs in all sequenced Drosophila species and in their single vertebrate ortholog EED, consistent with its conserved binding to histone H3 (Tie et al, 2007). Their high degree of protein sequence similarity, particularly in the WD motifs, suggests that ESCL and ESC have similar functions. It was recently demonstrated that recombinant ESCL, like ESC, can be assembled into complexes in vitro with recombinant E(Z), SU(Z)12, and p55 and that these complexes can methylate K27 of histone H3 (H3K27) in vitro (Wang et al., 2006). This suggests that any differences in their biological functions in vivo are likely be due to features other than the shared substrate specificity of complexes containing either one.
ESCL is expressed ubiquitously throughout development
Western analyses using polyclonal antibodies that specifically recognize ESCL and ESC (see Materials and Methods) revealed that, unlike ESC, ESCL is detectable at all developmental stages from embryo to adult (Fig. 1). While both ESC and ESCL are present throughout embryogenesis, ESCL levels are much lower during embryogenesis than they are in larval and pupal stages, while ESC is only detected at very low levels in first and second instar larvae (Fig.1, lanes 6 and 7). In adults, ESC is detected only in females, consistent with previous observations, while ESCL is readily detectable in both males and females (Fig. 1, lanes 10 and 11). Staining of embryos and larval tissues with ESCL antibodies revealed that ESCL is expressed ubiquitously throughout development and is present in all larval tissues examined. Representative embryo, salivary gland and eye disc are shown in Fig. 2. Like ESC, a substantial amount of the ESCL detected in embryos is nuclear and co-localizes with DNA (data not shown).
Fig. 1.

ESCL is expressed in all developmental stages. Western analyses of Drosophila samples in different developmental stages with anti-ESCL, ESC and E(Z) antibodies. α-Tubulin at bottom panel serves as a loading control.
Fig. 2.

ESCL is ubiquitously expressed and associates with chromosome. Immunostaining of embryos (top panel), eye imaginal discs (2nd panel) and salivary gland (3rd panel) with anti-ESCL antibodies (left). DNA was stained by DAPI in red (middle). Yellow color in merged images (right) indicates co-localization of ESCL and chromosome. Immunostaining of salivary gland without primary antibodies (bottom) serves as control.
ESCL binds to E(Z) and is physically associated with E(Z) complex components in vivo
We previously showed that the highly conserved WD region of ESC (residues 61-425) mediates its direct binding to the N-terminus of E(Z) (Tie et al., 1998). A GST-E(Z) fusion protein containing the N-terminal 73 residues of E(Z), the same region that binds ESC, also pulled down in vitro translated ESCL (Fig. 3A, lane 3 in bottom panel). Conversely, a GST-ESCL fusion protein containing just the ESCL WD region (residues 95-462), pulled down in vitro translated E(Z) (Fig. 3A, lane 7), just as GST-ESC61-425 (WD region) does (Fig. 3A, lane 6), indicating that ESCL also binds to E(Z). GST-E(Z)1-73 also pulled down both ESC and ESCL from Kc cell extracts (Fig. 3B, lane 3).
Fig. 3.

ESCL directly binds to E(Z) and physically associates with ESC/E(Z) complex components. (A) In vitro translated proteins (35S-labeled) were pulled down by GST fusion protein (lane 3, 6, 7) or by GST alone (lane 2, 5, as negative control). (B) GST pull-down from Kc cell extracts. (C, D and E). Co-immunopricipitation (co-IP) from Kc cell extracts in C, from embryo nuclear extracts (NE) in D and from third instar larval extracts (LE) in E. Co-IPs using pre-immune guinea pig serum (lane 2 in C, D and E) and rabbit serum (lane 5 of D and E) serve as negative control.
To test whether ESCL is physically associated with other components of E(Z) complexes in vivo we performed co-immunoprecipitation (co-IP) experiments from embryo nuclear extracts and Kc cell extracts. The association of E(Z) and SU(Z)12 with ESCL was confirmed by co-IP of both proteins from Kc cell extracts with anti-ESCL antibodies (Fig. 3C). Anti-ESCL antibodies also immunoprecipitated E(Z), SU(Z)12, p55, RPD3, and also ESC from embryo nuclear extracts (Fig. 3D, lane 3). Reciprocal co-IP experiments show that anti-E(Z) and anti-PCL antibodies also immunoprecipitated ESCL from embryo nuclear extracts (Fig. 3D, lane 6 and 7). Co-IP assays using third instar larval extracts revealed that E(Z) was immunoprecipitated by anti-ESCL antibodies and that ESCL was immunoprecipitated by anti-E(Z) antibodies (lane 3 and lane 6 of Fig 3E). These results, together with the expression profiles of ESC and ESCL, indicate that ESCL is physically associated with components of PRC2 complexes in embryos and during postembryonic stages, when ESC is no longer detectable. They also suggest that ESC and ESCL may be present together in at least some complexes during embryogenesis.
To further confirm the association of ESCL with E(Z) complexes, we fractionated extracts from Kc cells (which are derived from 6-12 hour embryos), nuclear extracts from 16-24 hour embryos, and third instar larval extracts on a Superose 6 size exclusion column. As shown in Fig. 4, the ESCL in Kc cells elutes with E(Z) and SU(Z)12 in fractions 14-17 and free ESCL elutes in fraction 22. The ESCL elution profile (fractions 14-18) overlaps that of ESC but is not identical. A longer exposure of Western blots of Kc cell fractions reveals that ESCL co-fractionates with E(Z) in fractions 11-18 and co-fractionates with PCL in fractions 11-13, suggesting that ESCL is present in a large (~1 MDa) PCL/E(Z) complex and a smaller (~600 kDa) E(Z) complex, comparable to similar ESC-containing complexes present in embryos (Tie et al, 2003). Further confirmation of an ESCL/E(Z)/PCL complex in fractions 11-13 (~1 MDa) is provided by depletion of PCL in Kc cells, which eliminated ESCL from fractions 11-13, but not from fractions 14-18 (~600 kDa complex) (see profiles of E(Z) and ESCL with a long exposure in RNAi Kc cells in Fig. 4). ESCL is readily detectable in third instar larval extracts (Fig. 1, lane 8), when ESC is no longer detectable, and co-fractionates with E(Z) and SU(Z)12 in fractions 12-17, comparable to the ESC/E(Z) and ESCL/E(Z) complexes present in late (16-24 hour) embryo extracts. This strongly suggests that ESCL largely replaces ESC in larval E(Z) complexes, when ESC is no longer detectable.
Fig. 4.
