Abstract
We carried out confocal Ca2+ imaging in myocytes permeabilized with saponin in ‘internal’ solutions containing: MgATP, EGTA and fluo-3 potassium salt.
Permeabilized myocytes exhibited spontaneous Ca2+ sparks and waves similar to those observed in intact myocytes loaded with fluo-3 AM.
In the presence of ‘low’[EGTA] (0·05 mm), Ca2+ waves arose regularly, even at relatively low [Ca2+] (50–100 nm, free). Increasing [EGTA] resulted in decreased frequency and propagation velocity of Ca2+ waves. Propagating waves were completely abolished at [EGTA] > 0·3 mm.
The frequency of sparks increased as a function of [Ca2+] (50–400 nm range) with no sign of a high affinity Ca2+-dependent inactivation process.
The rate of occurrence of Ca2+ sparks was increased by calmodulin and cyclic adenosine diphosphate-ribose (cADPR).
In the mammalian heart, Ca2+ influx through voltage-dependent Ca2+ channels in the sarcolemma triggers Ca2+-induced Ca2+ release from the sarcoplasmic reticulum (SR; Bers, 1991; Stern & Lakatta, 1992; Eisner et al. 1998). Under various conditions that result in increased cellular Ca2+ content Ca2+ release can occur spontaneously in the form of regenerative Ca2+ waves (Kort et al. 1985; Wier et al. 1987; Takamatsu & Wier, 1990; Lipp & Niggli, 1994; Trafford et al. 1995; Engel et al. 1995; Wussling & Salz, 1996; Cheng et al. 1996; Lukyanenko et al. 1996, 1999). Spontaneous Ca2+ waves have been implicated in certain cardiac dysfunctions such as triggered arrhythmias (Lakatta, 1992; Ishide, 1996). Nevertheless, the mechanisms of generation of Ca2+ waves and their relationship to the Ca2+ release process during normal excitation-contraction (E-C) coupling are not precisely understood. Recent studies using confocal Ca2+ imaging revealed that Ca2+ release during both normal E-C coupling and Ca2+ waves is a result of the summation of elementary release events, Ca2+ sparks (Cannell et al. 1994; Lopez-Lopez et al. 1995; Cheng et al. 1996). Ca2+ sparks can arise spontaneously and in response to electrical stimulation of the cell. Under normal cellular Ca2+ loading conditions sparks remain localized. When the cellular Ca2+ content is elevated they give rise to propagating Ca2+ waves (Cheng et al. 1996; Lukyanenko et al. 1996, 1999). The factors which could potentially influence the activity of individual Ca2+ release sites as well as the interaction between adjacent sites include Ca2+ levels in both cytosolic and SR luminal compartments, intracellular Ca2+ buffering, Ca2+ activation and inactivation properties of the release channels, and the presence of intracellular modulatory agents.
Permeabilization that allows rapid equilibration of various substances between extracellular fluid and cytosol has been a valuable tool for studying E-C coupling in both skeletal and cardiac muscle cells. In particular, Fabiato, by measuring force and aequorin light signals in permeabilized cardiac myocytes, has defined the Ca2+ dependence of SR Ca2+ release activation and inactivation (Fabiato, 1985), laying the foundation of the theory of Ca2+-induced Ca2+ release. In the present study we used confocal Ca2+ imaging to explore local Ca2+ signalling in permeabilized cardiac cells. We investigated the role of such factors as Ca2+ buffering, [Ca2+]i as well as calmodulin and cADPR in the modulation of local release events (Ca2+ sparks) and propagating Ca2+ waves.
METHODS
Cell isolation, permeabilization and experimental solutions
Adult Sprague-Dawley rats (200–300 g) were killed by lethal injection of Nembutal (Abbott Laboratories, 100 mg kg−1, i.p.), and single ventricular myocytes were obtained by enzymatic dissociation as described previously (Györke et al. 1997). The cells were loaded with fluo-3 by a 20 min incubation with 5 μm fluo-3 AM (acetoxymethyl ester form, Molecular Probes) at room temperature. The Tyrode solution contained (mm): 140 NaCl, 5.4 KCl, 0.5 MgCl2, 1–5 CaCl2, 10 Hepes, 0.25 NaH2PO4, 5.6 glucose; pH 7.3. The cardiac myocytes were permeabilized with saponin (0.01 % for 45–60 s) in an ‘internal’ solution containing (mm): 120 potassium aspartate, 3 MgATP (free [Mg2+]∼ 1 mm), 0.1 EGTA, 10 phosphocreatine, 5 U ml−1 creatine phosphokinase, and 8 % dextran (40 000, to prevent osmotic swelling of the cells); pH 7.2. The control experimental solution contained (mm): 120 potassium aspartate, 3 MgATP, 0.5 EGTA, 0.114 CaCl2 (free [Ca2+]∼ 100 nm), 10 phosphocreatine, 0.03 fluo-3 potassium salt (TefLabs, Austin, TX, USA) and 5 U ml−1 creatine phosphokinase; pH 7.2. Solutions with different buffering strengths and free [Ca2+] were prepared by adding appropriate amounts of K2EGTA and CaCal2. The free [Ca2+] at given total Ca2+, Mg2+, ATP and EGTA concentrations was calculated using a computer program (WinmAXC 1.80, Stanford University, CA, USA) and verified by measurements with a spectrofluorometer D-Scan (PTI, Monmouth Junction, NJ, USA) and the Ca2+ indicator fura-2 (TefLabs, Austin, TX, USA). The drugs were applied through a gravity-driven perfusion system. All experiments were performed at room temperature (21–23°C). All chemicals except fluo-3 and fura-2 were from Sigma.
