Abstract
Cell-attached single-channel recordings of NMDA channels were carried out in human dentate gyrus granule cells acutely dissociated from slices prepared from hippocampi surgically removed for the treatment of temporal lobe epilepsy (TLE). The channels were activated by l-aspartate (250–500 nm) in the presence of saturating glycine (8 μm).
The main conductance was 51 ± 3 pS. In ten of thirty granule cells, clear subconductance states were observed with a mean conductance of 42 ± 3 pS, representing 8 ± 2% of the total openings.
The mean open times varied from cell to cell, possibly owing to differences in the epileptogenicity of the tissue of origin. The mean open time was 2.70 ± 0.95 ms (range, 1.24–4.78 ms). In 87% of the cells, three exponential components were required to fit the apparent open time distributions. In the remaining neurons, as in control rat granule cells, two exponentials were sufficient. Shut time distributions were fitted by five exponential components.
The average numbers of openings in bursts (1.74 ± 0.09) and clusters (3.06 ± 0.26) were similar to values obtained in rodents. The mean burst (6.66 ± 0.9 ms), cluster (20.1 ± 3.3 ms) and supercluster lengths (116.7 ± 17.5 ms) were longer than those in control rat granule cells, but approached the values previously reported for TLE (kindled) rats.
As in rat NMDA channels, adjacent open and shut intervals appeared to be inversely related to each other, but it was only the relative areas of the three open time constants that changed with adjacent shut time intervals.
The long openings of human TLE NMDA channels resembled those produced by calcineurin inhibitors in control rat granule cells. Yet the calcineurin inhibitor FK-506 (500 nm) did not prolong the openings of human channels, consistent with a decreased calcineurin activity in human TLE.
Many properties of the human NMDA channels resemble those recorded in rat hippocampal neurons. Both have similar slope conductances, five exponential shut time distributions, complex groupings of openings, and a comparable number of openings per grouping. Other properties of human TLE NMDA channels correspond to those observed in kindling; the openings are considerably long, requiring an additional exponential component to fit their distributions, and inhibition of calcineurin is without effect in prolonging the openings.
The activation of NMDA receptor channels by agonist appears to be functionally different from that of nicotinic ACh receptors, with the most striking difference being the considerably longer duration of receptor activation (Gibb & Colquhoun, 1992). This lasting activation of NMDA receptors following ligand binding is responsible for the long duration of synaptic events mediated by these receptors (Lester et al. 1990; Edmonds et al. 1995; Wyllie et al. 1998). The prolonged synaptic responses, and the negative slope conductance provided by the voltage-dependent Mg2+ block, coupled with the Ca2+ permeability of the NMDA receptors, places these channels in a pivotal position for coincidence detection, regulation of Ca2+-dependent neuronal plasticity or degeneration, and aberrant neuronal discharges characteristic of epilepsies (Collingridge & Watkins, 1994; McBain & Mayer, 1994; Mody, 1998).
NMDA receptor channels are abundant in the human brain (Huntley et al. 1994; Scherzer et al. 1998), and appear to participate in glutamatergic synaptic transmission (Urban et al. 1990; Isokawa & Levesque, 1991; Masukawa et al. 1991; Hwa & Avoli, 1992; Isokawa et al. 1997), but little is known about the gating behaviour of the NMDA channel in human central nervous system (CNS) neurons. The cloned human NR1 subunit differs from that found in the rat by only seven of its 938 amino acids (Karp et al. 1993). Accordingly, the common features of homomeric human NR1 channels expressed in Xenopus oocytes include Ca2+ permeability, voltage-dependent block by Mg2+, antagonism by Zn2+ and other competitive and non-competitive antagonists (Karp et al. 1993; Planells-Cases et al. 1993). Biochemical studies of human NMDA channel properties have described similarities to rodent receptors in the modulation of channel activity by polyamines (Subramaniam et al. 1994), and channels comprising human NR1a/NR2A and NR1a/NR2B subunits permanently transfected into mouse fibroblasts have comparable electrophysiological properties to their rodent counterparts. However, expression systems may not accurately reflect the properties of NMDA channels, in the absence of several post-translational modifications present in native neurons (Sucher et al. 1996).
Studies of NMDA channels in native human cells have included recordings of whole-cell NMDA currents in cultured fetal neocortical and cerebellar neurons (Sah, 1995), but the immaturity of the tissue and the use of cultured neurons precludes analysis of cell type-specific NMDA channel characteristics. There are no reports on single NMDA channel gating in mature human CNS neurons. We have thus undertaken the present study to characterize the openings of ion channels gated by NMDA receptors in human dentate gyrus granule cells. We used granule cells acutely dissociated from slices prepared from hippocampi of adult temporal lobe epilepsy (TLE) patients subjected to therapeutic surgical resection of the hippocampal formation (Engel, 1993; Spencer & Spencer, 1994). Consequently, our data consist solely of recordings obtained from dissociated neurons of epileptic patients, since there is little chance for recording from ‘control’ adult human nerve cells. Nevertheless, our results allow a rigorous comparison to be made between the gating of NMDA channels recorded in live adult human neurons and the characteristic openings of NMDA channels in rodent hippocampal neurons, including those observed in animal models of TLE. Consistent with the large number of studies showing an enhanced NMDA component of synaptic responses in the human TLE dentate gyrus (Urban et al. 1990; Isokawa & Levesque, 1991; Masukawa et al. 1991; Isokawa et al. 1997), our findings reveal the openings of NMDA channels in human TLE granule cells to be more like those seen in neurons dissociated from animal models of the disease (Köhr et al. 1993). Importantly, the conductance and the kinetic behaviour of the channels resembles in many respects that found in lower mammalian preparations. Furthermore, as the single-channel properties of synaptic and extrasynaptic NMDA receptors appear to be comparable (Lester et al. 1990; Clark et al. 1997), our studies of the functional properties of extrasynaptic NMDA receptor channels in the cell-attached configuration can be used to understand how the behaviour of these receptors gives rise to the unique features of NMDA receptor-mediated synaptic events in TLE. Part of this work has been previously published as an abstract (Lieberman et al. 1996).
METHODS
Preparation of brain slices and dissociation of human dentate gyrus granule cells
Human hippocampal tissue was obtained from en bloc resections of mesial temporal lobes, surgically removed by Dr Itzhak Fried (Neurosurgery, UCLA School of Medicine), from pharmaco-resistant TLE patients. The study was conducted under the guidance of the Declaration of Helsinki (1989) and the informed consent was approved by the UCLA Human Subject Protection Committee (protocol HSPC #93-11-642-11). Patient selection was based on the guidelines established at the UCLA Medical Center and on the Consensus Statement on Surgery for Epilepsy prepared by the NIH (Isokawa & Levesque, 1991; Isokawa et al. 1997). The resected tissue was immediately immersed in ice-cold, oxygenated artificial cerebrospinal fluid (ACSF) and transverse hippocampal slices were prepared and maintained according to published procedures (Isokawa et al. 1997). Coronal slices including the hippocampus were prepared within 15 min from the time of tissue removal by Dr Masako Isokawa (Brain Research Institute, UCLA School of Medicine) on a vibratome, and were maintained at 32°C in an ACSF with the following composition (mM): 126 NaCl, 2.5 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose and 1 pyruvic acid; pH 7.3. The ACSF was constantly bubbled with a 95 % O2 and 5 % CO2 gas mixture.
Our previously published methods for the acute dissociation of neurons from rodent brain slices (Mody et al. 1989) have been adapted for use in adult human CNS tissue. For enzymatic digestion, the dentate gyrus was first microdissected from each 450 μm thick coronal hippocampal slice within 1-8 h from the time slices were cut, and then incubated for 20-25 min (32°C) in 2 ml of oxygenated ACSF with 1.75 mg ml−1 pronase E (protease Type XIV, Sigma). Two to four microsections of the isolated dentate gyrus were each triturated in separate test-tubes containing 2 ml of cold (4°C) Hepes-buffered ACSF (NaHCO3 was replaced by equimolar Hepes) using two fire-polished Pasteur pipettes of increasingly smaller tip diameter. The neuronal suspension was transferred into a tissue culture dish, the neurons were allowed to settle for 10-15 min, and the dish was then thoroughly washed with a nominally Mg2+-free solution. Recordings were performed 15 min to 2 h after this wash. Each dissociation yielded tens of viable granule cells suitable for patch-clamp recordings. Albeit more variable in shape and size than rat dentate gyrus granule cells (Seress & Mrzljak, 1987; Isokawa et al. 1991; Seress, 1992; von Campe et al. 1997), human granule cells can be recognized under visual inspection after dissociation. Granule cells can be identified by the characteristic acute angle of their dendritic branching, the shape and size of their somata, and the occasional presence of basal dendrites (L. Seress, personal communication).
