Summary
The polarized architecture of epithelia relies on an interplay between the cytoskeleton, the trafficking machinery, cell-cell and cell-matrix adhesion. Specifically, contact with the basement membrane (BM), an extracellular matrix underlying the basal side of epithelia, is important for cell polarity. However, little is known about how BM proteins themselves achieve a polarized distribution. In a genetic screen in the Drosophila follicular epithelium, we identified mutations in Crag, which encodes a conserved protein with domains implicated in membrane trafficking. Follicle cells mutant for Crag lose epithelial integrity and frequently become invasive. The loss of Crag leads to the anomalous accumulation of BM components on both sides of epithelial cells without directly affecting the distribution of apical or basolateral membrane proteins. This defect is not generally observed in mutants affecting epithelial integrity. We propose that Crag plays a unique role in organizing epithelial architecture by regulating the polarized secretion of BM proteins.
Introduction
Epithelia are organized as sheets of tightly adherent cells with distinct apicalbasal polarity (Muller and Bossinger, 2003). Intact tissue architecture is vital for their function and a loss of epithelial organization is often associated with carcinoma progression and tumor metastasis (Thiery, 2002). The polarized architecture of epithelial cells is evident from the presence of distinct apical and basolateral membrane domains that have different lipid and protein compositions (Mostov et al., 2003; Muller and Bossinger, 2003). To establish and maintain these separate membrane domains newly synthesized and recycled proteins need to be delivered to the correct location, a process that requires the sorting of proteins into different apical and basolateral transport vesicles, followed by their transport, targeting and fusion with specific regions of the plasma membrane. Protein localization is subsequently maintained through interactions with the underlying cytoskeleton and other cortical protein complexes and through intercellular junctions that inhibit diffusion between the apical and basolateral membrane domains (Mostov et al., 2003; Rodriguez-Boulan et al., 2005).
In order for apical and basolateral transport vesicles to reach and fuse with the correct membrane region, initial cell surface asymmetries need to be established. This is achieved by a combination of external cues including sites of contact with neighboring cells and with the extracellular matrix (ECM) (Nelson, 2003; O'Brien et al., 2002). Of particular interest in this light is the basement membrane (BM), a specialized sheet of ECM contacting the basal side of epithelial tissues. The BM is composed primarily of the secreted glycoproteins Laminin, Collagen IV and Nidogen and the heparan sulfate proteoglycan Perlecan. Interactions between the BM and epithelial cells are mediated by a variety of cell surface receptors including integrins and Dystroglycan (Yurchenco et al., 2004). Evidence from genetic analyses in model organisms and experiments in cultured cells have indicated an important role for the BM in generating epithelial cell polarity (Li et al., 2003; O'Brien et al., 2002). Therefore, the accumulation of BM on the basal side of the epithelium only, is crucial to convey the positional information necessary to maintain a polarized architecture.
Even though substantial progress has been made in understanding the mechanisms that regulate the polarized trafficking of basolateral membrane proteins (Mostov et al., 2003; Rodriguez-Boulan et al., 2005), very little is known about how basally secreted proteins, such as BM components, are sorted and delivered specifically to the basal side of epithelial cells. Different pathways for the polarized trafficking of basolateral membrane and secreted proteins have been proposed, yet no components specifically regulating the polarized secretion of BM proteins have been identified (Boll et al., 1991; Caplan et al., 1987; Cohen et al., 2001; De Almeida and Stow, 1991).
To study the establishment and maintenance of epithelial architecture we use the follicular epithelium (FE) in Drosophila melanogaster, which forms a monolayer of somatic cells surrounding the germ line during oogenesis (Horne-Badovinac and Bilder, 2005). The follicle cells (FC) that make up the FE display a distinct apical-basal polarity, apparent in the presence of different membrane and cortical domains, apically localized adherens junctions and the polarized organization of the cytoskeleton (Muller and Bossinger, 2003). As in other epithelia, the basal side of the FE is in contact with the BM. The apical side, however, contacts the germ line rather than a lumen or the external environment. Cues on the basal, apical and lateral sides of the FC are required to establish and maintain a fully polarized epithelial phenotype (Abdelilah-Seyfried et al., 2003; Bilder et al., 2000; Cox et al., 2001; Goode and Perrimon, 1997; Manfruelli et al., 1996; Muller and Bossinger, 2003; Tanentzapf et al., 2000).
To further elucidate the molecular mechanisms controlling epithelial organization we took advantage of the stem cell derived nature of the FE and performed a genetic mosaic screen to identify genes required for this process. We identified multiple alleles of Crag (Calmodulin binding protein related to a Rab3 GDP-GTP exchange protein), an evolutionarily conserved gene for which no biological function had previously been described (Semova et al., 2003; Xu et al., 1998). We show that FC mutant for Crag often lose epithelial characteristics and become motile and invasive. Strikingly, prior to inducing a loss of epithelial integrity the absence of Crag causes the aberrant accumulation of the BM components Perlecan, Laminin and Collagen IV on both sides of the epithelium, a defect we did not generally observe in mutants disrupting epithelial structure. We propose that Crag plays a unique role in regulating epithelial architecture by specifically controlling the polarized secretion of BM proteins. While it has been suggested that BM components need specific factors to ensure their accurate basal secretion, Crag is the first protein identified to be required for such a process.
Results
CJ mutant follicle cells lose epithelial integrity
The Drosophila follicular epithelium (FE) forms a monolayer of regularly shaped cuboidal cells with their apical sides facing the developing germline (Figure 1A). To identify novel regulators of epithelial architecture we performed a genetic mosaic screen in the FE using a system in which clones of mutant cells are readily identified by the absence of GFP (Figure 1B). One complementation group we isolated in this screen consists of twelve mutant alleles that cause defects in epithelial organization in follicle cell (FC) clones. We named this complementation group CJ, after a representative allele, CJ101. Instead of forming a regular epithelial monolayer, CJ mutant FC accumulate in multiple layers in which cells adopt round or irregular morphologies rather than their normal cuboidal or columnar shapes (Figures 1A, B). In addition, CJ mutant FC frequently become motile and invade the germ line cluster (Figure 1C). In a considerable number of clones, defects appeared less severe and a bilayer of cells with epithelial characteristics could be observed (Figure 1D, 45% of clones with abnormalities, n=110). Interestingly, we noticed that the bilayered, multilayered and invasive regions regularly comprised a mix of mutant and wild type cells, suggesting that mutant cells can cause a loss of epithelial structure in the surrounding wild type tissue (Figures 1B, C, E, F). The defects in epithelial architecture in CJ mutant clones were only detected in FC at the poles of an egg chamber (e.g. arrow in Figure 1B). Mutant cells on the side of an egg chamber were indistinguishable from wild type cells (Figure 1B, arrowhead, n >100). A differential response of FC reflecting their position within the epithelium is commonly observed in mutants affecting epithelial architecture (Abdelilah-Seyfried et al., 2003; Goode and Perrimon, 1997; Goode et al., 2005; Lee et al., 1997; Tanentzapf et al., 2000).
Figure 1. CJ mutant FC lose epithelial integrity.

(A) Wild type egg chamber stained for F-actin (red). The follicle cells (fc) have a regular morphology and epithelial monolayer organization and surround the germline cells (gc). Apical (a) and basal (b) sides are marked. (B–D) Egg chambers containing CJ101 mutant follicle cell clones marked by the absence of GFP (green) and stained for F-actin (red). (B) CJ101 mutant FC at the poles (arrow) are irregular in shape and pile up in multiple layers. CJ101 mutant cells on the lateral sides of the egg chamber (arrowhead) are indistinguishable from surrounding wild type cells or cells in wild type egg chambers. (C) Stage 6 egg chamber in which FC have invaded the germline cluster. Note that a mix of mutant and wild type cells are found within the group of invading cells (arrow). (D) CJ101 mutant cells at the posterior pole forming two layers maintaining regular cell shape. (E–H) Egg chambers containing CJ mutant follicle cell clones marked by the absence of GFP (green) and stained for aPKC (E), DE-Cadherin (F), FasII (G) or Baz and Arm (H). (E) CJ101 mutant cells show a loss of cortical apical staining of aPKC (E’, red in E) when cells are multilayered (arrow) but not when mutant cells have normal epithelial architecture (arrowhead). Wild type (GFP positive) cells are commonly found intermingled with mutant cells in cases where severe multilayering is observed. Note that some mutant cells still accumulate high levels of cortical aPKC equivalent to apical levels in wild type cells. (F) DE-Cadherin levels (D’, red in D) in multilayered CZ085 mutant FC are similar to the levels at the lateral plasma membrane below the junctions in wild type cells. Occasionally, high levels of DE-Cadherin staining can be seen (arrow). In clones at the lateral sides of the cyst DE-Cadherin distribution appears identical to that in wild type cells (arrowhead). (G) CJ101 mutant cells that have lost epithelial morphology show membrane staining of FasII (G’, red in G) along their entire cell circumference (arrow) whereas mutant cells with a normal morphology show a lateral restriction of FasII (arrowheads) as in wild type cells. (H) CJ101 mutant clones forming two layers, each maintaining the polarized distribution of the apical cortical protein Baz (H’, red in H) and the junctional protein Arm (H’’, blue (pink in merge) in H). Scale bars in this and all subsequent figures represent 10 µm.