Confocal microscope
Experiments were performed as described previously (Györke et al. 1997), using an Olympus laser scanning confocal microscope (LSM-GB200) equipped with an Olympus × 60, 1.4 NA objective. Fluo-3 was excited by light at 488 nm using a 25 mW argon laser with intensity attenuated to 1–3 %. Fluo-3 fluorescence was measured at wavelengths of > 515 nm. Images were acquired in the line-scan mode of the microscope at a rate of 2.1 or 8.3 ms per scan, with the scan line oriented along the longitudinal axis of the cell. An analog recording of fluorescence intensity was digitized into 640 pixels, giving a nominal pixel dimension of 0.41 μm. To reduce cell damage by the laser illumination, the position of the line scan was changed after acquiring three to six images from each particular location. Thus, in a typical cell the measurements could be performed for 10–15 min without significant alterations in Ca2+ spark properties.
Ca2+ sparks were detected and measured using a computer algorithm similar to that described previously (Song et al. 1997; Cheng et al. 1999). The program defines Ca2+ sparks as regions of elevated fluorescence relative to the standard deviation (s.d.) of background noise of the fluorescence image. The performance of the program at different event detection threshold settings was tested by using standard Ca2+ sparks of various intensities contaminated with appropriate amounts of random noise (Song et al. 1997). With the detection threshold set at a level of 2.6 ×s.d., the amplitude of events detected with 50 % efficiency was about 1.3F/Fo (where F is the recorded fluorescence intensity and Fo is background fluorescence) while the probability of false events was 1–2 %. The average propagation velocity of Ca2+ waves was determined by fitting a linear function to the position of the wave (defined at half-maximal amplitude) in the x–t plane (Lukyanenko et al. 1999). Image processing and analysis were performed using NIH Image (NIH, Bethesda, MD, USA) and IDL software (Research Systems Inc., Boulder, CO, USA).
RESULTS
Effects of permeabilization
Figure 1 illustrates the main steps of a permeabilization experiment in a single isolated rat ventricular myocyte pre-loaded with fluo-3 AM. The series of line-scan images in B were acquired before permeabilization (a), after permeabilization with 0.01 % saponin for 1 min in an internal solution containing no dye (b) and after addition to the internal solution of 30 μm fluo-3 potassium salt in the presence of 0.1 mm (c) or 0.5 mm EGTA (d). Photographic wide field images of the myocyte before and after permeabilization are illustrated at the top (A). As described previously (Cheng et al. 1993, 1996; Lukyanenko et al. 1996), the intact cell spontaneously exhibited sporadic Ca2+ sparks and occasional Ca2+ waves. Permeabilization was confirmed by the disappearance of all the fluorescence signals in the bathing solution containing no dye and re-emergence of the signals after introduction of the free acid form of the dye into the bathing solution. Note that the overall appearance of the cell and the Ca2+ signals before and after permeabilization are very similar. Figure 1C shows surface plots of Ca2+ sparks in intact and permeabilized cells in the presence of 0.1 or 0.5 mm EGTA. Table 1 summarizes spark statistics for the same conditions. As can be seen, permeabilization had no significant impact on the frequency, amplitude, width or length of the events (for all groups, P > 0.05). The similarities in the spatio-temporal properties of Ca2+ sparks at 0.1 and 0.5 mm EGTA are likely to be due to the slow rate of Ca2+ binding by EGTA. The overall similarities between Ca2+ sparks and waves in intact and permeabilized cells suggest that our permeabilization procedure does not significantly alter the Ca2+ signalling mechanisms in the cell.
Figure 1. The effects of permeabilization on Ca2+ sparks and Ca2+ waves in ventricular myocytes.
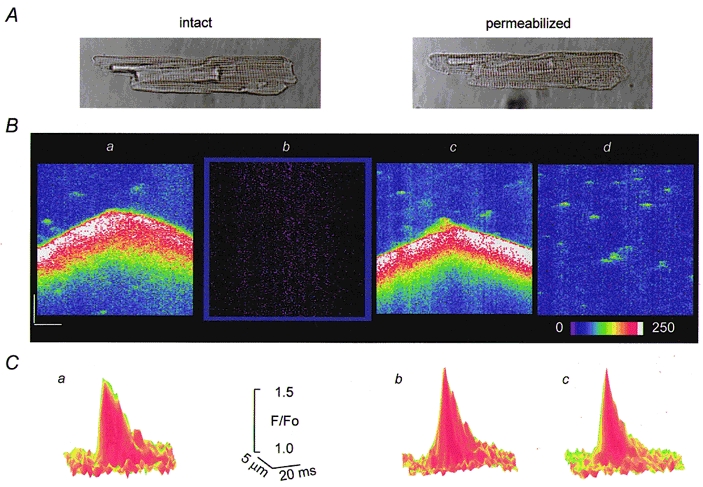
A, images of a cardiac myocyte obtained in transmitted light before and after permeabilization with saponin. B, line-scan images of fluorescence in a portion of the same cell pre-loaded with fluo-3 AM measured before permeabilization (a, [Ca2+]o= 5 mm), after permeabilization in an internal solution with no dye (b) and after addition to the internal solution 30 μm fluo-3 potassium salt in the presence of 0.1 (c) or 0.5 mm EGTA (d) (pCa 7). Calibration bars: horizontal 10 μm, vertical 0.4 s, the colour bar represents changes in units of absolute fluorescence. C, surface plots of Ca2+ sparks measured before permeabilization (a) and after permeabilization in the presence of 0.1 or 0.5 mm EGTA (b and c, respectively). Each plot was obtained by averaging 10 individual events.