Cell-attached recordings of NMDA channel openings
Thin-walled borosilicate glass (1.50 mm o.d., 1.12 mm i.d. with filament; Garner Glass Co., Claremont, CA, USA) pulled in two stages on a Narishige PP-83 electrode puller was used for patch electrodes. Pipettes were fire polished and coated with Sylgard resin and had resistances of 8-12 MΩ after being filled with recording solution. High-resistance pipettes were used to avoid recording from multiple channels. Cell-attached recordings were performed using an Axopatch 200A integrating patch-clamp amplifier (Axon Instruments) at 22-25°C. The extracellular solution contained (mM): 110 Na2SO4, 5 Cs2SO4, 1.8 CaCl2, 10 Hepes, 10 glucose, 1 pyruvic acid and 0.001 tetrodotoxin (TTX; Calbiochem). The SO42− salts were used to provide quieter recordings and to diminish the spontaneous openings of chloride channels (McLarnon & Curry, 1990), but reduced the Ca2+ activity to 0.4 mM as measured with ion-sensitive electrodes. The pH was adjusted to 7.25 with NaOH and the osmolarity varied between 285 and 305 mosmol l−1. Stock solutions of L-aspartic acid (1 mM) and glycine (1 mM) were freshly prepared and used at proper dilutions. All chemicals, except those indicated otherwise, were purchased from Fluka (Buchs, Switzerland).
Cell-attached recordings of steady-state NMDA channel activity were obtained by using 250-500 nM L-aspartic acid and 8 μM glycine in the recording pipettes. The recording electrodes were filled with the agonist dissolved in the extracellular solution. The use of low agonist concentrations ensured that channel activations were clearly separated from each other, prevented pronounced desensitization of the channel, and minimized non-NMDA receptor openings (Gibb & Colquhoun, 1992; Köhr et al. 1993). Estimates of the NMDA receptor channel reversal potential gave a resting potential of -50 to -60 mV and a conductance of around 50 pS of the main conductance state. This was determined from the slope of the I-V plot based on at least two different holding potentials at least 20 mV apart from each other.
Data analysis
Recordings were filtered through the 10 kHz Bessel filter (4-pole, -3 dB) of the amplifier at a gain of 100 mV pA−1, and were stored in a pulse-code modulated digitized form (88 kHz; Neurodata, New York, NY, USA) on videotapes. Off-line, the continuous records were filtered at 2 kHz (8-pole Bessel, -3 dB; model 902LPF, Frequency Devices, Haverhill, MA, USA), giving a combined cut-off frequency of 1.96 kHz. The analog signals were sampled at 20 kHz (DT 2821 A/D board, Data Translation, Marlboro, MA, USA) using an Intel Pentium-based computer. Recordings were analysed off-line with the PAT program of the Strathclyde Electrophysiology Software (courtesy of J. Dempster, University of Strathclyde, Glasgow, UK) using a 50 % threshold crossing algorithm for event detection. Any event that surpassed this level of detection was considered a full opening. Likewise, any closure to a level below threshold was considered complete. Thus openings either to or from another substate, and closures to or from substates, were not noted. For most of the time (∼90 %) the channels spent in the open state, the openings were to the highest conductance level, with substates accounting for the rest of the time the channels were open. Given the sampling rates and the cut-off frequencies described above, we imposed a fixed minimal resolution of 100 μs for both open and closed times, as sojourns of shorter than this duration would not have reached 50 % of their real amplitudes. The standard deviation of the baseline noise under our recording conditions ranged between 0.20 and 0.35 pA.
The analysis of dwell time distributions was done using software written and developed by Y. DeKoninck and I. Mody. Each exponential component of the closed time distribution of NMDA channels recorded with low agonist concentrations corresponds to a shut interval that separates groupings of NMDA channel openings. Shut time distributions were obtained first by fitting multiple exponential distributions (McManus et al. 1987) with a Simplex-based maximum likelihood method to log binned histograms (9 bins per decade) plotted on a square root ordinate (Sigworth & Sine, 1987). Critical closed times (Tc) for determination of burst, cluster and supercluster durations were calculated from the respective time constants of the shut time distributions (Colquhoun & Sakmann, 1985) by solving the following equation for Tc:
where τi is the i th time constant from the distribution of shut times, and i= 2, 3 or 4. Calculating Tc in this manner equalizes the percentage of gaps belonging to a long gap distribution misclassified as short, and the percentage of gaps belonging to a short distribution misclassified as long (Colquhoun & Sakmann, 1985; Edmonds & Colquhoun, 1992). The groupings of NMDA channel openings were classified according to published methods (Lieberman & Mody, 1994; Edmonds et al. 1995). Data are expressed as means ±s.e.m., unless otherwise noted.
RESULTS
Viable granule cells suitable for cell-attached NMDA channel recordings were dissociated from hippocampal slices obtained from ten TLE patients (six males and four females) with a mean age (±s.d.) of 27.4 ± 11.4 years (range, 14.33-50.25 years) at the time of surgery. The granule cells were visually identified prior to the recordings based on their characteristic morphology (see Methods) (Seress & Mrzljak, 1987; Isokawa et al. 1991; Seress, 1992; von Campe et al. 1997), and a total of thirty recordings of NMDA channel activity were included in the analysis based on the stability and the number of channel openings. The average number of openings used for analysis was 1265.
Multiple conductance states of NMDA channels
Single-channel recordings from cultured embryonic (Ascher et al. 1988; McBain & Mayer, 1994), neonatal (Howe et al. 1988, 1991), as well as adult rodent central neurons (Gibb & Colquhoun, 1991, 1992) have shown the activation of multiple conductances by low agonist concentrations applied to NMDA receptor channels. Noting that the external Ca2+ concentration has a strong inhibitory effect on the size of single-channel conductances (Ascher & Nowak, 1988; Jahr & Stevens, 1993), in acutely dissociated neurons from the CA1 region, Gibb & Colquhoun (1992) found conductance levels of 51 and 37 pS in nominally Mg2+-free extracellular solution containing 1.0 mM Ca2+, and 42 and 33 pS main conductance states in 2.5 mM external Ca2+. Our previous results in rat dentate gyrus granule cells, recorded under conditions similar to the present experiments, have not indicated the presence of any significant subconductance states (Köhr et al. 1993; Lieberman & Mody, 1995). This observation held true whether the cells originated from a control preparation or from an experimental model of TLE (kindling).
In contrast to the rat granule cells, in ten of the thirty human granule cells clear subconductance states could be recorded. The lower conductance state was 42 ± 3 pS and when present, it comprised 8 ± 2 % of the total time spent in the open state. The main conductance state was 51 ± 3 pS. An example of direct transitions between the two conductance states is given in Fig. 1. Considering the activity of Ca2+ in our extracellular solution (0.4 mM) and the relationship between single NMDA channel conductance and extracellular Ca2+ (Jahr & Stevens, 1993), the measured main conductance level is in good agreement with the calculated value of 54 pS.
Figure 1.