Epithelial polarity in CJ mutant follicle cells
Defects in epithelial organization, as observed in CJ mutant FC are typically associated with a loss of apical-basal polarity. To assess epithelial polarity in CJ mutant cells, we analyzed the distribution of cortical and transmembrane proteins that normally adopt asymmetric distributions along the apical-basal axis of the epithelium. We examined the distribution of the apical markers atypical Protein Kinase C (aPKC), Bazooka (Baz), Par-6 and dPatj, the adherens junction components Armadillo (Arm) and DE-Cadherin, and the basolateral proteins Discs large (Dlg), Lethal giant larvae (Lgl), Fasciclin II and β-spectrin. In multilayered CJ mutant clones aPKC, Baz, Par6 and dPatj failed to localize to the cortex (Figure 1E, arrow, and data not shown), indicating a loss of apical membrane identity. Occasionally, cortical foci accumulating apical markers were detected, possibly reflecting remnants of the apical membrane domain (Figure 1E). In multilayered CJ mutant cells Arm and DE-Cadherin were found at the membrane at low levels, similar to those at the lateral membrane below the junctions of wild-type cells (Figure 1F and data not shown). Scattered foci accumulating Arm and DE-Cadherin at high levels, similar to those at the adherens junctions of wild type cells were also observed (Figure 1F). Finally, basolateral proteins were found to maintain their cortical localization but were aberrantly distributed along the entire membrane in multilayered CJ mutant cells (Figure 1G, arrow, and data not shown). In summary, CJ mutant FC that have completely lost epithelial integrity mislocalize apical, junctional and basolateral proteins.
However, CJ mutant clones in which cells maintained their normal epithelial morphology and monolayer organization displayed a wild type distribution of apical, junctional and basolateral markers (Figures 1E–G, arrowheads, and data not shown). Similarly, in mutant clones adopting a bilayer organization, both layers displayed discrete apical and basolateral membrane domains without obvious defects in junctional integrity (Figure 1H).
Given that the anomalous distribution of apical and basolateral proteins is only observed in cases where epithelial architecture is severely perturbed, the disruption of the apical and basolateral membrane domains may not be the primary defect in CJ mutant cells.
The epithelial defects in CJ mutants are caused by mutations in the DENN domain protein Crag
We used a combination of meiotic recombination mapping and complementation analysis with Duplications and Deficiencies to map the lethal phenotype of CJ mutants and identified the genomic region around 7F7-8 as the region containing the lethal mutation. Sequencing of candidate genes in this region identified point mutations in the coding region of a gene named Crag (for Calmodulin binding protein related to a Rab3 GDP/GTP exchange protein) (Xu et al., 1998) in which, prior to our study, no mutations had been isolated. The Crag locus encodes two protein isoforms, Crag-PA and Crag-PB, of 1671 and 1644 amino acids respectively (Figure 2A; Berkeley Drosophila Genome Project). The strong alleles CragGG43, CragCJ101 and CragCZ085 contain premature stop codons at aa 161, aa 367 and 659 respectively. CragCB188, a weaker temperature sensitive allele, contains a missense mutation in aa 469 changing a glycine residue to an aspartate. The early stop codons in the CragGG43 and CragCJ101 alleles truncate the protein before or within conserved protein domains (Figure 2A and see below). Moreover, CragGG43, CragCJ101 and CragCZ085 homozygotes or hemizygotes die in early larval stages similarly to CragCJ101/Df(1)KA14 transheterozygotes, providing evidence that these alleles represent a strong or complete loss of Crag function. A genomic rescue construct, including the Crag gene and ~1.5 kb upstream and downstream sequences, as well as a HA-tagged Crag-PA and Crag-PB transgenes expressed using the GAL4/UAS system, fully rescued the lethality and the phenotypes in the FE. This confirms that the observed phenotypes are caused by the absence of Crag function (Figures 2B, C).
Figure 2. Epithelial defects in CJ mutant FC are caused by mutations in Crag.

(A) Schematic representation of the domain organization of the Crag protein (top) and table depicting the percentages of similarity between conserved regions in Crag, representative Crag homologues in different species and the Drosophila homolog of the DENN containing protein Rab3-GEF (bottom). Crag contains an amino-terminal DENN, uDENN and dDENN domain (red, 1–3) followed by a region responsible for Calmodulin binding (blue, 4, CaM) and a conserved C-terminal region (green, 5, Cterm). The location of the nonsense mutations found in the strong alleles CragGG43, CragCJ101 and CragCZ085 and the missense mutation present in the hypomorphic allele CragCB188 are depicted with arrows below the protein (top). The percentages of similarity were calculated using the EMBOSS global or local pairwise alignment tool. H. sapiens Crag is human DENND4A (c-myc promoter binding protein, Acc: NP_005839), one of three Crag homologues in humans. D. rerio Crag is a zebrafish Crag homolog (Acc: CAK03709). C. elegans. Crag is the protein F52C12.4. Note that CaM and Cterm are not present in Rab3-GEF (n.p.). (B) Ovariole of a CragCZ085 homozygous female containing a Crag genomic rescue construct stained for F-actin (red) and DNA (blue). The FE looks entirely normal. (C) Stage 6 egg chamber containing a CragCJ101 mutant clone encompassing the entire epithelium and expressing a HA-Crag transgene. No epithelial defects are observed. The clone is marked by the absence of GFP (green) and the egg chamber is stained for F-actin (red) and HA-Crag (blue).
Crag was originally identified as a Calmodulin binding protein (Xu et al., 1998) and is highly conserved in vertebrates and invertebrates (Figure 2A) (Semova et al., 2003); GenBank). At their amino-terminus Crag proteins contain a DENN (for differentially expressed in normal and neoplastic cells), uDENN (upstream of DENN) and dDENN (downstream of DENN) domain (Levivier et al., 2001; Semova et al., 2003). A large family of proteins present in most eukaryotes contains this set of domains (Pfam: PF02141). The function of the DENN domain is as yet unknown but its presence in proteins that bind to regulators of vesicle trafficking, most notably to a number of Rab GTPases, has raised the possibility that DENN-containing proteins play a role in membrane trafficking (Levivier et al., 2001). The missense mutation in CragCB188 in a glycine residue that is conserved in nearly all DENN domains suggests that this domain is critical for Crag function.
The DENN domain in Crag is followed by a region that mediates Calmodulin binding in vitro (Xu et al., 1998). To ask if Crag also binds Calmodulin in vivo in the FC we performed co-immunoprecipitations between HA-Crag and Calmodulin and found that Crag can pull down Calmodulin and vice versa in a Ca2+- dependent manner (Figure S1 in the Supplemental Data available with this article online). At its carboxy-terminus, Crag contains a domain that is conserved among Crag homologues but is not found in other proteins.
Crag localizes to the plasma membrane and endosomal compartments
The presence of a DENN domain in the Crag protein may indicate a role for Crag in regulating protein trafficking. To ask whether Crag protein distribution is consistent with such a role, we raised antibodies against Crag and in addition, expressed tagged transgenes. We found that Crag is expressed in the FE as it is first formed in the germarium and maintains expression in the entire epithelium throughout its development (Figures 3A, B and data not shown). The specificity of Crag protein expression was validated by the absence of staining in Crag mutant clones (Figure 3C).
Figure 3. Crag expression pattern and subcellular distribution.