Table 1.
Spatio-temporal properties of Ca2+ sparks in intact and permeabilized ventricular cells
| Frequencya | Amplitude (F/Fo) | Duration (ms)b | Width (μm)c | ||
|---|---|---|---|---|---|
| Intact cells | [Ca2+]o= 1 mm | 4.60 ± 0.48 | 1.69 ± 0.02 | 27.7 ± 1.1 | 1.75 ± 0.07 |
| Skinned cells | [EGTA] = 0.1 mm | 4.25 ± 0.88 | 1.72 ± 0.02 | 26.1 ± 0.7 | 1.85 ± 0.05 |
| [EGTA] = 0.5 mm | 6.36 ± 0.65 | 1.71 ± 0.03 | 25.3 ± 0.6 | 1.96 ± 0.06 |
Data represented as means ±s.e.m. of measurements, n= 30–235.
Spark frequency was defined as the number of events per second per 100 μm line scanned.
Duration
width of spark were measured at half-maximal amplitude.
Effects of EGTA
Theoretical studies predict that soluble intracellular Ca2+ buffers must have a strong effect on interaction between release sites by lowering the rate of effective diffusion of Ca2+ (Keizer et al. 1998). We experimentally tested the effects of intracellular Ca2+ buffering on Ca2+ waves in permeabilized cardiac myocytes. In these experiments Ca2+ buffering strength was varied by addition of different concentrations of EGTA to the bathing solution, while adjusting basal Ca2+ to a constant value of 100 nm. In internal solutions with low Ca2+ buffering strength (0.05 mm EGTA), Ca2+ waves arose relatively frequently (0.06 ± 0.01 s−1, n= 21) and propagated typically through the entire cell with a velocity of about 70 μm s−1 (66 ± 6 μm s−1, n= 21; Fig. 2A). Elevation of EGTA concentration to 0.1 mm resulted in decreased frequency and propagation velocity of waves (0.03 ± 0.01 s−1 and 53 ± 1 μm s−1, respectively, n= 12; Fig. 2B). Further increase in [EGTA] resulted in fragmentation of Ca2+ waves into abortive responses (Fig. 2C and D). Images obtained at high [EGTA] also clearly show that Ca2+ waves arise from sequential activation of discrete release events, revealing the saltatory nature of wave propagation. Propagating Ca2+ waves were completely abolished at [EGTA] > 0.3 mm. The fast Ca2+ buffer BAPTA prevented Ca2+ waves even at lower concentrations (∼0.1 mm, not shown). These results indicate that Ca2+ buffering has a profound influence on Ca2+ waves.
Figure 2. Effects of calcium buffering on Ca2+ waves in saponin-permeabilized myocytes.

A, B, C and D, representative line-scan images of fluorescence recorded in a permeabilized myocyte in the presence of 0.05 mm (A), 0.1 mm (B), 0.2 mm (C) and 0.3 mm EGTA (D). Free [Ca2+] in all cases was adjusted to 100 nm. Calibration bars: horizontal 15 μm, vertical 0.35 s.
Effects of [Ca2+]
Ca2+ is the principal regulator of the activity of ryanodine receptors (RyRs) in the heart. Although the Ca2+ dependence of RyR activation and inactivation has been described in in vitro experiments (Coronado et al. 1994), the Ca2+ dependency of RyRs in situ remains uncertain because of the difficulties in measuring and controlling [Ca2+] in the diadic cleft during E-C coupling. We took advantage of our permeabilized myocyte preparation to investigate the relationship between Ca2+ and the activity of Ca2+ release sites. Figure 3A illustrates the effects of increasing [Ca2+] from 50 nm to 100, 150 or 200 nm in the presence of 0.1 mm EGTA. Elevating [Ca2+] resulted in increased frequency of Ca2+ waves. In addition, at elevated [Ca2+] multi-focal Ca2+ waves arising simultaneously from several independent sites became evident. Quantitative assessments of spark properties at high [Ca2+] were impaired by the presence of Ca2+ waves and elevated background fluorescence. Increases in background fluorescence also limited the Ca2+ concentrations in the experimental solution to a rather limited range because of saturation of the photomultiplier at high [Ca2+]i. Therefore, we employed bathing solutions containing high [EGTA] to abolish spontaneous Ca2+ waves. We also corrected the images for changes in background fluorescence at different [Ca2+]i by adjusting the fluorescence of the bathing solution outside the permeabilized cells to the same level. Figure 3B shows representative images from such an experiment recorded during successive increases of [Ca2+] from 100 to 150, 250 and 400 nm and a subsequent return to 100 nm. The effects of [Ca2+] on the frequency and amplitude of Ca2+ sparks from the same experiment are documented in Fig. 3C. Increasing [Ca2+] in the range 100–400 nm resulted in a gradual increase in frequency of sparks. Changing back to the original solution with 100 nm Ca2+ resulted in restoration of spark frequency to the control level, thus indicating no significant signs of a run-down in sparking activity. The changes in the frequency of sparks were accompanied only by insignificant alterations in the amplitude of sparks (10 % at 150 nm[Ca2+], grey bars). The results of five experiments are summarized in Fig. 3D, which plots the frequency of Ca2+ sparks as a function of [Ca2+]. It is unlikely that the amplitude of Ca2+ sparks was saturated at elevated [Ca2+]. The KD of fluo-3 in the myoplasm has been estimated to be near 1 μm (Harkins et al. 1993). Ca2+ sparks with an amplitude of 200–300 nm would rise above a background [Ca2+] of 400 nm only to a level of 600–700 nm, which still would be in the linear range of the indicator.