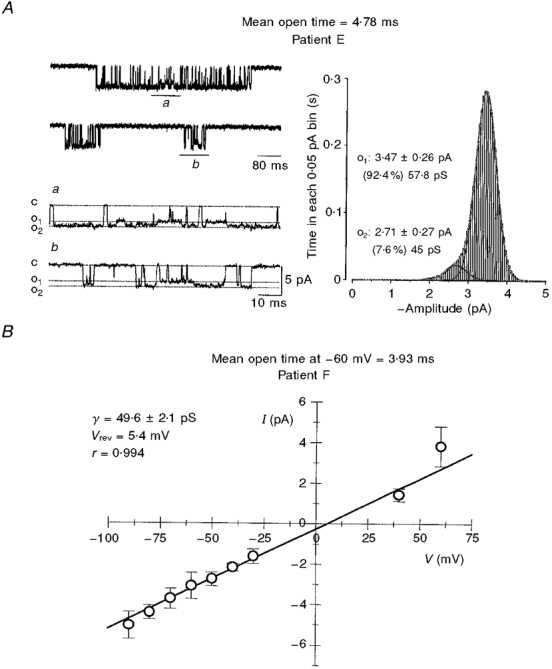
Conductance of single human NMDA channels
A, single-channel currents activated by L-aspartate and glycine in a cell-attached patch. Examples of channel openings (mean open time, 4.78 ms) are shown as downward deflections from the closed state (c) to two different conductance levels (o1 and o2). The dotted lines indicate the two current levels identified from the fit to the amplitude distribution shown on the right. The lower traces marked a and b in the left-hand panel are expansions of the corresponding segments marked on the raw traces shown in the upper panel. The vertical calibration bar is the same for both sets of traces. The channel spends less than 10 % of the time in the lower (45 pS) conductance state, while the larger (58 pS) conductance level is occupied over 90 % of the time. The right-hand panel is an all-points amplitude histogram for open events from the same patch, binned according to the time (s) spent in each 0.05 pA bin. Gaussian fits to the open state amplitudes are shown separately and also as a sum. B, relationship between single-channel current amplitudes (I) and patch potential (V). The mean single-channel current was estimated in this cell from Gaussian fits to the amplitude distribution for the main conductance state obtained at each membrane potential. The continuous line gives the least-squares fit, with a 49.6 pS slope conductance (γ) and a reversal potential (Vrev) of 5.4 mV. The error bars represent the s.d. of the fitted Gaussian distributions at each of the patch potentials. The mean open time for this cell is 3.93 ms.
In the nominal absence of extracellular Mg2+, keeping in mind that contaminants from various other salts cause such solutions to contain sub- or low micromolar levels of the cation (Gibb & Colquhoun, 1992), there was a linear dependence between the amplitude of the opening and the holding potential of the patch (Fig. 1B). The reversal potential for the six cells in which extensive voltage ranges could be obtained was between -1 and +6.2 mV. In the remaining cells, slope conductances were determined based on recordings done at fewer holding potentials, and were thus not included in our estimates of the reversal potential.
Heterogeneity of apparent open times
In sharp contrast to the similarities between the conductances of human and rat NMDA channels, there was a marked diversity of the apparent open times recorded in human granule cells held at similar membrane potentials. Variability was found among different patients, but a clear heterogeneity was present even within NMDA channel openings recorded in different granule cells of a given patient. This variability is clearly illustrated on the cumulative probability plot of all the mean open times (Fig. 2A). Since great care was taken to record from morphologically identified granule cells according to our strict anatomical criteria, the diversity in channel openings is unlikely to result from a heterogeneity of the recorded cell type. Rather, the diversity of channel openings may stem from the degree of TLE-associated alterations in a given granule cell, or from the extent of hippocampal sclerosis. The loss of dentate granule cells compared with autopsy controls ranged between 16 and 84 % (mean ±s.d., 60 ± 20 %) in the dorsal blade of the dentate gyrus and between 38 and 76 % (mean ±s.d., 60 ± 13 %) in the ventral blade. These data on the cell loss observed in the same hippocampal specimens were kindly provided by Dr Gary W. Mathern (Department of Neurology, UCLA School of Medicine). However, there was no clear correlation between the amount of cell loss in the granule cell layer and the mean open time of the NMDA channels. This is illustrated by two extreme cases (patients F and J). The range of the mean open times in patient F was 1.24-4.29 ms (mean, 2.75 ms), and this patient had a combined granule cell loss of 80.5 %. Similarly, in patient J, in spite of a combined granule cell loss of just 31 %, the mean open times ranged between 1.09 and 2.99 ms (mean 2.03 ms). Clearly, the neurons used in our recordings probably also varied in their participation in the ‘epileptic’ network, so epilepsy-induced alterations in channel kinetics would not be expected to be comparable between cells. Nevertheless, the overall distribution of mean open times (mean, 2.7 ms; s.d., 0.95 ms; range, 1.24-4.78 ms) for the thirty cells recorded from ten patients resembles the distribution of mean open times recorded in granule cells of kindled rats, an animal model of TLE. In this latter preparation, NMDA channel openings recorded 28 days following the last stimulation/seizure were considerably longer (mean, 3.07 ms; s.d., 1.23 ms; range, 1.41-5.88 ms; n= 33) than those in control granule cells (mean, 2.02 ms; s.d., 0.61 ms; range, 1.41-2.92 ms; n= 11; Mody & Lieberman, 1998). Interestingly, in 20 % of the human granule cells the mean open times of NMDA channels match quite well the range of open times obtained from control rat granule cells (Köhr et al. 1993; Lieberman & Mody, 1994; Mody & Lieberman, 1998). This suggests that the variability of open times recorded from the human population under study may represent differences in the extent to which epileptogenicity has altered the structure/function of the NMDA receptor (Mody, 1998), with more ‘control-like’ NMDA receptors being at the lower end and more ‘epileptic-like’ receptors at the upper end of the range.
Figure 2.

Variability of mean open times across patients and the stability of recordings from a given patch
A, cumulative probability distribution of mean apparent open times recorded in 30 granule cells from 10 TLE patients. The sigmoidal shape of the distribution suggests the presence of a single normally distributed population. Note the large variance of open times among different patients, but also within cells obtained from the same patient (e.g. patient F). All patients had marked hippocampal sclerosis upon pathological examination of the resected tissue specimens. B, stability plots of the mean open time and Popen in a cell with a mean open time close to the average of the overall distribution. The mean (±s.d.) of the 9440 openings recorded in this cell over a 15 min period was 2.99 ± 4.98 ms. The mean open times and the values for Popen were calculated over 10 s epochs corresponding to a single bin in the plots. The lines represent 6 point (1 min) running averages.
Due to this large variability of mean open times, the mean open time and the respective patient's code are identified in the individual figures of this study. We restricted our analysis to stable recordings (e.g. Fig. 2B), by excluding cells with conspicuous drifts of the dwell time characteristics.
Distribution of apparent shut times
Similar to the recordings of single NMDA channel openings in rat dentate gyrus granule cells (Köhr et al. 1993; Lieberman & Mody, 1994) and CA1 neurons (Gibb & Colquhoun, 1992), the shut time distributions in human dentate gyrus granule cells were best fitted with five exponential components. Figure 3 illustrates three examples of such distributions obtained in different cells with short, intermediate and long mean open times, respectively. The longest shut time components (> 1000 ms) were only found in cells with mean open times > 2.0 ms. The overall mean of the shut time distribution was 642 ± 127 ms.
Figure 3.

Distributions of shut times and open times
Each cell was chosen because its mean open time falls at three distinct points along the range of the recorded values. The top panels are taken from a cell with a 1.24 ms mean open time, which was the lowest measured. The middle panels are from a cell with a 2.55 ms mean, which is close to the 2.7 ms mean value of the pooled data. The bottom panels are from the cell also shown in Fig. 1A with the longest mean open time measured in our recordings. The panels depict the shut (left) and open (right) dwell-time histograms. The values were log-binned at 9 bins per decade and are shown on a square root abscissa. The time constants and the relative areas for each distribution are indicated in each panel. Note that there are 5 exponential components in each of the shut time distributions. The number of exponential components in the open time distributions is 2 for the channel with the shortest mean opening, but increases to 3 for the other channels.
The five exponential components of the shut times allowed the determination of the three critical shut times (Tc; see Methods for details) necessary for separation of bursts, clusters and superclusters (see below).