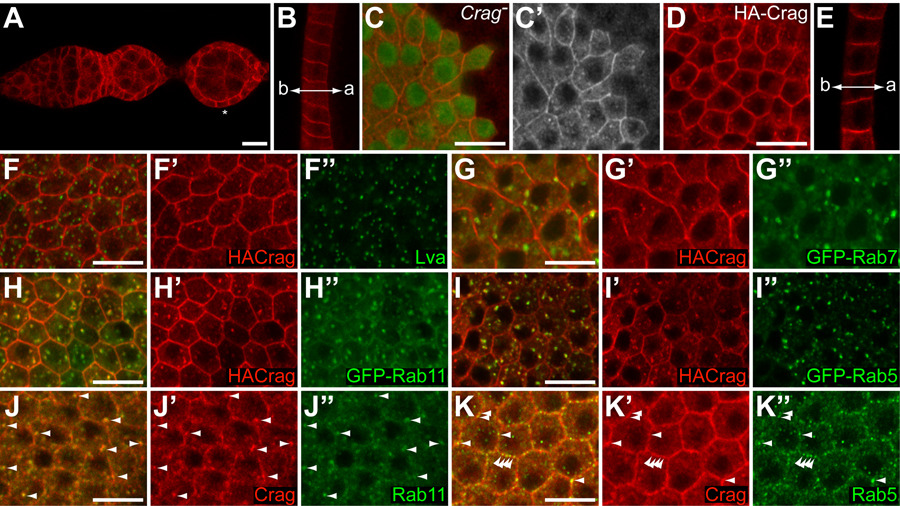
(A, B) Wild type egg chambers stained with an antibody against the carboxy-terminal half of Crag (red). (A) Germarium and early stages of oogenesis showing Crag expression in all FC and FC precursors. Crag is also present in the germline. Note the absence of Crag staining at the basal cortex (asterisk). (B) Cross section through the FE of a stage 10 egg chamber shows that Crag accumulates at the lateral cortex. Low levels of Crag protein can also be observed at the apical (a) cortex but no Crag is present at the basal (b) cortex. (C) Optical section through the FE of a stage 9 egg chamber with a CragCJ101 mutant clone showing accumulation of Crag at the cortex and in intracellular punctae in wild type cells (C’, red in C). The specificity of the Crag antibody is evident from the absence of staining in the mutant clone (marked by the absence of GFP, green). (D, E) Overexpressed HA-Crag localizes in the cytoplasm and accumulates at the cortex and in punctate structures similarly to endogenous Crag. (E) HA-Crag is enriched at the lateral cortex. (F–I) Optical sections through the FE of stage 8 or 9 egg chambers expressing HA-Crag (red) and GFP-Rab7 (G), GFP-Rab11 (H), GFP-Rab5 (I) or HACrag alone (F). No significant colocalization is observed between HA-Crag and the Golgi protein Lva (F, green) or between HA-Crag and Rab7-GFP (G, green). Substantial overlap is seen between HA-Crag and GFP-Rab11 (H, green) and between HA-Crag and GFP-Rab5 (I, green). (J, K) Optical section through the FE of wild type stage 9 egg chambers stained for endogenous Crag (red) and Rab11 (J, green) or Rab5 (K, green). A clear overlap is observed between Crag and Rab11 positive intracellular vesicles (J, arrowheads). Colocalization between Crag and Rab5 protein is most obvious at the plasma membrane (K, arrowheads).
Strikingly, in wild type cells Crag protein accumulates at the plasma membrane of FC and is found in punctate structures throughout the cytoplasm (Figure 3C). In early stages of oogenesis, Crag is present along the apical and lateral cell cortex (Figure 3A). In later stages, a bias to the lateral cortex becomes apparent although Crag staining at or just below the apical surface can be observed (Figure 3B and data not shown). In all stages, Crag is clearly absent from the basal cortex (Figures 3A, B). Overexpressed HA-tagged transgenes show a comparable (apico)-lateral cortical enrichment and punctate staining in the cytoplasm (Figures 3D, E).
To analyze the nature of the punctate cytoplasmic Crag staining, we performed colocalization studies with markers for various membrane compartments. We did not detect significant colocalization between HA-Crag and the Golgi marker Lava Lamp (Lva, Sisson et al., 2000) or the late endosomal marker GFP-Rab7 (Entchev et al., 2000) (Figures 3F, G). We did however observe a substantial overlap between HA-Crag and GFP-Rab11, which localizes to the recycling endosome (Emery et al., 2005) (Figure 3H). We also observed colocalization with the early endosomal marker GFP-Rab5 (Wucherpfennig et al., 2003) (Figure 3I). To ensure that the observed overlap between HA-Crag and GFP-Rab5 and GFP-Rab11 was not an artifact of protein overexpression we stained for endogenous Crag, Rab5 and Rab11 proteins and found that Crag colocalizes strongly with Rab11 in intracellular punctae (Figure 3J). Overlap between Rab5 and Crag was predominantly observed in punctae at the cell cortex (Figure 3K) but was less compelling in cytoplasmic vesicles.
Taken together, the presence of Crag protein at the cell cortex and in endosomal compartments is consistent with a putative role in trafficking.
Crag mutant follicle cells show specific defects in the polarized distribution of BM components
The polarized sorting and delivery of newly synthesized and endocytosed membrane proteins are crucial for epithelial architecture (Mostov et al., 2003; Rodriguez-Boulan et al., 2005). As other DENN domain containing proteins have been implicated in the regulation of trafficking and Crag localization is consistent with such a role, we asked whether the epithelial defects in Crag mutant FC were due to a failure in polarized trafficking. We therefore analyzed the distribution of a number of transmembrane proteins that are normally present on specific membrane domains in the epithelium. We focused our analysis on Crag mutant cells that maintained a normal morphology and monolayer organization to avoid secondary effects due to a loss of epithelial structure. We found that the distributions of the apical receptor Notch, the adherens junction protein DE-Cadherin and the basolateral adhesion molecules Fasciclin II, Fasciclin III and Neuroglian were indistinguishable in wild type and Crag mutant FC that maintained a normal morphology (Figures 4A–C and data not shown, n>20 for each). Since we did not observe obvious defects in the polarized distribution of apical, junctional or basolateral transmembrane proteins, Crag most likely does not directly regulate the polarized trafficking of these proteins.
Figure 4. Crag mutant FC specifically disrupt the polarized distribution of BM proteins.

(A–C) Cross section through the FC layer of an egg chamber containing CragCJ101 clones, marked by the absence of GFP (green) and stained for Notch (A), DE-Cadherin (B) or FasIII (C) (red in merged images (top panels), grayscale in bottom panels). (A) Notch accumulates at the apical plasma membrane and in vesicles in control (green) and mutant cells. (B) DE-Cadherin localizes faintly to the lateral membrane and at higher levels at the adherens junctions. No differences were observed between control and mutant FC. (C) The localization of FasIII to the (baso)lateral membrane is unaltered in the absence of Crag. (D–H) Control egg chamber (D), egg chambers with CragCJ101 clones (E–G) or egg chamber with CragCJ101 clones co-expressing HA-Crag (H) stained for Pcan (red in merged images, grayscale in single channel images). (D) In control egg chambers without clones, Pcan is seen in intracellular punctae in the FC, and accumulates exclusively on the basal side of the epithelium. (E, E’) In Crag mutant clones, Pcan accumulates strongly on the apical and basal sides of the FE (arrow), in contrast to the surrounding wild type cells (arrowhead) where Pcan is present solely on the basal side. Note that the mutant cells accumulating Pcan apically still have a normal morphology and that apical Pcan is observed at the anterior pole as well as on the side of the egg chamber. (F) Pcan accumulates apically of a CragCJ101 mutant clone at the posterior pole of an egg chamber. Note also that the epithelium is bi-layered and that Pcan is present between the two layers. (G) Even very small clones show anomalous apical localization of Pcan. Apical Pcan can also be seen in wild type cells adjacent to the clone (arrow, boundaries of clone are marked with dashed lines). (H) No apical accumulation of Pcan is detected in a Crag mutant clone when HA-Crag is co-expressed. The boundary of the clone is marked with dashed lines in the bottom panel. No difference in Pcan staining is observed between cells inside or outside the clone. (I–K) Cross sections through egg chambers with CragCJ101 clones stained for Lam (I, red in top panel, grayscale in bottom panel), Coll IV (J, red) or co-expressing Coll IVα2(Vkg)-GFP (K, green). Clones are marked by the absence of nuclear and cytoplasmic GFP (green). (I) Lam accumulates strongly on the apical side of CragCJ101 FC (arrows). Note however that not all cells within the clone show apical Lam staining (arrowheads). (J) Even a small CragCJ101 clone exhibits apical accumulation of Coll IV. (K) Vkg-GFP accumulates on the apical side of CragCJ101 FC (arrowhead) and of adjacent wild type cells (arrow). (L) Egg chamber with CragCJ01 clones stained for Dystroglycan (Dg, red). Dg accumulates at the basal and apical surface in the absence of Crag. (L’, L”) Higher magnification of two regions at the boundary between wild type and mutant cells depicted in K. Dg levels are increased in mutant cells and in wild type cells adjacent to the mutant clones (arrows, boundaries of the clones are marked with a dashed line).