Figure 3. Effects of [Ca2+]i on Ca2+ sparks and Ca2+ waves in saponin-permeabilized myocytes.

A, representative line-scan images of fluorescence recorded in a permeabilized myocyte at various [Ca2+]i levels (indicated at the top of the respective images) in the presence of 0.1 mm EGTA. B, line-scan images of Ca2+ sparks corrected for increases of background fluorescence at various [Ca2+]i levels (indicated at the top of the respective images) in the presence of 0.5 mm EGTA. Calibration bars: horizontal 15 μm (A) and 20 μm (B), vertical 0.5 s (A) and 0.1 s (B). C, Ca2+ spark frequency (blue) and amplitude (light grey) as a function of time before and after elevating [Ca2+]i to indicated levels for the experiment shown in B. D, Ca2+ spark frequency as a function of [Ca2+]i. The values are represented as means ±s.e.m. obtained in 5 experiments. The lines were obtained by fitting the data according to the equation f=fmax{[Ca2+]n/([Ca2+]n+KDn)}, where fmax= 10 000 events s−1 (100 μm)−1, KD= 9.9 μm and n= 1.6 (blue line); fmax= 20 000 events s−1 (100 μm)−1; KD= 15 μm and n= 1.6 (red line); and fmax= 30 000 events s−1 (100 μm)−1; KD= 20 μm and n= 1.6 (green line).
The observed potentiation of Ca2+ sparks could be attributed also to a possible increase in the SR Ca2+ content (Fabiato, 1992; Orchard et al. 1998) affecting the activity of the Ca2+ release channels at luminal sites (Györke & Györke, 1998). We assessed the potential role of this mechanism, by using thapsigargin to prevent accumulation of extra Ca2+ into the SR upon elevation of cytosolic Ca2+. Representative images from such an experiment are shown in Fig. 4A. The effects of [Ca2+] on spark frequency in the same experiment are quantified in Fig. 4B. We applied 10 μm thapsigargin for 1 min before elevating [Ca2+] from 100 nm to 250 nm. At this concentration and exposure time thapsigargin inhibits SR Ca2+ uptake without causing a significant loss in the SR Ca2+ content (Bassani et al. 1993; Lukyanenko et al. 1999). Under these experimental conditions increasing [Ca2+]i resulted in an about 6-fold (from 7.1 ± 0.9 to 39.8 ± 1.9 event s−1 (100 μm)−1, n= 5) increase in sparking frequency, which is similar to that observed in experiments without thapsigargin. Following more than 2–3 min of continuous exposure to thapsigargin, the frequency of sparks gradually decreased, apparently due to a loss of Ca2+ from the SR (Bassani et al. 1993; Lukyanenko et al. 1999). Taken together, these results suggest that under our experimental conditions, the increase in sparking activity cannot be attributed to increased SR Ca2+ load.
Figure 4. The effects of elevating [Ca2+]i on sparking activity in the presence of thapsigargin.

A, representative line-scan images of fluorescence acquired before (a and b) and at different times (2 and 3 min) after increasing [Ca2+]i from 80 nm to 250 nm in the presence of thapsigargin (c and d, respectively). Thapsigargin (10 μm) was introduced into the bathing solution 1 min before elevating [Ca2+]i. Calibration bars: horizontal 20 μm, vertical 0.15 s. B, Ca2+ spark frequency as a function of time before and after the addition of thapsigargin into the bathing solution in the same experiment. The experimental protocol is presented schematically at the top.
Effects of calmodulin and cADPR
We tested the effects on release site activity of certain membrane-impermeable putative regulators of the SR Ca2+ release such as cyclic adenosine diphosphate-ribose (cADPR, MW 541.3) and calmodulin (MW 16 680), which would be difficult to study in cells with an intact sarcolemma. Figure 5 illustrates representative images of Ca2+ sparks before and after exposure of two permeabilized cells to calmodulin (A, 5 μm) and cADPR (C, 5 μm), while Table 2 summarizes the effects of these drugs on Ca2+ spark properties. Both drugs caused dramatic increases in the frequency of Ca2+ sparks with no significant changes in the amplitude and spatio-temporal properties of the events. The effects of cADPR developed over a period of 2–6 min, and they were readily reversible. The onset of potentiation of Ca2+ sparks by calmodulin was considerably slower, 10–15 min. In addition, the cells showed no significant recovery upon reverting to the control solution during a period of 5–10 min. The slow onset and lack of reversibility of calmodulin effects could be attributed to the slow diffusion rate of this high molecular weight agent as well as to the fact that the effects are likely to involve long-lasting biochemical changes (phosphorylation of SR proteins). Incubation of the cells with calmodulin also resulted in a significant increase in the amplitude of caffeine-induced Ca2+ transients (31.2 ± 4.4 %, n= 5; Fig. 5B), suggesting that the effects of this agent on Ca2+ sparks might be mediated by changes in the SR Ca2+ load. These results further illustrate our ability to manipulate the environment of the release sites in ways not possible in intact cells.