Distribution of apparent open times
The exponential fitting of the open time distributions revealed that a large number of short openings were missed as a result of the limited temporal resolution of our recordings. The mean open times given in Fig. 2A, and those indicated in other figures, were calculated from the arithmetical mean of the resolved openings, and therefore overestimate the duration of NMDA channel openings because of exclusion of the missed events. The overall mean of the resolved openings was 2.71 ± 0.17 ms. Of all openings, the mean duration of resolved single openings was 1.93 ± 0.13 ms, significantly (P < 0.05, two-tailed t test) shorter than the value of 3.21 ± 0.20 ms of the resolved openings, which occurred in groups of two or more.
In individual neurons, the distribution of open times could be fitted with two or three exponential components. The distributions in the four cells with the shortest mean open times (see Fig. 2A) were all described with two exponential components (Fig. 3), much like the NMDA channels in control rat granule cells (Köhr et al. 1993; Lieberman & Mody, 1994). In the remaining twenty-six neurons, the distributions of open times were best fitted with three exponential components (Fig. 3), which regularly included a time constant longer than 8 ms. Such distributions are not normally seen in control rat granule cells, but are readily observed after kindling-induced epilepsy (Köhr et al. 1993; Lieberman & Mody, 1995).
The average open probability of the NMDA channels was 1.35 (± 0.4) × 10−2.
Voltage dependence of the resolved open times
In four cells, a sufficient number of openings could be obtained at different membrane potentials to evaluate the relationship between the duration of openings and the holding potential. As illustrated in Fig. 4 for a cell with a long mean open time at -60 mV, the apparent open times were clearly dependent on membrane potential. The open times were shortened by hyperpolarizations, and the relationship between membrane voltage and open time was comparable to that described in adult rat CA1 neurons by Gibb & Colquhoun (1992), albeit with a slope of less than e-fold per 34 mV depolarization. We had no opportunity to study the voltage dependence of openings in the total absence of extracellular Ca2+ or Mg2+, a condition that obviates the dependence of open times on membrane voltage (Gibb & Colquhoun, 1992). In two of the four neurons, one of which is illustrated in Fig. 4, we observed a sharp decrease in the open duration with depolarizations beyond 0 mV. The NMDA channel openings at such extreme depolarizing potentials have not been studied to date in cell-attached recordings, and the low values may be due to inadequate resolution of openings, as increase in patch noise is inherent to recordings at these excessive depolarizing potentials.
Figure 4.

Voltage dependence of open durations
The mean apparent open times are plotted as a function of membrane potential for the openings underlying the larger conductance state events used to generate the current-voltage relationship in the cell shown in Fig. 1B. Because of the exponential distribution of the open times, the large variances have been omitted for clarity from the figure. The decline in mean open time at positive membrane potentials may result in part from the increase in patch noise at these depolarized potentials. Note, however, that there is a steep linear relationship between open time and voltage near resting membrane potentials (-90 to -60 mV).
Burst, cluster and supercluster distributions
Bursts are groupings of channel openings, including single events, which are separated by a critical gap derived from the second and third shut time components. The average Tc for defining bursts in our recordings was 2.5 ± 0.24 ms, while the average duration of bursts was 6.66 ± 0.9 ms. The distribution of bursts was fitted with three exponential components (Fig. 5), with the exception of the four cells with the shortest mean open times, for which the open time distributions were also fitted with two exponential distributions. The opening probability during bursts (burst Popen) was high: 0.936 ± 0.004.
Figure 5.

Distribution of burst and cluster durations
The panels depict the burst durations (left), and cluster durations (right) from the same cells shown in Fig. 3. The values were log-binned at 9 bins per decade and are shown on a square root abscissa. The time constants and the relative areas for each distribution are indicated in each panel. The number of exponential components in the burst time distributions is 2 for the channel with the shortest mean opening, but increases to 3 for the other channels. Similarly, the number of exponential components in the distribution of clusters increases from 3 to 4.
Clusters of openings result from the complex groupings of bursts separated by a critical shut time calculated from the third and fourth shut time components (Gibb & Colquhoun, 1992; Köhr et al. 1993; Lieberman & Mody, 1994). The critical cluster-defining Tc was 22.2 ± 2.0 ms, and the average cluster duration was 20.1 ± 3.3 ms. In 26/30 cells, the distribution of these long groupings of openings could be best described by four exponential components (Fig. 5). In the remaining four cells with the shortest of openings, three exponentials were sufficient to describe the cluster distributions. Figure 5 also illustrates an example of such a distribution. The cluster Popen was 0.775 ± 0.013.
The longest of the complex NMDA channel openings termed superclusters are considered to constitute a single ‘activation’ of the channel (Gibb & Colquhoun, 1991, 1992; Edmonds et al. 1995; Wyllie et al. 1998). In steady-state recordings it is not possible to determine the exact times at which agonist binds and unbinds. Nevertheless, the prolongation of shut times separating superclusters upon lowering the agonist concentration (Gibb & Colquhoun, 1991) is consistent with sojourns in a supercluster representing openings and closures of the channel in an agonist bound state. Since glutamate is only available for binding to postsynaptic receptors for a very short time following release (Clements et al. 1992), it is unlikely that individual NMDA receptors are activated more than once during a single synaptic event (Wyllie et al. 1998). Similarly, the use of low agonist concentrations in steady-state recordings helps to promote the study of these single activations as superclusters extracted from the single channel records.
Although a large number of openings are required to adequately resolve such events, we observed superclusters in twenty-seven of the thirty recorded human granule cells. The gaps defining the separations between superclusters were obtained from the last two exponential components of the shut times, which yielded a Tc of 307.9 ± 57 ms. Four exponential components were used to fit the distribution of superclusters, which had a mean duration of 116.7 ± 17.5 ms. The average supercluster Popen, calculated for individual superclusters as the total open time during the supercluster divided by the supercluster's length, was 0.577 ± 0.102. Because of the inclusion of single openings into superclusters, this value is somewhat higher than that expected from the average total open time per supercluster of 18.24 ± 3.14 ms.
Distribution of the number of apparent openings per bursts and clusters
As discussed by Colquhoun & Hawkes (1982), theoretically there should be a one-to-one correspondence between the number of exponential components describing the open time and total open time per burst distribution and the number of components in the geometrical distributions of the number of openings per burst. In practice, however, as noted also by Gibb & Colquhoun (1992), some components of the geometric distributions are easily missed. As illustrated in Fig. 6, the number of geometric components describing the number of apparent openings per burst was one in all but the cells with the longest open time distributions. The mean value for all the recordings was 1.74 ± 0.09. Similarly, the geometrical distributions of the number of openings per cluster contained only two components (Fig. 6), instead of the three or four expected from the number of respective exponential components. The average number of openings per cluster was 3.06 ± 0.26. However, as pointed out for the NMDA channel openings recorded in rat CA1 neurons by Gibb & Colquhoun (1992), the proportion of all bursts consisting of a single opening (> 0.60 in the case of our recordings in human neurons) was considerably larger than the proportion of clusters with a single apparent opening (< 0.47), indicating that many single opening bursts are in fact part of clusters with more than one opening.
Figure 6.

Distribution of the number of apparent openings in bursts and clusters
The left, middle and right panels in this figure are taken from openings observed in the same cells shown in Figs 3 and 5. A, the distribution of the number of apparent openings per burst is fitted with either one or two geometric components. The fitted geometric components are shown as continuous lines superimposed on the histograms. B, the distribution of the number of apparent openings per cluster are fitted with two geometric components. The mean and relative percentage for each component are indicated for both A and B.
Distribution of open times adjacent to shut times of given duration
The durations of adjacent open and shut intervals have been described as being inversely related for skeletal muscle Cl− channels and a large conductance Ca2+-activated K+ channel. Shorter open intervals were found to be adjacent to longer shut intervals (McManus et al. 1985). This type of analysis can provide insight into the gating mechanism of the single channels (Colquhoun & Hawkes, 1987; Blatz & Magleby, 1989; McManus & Magleby, 1989). The relationship between the mean open time of NMDA channels and the adjacent shut time recorded in rat hippocampal CA1 neurons shows a negative slope (Gibb & Colquhoun, 1992), indicating that long openings are flanked by short closures and vice versa. Such dependence of the rate constants on previous channel activity would contradict Markovian kinetic assumptions about the gating of NMDA channels. In reality, however, the open time constants of NMDA channels recorded in rat hippocampal neurons did not vary with previous channel opening/closing history. Since only the relative areas of the open time constants varied as a function of adjacent shut times, Markovian kinetic assumptions were not violated.