Considering the importance of contact between epithelial cells and the BM in controlling epithelial architecture (Li et al., 2003; Yurchenco et al., 2004), we examined the localization of Perlecan (Pcan), a secreted heparan sulfate proteoglycan and major component of the BM (Yurchenco et al., 2004). In wild type egg chambers Pcan accumulates exclusively on the basal side of the FE (Figure 4D and Schneider et al., 2006). Strikingly, Crag mutant FC deposit Pcan on both their basal and apical sides (Figures 4E–G, 100% of stage 6–9 egg chambers with clones, n>100). This aberrant localization was seen even in very small clones, at the poles as well as on the sides of the egg chamber (Figures 4E–G). Occasionally, wild type cells adjacent to cells lacking Crag accumulated Pcan on their apical side, presumably due to local diffusion of Pcan secreted by the mutant cells (Figure 4G, arrow). Abnormal apical Pcan staining was observed with several Crag alleles, was fully suppressed upon expression of a genomic rescue construct (0% of stage 6–9 egg chambers showed apical Pcan, n>100) and partially rescued when a HA-tagged Crag was expressed in cells mutant for Crag (64% of egg chambers with clones showed no apical Pcan, 36% showed residual apical Pcan in a fraction of mutant cells, n=50) (Figure 4H and data not shown). These data confirm that the presence of the BM component Pcan on both sides of the epithelium is due to the absence of Crag activity.
To examine whether the aberrant apical localization of Pcan in Crag mutant FC was also observed for other BM proteins, we analyzed the distribution of two other major BM components, the secreted glycoproteins Laminin (Lam) and Collagen IV (Coll IV). In contrast to wild type epithelia where Lam and Coll IV are present only basally (Gutzeit et al., 1991; Lunstrum et al., 1988), both proteins were found on the basal as well as the apical side of follicular epithelia with Crag mutant clones (Figure 4I–K, 87% of egg chambers with clones showed apical Lam, n=93, 99% or 100% showed apical Coll IV as visualized by antibody staining (n=75) or by Coll IVα2 (=Vkg)-GFP expression (n=60) respectively). As for Pcan, this defect was observed even when only a few cells lacked Crag activity (e.g. Figure 4K) and was seen both at the poles and on the sides of the egg chamber. However, differences between the distribution of Pcan on the one hand and that of Lam and Coll IV on the other hand were apparent. First, we noticed a greater non-autonomy in aberrant Lam and Coll IV localization, where both proteins were often observed on the apical side of wild type cells several cell diameters away from a clone (Figure 4J and data not shown). Second, whereas strong apical Pcan staining was typically observed on all cells within a Crag mutant clone, not all cells in a clone accumulated Lam or Coll IV on their apical side (Figure 4I). Both observations may be explained by a stronger binding of Pcan to surface receptors on the apical side of the FC allowing it to accumulate strongly upon secretion and preventing it from diffusing over longer distances.
To determine whether the anomalous apical deposition of BM proteins in Crag mutant cells was a specific feature of the FE, or was a more general defect seen in other epithelia, we examined Pcan distribution in embryos lacking both maternal and zygotic Crag expression. Consistent with a more general role for Crag in regulating the accurate deposition of BM proteins in epithelia, we found that in late stages of embryogenesis Pcan accumulated aberrantly on the apical side of the epidermis in Crag mutants (Figure S2).
To ask whether the absence of Crag also caused defects in the distribution of BM receptors we analyzed the localization of Dystroglycan and the βPS integrin subunit. Both receptors are present at low levels at the apical plasma membrane of wild type FC in addition to their basal localization (Deng et al., 2003; Fernandez-Minan et al., 2007). No obvious differences in the localization of βPS were detected in the absence of Crag (data not shown). Conversely, Dystroglycan appeared to accumulate at higher levels at the apical plasma membrane of Crag mutant FC (100% of egg chambers with clones, n = 48) (Figure 4L). However, this increase in apical localization was not always cell-autonomous and is therefore likely to represent a secondary effect through stabilization of low levels of apical protein upon binding to BM proteins.
Taken together, our results demonstrate that Crag is required to ensure the accumulation of BM components exclusively on the basal side of epithelia, possibly by regulating their accurate polarized trafficking. Because this defect is observed in mutant cells irrespective of their morphology, it is not a consequence of the loss of epithelial structure but may instead be the primary defect that causes the observed disruption of epithelial organization in Crag mutant FC.
Mislocalization of BM components is not generally observed in mutants affecting epithelial organization
We next wanted to address whether the aberrant accumulation of BM proteins on the apical side of the FE is a defect caused specifically by the absence of functional Crag protein or whether it is more generally observed in the absence of proteins that regulate epithelial architecture. We therefore examined the localization of Pcan in FC lacking components of three major classes of polarity regulators: 1) the Baz/Par-6 complex, 2) the Crumbs (Crb) complex and 3) the Dlg group (Muller and Bossinger, 2003). Mutual antagonistic interactions between proteins of these different classes have been shown to lead to the formation of separate apical and basolateral membrane domains (Bilder et al., 2003; Tanentzapf and Tepass, 2003). As for Crag mutant clones, we restricted our analysis to mutant cells that maintained a regular monolayer organization to avoid secondary effects once epithelial integrity was compromised. In FC mutant for the null allele par6Δ226, Pcan was never found to accumulate on the apical side (n = 25, Figure 5A). Similarly, in crb11A2 or dlgm52 mutant FC Pcan was not found apically (n = 25, n = 26 respectively, Figures 5B, C). Hence, mistargeting of BM components to the apical side of the FE does not appear to be a general defect observed in mutants affecting epithelial architecture but is instead specifically caused by the absence of Crag function. Crag therefore appears to act in a unique and novel pathway, distinct from the Baz, Crb and Dlg groups of proteins, to regulate epithelial organization.
Figure 5. Mislocalization of Pcan is not a general defect in FC mutant for regulators of epithelial organization.
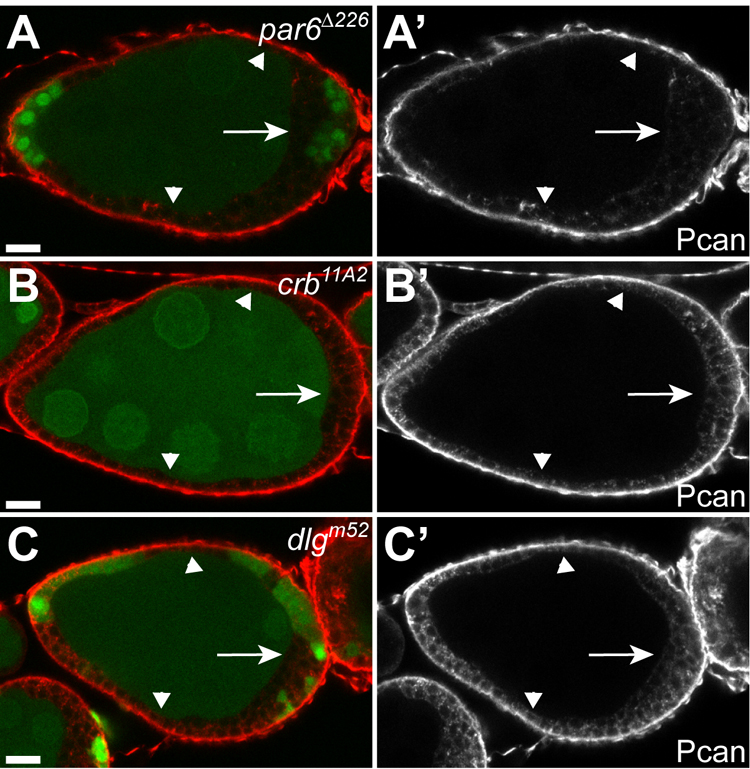
(A–C) Egg chambers containing par6Δ226 (A), crb11A2 (B) or dlgm52 (C) mutant clones, marked by the absence of GFP (green), stained for Pcan (red). Pcan accumulates exclusively on the basal side of par6, crb or dlg mutant cells that maintain a regular monolayer organization (arrowheads) or have formed multiple layers of irregularly shaped cells (arrows). Aberrant apical Pcan was never observed.