Figure 5. Effects of calmodulin and cADPR on Ca2+ sparks.

A, representative line-scan images of fluorescence changes acquired under control conditions (left-hand panel), 15 min after exposure of the cells to 5 μm calmodulin (middle panel), and 10 min after changing back to the control solution (right-hand panel). Calibration bars: horizontal 10 μm, vertical 0.2 s. B, caffeine-induced Ca2+ transients measured in the same cell at the same stages of the experiment as in A. Caffeine (20 mm) was applied for 2 s. C, representative line-scan images of fluorescence changes measured under control conditions (left-hand panel), 2 min after exposure of the cells to 5 μm cADPR (middle panel), and 5 min after reverting back to the control solution (right-hand panel). Calibration bars: horizontal 10 μm, vertical 0.2 s.
Table 2.
Spatio-temporal properties of Ca2+ sparks in permeabilized ventricular cells before and after addition of calmodulin or cADPR
| Frequencya | Amplitude (F/Fo) | Duration (ms)b | Width (μm)c | ||
|---|---|---|---|---|---|
| [Calmodulin] | 0 7 | 7 ± 1.2 | 1.8 ± 0.02 | 26 ± 0.9 | 2.02 ± 0.06 |
| 5 μm | 15.5 ± 2.2* | 1.9 ± 0.03 | 27 ± 0.6 | 1.93 ± 0.04 | |
| [cADPR] | 0 | 5.1 ± 1.1 | 1.5 ± 0.02 | 29 ± 1.4 | 1.92 ± 0.11 |
| 5 μm | 8.9 ± 1.8* | 1.5 ± 0.01 | 29 ± 1.3 | 1.94 ± 0.09 |
Data represented as means ±s.e.m. of measurements, n= 24–329.
Spark frequency was defined as the number of events per second per 100 μm line scanned.
Duration
width of spark were measured at half-maximal amplitude.
Significantly different from control at P < 0.05.
DISCUSSION
In the present study we investigated the Ca2+-releasing activity of the SR in saponin-permeabilized cardiac myocytes using confocal Ca2+ imaging. In essential internal solutions designed to maintain the basic functional integrity of Ca2+ stores, permeabilized cardiac myocytes exhibited Ca2+ sparks and Ca2+ waves similar to those observed in intact cells. During permeabilization, the content of the cells is diluted in the internal bathing solution (∼100 000-fold dilution). Thus, it appears that no endogenous factors such as cytosolic kinases and phosphatases that could be lost through equilibration with the bathing solution are essential to maintaining basic activation and inactivation properties of the release sites.
We described explicitly the dependency of spark frequency upon [Ca2+] in the range 50–400 nm. It is interesting to speculate how these results might relate to the overall Ca2+ dependency of Ca2+ spark activation in cardiomyocytes. If we assume that all of the the approximately 200 release units (Sommer, 1995; Franzini-Armstrong & Protasi, 1997) contained in the volume of a rectangular block of approximately 1 μm width (diameter of the laser beam spot), 1 μm height (depth of field of the microscope) and 100 μm length scanned along a myocyte can be activated within 10 ms (Cannell et al. 1994), the maximal frequency of sparks would be 20 000 events s−1 (100 μm)−1. This maximal sparking rate would be consistent with the estimated frequency of sparks during action potential stimulation, when presumably most of the release units become activated (∼15 000 events s−1 (100 μm)−1, Cannel et al. 1994). We fitted our data presented in Fig. 3D to Hill functions with three different maximal sparking rates of 10 000, 20 000 and 30 000 events s−1 (100 μm)−1 (Fig. 3D). The best fits to the data for the three specified maximal sparking rates yielded Ca2+ dissociation constants of 9.9, 15.2 and 19.6 μm and Hill coefficients equal to 1.6 in all cases. The estimated values of KD of Ca2+ sensitivity of release sites are in the range of Ca2+ dependency of RyR open probability measured in lipid bilayer experiments in the presence of physiological concentrations of Mg2+ and ATP (KD≈ 30 μm; Györke & Györke, 1998). The relatively low KD and supra-linear dependency of release site activation on [Ca2+] could explain why most release events normally remain localized and do not initiate calcium-induced calcium release (CICR) in the neighbouring release sites (Stern et al. 1999). We should point out, however, that because of the limited range of [Ca2+] over which the sparks could be measured the obtained characteristics of Ca2+ dependency of release sites represent only approximate estimates.