We examined the Markovian nature of the gating process of NMDA channels in human granule cells and compared it with that previously reported in rat neurons. We looked at the relationship between easily distinguishable ranges of channel shut times and the mean durations of the adjacent channel openings (McManus & Magleby, 1989; Gibb & Colquhoun, 1992). The linear two-dimensional inverse correlation between the adjacent dwell times is shown in Fig. 7A in a granule cell with one of the longest recorded mean open times. Figure 7B shows the three-dimensional distribution of this relationship (Magleby & Song, 1992). As expected from the NMDA channel openings recorded in rat hippocampal neurons (Gibb & Colquhoun, 1992), the open time constants were not dependent on the duration of adjacent shut times. Figure 8 illustrates that the three exponential components of the open time distributions remained similar with respect to the time constants of the distributions, regardless of the duration of the adjacent shut times. The negative correlation seen in Fig. 7A is a result of the relative areas of the fast, intermediate and slow time constants (Fig. 8), which indeed change with various durations of the adjacent shut times. As in rat hippocampal neurons (Gibb & Colquhoun, 1992), the area of the intermediate component was the least dependent on the length of the adjacent closures (Fig. 8).
Figure 7.

Adjacent dwell-time distributions
A, relationship between the mean durations of adjacent open and shut intervals. The graph shows the mean open time for each average adjacent shut time range for the channel shown in Fig. 1A. The openings were separated into groups based on the length of the adjacent closed times. The six ranges used (I, 50-100 μs; II, 100 μs to 1 ms; III, 1-10 ms; IV, 10-100 ms; V, 100 ms to 1 s; VI, 1-10 s) show that long openings occur adjacent to brief closures, while brief openings occur adjacent to long closures. The continuous straight line gives the linear regression (r= 0.91), while the other two lines indicate the 95 % confidence intervals. B, three-dimensional plot demonstrating the relationship between adjacent open and shut intervals. The most frequently observed occurrence is of brief shut times adjacent to long openings. This large number of long openings accounts for the prolonged mean open time. Note the relative absence of long openings flanked by long closed times that separate the superclusters.
Figure 8.

Mean time constants (left) and areas (right) of the exponential components describing conditional open time distributions
The apparent open times were taken from distributions II-VI of the adjacent shut time ranges indicated in Fig. 7A. For each range of shut times, three exponential components were fitted to the distributions of adjacent open times. The three open time constants (left) for the fast (□), intermediate (○) and slow (▵) components and their respective areas (right) are plotted against the average values for each of the five ranges of adjacent shut times.
Alterations in the activity of calcineurin may contribute to the epileptic phenotype
Based on the comparison of the NMDA channel openings in human TLE neurons with those recorded in granule cells of epileptic rats (Köhr et al. 1993; Mody & Lieberman, 1998), there are strong indications that a post-translational modification of the NMDA receptor channel may underlie its altered function in TLE. Since protein kinases and phosphatases are constantly involved in the control NMDA receptor function, a shift in the balance of phosphorylation/dephosphorylation towards the more phosphorylated state would lead to an up-regulation of NMDA channel activity. Pharmacological inhibition of calcineurin, a Ser/Thr phosphatase that normally provides a Ca2+-calmodulin-dependent negative feedback on NMDA channel openings in control rat dentate granule cells, leads to increases in NMDA channel open duration by promoting phosphorylation (Lieberman & Mody, 1994). Our preliminary data indicate a total lack of effect of calcineurin inhibitors on the openings of kindled rat NMDA channels (Mody & Lieberman, 1998). Thus the potential loss of such negative feedback by the effect of calcineurin on NMDA channels would result in the long duration of NMDA channel openings seen in both kindled rat and human TLE neurons.
Substantial calcineurin levels are present in the principal cells of the human hippocampus, including dentate granule cells, obtained from autopsy controls and from non-sclerotic surgically removed epileptic specimens (Lie et al. 1998). However, consistent with our hypothesis on the loss of the calcineurin feedback, this study also found much reduced calcineurin levels in dentate granule cells of TLE patients with Ammon's horn sclerosis. Therefore, we wanted to investigate the possibility of a functional decrease in calcineurin activity in human dentate granule cells from the TLE patient tissue used in our study.
After sufficient baseline recording of NMDA channel activity, seven of the thirty neurons were exposed to FK-506, a potent calcineurin inhibitor that prolongs NMDA channel openings in control rat neurons (Lieberman & Mody, 1994; Sik et al. 1998), but not in kindled rat granule cells (Mody & Lieberman, 1998). As in kindled neurons, the NMDA channel openings recorded in the TLE human dentate granule cells are also insensitive to 500 nM FK-506 (Fig. 9A). Overall, treating cells with FK-506 caused no significant change in any of the simple or complex groupings of channel openings (P > 0.5, paired t test, n= 7, Table 1). The plots in Fig. 9B also illustrate that in the seven cells exposed to FK-506 there was a one-to-one correspondence between the duration of openings or bursts under control conditions and those recorded in the presence of FK-506. The slopes of the regression lines (Fig. 9B) are not significantly different from 1.0, indicating the absence of a change caused by calcineurin inhibition. More data points would be necessary for a proper cluster analysis to establish whether there may be some residual calcineurin activity in human dentate granule cells, and whether this activity is present exclusively in cells that have short duration openings under control conditions.
Figure 9.

Pharmacological inhibition of the Ca2+-calmodulin-dependent protein phosphatase calcineurin by FK-506 fails to prolong NMDA channel openings in human TLE neurons
A, raw records of cell-attached channel openings taken from a cell before and after addition of FK-506 to the bath. Scale bars represent 25 ms/5 pA. B, the mean open times and the mean burst durations recorded in the 7 cells are plotted such that each point's abscissa is defined by the value of the dwell time measured during the control period (‘In control’), while the ordinate represents the dwell time obtained in the presence of FK-506 (‘In FK-506′). A linear regression of the form f (x) =ax was fitted to the points, and the values of the regression coefficients are indicated. The values of a are also given (±s.e.m.). Neither is significantly different from 1.0, indicating no change induced by FK-506 (also see Table 1) in mean open times and mean burst durations. The dotted lines correspond to the 95 % confidence intervals of the fits.
Table 1.
Summary of effects of calcineurin inhibition following perfusion of FK-506 (500 nM) on the mean (±s.e.m.) dwell time characteristics of human NMDA receptor channels in seven granule cells of temporal lobe epilepsy patients
| Control | 500 nm FK-506 | n | |
|---|---|---|---|
| Shut time (ms) | 464.02 ± 249.81 | 563.60 ± 171.92 | 7 |
| All openings (ms) | 2.65 ± 0.19 | 2.91 ± 0.34 | 7 |
| Openings in bursts with 1 opening (ms) | 2.10 ± 0.12 | 2.40 ± 0.31 | 7 |
| Openings in bursts with ≥ 2 openings (ms) | 2.97 ± 0.23 | 3.22 ± 0.38 | 7 |
| Burst length (ms) | 5.12 ± 0.59 | 5.65 ± 0.99 | 7 |
| Total open per burst (ms) | 4.62 ± 0.55 | 5.13 ± 0.91 | 7 |
| Number of openings per burst | 1.73 ± 0.11 | 1.75 ± 0.15 | 7 |
| Cluster length (ms) | 15.08 ± 1.65 | 12.85 ± 2.00 | 7 |
| Total open per cluster (ms) | 8.09 ± 1.13 | 8.28 ± 1.71 | 7 |
| Number of openings per cluster | 3.02 ± 0.28 | 2.73 ± 0.28 | 7 |
| Supercluster length (ms) | 109.63 ± 26.19 | 121.07 ± 54.72 | 7 |
| Total open per supercluster (ms) | 15.29 ± 2.45 | 12.60 ± 2.88 | 7 |
| Number of openings per supercluster | 5.64 ± 0.54 | 4.13 ± 0.51 | 7 |
DISCUSSION
This paper describes the properties of NMDA channel openings in adult human dentate gyrus granule cells. These channels are present in the human brain (Huntley et al. 1994; Scherzer et al. 1998), and have been implicated in numerous physiological and pathological processes in the nervous system (Collingridge & Watkins, 1994). Yet, in the absence of any high resolution recordings of single NMDA channel activity in adult human nerve cells, the properties of these channels have been merely inferred from those recorded in lower mammals. Our results fill this gap, but it needs to be pointed out that our recordings were obtained from granule cells acutely dissociated from hippocampi of TLE patients, and thus cannot be considered as ‘controls’. Nevertheless, there are a considerable number of similarities between the activation of NMDA channels in human neurons and those recorded in hippocampal neurons of adult rodents. However, there are some clear differences, including the duration and distribution of openings that more closely resemble the channel openings recorded in granule cells obtained from hippocampi of kindled rats used as an experimental model of TLE (Köhr et al. 1993). These key differences may thus provide insights into some deleterious alterations in neuronal excitability leading to an enhanced activation of NMDA channels in epileptic neurons (Mody, 1998).