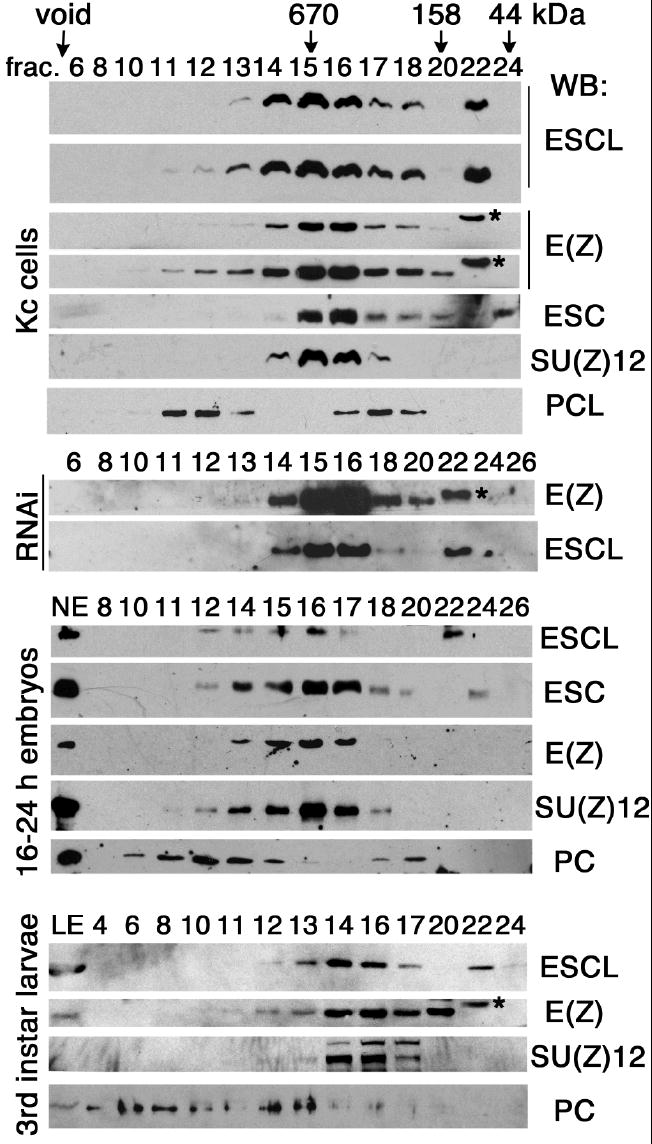
ESCL co-fractionates with E(Z) in different stages. Fractionation of Kc cell extracts (top), PCL-depleted Kc cell extracts (middle panels indicated by RNAi), nuclear extracts (NE), from 16-24 hour embryos, at middle panels, and third instar larval extracts (bottom) were carried out on a Superose 6 column. Fraction number is indicated at top of each lane. Proteins in fractions were analyzed by Western blots (WB). ESCL and E(Z) were detected with regular and long exposure by WB in Kc cell extracts (top), showing identical profile in fraction 11-18. Note free ESCL in fraction 22 and free ESC in fraction 24. A non-specific band appeared above E(Z) in fraction 22 is marked by an asterisk (also see E(Z) in Fig. 5 A and C). Fractionation of PC-depleted Kc cell extracts, which served as a control and has no effect on PCL/ESCL/E(Z) complex in fraction 11-13, is not shown.
Either ESC or ESCL is necessary and sufficient for methylation of H3K27 in vivo
ESC is required for methylation of nucleosomal H3 by purified ESC/E(Z) complex in vitro (Czermin et al., 2002; Nekrasov et al., 2005). Consistent with this, 2mH3K27 and 3mH3K27 levels are greatly reduced in embryo extracts from an esc null mutant (Ketel et al., 2005). It was recently reported that recombinant E(Z) complexes containing either ESC or ESCL can methylate H3K27 in vitro (Wang et al., 2006). This study did not distinguish between mono-(1m), di- and trimethylated isoforms of H3K27, so it is not known whether ESC- and ESCL-containing complexes might differ in their ability to produce different methyl-H3K27 isoforms. To assess whether ESCL plays a similar role to ESC in E(Z)-dependent HMTase activity in vivo, we used RNAi to knock down ESC and ESCL either individually or simultaneously. Drosophila S2 cells and Kc cells, which contain both proteins, were subjected to RNAi treatment and then examined for depletion of these proteins and the effects on H3K27 methylation.
After 7-day dsRNA treatment, targeted proteins were analyzed by Western blots to confirm depletion of each by RNAi (Fig. 5 A and C) (see Supplementary data for discussion of effects of depletion of E(Z), ESC and ESCL on other PRC2 proteins). We then examined the effects of protein depletion on the methylation of H3K27 (Fig 5 B and D). Total histone H3 (bottom panel) and its mono-, di-, and tri-methylated isoforms were detected by Western in untreated and control RNAi treated (mtm1 dsRNA) S2 cells (lanes 1 and 2).
In E(Z)-depleted S2 cells (lane 3), 1m-, 2m- and 3mH3K27 were reduced or eliminated (compared to lanes 1 and 2), but levels of dimethylated H3K4 and H3K9 (2mH3K4, 2mH3K9) were unchanged, consistent with E(Z) being responsible for most if not all methylation of H3K27 in S2 cells, and consistent with results from immunostaining of polytene chromosomes from an E(z) null mutant (Ebert et al., 2004). Although it was substantially reduced, 1mH3K27 was always detectable after knockdown of E(Z) (lane 3, Fig 5B). Montgomery et al. (Montgomery et al., 2005) also observed residual 1mH3K27 in eed mutant mouse cells. It is possible that 1mH3K27 might require only low levels of E(Z) that are not completely eliminated by RNAi treatment. We also cannot exclude the possibility that in addition to E(Z), another HMTase may be responsible for synthesis of some 1mH3K27. Interestingly, mouse ES cells that are mutant for the histone methyltransferase Suv39 have been reported to lose 1mH3K27 staining on heterochromatin (Peters et al., 2003).
In contrast to E(Z) knockdown, knockdown of ESC alone or ESCL alone (Fig 5B, lanes 5 and 6), had little or no effect on 2m- and 3mH3K27 levels. However, after simultaneous knockdown of both ESC and ESCL 2m- and 3mH3K27 were barely detectable (lanes 7 and 8) while 1mH3K27 was readily detected, and E(Z) and SU(Z)12 remained detectable. These results indicate that the presence of either ESC or ESCL is sufficient for apparently normal levels of 2m- and 3mH3K27 in bulk chromatin of S2 cells. To confirm that this apparent functional redundancy of ESC and ESCL is not specific to the S2 cells we used, we also knocked down ESC and ESCL in the Drosophila Kc167 cell line (which is derived from 6-12 hour embryos). As shown in Fig. 5C and D, similar effects on H3 K27 methylation were observed upon protein depletion.
In a previous study we found that the 3mH3K27 level was more sensitive than the 2mH3K27 or 1mH3K27 levels to knockdown of ESCL and ESC by RNAi (Tie et al., 2007). Consistent with this, depletion of ESC and partial depletion of ESCL by treatment of S2 and Kc cells with the 700 bp esc dsRNA, which also reduced E(Z) and SU(Z)12 levels (lane 4 in Fig. 5 A and C), resulted in the loss of 3mH3K27 (lane 4 in panel 3 of Fig. 5 B and D) but little change on 1m- or 2mH3K27. Interestingly, in both S2 and Kc cells, depletion of ESC slightly reduced the 3mH3K27 level while depletion of ESCL did not, (compare lanes 5 to lane 6, panel 3 of Fig. 5 B and D). This occurred despite the fact that the ESCL level increases substantially when ESC is depleted (see below). While differences in efficiency of knockdown might contribute to this difference, it may also indicate that more ESCL is required to produce the same level of 3mH3K27 achieved with less ESC, i.e., ESC-containing complexes may be more efficient at tri-methylation than ESCL-containing complexes. A possible basis for this is suggested by our previous observation that the N-termini of both ESC and ESCL bind to histone H3, but ESCL binds somewhat more weakly (Tie et al. 2007). Since H3 binding by ESC is required for trimethylation of H3K27 by E(Z) in vivo (Tie et al. 2007), weaker H3 binding by ESCL could reduce the HMTase activity of ESCL-containing complexes relative to ESC/ESCL complexes.