In mechanically skinned Purkinje cells, Fabiato showed that sub-micromolar basal Ca2+ resulted in a substantial decrease in the macroscopic CICR, suggesting that Ca2+ release is inhibited at a high affinity inactivation site (Fabiato, 1985). Under our experimental conditions, sparking activity does not appear to be influenced by such a high Ca2+ affinity inactivation process because spark frequency only increased steadily upon elevations of [Ca2+]. Our results, however, do not rule out the possibility that local release events are controlled by high local Ca2+ through a low-affinity inactivation process. Inhibition of RyRs by Ca2+ in a range of 50 μm to 10 mm has been reported in both lipid bilayer (Laver et al. 1995; Copello et al. 1997; Györke & Györke, 1988; Marengo et al. 1998) and vesicle flux experiments (Chamberlain et al. 1984; Zimanyi & Pessah, 1991; Chu et al. 1993). Such a low affinity inactivation, as well as a possible use-dependent inactivation induced by prior activation of the channel (Sham et al. 1998; Zahradnikova et al. 1999), could contribute to local release termination without influencing the stationary frequency of sparks. Indeed, at the sub-micromolar [Ca2+] employed in our experiments, the probability of reactivation of individual release units must be quite low (P= 0.09 assuming 200 independent release units sparking at a rate of 60 events s−1 (100 μm)−1) for time periods compatible with the time of recovery from such inactivated conditions (∼1 s).
The propagating Ca2+ waves were profoundly influenced by Ca2+ buffering. At low Ca2+ buffering strength (EGTA < 100 μm), waves arose regularly even at low [Ca2+]. Increasing EGTA resulted in decreased wave generation. Propagating Ca2+ waves were completely abolished at EGTA > 0.3 mm. These results reveal the importance of intracellular Ca2+ buffering in the confinement of CICR in cardiac cells. The high sensitivity of Ca2+ wave propagation to Ca2+ buffering is consistent with a saltatory mechanism of Ca2+ wave propagation (Keizer et al. 1998; Lukyanenko et al. 1999). In contrast to a continuous model of Ca2+ waves in a saltatory model, the release sites are spatially separated and thus are sensitive to interventions impairing the free diffusion of Ca2+. In fact, site-to-site propagation by sequential activation of Ca2+ sparks was evident in the presence of intermediate EGTA concentrations (Fig. 2C and D).
The activity of release sites was affected by the endogenous signalling molecules calmodulin and cADPR. Interestingly, calmodulin, which has been shown to inhibit RyRs (by direct interaction or through the activation of Ca2+-calmodulin-dependent protein kinase) in most RyR reconstitution studies (Meissner & Henderson, 1987; Takasago et al. 1991; Lokuta et al. 1995; Hain et al. 1995), enhanced sparking activity in cardiac cells. We attribute this potentiation to increased accumulation of Ca2+ within the SR due to stimulation of the SR Ca2+-ATPase (Narayanan & Xu, 1997) and subsequent activation of RyRs by Ca2+ at luminal sites (Györke & Györke, 1988). Indeed, exposure of the cells to calmodulin resulted in a significant increase in the SR Ca2+ content (Fig. 5B). These results are in line with the previous studies suggesting that luminal Ca2+ is a critical determinant of the functional state of Ca2+ release in cardiac muscle (Bassani et al. 1995; Györke et al. 1997; Eisner et al. 1998). The demonstrated potentiation of Ca2+ sparks by cADPR supports the previous studies suggesting that cADPR can enhance Ca2+ release presumably through potentiation of RyRs (Meszaros et al. 1993; Iino et al. 1997; Galione et al. 1998). Taken together, our results show that saponin-permeabilized cells can be a useful model system for studying both spatial and temporal aspects of Ca2+ signalling in the heart under conditions in which the environment surrounding the Ca2+ release channels can be controlled precisely.
Acknowledgments
This work was supported by the National Institutes of Health (HL 63043, HL 52620, HL 03739). S. Györke is an Established Investigator of the American Heart Association.