Comparison of human and rodent NMDA channel properties
The conductance of NMDA channels in adult human central neurons was similar to that found in many other animal species. The main conductance state was the characteristic ‘50 pS’ level (McBain & Mayer, 1994). One difference between human and rat dentate gyrus granule cells is the presence of many discernible openings to subconductance states in the human neurons.
The distribution of openings and closures, and the number of exponential components in the distributions compare remarkably well with those observed in rat neurons prepared in an identical manner, and recorded under similar conditions (Köhr et al. 1993; Lieberman & Mody, 1994). Similar to NMDA channel openings observed in rodent dentate gyrus granule cells using low concentrations of L-aspartate and saturating concentrations of glycine, five exponential shut time components were needed to best describe the shut time distributions (Köhr et al. 1993; Lieberman & Mody, 1994). The number of shut time components is also shared with NMDA channels recorded in the rat CA1 region (Gibb & Colquhoun, 1991, 1992), and is consistent with the existence of at least five shut states of the channel. The similarities with the open time distributions are more equivocal.
In contrast to the two exponentials that adequately describe the openings in control rat granule cells (Köhr et al. 1993; Lieberman & Mody, 1994), the NMDA channel openings in all but four cells (which also possessed the shortest mean open times) of the thirty human neurons could be best fitted by the sum of three exponentials. This finding, however, agrees well with the distribution of NMDA channel openings in kindled (epileptic) rat granule cells (Köhr et al. 1993; Lieberman & Mody, 1995; Mody & Lieberman, 1998). The average duration of the apparent openings (2.71 ms), not corrected for missed events, also matches the values found in kindled rat neurons (Köhr et al. 1993) rather than those recorded in control rat granule cells (Köhr et al. 1993; Lieberman & Mody, 1994; Sik et al. 1998) or CA1 neurons (Gibb & Colquhoun, 1991, 1992; Sik et al. 1998). In spite of the semblance with the properties of kindled rat NMDA channel openings, the cell-to-cell variability of channel openings in human TLE granule cells was large (Fig. 2A). Even within one patient, different neurons had channels with widely varying mean open times. As all of the patients had significant granule cell loss, it is possible that some of the surviving neurons may have retained their control-like properties, while others have been altered during the process of TLE (Mody, 1998).
The characteristic openings of NMDA channels in bursts, clusters and superclusters were also present in human neurons. Of these, the duration of superclusters is of particular interest, because these types of openings may represent single activations of NMDA channels by agonist (Gibb & Colquhoun, 1991), and thus may underlie shaping of the synaptic currents mediated by NMDA receptors (Lester et al. 1990; Edmonds et al. 1995; Wyllie et al. 1998). The average supercluster duration recorded in the human neurons was 116.7 ms, which may correspond to the long decay (178 ms) of NMDA receptor-mediated synaptic currents recorded in human dentate gyrus granule cells in slice preparations (M. Isokawa & I. Mody, unpublished observations). There are several reports consistent with an enhanced NMDA receptor-mediated synaptic excitability in human tissue obtained from medically intractable epileptic patients who underwent therapeutic surgical resection of the hippocampus (Urban et al. 1990; Isokawa & Levesque, 1991; Masukawa et al. 1991; Isokawa et al. 1997). Thus the possible prolongation of NMDA channel openings in dentate gyrus granule cells may be a key factor in the observed hyperexcitability. The voltage-dependent prolongation of channel openings observed in the human neurons may further enhance the hyperexcitability at depolarized potentials.
Are NMDA channels altered in human epileptic neurons?
At this point, in the absence of recordings from control human neurons, this question is impossible to answer. However, available anatomical and biochemical data comparing NMDA receptor expression in the dentate gyrus of TLE patients with that in autopsy controls remains equivocal concerning the appearance of structural alterations of the NMDA channel. Early studies examining the distribution of the mRNA for NR1 described a loss of NR1-positive cells, which could be explained entirely by the degree of cell loss in the granule cell layer (Bayer et al. 1995). Using autoradiography to detect the binding of radiolabelled agonists, decreases in NMDA receptor expression were detected in CA3 and CA1 subfields of the hippocampus of TLE patients (Geddes et al. 1990; Hosford et al. 1991), although modest increases were seen in the subiculum and dentate molecular layer (Geddes et al. 1990) and in the CA1 and hilar regions (Brines et al. 1997). Using antibodies against glutamate receptor subtypes, Blümcke et al. (1996) found an enhanced expression of the GluR2(B) subunit in the molecular layer of the dentate gyrus of patients with TLE-associated Ammon's horn sclerosis, but no change in the levels of the NMDA receptor NR1 subunit. Furthermore, biochemical studies find no change in the polyamine-dependent modulation of [3H] MK-801 binding to NMDA receptors in human epileptic tissue (Subramaniam et al. 1994). On the other hand, recent studies have indicated an enhanced expression of NR2A/B subunits in the dentate gyrus using in situ hybridization (Mathern et al. 1997) and immunocytochemistry (Mathern et al. 1998).
While the issue of structural alterations of the NMDA receptor channel remains controversial, recent data strongly suggest that functional alterations of the NMDA channel may contribute to an enhanced activation of NMDA channels in human TLE granule cells (Mody, 1998). Recent immunohistochemical studies have compared control and non-sclerotic TLE tissue with sclerotic TLE hippocampi, and have demonstrated both a significant increase in the expression of the Ca2+-calmodulin-dependent protein kinase CaMKII and a significant reduction in the level of calcineurin in dentate granule cells from epileptic human hippocampus (Lie et al. 1998). Since both of these enzymes modulate NMDA receptor function (Lieberman & Mody, 1994, 1999), a TLE-induced imbalance between protein kinase and phosphatase activities may favour the more active, phosphorylated state of the NMDA channel, contributing to the prolonged NMDA channel openings observed in TLE. If a decrease in calcineurin levels or its activity underlies the increases in NMDA channel open durations in epileptic neurons, then the ability of exogenously applied calcineurin inhibitors to prolong NMDA channel openings should be greatly reduced (Mody & Lieberman, 1998). Indeed, we observed such an ‘occlusion’ of calcineurin inhibition in these human TLE granule cell recordings, i.e. FK-506 was ineffective in prolonging NMDA channel openings, perhaps because calcineurin activity was already reduced during the development of epilepsy.
Because of the inherent difficulty in obtaining untainted control human hippocampal neurons, our conclusions about possible changes in signal transduction pathways of epileptic neurons can be relied upon only in combination with pharmacological and anatomical findings. Future studies will need to address more closely the precise nature of the alterations leading to possible changes in NMDA channel properties. As we better characterize the anatomical and physiological properties of human hippocampal neurons from autopsy controls and non-epileptic resections of human CNS neurons, our ability to understand the structural and functional alterations that underlie TLE will continue to improve.