ESCL replaces ESC in ESC/E(Z) complexes in ESC-depleted S2 cells
The level of ESCL protein was markedly elevated in S2 cells treated with the 490 bp esc dsRNA (see ESCL in Fig. 5A, lane 5 and Fig. 6A, lane 2). We examined the 600 kDa E(Z) complexes in these RNAi-treated and untreated S2 cells. Fractionation of untreated S2 cell extracts on a size exclusion column revealed that approximately 60% of the detectable ESC protein is present in the 600 kDa E(Z) complex (Fig. 6B, panel e, fractions 15-16), while most of the ESCL is present in free form (panel b, fraction 22 in Fig. 6B) and only a small portion (< 10%) is detectable in the 600 kDa E(Z) complex (fractions 15-16). This suggests that the 600 kDa complexes contain predominantly ESC when both proteins are present in S2 cells. However, in extracts from ESC-depleted S2 cells, most of the detectable ESCL protein is present in the 600 kDa complex (see ESCL in panel c and E(Z) in panel d, fractions 14-20, Fig. 6B). This suggests that the elevated steady state level of ESCL in these cells is likely due to its increased stability when incorporated into E(Z) complexes. In contrast, depletion of ESCL barely affected the fraction of ESC present in the 600 kD complex (see ESC in panel e and f, Fig. 6B, fractions 15-16), presumably due to the very limited proportion of total ESCL normally present in E(Z) complexes in untreated cells (Fig. 6B, panel b, fractions 15-16). These data strongly suggest that ESCL replaces ESC in the 600 kDa E(Z) complex in vivo when ESC is absent or present at low levels. Conversely, they also suggest that ESC out-competes ESCL for assembly into E(Z) complexes when both proteins are present, such as during embryogenesis, when ESC is most abundant.
Fig. 6.
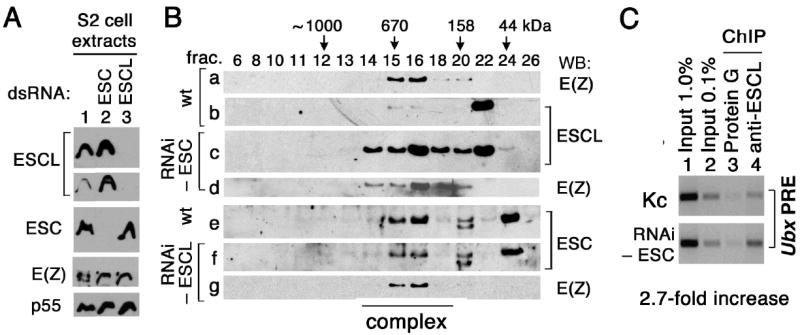
ESCL replaces ESC in the 600-kDa complex in ESC-depleted cells. (A) Western analyses of extracts from normal (lane 1), and ESC-, ESCL-depleted S2 cells (lanes 2 and 3). (B) Cell extracts were fractionated on a Superose 6 column and analyzed by Western blots. Fraction numbers and their correlated size were labeled on the top. (C) ESCL binding to Ubx PRE is enhanced in ESC-depleted Kc cells. Chromatin immunoprecipitation (ChIP) was carried out with or without anti-ESCL antibody in normal or ESC-depleted Kc cells. The Ubx PRE (150 bp) was detected by PCR in a linear range. Lanes 1 and 2 respectively show 1.0% and 0.1% of total DNA. ChIP efficiency (normalized to input) is ~ 2.7 fold higher in ESC ESC-depleted Kc cells.
During embryogenesis when ESC is present at its highest levels, some ESCL is also present in E(Z) complexes (Fig. 4). To determine whether these ESCL-containing complexes associate with PREs and participate in silencing of homeotic genes, we used chromatin immunoprecipitation to test whether ESCL is bound to the Ubx PRE in Kc cells, which are of embryonic origin but contain a somewhat greater proportion of ESCL in E(Z) complexes than S2 cells (compare ESCL levels in fraction 14-16, top panel of Fig. 4 to fraction 15-16, second panel of Fig. 6B). As shown in Fig. 6C, the Ubx PRE was specifically immunoprecipitated by anti-ESCL antibodies (top panel, lane 4) but not when antibody was absent (lane 3). ChIP efficiency appears low (less than 0.1% of input), possibly due to the presence of ESC-containing E(Z) complexes that compete for binding to the same PRE (Tie et al., 2007). Consistent with this possibility, in ESC-depleted Kc cells ChIP efficiency with anti-ESCL antibodies increased 2.7-fold (bottom panel, lane 4), reflecting the increased amount of ESCL present in E(Z) complexes when ESC is depleted (Fig. 6B). These data indicate that some ESCL-containing complexes are bound to the Ubx PRE in Kc cells.
An escl mutation enhances esc mutant phenotypes
The escld01514 line, generated by Exelixis (Thibault et al., 2004) contains an insertion of an XP element 5’ of codon 2 of the escl coding sequence, separating the 5’ regulatory region and initiation codon of escl from the rest of the gene. The last three bases of the P element (ATG) restore an intact escl protein coding sequence. A spliced escl RNA can be detected in homozygous escld01514 embryos by RT-PCR at approximately 7.7% of the wild type level (Fig. 7A). An ESCL protein, indistinguishable in size from the wild type protein, is present at approximately 10% of the wild type level in late embryos or adults (Fig. 7B).
Fig. 7.

ESCL expression levels in escld01514 homozygotes are reduced dramatically. (A) RNAs were measured by RT-PCR in wild-type (WT) or escld01514 / escld01514 embryos (escl / escl). Lanes 2 and 4 show RT-PCR omitted reverse transcriptase (RTase) and serve as control. Note that escl RNA level in escld01514 / escld01514 embryos is 7.7% of that in wild type. (B) Adult flies were homogenized and used for Western analysis of ESCL (top) and CYP33 (bottom, loading control). Note that the ESCL level in escld01514 / escld01514 flies (lane 2) is approximately 10% of that in wild-type (lane 1). w; escld01514 / escld01514 flies were crossed to an αtub GAL4 driver, w; P{w+ α̃tubulin GAL4}/TM3. The progeny from this cross, w; escld01514 /+; P{w+ α̃tubulin GAL4}/+ (lane3) and w; escld01514 /+; TM3/+ (lane 4) were compared to each other.