The presence of Pcan on the apical side of Crag mutant cells does not depend on transcytosis from the basal side
Different mechanisms could lead to the presence of BM components on the apical side of the epithelium in Crag mutant cells. Newly synthesized BM proteins could be targeted aberrantly to the apical side in the absence of Crag activity. Alternatively, BM components could be transported from the basal to the apical side through transcytosis in mutant cells. It was shown that the latter mechanism is responsible for the formation of a small apical BM cap over anterior polar cells at stage 8 of oogenesis (Medioni and Noselli, 2005). To distinguish between these two scenarios, we generated FC clones lacking both Crag and Pcan (referred to as pcan−Crag− clones). pcan− and pcan−Crag− clones lose the punctate intracellular Pcan staining observed in wild type cells (Figures 6A, B). This punctate staining may therefore reflect Pcan in the biosynthetic pathway. However, small or medium sized pcan− and pcan−Crag− clones maintain basal Pcan localization, either due to the diffusion of Pcan synthesized and secreted by neighboring wild type cells or to a low turnover of Pcan protein once assembled in the BM (Figures 6A, B). In contrast to Crag mutant cells, pcan−Crag− FC never show Pcan staining on their apical side (n > 50, compare Figures 6B and C). Because Pcan is still present on the basal side of these clones, this suggests that apical Pcan in Crag mutant cells does not originate from transcytosed basal material but rather from newly synthesized protein.
Figure 6. The accumulation of Pcan on the apical side of Crag mutant FC originates from newly synthesized protein.
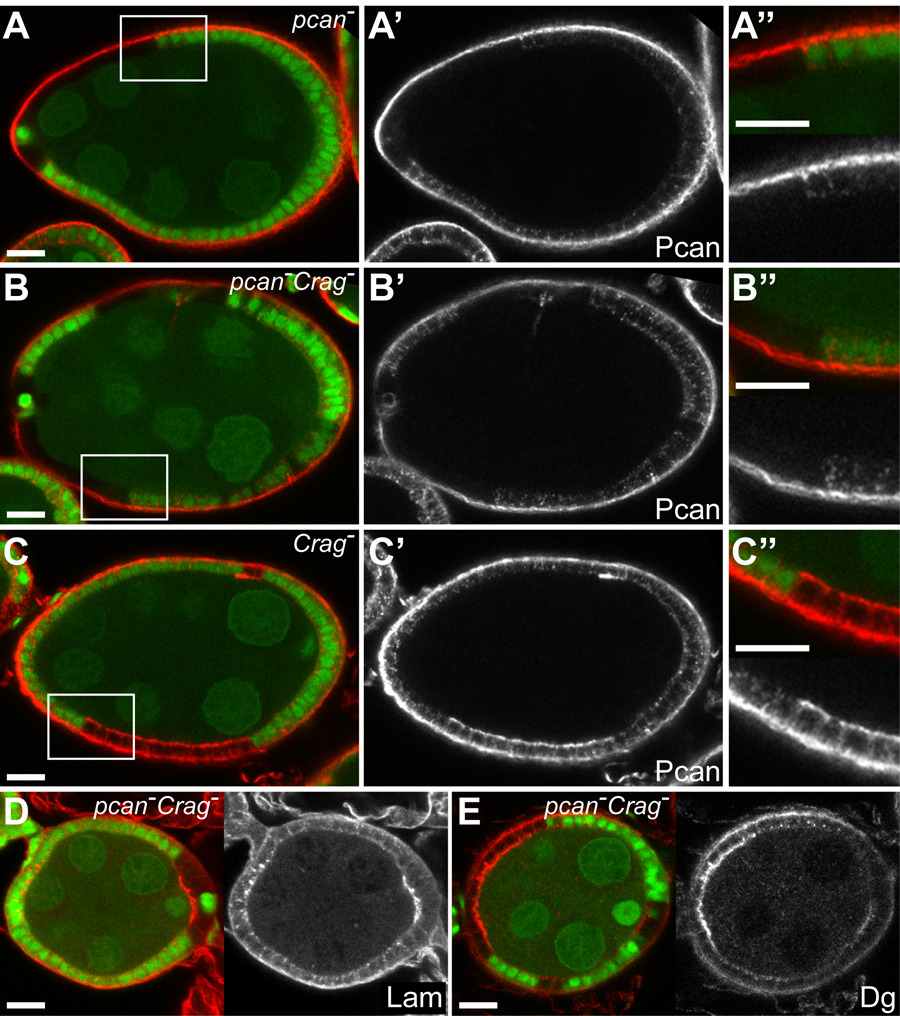
(A–C) Egg chambers with pcannull (A), pcannull CragCJ101 (B) or CragCJ101 (C) clones marked by the absence of GFP (green) and stained for Pcan (red). Higher magnifications of the boxed areas in A–C are depicted in A”–C”. (A) Whereas Pcan can be detected in intracellular punctae in wild type FC (green), pcannull clones show no intracellular Pcan staining. In contrast, Pcan is present on the basal side of the FE in control and pcannull cells. (B) Pcan staining in pcannullCragCJ101 FC looks similar to that in pcannull clones. Note that even though basal Pcan can readily be observed no apical accumulation is visible. (C) Conversely, a striking apical Pcan localization is observed in CragCJ101 clones. (D, E) Egg chambers with pcannullCragCJ101 clones marked by the absence of GFP (green) and stained for Lam (D, red) or Dystroglycan (E, red). (D) Apical Lam staining is observed in pcannullCragCJ101 clones similarly to CragCJ101 clones. (E) Dystroglycan accumulates strongly at the apical surface in pcannull CragCJ101 clones.
FC lacking both Pcan and Crag were identical to Crag mutant FC, but differed from pcan mutant clones. This was apparent from their general morphology and organization and from the localization of Laminin and Dystroglycan (Figure 6D, E and data not shown). Whereas Laminin localization is not obviously affected in pcan mutant clones (n > 50, and (Schneider et al., 2006)), ectopic apical Laminin can readily be observed in pcan−Crag− clones, as in Crag mutant FC (93% of egg chambers with pcan−Crag− clones showed apical Laminin, n = 84) (Figure 6D). Similarly, Dystroglycan is found at increased levels at the apical surface in pcan−Crag− clones (Figure 6E). These results indicate that the presence of Pcan on the apical side of the FC is not a prerequisite for the presence of apical Laminin and Dystroglycan and the disruption of epithelial architecture in Crag mutant cells. The presence of Laminin and/or other BM components may suffice to induce the epithelial defects observed in Crag mutant cells.
Discussion
The maintenance of epithelial architecture relies on the polarized trafficking of apical and basolateral proteins to their respective membrane domains (Mostov et al., 2003; Rodriguez-Boulan et al., 2005). Whereas considerable progress has been made in elucidating the molecular mechanisms regulating the polarized trafficking of basolateral transmembrane proteins, little is known about how basolaterally secreted proteins, such as components of the BM, obtain their polarized distribution. Here, we report the first functional characterization of the evolutionarily conserved protein Crag. We show that Crag mutant FC lose epithelial integrity and become invasive. We propose that Crag plays a unique and novel role in the organization of epithelial structure by specifically regulating the polarized secretion of BM components.
First, we have shown that Crag mutant cells lose the polarized distribution of the BM components Pcan, Lam and Coll IV. Instead of being present exclusively on the basal side of the epithelium, these BM proteins were found to accumulate both basally and apically in the absence of Crag. This was true for all mutant clones analyzed, regardless of their position within the epithelium and even in the absence of any other discernible phenotypes. This contrasts with apical, junctional and basolateral membrane proteins, which have altered distributions in Crag mutant cells only when epithelial structure is severely disturbed. The failure to restrict the accumulation of BM components to the basal side of the FE is therefore likely the primary defect in Crag mutant cells. Second, the mistargeting of BM components in Crag mutant cells appears to be a specific consequence of the loss of Crag function and not a general defect observed in the absence of other proteins known to regulate epithelial organization. FC mutant for par-6, crb or dlg and thus lacking the activity of either one of three major polarity complexes show no alterations in the accumulation of BM proteins. Crag may therefore be the first factor identified to specifically control the accurate deposition of BM on the basal side of epithelial cells.