Acknowledgments
This study was supported by NIH/NINDS grant NS 36142 and the Coelho Endowment to I. M. D. N. L. was supported by a Hughes Predoctoral Fellowship and a Fellowship from the Epilepsy Foundation of America. The human tissue was kindly provided by Dr Itzak Fried (Division of Neurosurgery, UCLA School of Medicine) and Dr Masako Isokawa (Brain Research Institute, UCLA School of Medicine), both members of the NINDS Program Project ‘A Clinical Research Program for the Partial Epilepsies’ (Dr Jerome Engel, Jr, Program Director, Department of Neurology, UCLA School of Medicine). We would like to thank Dr László Seress (Department of Anatomy, University of Pécs, Hungary) for advice on the anatomical identification of acutely dissociated human dentate gyrus granule cells. We appreciate Brian Oyama's technical assistance.
References
- Ascher P, Bregestovski P, Nowak L. N-methyl-D-aspartate-activated channels of mouse central neurones in magnesium-free solutions. The Journal of Physiology. 1988;399:207–226. doi: 10.1113/jphysiol.1988.sp017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P, Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. The Journal of Physiology. 1988;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer T A, Wiestler O D, Wolf H K. Hippocampal loss of N-methyl-D-aspartate receptor subunit 1 mRNA in chronic temporal lobe epilepsy. Acta Neuropathologica (Berlin. 1995;89:446–450. doi: 10.1007/BF00307650. [DOI] [PubMed] [Google Scholar]
- Blatz A L, Magleby K L. Adjacent interval analysis distinguishes among gating mechanisms for the fast chloride channel from rat skeletal muscle. The Journal of Physiology. 1989;410:561–585. doi: 10.1113/jphysiol.1989.sp017549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümcke I, Beck H, Scheffler B, Hof P R, Morrison J H, Wolf H K, Schramm J, Elger C E, Wiestler O D. Altered distribution of the α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor subunit GluR2 (4) and the N-methyl-D-aspartate receptor subunit NMDAR1 in the hippocampus of patients with temporal lobe epilepsy. Acta Neuropathologica (Berlin. 1996;92:576–587. doi: 10.1007/s004010050564. 10.1007/s004010050564. [DOI] [PubMed] [Google Scholar]
- Brines M L, Sundaresan S, Spencer D D, De L N. Quantitative autoradiographic analysis of ionotropic glutamate receptor subtypes in human temporal lobe epilepsy: upregulation in reorganized epileptogenic hippocampus. European Journal of Neuroscience. 1997;9:2035–2044. doi: 10.1111/j.1460-9568.1997.tb01371.x. [DOI] [PubMed] [Google Scholar]
- Clark B A, Farrant M, Cull-Candy S G. A direct comparison of the single-channel properties of synaptic and extrasynaptic NMDA receptors. Journal of Neuroscience. 1997;17:107–116. doi: 10.1523/JNEUROSCI.17-01-00107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J D, Lester R A, Tong G, Jahr C E, Westbrook G L. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Oxford: Oxford University Press; The NMDA Receptor. [Google Scholar]
- Colquhoun D, Hawkes A G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philosophical Transactions of the Royal Society of London. 1982;300:1–59. doi: 10.1098/rstb.1982.0156. B. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Hawkes A G. A note on correlations in single ion channel records. Proceedings of the Royal Society of London. 1987;230:15–52. doi: 10.1098/rspb.1987.0008. B. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. The Journal of Physiology. 1985;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds B, Colquhoun D. Rapid decay of averaged single-channel NMDA receptor activations recorded at low agonist concentration. Proceedings of the Royal Society of London. 1992;250:279–286. doi: 10.1098/rspb.1992.0160. B. [DOI] [PubMed] [Google Scholar]
- Edmonds B, Gibb A J, Colquhoun D. Mechanisms of activation of glutamate receptors and the time course of excitatory synaptic currents. Annual Review of Physiology. 1995;57:495–519. doi: 10.1146/annurev.ph.57.030195.002431. 10.1146/annurev.ph.57.030195.002431. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr . Surgical Treatment of the Epilepsies. New York: Raven Press; 1993. [Google Scholar]
- Geddes J W, Cahan L D, Cooper S M, Kim R C, Choi B H, Cotman C W. Altered distribution of excitatory amino acid receptors in temporal lobe epilepsy. Experimental Neurology. 1990;108:214–220. doi: 10.1016/0014-4886(90)90125-c. 10.1016/0014-4886(90)90125-C. [DOI] [PubMed] [Google Scholar]
- Gibb A J, Colquhoun D. Glutamate activation of a single NMDA receptor-channel produces a cluster of channel openings. Proceedings of the Royal Society of London. 1991;243:39–45. doi: 10.1098/rspb.1991.0007. B. [DOI] [PubMed] [Google Scholar]
- Gibb A J, Colquhoun D. Activation of N-methyl-D-aspartate receptors by L-glutamate in cells dissociated from adult rat hippocampus. The Journal of Physiology. 1992;456:143–179. doi: 10.1113/jphysiol.1992.sp019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosford D A, Crain B J, Cao Z, Bonhaus D W, Friedman A H, Okazaki M M, Nadler J V, McNamara J O. Increased AMPA-sensitive quisqualate receptor binding and reduced NMDA receptor binding in epileptic human hippocampus. Journal of Neuroscience. 1991;11:428–434. doi: 10.1523/JNEUROSCI.11-02-00428.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J R, Colquhoun D, Cull-Candy S G. On the kinetics of large-conductance glutamate-receptor ion channels in rat cerebellar granule neurons. Proceedings of the Royal Society of London. 1988;233:407–422. doi: 10.1098/rspb.1988.0030. B. [DOI] [PubMed] [Google Scholar]
- Howe J R, Cull-Candy S G, Colquhoun D. Currents through single glutamate receptor channels in outside-out patches from rat cerebellar granule cells. The Journal of Physiology. 1991;432:143–202. doi: 10.1113/jphysiol.1991.sp018381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley G W, Vickers J C, Janssen W, Brose N, Heinemann S F, Morrison J H. Distribution and synaptic localization of immunocytochemically identified NMDA receptor subunit proteins in sensory-motor and visual cortices of monkey and human. Journal of Neuroscience. 1994;14:3603–3619. doi: 10.1523/JNEUROSCI.14-06-03603.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa G G, Avoli M. Excitatory synaptic transmission mediated by NMDA and non-NMDA receptors in the superficial/middle layers of the epileptogenic human neocortex maintained in vitro. Neuroscience Letters. 1992;143:83–86. doi: 10.1016/0304-3940(92)90238-3. 10.1016/0304-3940(92)90238-3. [DOI] [PubMed] [Google Scholar]
- Isokawa M, Avanzini G, Finch D M, Babb T L, Levesque M F. Physiologic properties of human dentate granule cells in slices prepared from epileptic patients. Epilepsy Research. 1991;9:242–250. doi: 10.1016/0920-1211(91)90058-n. 10.1016/0920-1211(91)90058-N. [DOI] [PubMed] [Google Scholar]
- Isokawa M, Levesque M, Fried I, Engel J., Jr Glutamate currents in morphologically identified human dentate granule cells in temporal lobe epilepsy. Journal of Neurophysiology. 1997;77:3355–3369. doi: 10.1152/jn.1997.77.6.3355. [DOI] [PubMed] [Google Scholar]
- Isokawa M, Levesque M F. Increased NMDA responses and dendritic degeneration in human epileptic hippocampal neurons in slices. Neuroscience Letters. 1991;132:212–216. doi: 10.1016/0304-3940(91)90304-c. 10.1016/0304-3940(91)90304-C. [DOI] [PubMed] [Google Scholar]
- Jahr C E, Stevens C F. Calcium permeability of the N-methyl-D-aspartate receptor channel in hippocampal neurons in culture. Proceedings of the National Academy of Sciences of the USA. 1993;90:11573–11577. doi: 10.1073/pnas.90.24.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp S J, Masu M, Eki T, Ozawa K, Nakanishi S. Molecular cloning and chromosomal localization of the key subunit of the human N-methyl-D-aspartate receptor. Journal of Biological Chemistry. 1993;268:3728–3733. [PubMed] [Google Scholar]