The escld01514 mutation is viable and fertile when homozygous or hemizygous (over three different deletions), and exhibits no obvious morphological phenotypes, regardless of whether the parents were homozygous or heterozygous for the mutation (or for a deletion removing escl). Hemizygotes are phenotypically indistinguishable from homozygotes, suggesting that escld01514 is at least a strong hypomorpic allele if not a null allele. The grossly wild type appearance of adult homozygotes suggests that ESCL is dispensable for development of normal adult morphology, although it remains possible that the small amount of wild type protein produced is sufficient for some level of ESCL function and that a mutation eliminating all ESCL protein might exhibit some visible phenotype.
If ESCL and ESC play similar roles in E(Z) complexes, escl mutations would be expected to enhance the phenotypes of esc mutants. Wang et al. (2006) showed that a heterozygous deletion that removes escl can enhance the phenotypes of several esc mutations, and that an escl transgene rescues this enhancement. To test the effect of the escld01514 mutation on adult esc mutant phenotypes, we constructed a recombinant chromosome containing the null esc6 and the escld01514 mutations and crossed it to different esc alleles. A substantial majority of the homozygous esc progeny of heterozygous esc females survive to adulthood, due to “maternal rescue”, and exhibit only mild Polycomb phenotypes, most frequently partial transformations of second and third thoracic segments (T2 and T3) to first thoracic (T1) segment identity, evidenced in males by the presence of partial to complete “extra” sex combs on the second and third legs (Hannah-Alava et al., 1958; Struhl 1981; Struhl 1983). When heterozygous, the escld01514 mutation strongly enhances the adult Polycomb phenotypes of all the heteroallelic esc mutant genotypes tested (see Table 1). For example, esc6 escld01514 / esc5 adult males typically exhibited strong transformations of T2 and T3 legs to T1 legs, evidenced by the presence of complete sex combs on T2 and T3 legs (see arrows in E and F, Fig. 8), transformation of T2 coxa to T1 (see arrow head in E and F, Fig. 8) as well as presence of the transverse row bristles on T2 and T3 legs in males and females (see asterisk in G and H in Fig. 8). T2 segments also displayed reduced or absent apical bristles (compare Fig. 2-8E and F, arrow head), missing mesoplural bristles (compare G to H in Fig. 8, asterisk), a reduced sternopleural region and missing sternopleural bristles (not shown), all of which are indicative of transformation towards T1 (Hannah-Alava et al., 1958). Partial transformations of wing to haltere (see arrow in A and B, Fig. 8), which were never seen in esc homozygotes, characterized by inflation, reduction in wing size with disproportionate reduction in the posterior compartment, were also observed. Pharate adults were also scored after removal from the pupal case. Antenna to leg transformations (see arrow in C and D , Fig. 8) and transformations of anterior abdominal segments to more posterior abdominal segment identities (not shown) were also observed in esc6 escld01514 / esc4 flies. Similar results were obtained when comparable genotypes were generated by crossing esc6 to deletions that remove both esc and escl, (Df(2L)esc-P3-0 and Df(2L)Prl nub) (Table 1), consistent with Wang et al. (2006).
Table 1.
Effects of loss and overexpression of escl on esc phenotypes
| Genotype | Mean Score† | St. Dev. | N | obs. / exp. ‡ |
|---|---|---|---|---|
| esc4 / esc6 | 1.8* | 2.5 | 39 | 18% (76/427) |
| esc4 / esc6 escl | 20* | 0 | 4 | 2% (6/243) |
| esc4 / esc6 escl; + / αtub GAL4 | 0 | 0 | 75 | 98% (179/181) |
| esc6 / esc5 | 2.6* | 2.7 | 58 | 84% (132/158) |
| esc6 escl/ esc5 | 16* | 2 | 15 | 13% (19/148) |
| esc6 escl/ esc5 ; αtub GAL4/+ | 0 | 0 | 76 | 125% (152/121) |
| esc6 / esc2 | 3* | 3 | 67 | 61% (135/221) |
| esc6 escl / esc2 | 20* | 0 | 21 | 16% (37/231) |
| esc6 / Df(2L) escP3-0 | 13.1 | 3.9 | 9 | 37% (20/54) |
| esc6 escl / Df(2L)escP3-0 | NA | 0% (0/189) | ||
| esc6 escl / Df(2L)escP3-0 ; αtubGAL4/+ | 0 | 0 | 40 | 109% (81/74) |
| esc6 / Df(2L)Prl | 13.4 | 1.6 | 14 | 39% (20/51) |
| esc6 escl / Df(2L)Prl | NA | 0% (0/209) | ||
| esc6 escl / Df(2L)Prl; αtubGAL4/+ | 0 | 0 | 14 | 100% (21/21) |
| esc6 escl / escl | 0 | 0 | 48 | 106% (103/97 |
| esc6 escl / esc6 | 18 | 0 | 3 | 3% (41/155) |
| esc6 escl / esc6 escl | NA | 0% (0/200) | ||
| esc6 escl / esc6 escl; αtub GAL4/+ | 0 | 0 | 107 | 90% (107/118) |
For all genotypes, the maternally contributed chromosome is listed first.
Mean scores of the pairs of genotypes are significantly different P > 0.0001.
Mean scores are for males only.
Observed (obs.) represents both male and female of the given genotype.
Expected (exp.) is the mean number of adult flies of the other genotypes from the same cross.
Fig. 8.

Reduction of escl enhances esc null phenotypes. A, C, E and G are wild type adults. B. D, F and H are esc6 escld01514 / esc5 adults that have strong Polycomb phenotypes. Adult wing in A is transformed to a haltere in B. Antennae, an arrow indicated in C, are partially transformed to legs in D. Legs in E have sex combs (arrow) and transverse rows (asterisk) on T1, and large apical bristles (arrowhead) on T2 in wild type. Posterior legs are transformed to anterior legs in F as evidenced by sex combs on T2 and T3 (arrow), presence of transverse rows on T2 (asterisk, transverse rows on T3 are not visible in this view) and reduced apical bristles on T2 (arrowhead). G and H are a ventral view of the thorax. Mesoplural bristles are present in wild type in G (asterisk) in T2 but are missing in H indicative of a T2 to T1 transformation, and T2 coxa (arrow in G and H) is transformed to T1 coxa (arrowhead).