Based on the well-established role of the BM in regulating epithelial organization, we propose that the presence of BM components on both sides of the FE is responsible for the loss of epithelial integrity in the absence of Crag. Studies in both model organisms and cultured cells have pointed to a central role for the BM in regulating epithelial polarity and tissue organization (Li et al., 2003; Miner and Yurchenco, 2004; Yurchenco et al., 2004). In particular, contact with the BM has been shown to direct the orientation of the apical-basal axis of epithelia, with the apical pole forming opposite to the side contacting the BM (O'Brien et al., 2002). Exposure of the apical surface of cultured epithelial cysts or monolayers to either collagen or laminin triggers a reversal of polarity (Chambard et al., 1981; Hall et al., 1982; O'Brien et al., 2001; Schwimmer and Ojakian, 1995; Wang et al., 1990; Yu et al., 2005; Zuk and Matlin, 1996). Polarity reversal is completed only after the BM present on the original basal side of the epithelium has been degraded, and is preceded by a loss of apical membrane identity, a dispersal of basolateral proteins and the formation of multiple cell layers (Schwimmer and Ojakian, 1995; Wang et al., 1990; Zuk and Matlin, 1996). This artificially induced situation shows noticeable similarities with the behavior of Crag mutant FC where BM components are found in contact with both sides of the epithelium. Cells form multiple layers, the cortical localization of apical proteins is lost and basolateral proteins are found along the entire cell surface. Thus, the presence of a BM on both sides of the FE in the absence of Crag can account for the observed phenotypes. Even though the significance of the BM in organizing epithelial architecture has been well established, our results underscore the importance of maintaining a polarized deposition of BM proteins on the basal side of epithelial cells.
Interestingly, epithelial disorganization is not restricted to Crag mutant cells but is also seen in neighboring wild type cells. This non-autonomy can be explained by the observation that Pcan, Lam and Coll IV were found on the apical side of wild type cells neighboring mutant cells, presumably due to their diffusion within the extracellular space.
How could the absence of Crag protein lead to the aberrant deposition of BM components on the apical side of epithelial cells? Previous work in tissue culture has suggested that epithelial cells secrete BM proteins predominantly from their basal side and has indicated the existence of an active sorting and targeting process mediating this polarized secretion (Boll et al., 1991; Caplan et al., 1987; Natori et al., 1992; Unemori et al., 1990). Furthermore, it has been suggested that different trafficking routes for basally secreted and basolateral transmembrane proteins exist (Boll et al., 1991; Caplan et al., 1987; Cohen et al., 2001; De Almeida and Stow, 1991). It is thus conceivable that Crag plays a role in specifically controlling the polarized secretion of BM proteins.
A direct role for Crag in regulating membrane trafficking is suggested by the presence of a conserved DENN domain at its amino-terminus. DENN domains are found in a large number of proteins in nearly all eukaryotes. The precise function of the DENN domain remains to be elucidated but its presence in a number of proteins involved in endocytosis or exocytosis, most notably in a number of Rab interactors, led to the hypothesis that the DENN domain regulates membrane trafficking (Allaire et al., 2006; Clague and Lorenzo, 2005; Falbel et al., 2003; Levivier et al., 2001; Miyoshi and Takai, 2004). Moreover, the localization of Crag to the cell cortex and to recycling and early endosome membranes is consistent with a role in membrane trafficking.
We showed that apical Pcan in Crag mutant cells originates from newly synthesized protein and not from transcytosed basal protein because Crag pcan double mutant clones that maintain basal Pcan do not show apical Pcan. Assuming that in wild type FC newly synthesized BM components are secreted exclusively on the basal side, Crag could act by ensuring one or more steps during their polarized secretion. Crag may facilitate the sorting of newly synthesized BM components. In the absence of Crag, inaccurate sorting would lead to the packaging of BM proteins in vesicles destined for both apical and basolateral secretion. The colocalization of Crag protein with the recycling endosome (RE) marker Rab11 may point to a role for Crag in protein sorting as the RE has been shown to be a hub for the sorting of both newly synthesized and endocytosed apical and basolateral proteins (Ang et al., 2004; Rodriguez-Boulan et al., 2005). Alternatively, Crag may act at a later step and direct the polarized transport, targeting or fusion of vesicles containing BM components specifically with the basal membrane. Interestingly, since Crag is absent from the basal cortex in FC, Crag would play a repulsive role, inhibiting the targeting or fusion of BM protein containing vesicles with apical and lateral membranes. In the absence of Crag, transport vesicles would be allowed to fuse with basolateral as well as apical membranes leading to the observed apolar distribution of Lam, Coll IV and Pcan.
Even though evidence from previous work points toward the active sorting and polarized secretion of BM proteins (Boll et al., 1991; Caplan et al., 1987; Natori et al., 1992; Unemori et al., 1990), the possibility remains that BM components are secreted both apically and basally in wild type FC but stabilized only on the basal side, or removed preferentially apically, for instance through degradation by localized matrix metalloproteases. While these scenarios require additional assumptions, Crag could mediate either of these processes and regulate the basal accumulation of BM proteins more indirectly, yet specifically.
In summary, we have uncovered a crucial and novel role for the conserved protein Crag in the regulation of epithelial architecture of the Drosophila FE. Crag appears to specifically affect the polarized trafficking of BM components. Even though the existence of different trafficking routes for basally secreted and basolateral transmembrane proteins has been suggested previously, Crag is – to our knowledge – the first specific component required to ensure the polarized accumulation of BM on the basal side of epithelial cells. It therefore serves to define this process and provides a clear entry point into its molecular analysis. It will be interesting to elucidate the molecular machinery through which Crag regulates the polarized deposition of BM components.
Experimental procedures
Fly stocks and genetics
Control flies used were y w FRT19A or OreR. Deficiency and Duplication lines and P-element lines used for mapping (Bellen et al., 2004) were obtained from Bloomington. The mutant lines dlgm52 FRT101 (Woods and Bryant, 1991), FRT82B crb11A2 (Bilder et al., 2003) and par6Δ226 FRT9-2 (Petronczki and Knoblich, 2001) were gifts from J. Zallen. pcannull FRT101 (Schneider et al., 2006) was a gift from S. Baumgartner. The transgenic lines UAS-GFP-Rab5 (Wucherpfennig et al., 2003), UAS-GFP-Rab7 (Entchev et al., 2000) and UAS-GFP-Rab11 (Emery et al., 2005) were gifts from M. Gonzalez-Gaitan. GR1-Gal4 was used for overexpression experiments. The Vkg-GFP line (CC00791) (Buszczak et al., 2007) was obtained from the Spradling lab through FlyTrap. Follicle cell clones were generated using the FRT/UAS-Flp/GAL4 system (Duffy et al., 1998). The genotypes of females used for the analyses can be found in the supplemental data.
Mosaic screen
Males of the genotype y w FRT19A were mutagenized and balanced as described by (Chen and Schupbach, 2006). Females from each lethal line were crossed to males of the genotype Ubi-GFP FRT19A/Y; e22c-Gal4, UAS-Flp/CyO. Female offspring of the genotype * FRT19A/Ubi-GFP FRT19A; e22c-Gal4, UAS-Flp/+ were dissected, fixed and stained with Phalloidin and Hoechst. Ovaries were mounted in Aquapolymount and scored for epithelial defects using an Axiophot epifluorescence microscope. In 2 separate screens a total of 12 Crag alleles were isolated.