- Köhr G, de Koninck Y, Mody I. Properties of NMDA receptor channels in neurons acutely isolated from epileptic (kindled) rats. Journal of Neuroscience. 1993;13:3612–3627. doi: 10.1523/JNEUROSCI.13-08-03612.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester R A, Clements J D, Westbrook G L, Jahr C E. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990;346:565–567. doi: 10.1038/346565a0. 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Lie A A, Blümcke I, Beck H, Schramm J, Wiestler O D, Elger C E. Altered patterns of Ca2+/calmodulin-dependent protein kinase II and calcineurin immunoreactivity in the hippocampus of patients with temporal lobe epilepsy. Journal of Neuropathology and Experimental Neurology. 1998;57:1078–1088. doi: 10.1097/00005072-199811000-00011. [DOI] [PubMed] [Google Scholar]
- Lieberman D N, Isokawa M, Fried I, Mody I. Properties of single NMDA channels in dentate granule cells of temporal lobe epilepsy patients. Epilepsia. 1996;37:79. [Google Scholar]
- Lieberman D N, Mody I. Regulation of NMDA channel function by endogenous Ca2+-dependent phosphatase. Nature. 1994;369:235–239. doi: 10.1038/369235a0. 10.1038/369235a0. [DOI] [PubMed] [Google Scholar]
- Lieberman D N, Mody I. Kindling-induced long-lasting prolongation of single NMDA channel openings occludes the effect of phosphatase inhibition. Society for Neuroscience Abstracts. 1995;21:1113. [Google Scholar]
- Lieberman D N, Mody I. Casein kinase-II regulates NMDA channel function in hippocampal neurons. Nature Neuroscience. 1999;2:125–132. doi: 10.1038/5680. 10.1038/5680. [DOI] [PubMed] [Google Scholar]
- McBain C J, Mayer M L. N-methyl-D-aspartic acid receptor structure and function. Physiological Reviews. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- McLarnon J G, Curry K. Single channel properties of the N-methyl-D-aspartate receptor channel using NMDA and NMDA agonists: on-cell recordings. Experimental Brain Research. 1990;82:82–88. doi: 10.1007/BF00230840. [DOI] [PubMed] [Google Scholar]
- McManus O B, Blatz A L, Magleby K L. Inverse relationship of the durations of adjacent open and shut intervals for Cl and K channels. Nature. 1985;317:625–627. doi: 10.1038/317625a0. [DOI] [PubMed] [Google Scholar]
- McManus O B, Blatz A L, Magleby K L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflügers Archiv. 1987;410:530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- McManus O B, Magleby K L. Kinetic time constants independent of previous single-channel activity suggest Markov gating for a large conductance Ca-activated K channel. Journal of General Physiology. 1989;94:1037–1070. doi: 10.1085/jgp.94.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K L, Song L. Dependency plots suggest the kinetic structure of ion channels. Proceedings of the Royal Society of London. 1992;249:133–142. doi: 10.1098/rspb.1992.0095. B. [DOI] [PubMed] [Google Scholar]
- Masukawa L M, Higashima M, Hart G J, Spencer D D, O'Connor M J. NMDA receptor activation during epileptiform responses in the dentate gyrus of epileptic patients. Brain Research. 1991;562:176–180. doi: 10.1016/0006-8993(91)91205-f. [DOI] [PubMed] [Google Scholar]
- Mathern G W, Pretorius J K, Kornblum H I, Mendoza D, Lozada A, Leite J P, Chimelli L M, Fried I, Sakamoto A C, Assirati J A, Lévesque M F, Adelson P D, Peacock W J. Human hippocampal AMPA and NMDA mRNA levels in temporal lobe epilepsy patients. Brain. 1997;120:1937–1959. doi: 10.1093/brain/120.11.1937. [DOI] [PubMed] [Google Scholar]
- Mathern G W, Pretorius J K, Mendoza D, Lozada A, Leite J P, Chimelli L, Fried I, Sakamoto A C, Assirati J A, Adelson P D. Increased hippocampal AMPA and NMDA receptor subunit immunoreactivity in temporal lobe epilepsy patients. Journal of Neuropathology and Experimental Neurology. 1998;57:615–634. doi: 10.1097/00005072-199806000-00008. [DOI] [PubMed] [Google Scholar]
- Mody I. Ion channels in epilepsy. International Review of Neurobiology. 1998;42:199–226. doi: 10.1016/s0074-7742(08)60611-x. [DOI] [PubMed] [Google Scholar]
- Mody I, Lieberman D N. Lasting prolongation of NMDA channel openings after kindling. In: Corcoran M E, Moshe S L, editors. Kindling. Vol. 5. New York: Plenum Press; 1998. pp. 65–73. [Google Scholar]
- Mody I, Salter M W, MacDonald J F. Whole-cell voltage-clamp recordings in granule cells acutely isolated from hippocampal slices of adult or aged rats. Neuroscience Letters. 1989;96:70–75. doi: 10.1016/0304-3940(89)90245-0. [DOI] [PubMed] [Google Scholar]
- Planells-Cases R, Sun W, Ferrer-Montiel A V, Montal M. Molecular cloning, functional expression, and pharmacological characterization of an N-methyl-D-aspartate receptor subunit from human brain. Proceedings of the National Academy of Sciences of the USA. 1993;90:5057–5061. doi: 10.1073/pnas.90.11.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah D W. Human fetal central neurons in culture: voltage- and ligand-gated currents. Journal of Neurophysiology. 1995;74:1889–1899. doi: 10.1152/jn.1995.74.5.1889. [DOI] [PubMed] [Google Scholar]
- Scherzer C R, Landwehrmeyer G B, Kerner J A, Counihan T J, Kosinski C M, Standaert D G, Daggett L P, Veliçelebi G, Penney J B, Jr, Young A B. Expression of N-methyl-D-aspartate receptor subunit mRNAs in the human brain: Hippocampus and cortex. Journal of Comparative Neurology. 1998;390:75–90. doi: 10.1002/(sici)1096-9861(19980105)390:1<75::aid-cne7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Seress L. Morphological variability and developmental aspects of monkey and human granule cells: differences between the rodent and primate dentate gyrus. Epilepsy Research Supplement. 1992;7:3–28. [PubMed] [Google Scholar]
- Seress L, Mrzljak L. Basal dendrites of granule cells are normal features of the fetal and adult dentate gyrus of both monkey and human hippocampal formations. Brain Research. 1987;405:169–174. doi: 10.1016/0006-8993(87)91003-1. [DOI] [PubMed] [Google Scholar]
- Sigworth F J, Sine S M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophysical Journal. 1987;52:1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Hájos N, Gulácsi A, Mody I, Freund T F. The absence of a major Ca2+ signaling pathway in GABAergic neurons of the hippocampus. Proceedings of the National Academy of Sciences of the USA. 1998;95:3245–3250. doi: 10.1073/pnas.95.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D D, Spencer S S. Hippocampal resections and the use of human tissue in defining temporal lobe epilepsy syndromes. Hippocampus. 1994;4:243–249. doi: 10.1002/hipo.450040303. [DOI] [PubMed] [Google Scholar]
- Subramaniam S, O'Connor M J, Masukawa L M, McGonigle P. Polyamine effects on the NMDA receptor in human brain. Experimental Neurology. 1994;130:323–330. doi: 10.1006/exnr.1994.1210. [DOI] [PubMed] [Google Scholar]
- Sucher N J, Awobuluyi M, Choi Y B, Lipton S A. NMDA receptors: from genes to channels. Trends in Pharmacological Sciences. 1996;17:348–355. [PubMed] [Google Scholar]
- Urban L, Aitken P G, Friedman A, Somjen G G. An NMDA-mediated component of excitatory synaptic input to dentate granule cells in ‘epileptic’ human hippocampus studied in vitro. Brain Research. 1990;515:319–322. doi: 10.1016/0006-8993(90)90615-i. [DOI] [PubMed] [Google Scholar]
- von Campe G, Spencer D D, de Lanerolle N C. Morphology of dentate granule cells in the human epileptogenic hippocampus. Hippocampus. 1997;7:472–488. doi: 10.1002/(SICI)1098-1063(1997)7:5<472::AID-HIPO4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Wyllie D J, Behe P, Colquhoun D. Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. The Journal of Physiology. 1998;510:1–18. doi: 10.1111/j.1469-7793.1998.001bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