Survival of esc6 escld01514 / esc− + flies is also greatly reduced compared to esc mutants (which themselves are less than 100% viable). For example, 84% of esc6/esc5 progeny of heterozygous parents survive to adulthood, while just 13% of esc6 escld01514 / esc5 + do. Similar effects of heterozygous escld01514 were observed in other heteroallelic esc combinations. This indicates that even when heterozygous, the escld01514 mutation exacts a considerable toll on adult viability of “maternally rescued” esc mutant genotypes.
esc6 escld01514 double homozygotes die at the end of an extended larval period
esc6 escld01514 double homozygotes (or hemizygotes) derived from heterozygous esc6 escld01514 parents are 100% lethal, despite the presence of maternally deposited esc+ gene products in the mutant embryos. While many survive to third instar, they are developmentally delayed, persisting as larvae for up to 12 days. They become lethargic, do not leave the media to crawl up the wall of their container to pupariate, and eventually die without pupariating. Third instar esc6 escld01514 double homozygotes have small eye-antennal discs and wing discs (see C and D in Fig. 9) and apparently normal sized leg, haltere, and labial discs (see E, F, G, and H in Fig. 9). Their larval brain lobes are also much smaller, presumably due to the reduced size of the imaginal progenitors of the large adult optic lobes, which proliferate extensively throughout the larval period and account for much of the size of the late third instar brain lobes (A and B in Fig. 9). This small brain phenotype is also observed in E(z) and Su(z)12 mutants, which also have small imaginal discs (Birve et al., 2001; Phillips and Shearn, 1990; Shearn et al., 1978). The late larval lethality of the esc6 escld01514 double homozygotes indicates that the “maternal rescue” of homozygous esc progeny of heterozygous esc mothers to adulthood requires ESCL, at least during the larval period if not earlier. It could also be interpreted to suggest that the apparent critical early requirement for ESC might reflect a temporal division of labor between ESC and ESCL to provide the same function throughout development, viz., binding to histone H3 and stimulating the HMTase activity of E(Z). However, unless an unequivocal null escl allele proves to have mutant phenotypes, the adult viability and fertility of escld01514 homozygotes and hemizygotes suggests that under normal circumstances, when esc+ is expressed both maternally and zygotically, ESCL is dispensable or plays a yet to be identified role that is not essential for normal development.
Fig. 9.
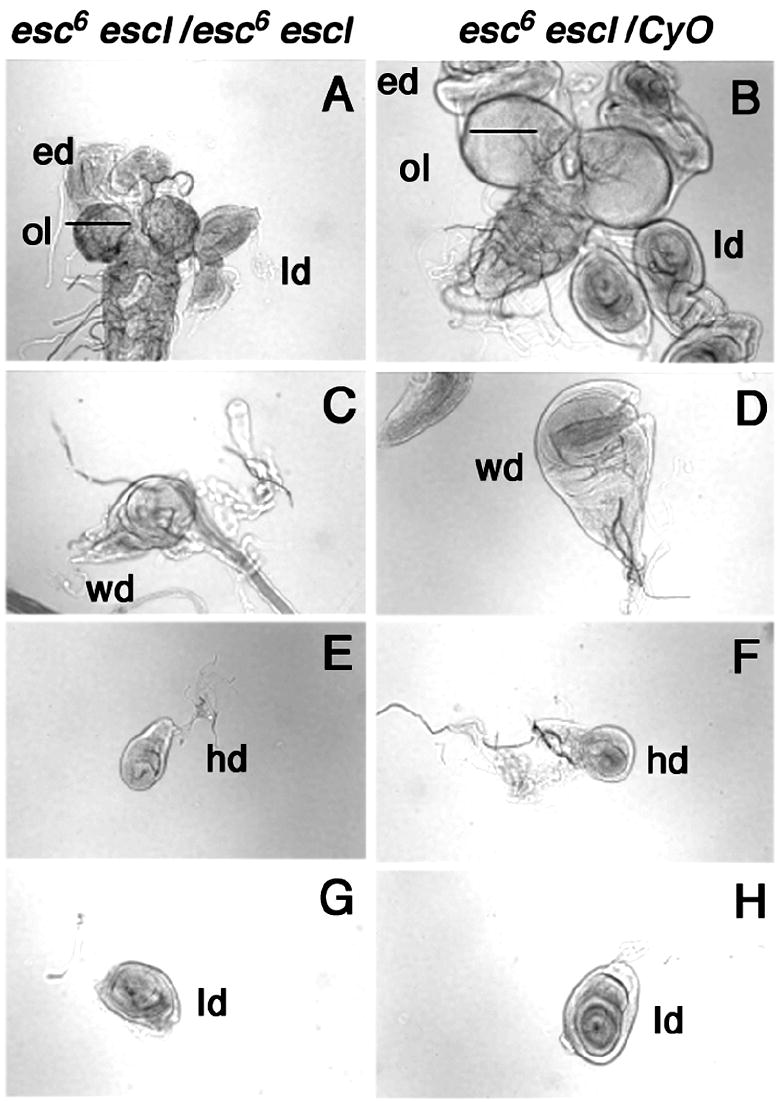
Comparison of esc6 escl d01514 double homozygotes and control imaginal discs. esc6 escl d01514/esc6 escl d01514 were detected by absence of GFP and compared to esc6 escld01514/CyO P{w+Kr GFP19} larvae from the same bottles. In image of A, esc6 escl d01514/esc6 escl d01514 (left) have smaller optical lobes (ol) and eye discs (ed) than esc6 escl d01514/CyO P{w+KrGFP19} (right). C and D are the wing discs (wd). Smaller size of esc6 escl d01514/esc6 escl d01514 may reflect transformation of the wing disc to a haltere disc. E and F are haltere discs (hd), and G and H are leg discs (ld). There is no obvious size difference between esc6 escl d01514/esc6 escl d01514 and controls, although a proliferation defect may not be visible in these smaller sized discs.
ESCL can substitute for ESC in vivo
To test whether ESCL is functionally equivalent to ESC in vivo, we took advantage of the fact that the XP element insertion before codon 2 in escld01514 restores the wild type coding sequence and can thus be used to induce escl over-expression from its GAL4-regulated minimal promoter when combined with a GAL4 driver. The zygotically expressed driver used, α tubulin(84B)-GAL4 ( αtub-GAL4) (see Materials and Methods) promotes substantial over-expression of ESCL throughout development (Fig. 7B, lanes 3 and 4) and this is sufficient to completely rescue the extra sex combs phenotype of “maternally rescued” esc6 / esc4 and esc6 / esc5 adults as well as esc6 hemizygotes. These same esc mutant genotypes also have reduced survival to adulthood that was also fully rescued to wild type levels (Table 1). These data demonstrate that zygotic over-expression of ESCL can compensate for the loss of zygotically expressed ESC.
A more stringent test of their functional equivalence was also conducted by determining whether constitutive expression of escl both maternally and zygotically can rescue the lethality of embryos that contain no ESC protein (i.e., homozygous esc progeny of homozygous esc females). For this purpose, we constructed a genotype that expresses ESCL both zygotically and maternally by combining the αtub-GAL4 and matα4-GAL-VP16,V37 (αmat-GAL4) drivers respectively. The latter drives exclusively maternal expression (from the 67C α-tubulin promoter), primarily in nurse cells, of a GAL4-VP16 fusion protein, which is loaded into the developing oocyte (Kaltschmidt et al., 2000; Theurkauf et al., 1986).