Mapping of Crag mutations
We used recombination with visible recessive markers to map the lethal mutation in Crag mutants to the region between cut (7B4) and vermillion (9F11). The lethal phenotype was rescued by duplications uncovering genomic regions 7A8; 8A5 and 7D; 8B3-D7 (Dp(1;2) sn[+]72d and Dp(1;Y)850 respectively) allowing us to use the Deficiencies Df(1)KA14 and Df(1)RA2 to further map the mutation between 7F1 and 8A4. This also allowed us to confirm that all Crag alleles isolated were allelic. Subsequent recombination mapping with P-element insertions placed the lethal mutation between BG02451 (7F4) and BG02681 (7F8). To identify the molecular lesions, the various Crag mutant lines were balanced over an FM7c, Kr>GFP balancer chromosome and genomic DNA from ~10–15 non-GFP expressing Crag−/Y first instar larvae was isolated using a method described in http://www.fruitfly.org/about/methods/inverse.pcr.html. PCR products covering the Crag gene region were sequenced and sequences were compared with those of FRT19A control products. For each allele, PCR products of at least 2 independent genomic preps were sequenced.
Immunofluorescence stainings
Ovaries were dissected in PBS, fixed in PBS with 4% paraformaldehyde for 20’ at room temperature and stained as described (Ashburner, 1989). Schneider’s medium was used instead of PBS for dissections and fixations in the case of Crag and HA-Crag stainings. Ovaries, mounted in Aquapolymount, were visualized using a Zeiss laser scanning confocal microscope (lsm510). Primary antibodies used were rat polyclonal CragC (this study, 1:200), rabbit aPKC (1:1000, Santa Cruz), mouse Arm (N2 7A1, 1:50, DSHB), rabbit Baz (1:500, Wodarz et al., 1999), rat DE-Cadherin (DCAD2, 1:20, DSHB), mouse CollagenIV (6G7, 1:10, Murray et al., 1995) rabbit DG-cyto (1:1000, Deng et al., 2003)), mouse FasII (1D4, 1:1000 conc., DSHB), mouse FasIII (7G10, 1:10, DSHB), mouse HA (F-7, 1:1000, Santa Cruz), rat HA (3F10, 1:50, Roche), rabbit Laminin (1:1000, Fessler et al., 1987) rabbit Laminin-A (293, 1:20, Gutzeit et al., 1991), mouse Notch (C17.9C6, 1:100, DSHB), rabbit Pcan (1:1000, (Friedrich et al., 2000)), rabbit Rab5 (1:50, Wucherpfennig et al., 2003), mouse Rab11 (1:20, BD Biosciences). Secondary antibodies were AlexaFluor488, 568, 647 conjugated (Molecular probes) and used at 1:1000. Phalloidin conjugates and Hoechst were from Molecular Probes.
Constructs and antibody production
To generate the Crag genomic rescue construct, BAC clone RP98-40O10 was digested with NheI/KpnI and a 12.4 kb fragment including the Crag gene, 1695 bp upstream and 1561 bp downstream sequences was cloned into pCasper4. To generate pUASp-HA-CragA and pUASp-HA-CragB the coding regions and 3’UTRs of Crag-PA and Crag-PB were cloned into pUASp in frame with an N-terminal triple HA tag. For Crag-PB we used EST clone AT30747. Because no full length cDNA encoding Crag-PA was available we constructed full length Crag-PA by exchanging a 1kb DraIII/FspI fragment from AT30747 with that of LD37492 which contains the Crag-PA specific exon 10. For antibody production a fragment encoding aa 919–1666 of Crag-PA was cloned into pGEX-4T-1. GST-CragC was purified on Ni-NTA agarose (Novagen) and Glutathione-Sepharose (GE Healthcare) and injected into rats to generate rat polyclonal antibodies (Pocono Rabbit Farm and Laboratory Inc.).
Supplementary Material
Acknowledgements
We thank S. Baumgartner, L. and J. Fessler, M. Gonzalez-Gaitan, H. Gutzeit, H. Ruohola-Baker, A. Wodarz, J. Zallen, the Developmental Studies Hybridoma Bank and the Bloomington stock center for providing flies and antibodies; J. Goodhouse for advice with confocal microscopy and members of the Schüpbach and Wieschaus labs for feedback and advice. We also thank S. De Renzis, C. Denef, F. Ulrich, Y. Yan, and J. Zallen for helpful comments on this or previous versions of the manuscript. This work was supported by the Howard Hughes Medical Institute and US Public Health Service Grants PO1 CA41086 and RO1 GM077620. N.D. was supported by a Long-Term Fellowship from the Human Frontier Science Program during part of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelilah-Seyfried S, Cox DN, Jan YN. Bazooka is a permissive factor for the invasive behavior of discs large tumor cells in Drosophila ovarian follicular epithelia. Development. 2003;130:1927–1935. doi: 10.1242/dev.00420. [DOI] [PubMed] [Google Scholar]
- Allaire PD, Ritter B, Thomas S, Burman JL, Denisov AY, Legendre-Guillemin V, Harper SQ, Davidson BL, Gehring K, McPherson PS. Connecdenn, a novel DENN domain-containing protein of neuronal clathrin-coated vesicles functioning in synaptic vesicle endocytosis. J Neurosci. 2006;26:13202–13212. doi: 10.1523/JNEUROSCI.4608-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang AL, Taguchi T, Francis S, Folsch H, Murrells LJ, Pypaert M, Warren G, Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M. A laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, Evans-Holm M, Hiesinger PR, Schulze KL, Rubin GM, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167:761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- Bilder D, Schober M, Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol. 2003;5:53–58. doi: 10.1038/ncb897. [DOI] [PubMed] [Google Scholar]
- Boll W, Partin JS, Katz AI, Caplan MJ, Jamieson JD. Distinct pathways for basolateral targeting of membrane and secretory proteins in polarized epithelial cells. Proc Natl Acad Sci U S A. 1991;88:8592–8596. doi: 10.1073/pnas.88.19.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M, Paterno S, Lighthouse D, Bachman J, Planck J, Owen S, Skora AD, Nystul TG, Ohlstein B, Allen A, et al. The carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics. 2007;175:1505–1531. doi: 10.1534/genetics.106.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan MJ, Stow JL, Newman AP, Madri J, Anderson HC, Farquhar MG, Palade GE, Jamieson JD. Dependence on pH of polarized sorting of secreted proteins. Nature. 1987;329:632–635. doi: 10.1038/329632a0. [DOI] [PubMed] [Google Scholar]
- Chambard M, Gabrion J, Mauchamp J. Influence of collagen gel on the orientation of epithelial cell polarity: follicle formation from isolated thyroid cells and from preformed monolayers. J Cell Biol. 1981;91:157–166. doi: 10.1083/jcb.91.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Schupbach T. The role of brinker in eggshell patterning. Mech Dev. 2006;123:395–406. doi: 10.1016/j.mod.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Clague MJ, Lorenzo O. The myotubularin family of lipid phosphatases. Traffic. 2005;6:1063–1069. doi: 10.1111/j.1600-0854.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- Cohen D, Musch A, Rodriguez-Boulan E. Selective control of basolateral membrane protein polarity by cdc42. Traffic. 2001;2:556–564. doi: 10.1034/j.1600-0854.2001.20805.x. [DOI] [PubMed] [Google Scholar]
- Cox DN, Seyfried SA, Jan LY, Jan YN. Bazooka and atypical protein kinase C are required to regulate oocyte differentiation in the Drosophila ovary. Proc Natl Acad Sci U S A. 2001;98:14475–14480. doi: 10.1073/pnas.261565198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida JB, Stow JL. Disruption of microtubules alters polarity of basement membrane proteoglycan secretion in epithelial cells. The American journal of physiology. 1991;261:C691–C700. doi: 10.1152/ajpcell.1991.261.1.C691. [DOI] [PubMed] [Google Scholar]
- Deng WM, Schneider M, Frock R, Castillejo-Lopez C, Gaman EA, Baumgartner S, Ruohola-Baker H. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development. 2003;130:173–184. doi: 10.1242/dev.00199. [DOI] [PubMed] [Google Scholar]
- Duffy JB, Harrison DA, Perrimon N. Identifying loci required for follicular patterning using directed mosaics. Development. 1998;125:2263–2271. doi: 10.1242/dev.125.12.2263. [DOI] [PubMed] [Google Scholar]
- Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, Gaitan MG, Knoblich JA. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122:763–773. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Entchev EV, Schwabedissen A, Gonzalez-Gaitan M. Gradient formation of the TGF-beta homolog Dpp. Cell. 2000;103:981–991. doi: 10.1016/s0092-8674(00)00200-2. [DOI] [PubMed] [Google Scholar]