References
- Bassani JW, Bassani RA, Bers DM. Twitch-dependent SR Ca accumulation and release in rabbit ventricular myocytes. American Journal of Physiology. 1993;265:C533–540. doi: 10.1152/ajpcell.1993.265.2.C533. [DOI] [PubMed] [Google Scholar]
- Bassani JW, Yuan W, Bers DM. Fractional SR Ca2+ release is regulated by trigger Ca2+ and SR Ca2+ content in cardiac myocytes. American Journal of Physiology. 1995;268:C1313–1329. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991. [Google Scholar]
- Cannell MB, Cheng H, Lederer WJ. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophysical Journal. 1994;67:1942–1956. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain BK, Volpe P, Fleischer S. Calcium-induced calcium release from purified cardiac sarcoplasmic reticulum vesicles. Journal of Biological Chemistry. 1984;259:7540–7546. [PubMed] [Google Scholar]
- Cheng H, Lederer W, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]i waves in cardiac myocytes. American Journal of Physiology. 1996;270:C148–159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- Cheng H, Song LS, Shirokova N, Gonzalez A, Lakatta EG, Rios E, Stern MD. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophysical Journal. 1999;76:606–617. doi: 10.1016/S0006-3495(99)77229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu A, Fill M, Stefani E, Entman ML. Cytoplasmic Ca2+ does not inhibit the cardiac muscle sarcoplasmic reticulum ryanodine receptor Ca2+ channel, although Ca2+-induced Ca2+ inactivation of Ca2+ release is observed in native vesicles. Journal of Membrane Biology. 1993;135:49–59. doi: 10.1007/BF00234651. [DOI] [PubMed] [Google Scholar]
- Copello JA, Barg S, Onoue H, Fleicher S. Heterogeneity of Ca2+ gating of skeletal muscle and cardiac ryanodine receptor. Biophysical Journal. 1997;73:141–156. doi: 10.1016/S0006-3495(97)78055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado R, Morrissette J, Sukhareva M, Vaughan DM. Structure and function of ryanodine receptors. American Journal of Physiology. 1994;266:C1485–1504. doi: 10.1152/ajpcell.1994.266.6.C1485. [DOI] [PubMed] [Google Scholar]
- Eisner DA, Trafford AW, Diaz ME, Overend CL, O'Neill SC. The control of Ca release from the cardiac sarcoplasmic reticulum: regulation versus autoregulation. Cardiovascular Research. 1998;38:589–604. doi: 10.1016/s0008-6363(98)00062-5. [DOI] [PubMed] [Google Scholar]
- Engel J, Sowerby AJ, Finch AE, Fechner M, Stier A. Temperature dependence of Ca2+ wave properties in cardiomyocytes: implications for the mechanism of autocatalytic Ca2+ release in wave propagation. Biophysical Journal. 1995;68:40–45. doi: 10.1016/S0006-3495(95)80196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced calcium release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. Journal of General Physiology. 1985;85:291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Two kinds of calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cardiac cells. In: Frank GB, editor. Excitation-Contraction Coupling in Skeletal, Cardiac and Smooth Muscle. New York: Plenum Press; 1992. pp. 245–262. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiological Reviews. 1997;77:699–729. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- Galione A, Cui Y, Empson R, Iino S, Wilson H, Terrar D. Cyclic ADP-ribose and the regulation of calcium-induced calcium release in eggs and cardiac myocytes. Cell Biochemistry and Biophysics. 1998;28:19–30. doi: 10.1007/BF02738307. [DOI] [PubMed] [Google Scholar]
- Györke I, Györke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophysical Journal. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S, Lukyanenko V, Györke I. Dual effects of tetracaine on spontaneous calcium release in rat ventricular myocytes. The Journal of Physiology. 1997;500:297–309. doi: 10.1113/jphysiol.1997.sp022021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hain J, Onoue H, Mayrleitner M, Fleischer S, Schindler H. Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from cardiac muscle. Journal of Biological Chemistry. 1995;270:2074–2081. doi: 10.1074/jbc.270.5.2074. [DOI] [PubMed] [Google Scholar]
- Harkins AB, Kurebayashi N, Baylor SM. Resting myoplasmic free calcium in frog skeletal muscle fibers estimated with fluo-3. Biophysical Journal. 1993;65:865–881. doi: 10.1016/S0006-3495(93)81112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino S, Cui Y, Galione A, Terrar DA. Actions of cADP-ribose and its antagonists on contraction in guinea pig isolated ventricular myocytes. Influence of temperature. Circulation Research. 1997;81:879–884. doi: 10.1161/01.res.81.5.879. [DOI] [PubMed] [Google Scholar]
- Ishide N. Intracellular calcium modulators for cardiac muscle in pathological conditions. Japanese Heart Journal. 1996;37:1–17. doi: 10.1536/ihj.37.1. [DOI] [PubMed] [Google Scholar]
- Keizer J, Smith G, Ponce-Dawson S, Pearson J. Saltatory propagation of Ca2+ waves by Ca2+ sparks. Biophysical Journal. 1998;75:595–600. doi: 10.1016/S0006-3495(98)77550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kort AA, Capogrossi MC, Lakatta EG. Frequency, amplitude, and propagation velocity of spontaneous Ca2+-dependent contractile waves in intact adult rat cardiac muscle and isolated myocytes. Circulation Research. 1985;57:844–855. doi: 10.1161/01.res.57.6.844. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Functional implications of spontaneous sarcoplasmic reticulum Ca2+ release in the heart. Cardiovascular Research. 1992;26:193–214. doi: 10.1093/cvr/26.3.193. [DOI] [PubMed] [Google Scholar]
- Laver DR, Roden LD, Ahern GP, Eager KR, Junankar PR, Dulhunty AF. Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. Journal of Membrane Biology. 1995;147:7–22. doi: 10.1007/BF00235394. [DOI] [PubMed] [Google Scholar]