When esc6 escld01514 / CyO ; αmat-GAL4 / TM3 males and females were crossed to each other (i.e., when both endogenous esc and GAL4-driven escl were expressed only maternally) no homozygous esc6 escld01514 adults were recovered. This confirms that the survival of homozygous esc mutants to adulthood (“maternally rescued” adults) specifically requires the presence of ESCL in postembryonic stages and not simply an increased level of maternally derived ESCL during embryogenesis. In contrast, when esc6 escld01514 / CyO ; αmat-GAL4 / TM3 females were crossed to esc6 escld01514 / CyO ; αtub / TM3 males (i.e., when both escl and esc were expressed maternally and escl was also expressed zygotically) homozygous esc6 escld01514 / esc6 escld01514 ; αmat-GAL4 / αtub-GAL4 adults were readily recovered, as expected. Since they possess both maternal and zygotic drivers in a homozygous esc6 null background, they can be crossed to each other to test whether maternal and zygotic expression of escl is sufficient to rescue the lethality of embryos that contain no ESC protein. Remarkably, this cross produces viable adult offspring that appear indistinguishable from wild type adults in all respects. Of fifty progeny examined in detail, one had a partial wing to haltere transformation and one had spots of pigmentation in abdominal segment 4, similar to a mild Mcp phenotype. These results demonstrate that ESCL can substitute for ESC at all times and suggest that ESCL is functionally equivalent to ESC, and that the differences in the amino acid sequences between ESC and ESCL, including the additional 33 residues at the ESCL N-terminus, do not reflect any obvious qualitative functional differences. However, the relatively high levels of ESCL produced, especially with the αtub-GAL4 driver, might compensate for any quantitative functional differences that might exist between the two proteins. The partial haltere to wing transformation seen in one adult might hint at this possibility. It also remains possible that there are some more subtle qualitative differences that were not revealed in this study.
Discussion
Similarities and differences between ESC and ESCL
The evidence presented above indicates that ESCL can perform the same functions as ESC in PRC2 complexes. In particular, the ability of ESCL to support the development of embryos that lack any ESC to normal appearing viable adults, strongly suggests that ESCL can carry out all the functions of ESC and that the two proteins are functionally equivalent, at least for normal development to adulthood. This does not mean that there are no differences between the two proteins. The rescue of embryos containing no ESC protein by ESCL over-expression did produce an occasional fly with a mild PcG phenotype, a hint that ESCL may not be quite as effective as ESC either in forming complexes with other PRC2 components or in promoting H3K27 methylation by PRC2 complexes.
Some of the RNAi results also hint that ESCL-containing complexes may not be quite as effective at H3K27 methylation as ESC-containing complexes. In particular, depletion of ESC reduced the 3mH3K27 level more than depletion of ESCL did, in both S2 and Kc cells (compare lanes 5 to lane 6 and lanes 1 and 2, panel 3 of Fig. 5 B and D). This occurred despite the fact that the ESCL level increases quite substantially when ESC alone is depleted. While differences in efficiency of knockdown might contribute to this, it may also indicate that more ESCL is required to produce the same level of 3mH3K27 achieved with less ESC, i.e., ESC-containing complexes may be more effective at H3 methylation than ESCL-containing complexes. A possible basis for such a difference is suggested in previous work demonstrating that ESC and ESCL both bind to histone H3 (Tie et al. 2007), in which we noticed that ESCL binding to H3 appears to be somewhat weaker.
ESCL dispensability
The results reported above also demonstrate that while ESCL is expressed throughout development and during adulthood, it is apparently dispensable, under normal culture conditions, at least for development of morphologically normal fertile adults. This is somewhat surprising in light of the complete lethality of esc escl double mutants, which clearly indicates that the well-known “maternal rescue” of the embryonic lethality of esc mutants is absolutely dependent on ESCL. esc escl double homozygotes die at the end of an extended larval period and have markedly smaller brain hemispheres, wing and eye-antennal discs, while the other discs appear to be similar in size to wild type discs. The normally large wing disc is likely to be smaller due to transformation into a haltere disc due to derepression of Ubx in the wing disc, a phenotype typically seen only as a partial transformation in adults of very strong Polycomb mutant genotypes. Transformation of the leg discs to other segmental identities, which would not be reflected in changes in disc size, is also likely in the esc escl mutants, since the signature “extra sex combs” phenotype seen in maternally rescued adult esc homozygotes is already enhanced when these adults are also heterozygous for escl. Similarly, the decrease in the size of the eye antennal disc is consistent with the partial antenna to leg transformations observed in the esc adults that are also heterozygous for the escl mutation. Mutations in E(z) and Su(z)12 (Birve et al., 2001; Shearn et al., 1978) have a similar late lethal phase and also exhibit small imaginal discs and larval brains, consistent with their functional collaboration in the same complexes. Why then is ESCL largely dispensable, given the impact of the escl mutation on development to adulthood in esc escl double mutants?
The demonstration that ESCL can substitute fully for ESC in vivo, provided it is expressed at adequate levels at all times, further suggests that the two proteins are qualitatively equivalent and that in the absence of the other, either one is necessary and sufficient for normal development to adulthood. Nonetheless, it appears that normally ESCL does not come into play in a substantial way unless ESC levels are compromised. A reason for this is suggested by the discovery that in S2 cells most ESCL protein is in free form when ESC is relatively abundant, and that the level of ESCL, along with the proportion of it found in the 600 kDa complex, increases dramatically in cells that have been depleted of ESC by RNAi. The amount of ESCL that is bound to the Ubx PRE also increases after ESC depletion. These observations suggest that ESCL levels are regulated in part by ESC levels through a mechanism involving competition for participation in E(Z) complexes, which serves to stabilize ESCL. Since we do not know the actual abundances of ESC and ESCL, we cannot determine to what extent this may be due to intrinsic differences in the affinity of the two proteins for these complexes or is simply a reflection of mass action favoring the more abundant of the two proteins. Abundance almost certainly plays a key role in early embryos, when the ESC level is highest and the ESCL level is at its lowest. It is harder to explain why ESCL does not become essential in postembryonic stages, when the ESC level is much lower and the ESCL level is at its highest. This might hint that even at low levels ESC has an intrinsic advantage over ESCL in competition for complex assembly. Alternatively, it is possible that the levels of ESC in postembryonic stages have been underestimated by measurements in bulk extracts and that in imaginal discs, critical tissues for development of the adult, ESC levels remain high enough to continue to out-compete ESCL for complex formation in those tissues. However, in bulk extracts from late larvae, a substantial fraction of ESCL is detected in the 600 kDa complex and ESCL is readily co-immunoprecipitated with E(Z) from these extracts. Whichever the case, it appears that ESCL has little or no role when ESC is present at normal levels. It remains possible that ESCL becomes more important in adults or under specific environmental conditions.
The results of RNAi-mediated knockdown in S2 and Kc cells clearly demonstrate that depletion of either ESC or ESCL has no appreciable effect on the levels of di-methyl and tri-methyl H3K27, while simultaneous depletion of both proteins greatly reduces the tri- and di-methyl isoforms. This strongly suggests that ESC and ESCL are functionally interchangeable for this function of PRC2 and indicates that each protein is produced at sufficient levels in S2 cells to promote normal levels of H3K27 methylation in the absence of the other. This also suggests that the common role of both proteins in PRC2 complexes, i.e., binding to histone H3 and stimulating E(Z)-mediated H3K27 methylation (Tie et al., 2007), is the basis of their interchangeability in vivo.