- Falbel TG, Koch LM, Nadeau JA, Segui-Simarro JM, Sack FD, Bednarek SY. SCD1 is required for cytokinesis and polarized cell expansion in Arabidopsis thaliana [corrected] Development. 2003;130:4011–4024. doi: 10.1242/dev.00619. [DOI] [PubMed] [Google Scholar]
- Fernandez-Minan A, Martin-Bermudo MD, Gonzalez-Reyes A. Integrin signaling regulates spindle orientation in Drosophila to preserve the follicular-pithelium monolayer. Curr Biol. 2007;17:683–688. doi: 10.1016/j.cub.2007.02.052. [DOI] [PubMed] [Google Scholar]
- Fessler LI, Campbell AG, Duncan KG, Fessler JH. Drosophila laminin: characterization and localization. J Cell Biol. 1987;105:2383–2391. doi: 10.1083/jcb.105.5.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MV, Schneider M, Timpl R, Baumgartner S. Perlecan domain V of Drosophila melanogaster. Sequence, recombinant analysis and tissue expression. Eur J Biochem. 2000;267:3149–3159. doi: 10.1046/j.1432-1327.2000.01337.x. [DOI] [PubMed] [Google Scholar]
- Goode S, Perrimon N. Inhibition of patterned cell shape change and cell invasion by Discs large during Drosophila oogenesis. Genes Dev. 1997;11:2532–2544. doi: 10.1101/gad.11.19.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode S, Wei J, Kishore S. Novel spatiotemporal patterns of epithelial tumor invasion in Drosophila discs large egg chambers. Dev Dyn. 2005;232:855–864. doi: 10.1002/dvdy.20336. [DOI] [PubMed] [Google Scholar]
- Gutzeit HO, Eberhardt W, Gratwohl E. Laminin and basement membrane-associated microfilaments in wild-type and mutant Drosophila ovarian follicles. J Cell Sci. 1991;100(Pt 4):781–788. doi: 10.1242/jcs.100.4.781. [DOI] [PubMed] [Google Scholar]
- Hall HG, Farson DA, Bissell MJ. Lumen formation by epithelial cell lines in response to collagen overlay: a morphogenetic model in culture. Proc Natl Acad Sci U S A. 1982;79:4672–4676. doi: 10.1073/pnas.79.15.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne-Badovinac S, Bilder D. Mass transit: epithelial morphogenesis in the Drosophila egg chamber. Dev Dyn. 2005;232:559–574. doi: 10.1002/dvdy.20286. [DOI] [PubMed] [Google Scholar]
- Lee JK, Brandin E, Branton D, Goldstein LS. alpha-Spectrin is required for ovarian follicle monolayer integrity in Drosophila melanogaster. Development. 1997;124:353–362. doi: 10.1242/dev.124.2.353. [DOI] [PubMed] [Google Scholar]
- Levivier E, Goud B, Souchet M, Calmels TP, Mornon JP, Callebaut I. uDENN, DENN, and dDENN: indissociable domains in Rab and MAP kinases signaling pathways. Biochem Biophys Res Commun. 2001;287:688–695. doi: 10.1006/bbrc.2001.5652. [DOI] [PubMed] [Google Scholar]
- Li S, Edgar D, Fassler R, Wadsworth W, Yurchenco PD. The role of laminin in embryonic cell polarization and tissue organization. Dev Cell. 2003;4:613–624. doi: 10.1016/s1534-5807(03)00128-x. [DOI] [PubMed] [Google Scholar]
- Lunstrum GP, Bachinger HP, Fessler LI, Duncan KG, Nelson RE, Fessler JH. Drosophila basement membrane procollagen IV. I. Protein characterization and distribution. J Biol Chem. 1988;263:18318–18327. [PubMed] [Google Scholar]
- Manfruelli P, Arquier N, Hanratty WP, Semeriva M. The tumor suppressor gene, lethal(2)giant larvae (1(2)g1), is required for cell shape change ofepithelial cells during Drosophila development. Development. 1996;122:2283–2294. doi: 10.1242/dev.122.7.2283. [DOI] [PubMed] [Google Scholar]
- Medioni C, Noselli S. Dynamics of the basement membrane in invasive epithelial clusters in Drosophila. Development. 2005;132:3069–3077. doi: 10.1242/dev.01886. [DOI] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Miyoshi J, Takai Y. Dual role of DENN/MADD (Rab3GEP) in neurotransmission and neuroprotection. Trends Mol Med. 2004;10:476–480. doi: 10.1016/j.molmed.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Mostov K, Su T, ter Beest M. Polarized epithelial membrane traffic: conservation and plasticity. Nat Cell Biol. 2003;5:287–293. doi: 10.1038/ncb0403-287. [DOI] [PubMed] [Google Scholar]
- Muller HA, Bossinger O. Molecular networks controlling epithelial cell polarity in development. Mech Dev. 2003;120:1231–1256. doi: 10.1016/j.mod.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Murray MA, Fessler LI, Palka J. Changing distributions of extracellular matrix components during early wing morphogenesis in Drosophila. Dev Biol. 1995;168:150–165. doi: 10.1006/dbio.1995.1068. [DOI] [PubMed] [Google Scholar]
- Natori Y, O'Meara YM, Manning EC, Minto AW, Levine JS, Weise WJ, Salant DJ. Production and polarized secretion of basement membrane components by glomerular epithelial cells. The American journal of physiology. 1992;262:F131–F137. doi: 10.1152/ajprenal.1992.262.1.F131. [DOI] [PubMed] [Google Scholar]
- Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- O'Brien LE, Zegers MM, Mostov KE. Opinion: Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- Petronczki M, Knoblich JA. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat Cell Biol. 2001;3:43–49. doi: 10.1038/35050550. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- Schneider M, Khalil AA, Poulton J, Castillejo-Lopez C, Egger-Adam D, Wodarz A, Deng WM, Baumgartner S. Perlecan and Dystroglycan act at the basal side of the Drosophila follicular epithelium to maintain epithelial organization. Development. 2006;133:3805–3815. doi: 10.1242/dev.02549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmer R, Ojakian GK. The alpha 2 beta 1 integrin regulates collagen-mediated MDCK epithelial membrane remodeling and tubule formation. J Cell Sci. 1995;108(Pt 6):2487–2498. doi: 10.1242/jcs.108.6.2487. [DOI] [PubMed] [Google Scholar]
- Semova N, Kapanadze B, Corcoran M, Kutsenko A, Baranova A, Semova A. Molecular cloning, structural analysis, and expression of a human IRLB, MYC promoter-binding protein: new DENN domain-containing protein family emerges smallstar, filled. Genomics. 2003;82:343–354. doi: 10.1016/s0888-7543(03)00103-4. [DOI] [PubMed] [Google Scholar]
- Sisson JC, Field C, Ventura R, Royou A, Sullivan W. Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J Cell Biol. 2000;151:905–918. doi: 10.1083/jcb.151.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G, Smith C, McGlade J, Tepass U. Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J Cell Biol. 2000;151:891–904. doi: 10.1083/jcb.151.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G, Tepass U. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat Cell Biol. 2003;5:46–52. doi: 10.1038/ncb896. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Unemori EN, Bouhana KS, Werb Z. Vectorial secretion of extracellular matrix proteins, matrix-degrading proteinases, and tissue inhibitor of metalloproteinases by endothelial cells. J Biol Chem. 1990;265:445–451. [PubMed] [Google Scholar]
- Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. II. Disassembly and assembly of plasma membrane domains during reversal of epithelial cell polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 1990;95(Pt 1):153–165. doi: 10.1242/jcs.95.1.153. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Kuchinke U, Knust E. Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature. 1999;402:544–547. doi: 10.1038/990128. [DOI] [PubMed] [Google Scholar]
- Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig T, Wilsch-Brauninger M, Gonzalez-Gaitan M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J Cell Biol. 2003;161:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XZ, Wes PD, Chen H, Li HS, Yu M, Morgan S, Liu Y, Montell C. Retinal targets for calmodulin include proteins implicated in synaptic transmission. J Biol Chem. 1998;273:31297–31307. doi: 10.1074/jbc.273.47.31297. [DOI] [PubMed] [Google Scholar]
- Yu W, Datta A, Leroy P, O'Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell. 2006;16:433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22:521–538. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Zuk A, Matlin KS. Apical beta 1 integrin in polarized MDCK cells mediates tubulocyst formation in response to type I collagen overlay. J Cell Sci. 1996;109(Pt 7):1875–1889. doi: 10.1242/jcs.109.7.1875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