- Lipp P, Niggli E. Modulation of Ca2+ release in cultured neonatal rat cardiac myocytes. Circulation Research. 1994;74:979–990. doi: 10.1161/01.res.74.5.979. [DOI] [PubMed] [Google Scholar]
- Lokuta AJ, Rogers TB, Lederer WJ, Valdivia HH. Modulation of cardiac ryanodine receptors of swine and rabbit by a phosphorylation-dephosphorylation mechanism. The Journal of Physiology. 1995;487:609–622. doi: 10.1113/jphysiol.1995.sp020904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lopez JR, Shacklock PS, Balke CW, Wier WG. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995;268:1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V, Györke I, Györke S. Regulation of calcium release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflügers Archiv. 1996;432:1047–1054. doi: 10.1007/s004240050233. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V, Subramanian S, Györke I, Wiesner T, Györke S. The role of luminal Ca2+ in generation of Ca2+ waves in rat ventricular myocytes. The Journal of Physiology. 1999;518:173–186. doi: 10.1111/j.1469-7793.1999.0173r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengo JJ, Hidalgo C, Bull R. Sulfhydryl oxidation modifies the calcium dependence of ryanodine-sensitive calcium channels of excitable cells. Biophysical Journal. 1998;74:1263–1277. doi: 10.1016/S0006-3495(98)77840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G, Henderson JS. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. Journal of Biological Chemistry. 1987;262:3065–3073. [PubMed] [Google Scholar]
- Meszaros LG, Bak J, Chu A. Cyclic ADP-ribose as an endogenous regulator of the non-skeletal type ryanodine receptor Ca2+ channel. Nature. 1993;364:76–79. doi: 10.1038/364076a0. [DOI] [PubMed] [Google Scholar]
- Narayanan N, Xu A. Phosphorylation and regulation of the Ca(2+)-pumping ATPase in cardiac sarcoplasmic reticulum by calcium/calmodulin-dependent protein kinase. Basic Research in Cardiology. 1997;92(suppl. 1):25–35. doi: 10.1007/BF00794065. [DOI] [PubMed] [Google Scholar]
- Orchard CH, Smith GL, Steele DS. Effects of cytosolic Ca2+ on the Ca2+ content of the sarcoplasmic reticulum in saponin-permeabilized rat ventricular trabeculae. Pflügers Archiv. 1998;435:555–563. doi: 10.1007/s004240050552. [DOI] [PubMed] [Google Scholar]
- Sham JS, Song LS, Chen Y, Deng LH, Stern MD, Lakatta EG, Cheng H. Termination of Ca2+ release by a local inactivation of ryanodine receptors in cardiac myocytes. Proceedings of the National Academy of Sciences of the USA. 1998;95:15096–15101. doi: 10.1073/pnas.95.25.15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer JR. Comparative anatomy: in praise of a powerful approach to elucidate mechanisms translating cardiac excitation into purposeful contraction. Journal of Molecular Cell Cardiology. 1995;27:19–35. doi: 10.1016/s0022-2828(08)80004-1. [DOI] [PubMed] [Google Scholar]
- Song LS, Stern MD, Lakatta EG, Cheng H. Partial depletion of sarcoplasmic reticulum calcium does not prevent calcium sparks in rat ventricular myocytes. The Journal of Physiology. 1997;505:665–675. doi: 10.1111/j.1469-7793.1997.665ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern MD, Lakatta E. Excitation-contraction in the heart: the state of the question. FASEB Journal. 1992;6:3092–3100. doi: 10.1096/fasebj.6.12.1325933. [DOI] [PubMed] [Google Scholar]
- Stern MD, Song LS, Cheng H, Sham JS, Yang HT, Boheler KR, Rios E. Local control models of cardiac excitation-contraction coupling. A possible role for allosteric interactions between ryanodine receptors. Journal of General Physiology. 1999;113:469–489. doi: 10.1085/jgp.113.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R, Williams AJ. Cyclic ADP-ribose and related compounds activate sheep skeletal sarcoplasmic reticulum Ca2+ release channel. American Journal of Physiology. 1995;268:C1235–1240. doi: 10.1152/ajpcell.1995.268.5.C1235. [DOI] [PubMed] [Google Scholar]
- Takamatsu T, Wier WG. Calcium waves in mammalian heart: quantification of origin, magnitude, waveform, and velocity. FASEB Journal. 1990;4:1519–1525. doi: 10.1096/fasebj.4.5.2307330. [DOI] [PubMed] [Google Scholar]
- Takasago T, Imagawa T, Furukawa K, Ogurusu T, Shigekawa M. Regulation of the cardiac ryanodine receptor by protein kinase-dependent phosphorylation. Journal of Biochemistry. 1991;109:163–170. doi: 10.1093/oxfordjournals.jbchem.a123339. (Tokyo) [DOI] [PubMed] [Google Scholar]
- Trafford AW, Lipp P, O'Neil CO, Niggli E, Eisner DA. Propagating calcium waves initiated by local caffeine application in rat ventricular myocytes. The Journal of Physiology. 1995;489:319–326. doi: 10.1113/jphysiol.1995.sp021053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier WG, Cannell MB, Berlin JR, Marban E, Lederer WJ. Cellular and subcellular heterogeneity of [Ca2+]i in single heart cells revealed by Fura-2. Science. 1987;235:325–328. doi: 10.1126/science.3798114. [DOI] [PubMed] [Google Scholar]
- Wussling MH, Salz H. Nonlinear propagation of spherical calcium waves in rat cardiac myocytes. Biophysical Journal. 1996;70:1144–1153. doi: 10.1016/S0006-3495(96)79715-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahradníková A, Dura M, Györke S. Modal gating transitions in cardiac ryanodine receptors during increases of Ca2+ concentration produced by photolysis of caged Ca2+ Pflügers Archiv. 1999;438:283–288. doi: 10.1007/s004240050911. [DOI] [PubMed] [Google Scholar]
- Zimanyi I, Pessah IN. Comparison of [3H]ryanodine receptors and Ca2+ release from rat cardiac and rabbit skeletal muscle sarcoplasmic reticulum. Journal of Pharmacology and Experimental Therapeutics. 1991;256:938–946. [PubMed] [Google Scholar]