In summary, while the complementary temporal expression patterns of ESC and ESCL during development initially suggested that ESCL might become essential during postembryonic development, the data presented here suggests otherwise, beginning with the lack of any obvious phenotypes in an strong escl mutant that makes little residual protein. The reason for the apparent dispensability of ESCL would appear to be due simply to the sufficiency of normal ESC levels at all times to promote normal development. Ironically, this is now made even clearer by the observed late larval lethality of the esc escl double mutants, since together with the viability and normal morphology of escl mutants, it demonstrates very clearly that, while ESCL is absolutely required for survival to adulthood in the absence of zygotic ESC expression, ESC alone is sufficient to promote survival to adulthood. Even the variable extra sex combs phenotype seen in maternally rescued esc mutant adults is unlikely to reflect a postembryonic requirement for ESCL, since the escl mutants themselves do not exhibit this phenotype even at low frequency. Unless a true protein null escl allele does, this means that esc by itself is sufficient for development into normal adults.
On the other hand, the data presented here do help to solve the puzzle posed by previous genetic evidence indicating that esc is unique among Polycomb Group proteins in being required only during early embryogenesis, and more recent biochemical evidence that ESC is essential for H3K27 methylation by E(Z), which is required continuously. While the maternal rescue of esc null embryos to adulthood by a single esc+ allele in the mother (Struhl, 1981) is remarkable and indicates that there is a critical early requirement for ESC, we can now see that this genetic result does not reflect a requirement for ESC only in the early embryo. It requires the back-up function provided by ESCL, which becomes required after the maternally deposited esc+ gene products are depleted in esc− embryos. The elevated level of ESCL and ESCL/E(Z) complexes caused by ESC depletion in S2 cells also suggests that this may involve compensatory elevation of ESCL/E(Z) complexes as maternally derived ESC disappears in esc− embryos. Similar considerations pertain to the experiments demonstrating rescue of esc null mutants by a brief pulse of zygotic esc+ expression from a heat-inducible hsp70-esc transgene during the first four hours of embryogenesis (Simon et al., 1995), which almost certainly also requires the back-up function of ESCL. At the time, these genetic experiments were interpreted to suggest that ESC was required only in the early embryo to ensure normal development to adulthood, except for the variable partial transformation of T2 and T3 legs to T1 identity. However, while it is now evident that the existence of ESCL and its continuous expression explains the apparent requirement for ESC only during embryogenesis, inferred from maternal rescue, these genetic experiments do not necessarily reveal a normal requirement for ESCL. On the contrary, the lack of obvious phenotypes in the escl mutant, together with the inability of maternally derived ESC alone to promote normal development to adults in the absence of ESCL, leads us to conclude that under normal circumstances, not only is ESC sufficient to promote normal development in the absence of ESCL, but also that esc expression during postembryonic stages is required for development of normal adult morphology. This conclusion was also arrived at almost 50 years ago based on genetic mosaic experiments in which esc mutant phenotypes were observed in clones of homozygous esc mutant cells induced at various times during the larval period (Hannah-Alava, 1958). The absence of phenotypes in the escl mutant, particularly the variable T2 and T3 extra sex combs phenotype seen in maternally rescued esc mutants, is consistent with the conclusion that postembryonic expression of ESC itself is required for development of normal adult morphology.
Origin and persistence of ESCL
Given the apparent redundancy of ESCL, at least for development of morphologically normal, fertile adults, why then has it persisted? All vertebrates for which complete genome sequences are available contain a single ESC/ESCL ortholog encoded by the EED gene, which is expressed continuously in all tissues (Schumacher et al., 1996). While there are several different EED isoforms arising from use of alternative translation initiation sites (Kuzmichev et al., 2004), it is not known whether their different N-termini make them functionally different. The C. elegans genome also contains a single ESC ortholog, mes-6 (Korf et al., 1998). Structural similarities between the esc and escl genes, particularly their intron-exon arrangements and the ESC and ESCL protein sequences, as well as the close proximity of the two genes on chromosome 2L, indicate that they arose by a gene duplication event. To date, outside of plants, the presence of two ESC/ESCL genes appears to be restricted to the Drosophilidae lineage. A single ortholog is present in the next most closely related lineages for which genome sequences are complete, including Culicidean Dipterans (three mosquitoes) Lepidopterans (Bombyx mori), Coleopterans (Tribolium casteneum) and Hymenopterans (Apis mellifera). All twelve Drosophila species for which complete genome sequence is available have two genes that are clearly recognizable ESC and ESCL orthologs. The most distant of these species diverged over 40 million years ago, sufficient time for one member of a duplicate gene pair to degenerate in the absence of selection for its function. A careful analysis of the changes that the esc and escl coding sequences have undergone in all these species should reveal whether or not the escl gene shows any signs of having begun to degenerate. However the qualitative functional equivalence of ESC and ESCL revealed in this study suggests that the persistence of ESCL and the conservation its function in H3K27 methylation by PRC2 complexes may be due to a fitness advantage conferred by ESCL for a function that has not yet been identified, perhaps a function it performs during adulthood.
A recent analysis of duplicate genes in the yeast S. cerevisiae that arose during a whole genome duplication event followed by massive gene loss (Guan et al., 2007) found that many surviving duplicates have not evolved distinct functions but have evolved divergent expression patterns, suggesting that the survival of both gene copies is driven by subsequent selection for retention of their common function in their distinct expression “spaces”. esc and escl might appear to approximate this situation, although their incompletely non-overlapping temporal expression profiles and functional requirements during development would likely diminish the selective advantage conferred by a more complete divergence of expression profiles. This is perhaps a further hint that ESCL might confer a fitness advantage for a function it plays in adults or under some conditions yet to be identified.
Supplementary Material
Acknowledgments
We thank previous and current members of Harte lab, especially Takehito Furuyama, Tom Breen, Rakhee Banerjee and Vincent Stepanik for their advice. Rabbit antibodies that specifically recognize trimethyl H3K27 were generously provided by Dr. Thomas Jenuwein. S2 cells (serum-dependent) and escl cDNAs were obtained from the Drosophila Genomics Resource Center. This work was supported by a grant (GM39255) from the National Institutes of Health to PJH and by an NIH predoctoral Training Grant award (T32HD007104) to RLK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beuchle D, Struhl G, Muller J. Polycomb Group proteins and heritable silencing of Drosophila Hox genes. Development. 2001;128:993–1004. doi: 10.1242/dev.128.6.993. [DOI] [PubMed] [Google Scholar]
- Birve A, Sengupta AK, Beuchle D, larsson J, Kennison JA, Rasmuson-Lestander Å, Muller J. Su(z)12, a novel Drosophila Polycomb group gene that is conserved in vertebrates and plants. Development. 2001;128:3371–3379. doi: 10.1242/dev.128.17.3371. [DOI] [PubMed] [Google Scholar]
- Cavener DR, Ray SC. Eukaryotic start and stop translation sites. Nucleic Acids Res. 1991;19:3185–3192. doi: 10.1093/nar/19.12.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V. Drosophila Enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell. 2002;111:185–196. doi: 10.1016/s0092-8674(02)00975-3. [DOI] [PubMed] [Google Scholar]
- Ebert A, Schotta G, Lein S, Kubicek S, Krauss V, Jenuwein T, Reuter G. Su(var) genes regulate the balance between euchromatin and heterochromatin in Drosophila. Genes Dev. 2004;18:2973–2983. doi: 10.1101/gad.323004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama T, Tie F, Harte PJ. Polycomb Group proteins ESC and E(Z) are present in multiple distinct complexes that undergo dynamic changes during development. Genesis. 2003;35:114–124. doi: 10.1002/gene.10173. [DOI] [PubMed] [Google Scholar]
- Guan Y, Dunham MJ, Troyanskaya OG. Functional analysis of gene duplications in Saccharomyces cerevisiae. Genetics. 2007;175:933–943. doi: 10.1534/genetics.106.064329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutjahr T, Frei E, Spicer C, Baumgartner S, White RAH, Noll M. The Polycomb-group gene, extra sex combs, encodes a nuclear member of the WD-40 repeat family. EMBO J. 1995;14:4296–4306. doi: 10.1002/j.1460-2075.1995.tb00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt JA, Davidson CM, Brown NH, Brand AH. Rotation and asymmetry of the mitotic spindle direct asymmetric cell division in the developing central nervous system. Nat Cell Biol. 2000;2:7–12. doi: 10.1038/71323. [DOI] [PubMed] [Google Scholar]
- Ketel CS, Andersen EF, Vargas ML, Suh J, Strome S, Simon JA. Subunit contributions to histone methyltransferase activities of fly and worm polycomb group complexes. Mol Cell Biol. 2005;25:6857–6868. doi: 10.1128/MCB.25.16.6857-6868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf I, Fan Y, Strome S. The Polycomb group in Caenorhabditis elegans and maternal control of germline development. Development. 1998;125:2469–2478. doi: 10.1242/dev.125.13.2469. [DOI] [PubMed] [Google Scholar]
- Kuzmichev A, Jenuwein T, Tempst P, Reinberg D. Different EZH2-containing complexes target methylation of histone H1 or nucleosomal histone H3. Mol Cell. 2004;14:183–193. doi: 10.1016/s1097-2765(04)00185-6. [DOI] [PubMed] [Google Scholar]
- Montgomery ND, Yee D, Chen A, Kalantry S, Chamberlain SJ, Otte AP, Magnuson T. The murine polycomb group protein Eed is required for global histone H3 lysine-27 methylation. Curr Biol. 2005;15:942–947. doi: 10.1016/j.cub.2005.04.051. [DOI] [PubMed] [Google Scholar]
- Nekrasov M, Wild B, Muller J. Nucleosome binding and histone methyltransferase activity of Drosophila PRC2. EMBO Rep. 2005;6:348–353. doi: 10.1038/sj.embor.7400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasini D, Bracken AP, Jensen MR, E LD, Helin K. Suz12 is essential for mouse development and for EZH2 histone methyltransferase activity. EMBO J. 2004;23:4061–4071. doi: 10.1038/sj.emboj.7600402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AH, Kubicek S, Mechtler K, O’Sullivan RJ, Derijck AA, Perez-Burgos L, Kohlmaier A, Opravil S, Tachibana M, Shinkai Y, Martens JH, Jenuwein T. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol Cell. 2003;12:1577–1589. doi: 10.1016/s1097-2765(03)00477-5. [DOI] [PubMed] [Google Scholar]
- Phillips MD, Shearn A. Mutations in polycombeotic, a Drosophila Polycomb-group gene, cause a wide range of maternal and zygotic phenotypes. Genetics. 1990;125:91–101. doi: 10.1093/genetics/125.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathe SS, Harte PJ. The Drosophila Extra sex combs protein contains WD repeats essential for its function as a repressor of homeotic genes. Mech Dev. 1995;52:77–87. doi: 10.1016/0925-4773(95)00392-e. [DOI] [PubMed] [Google Scholar]
- Schumacher A, Faust C, Magnuson T. Positional cloning of a global regulator of anterior-posterior patterning in mice. Nature. 1996;383:250–253. doi: 10.1038/383250a0. [DOI] [PubMed] [Google Scholar]
- Shearn A, Hersperger G, Hersperger E, Pentz ES, Denker P. Multiple allele approach to the study of genes in Drosophila melanogaster that are involved in imaginal disc development. Genetics. 1978;89:355–370. doi: 10.1093/genetics/89.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J, Bornemann D, Lunde K, Schwartz C. The extra sex combs product contains WD40 repeats and its time of action implies a role distinct from other Polycomb group products. Mech Dev. 1995;53:197–208. doi: 10.1016/0925-4773(95)00434-3. [DOI] [PubMed] [Google Scholar]
- Struhl G. A gene product required for correct initiation of segmental determination in Drosophila. Nature. 1981;293:36–41. doi: 10.1038/293036a0. [DOI] [PubMed] [Google Scholar]
- Struhl G. Role of the esc+ gene product in ensuring the selective expression of segment-specific homeotic genes in Drosophila. J Embryol Exp Morphol. 1983;76:297–331. [PubMed] [Google Scholar]
- Struhl G, Brower D. Early role of the esc+ gene product in the determination of segments in Drosophila. Cell. 1982;31:285–292. doi: 10.1016/0092-8674(82)90428-7. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE, Baum H, Bo J, Wensink PC. Tissue-specific and constitutive alpha-tubulin genes of Drosophila melanogaster code for structurally distinct proteins. Proc Natl Acad Sci U S A. 1986;83:8477–8481. doi: 10.1073/pnas.83.22.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault ST, Singer MA, Miyazaki WY, et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- Tie F, Furuyama T, Harte PJ. The Drosophila Polycomb-Group proteins ESC and E(Z) bind directly to each other and co-localize at multiple chromosomal sites. Development. 1998;125:3483–3496. doi: 10.1242/dev.125.17.3483. [DOI] [PubMed] [Google Scholar]
- Tie F, Furuyama T, Prasad-Sinha J, Jane EP, Harte PJ. The Drosophila Polycomb Group proteins ESC and E(Z) are present in a complex containing the histone-binding protein p55 and the histone deacetylase RPD3. Development. 2001;128:275–286. doi: 10.1242/dev.128.2.275. [DOI] [PubMed] [Google Scholar]
- Tie F, Prasad-Sinha J, Birve A, Rasmuson-Lestander A, Harte PJ. A 1 MDa ESC/E(Z) complex from Drosophila that contains Polycomblike and RPD3. Mol Cell Biol. 2003;23:3352–3362. doi: 10.1128/MCB.23.9.3352-3362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie F, Stratton CA, Kurzhals RL, Harte PJ. The N terminus of Drosophila ESC binds directly to histone H3 and is required for E(Z)-dependent trimethylation of H3 lysine 27. Mol Cell Biol. 2007;27:2014–2026. doi: 10.1128/MCB.01822-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, Zhang Y. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–878. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- Wang L, Njahren N, Vargas ML, Anderson EF, J B, J Z, L ME, S JR, Simon JA. Alternative ESC and ESC-like subunits of a Polycomb group histone methyltransferase complex are differentially deployed during Drosophila development. Mol Cell Biol. 2006;26:2637–2647. doi: 10.1128/MCB.26.7.2637-2647.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


