Abstract
Calcium-binding proteins dubbed KChIPs favour surface expression and modulate inactivation gating of neuronal and cardiac A-type Kv4 channels. To investigate their mechanism of action, Kv4.1 or Kv4.3 were expressed in Xenopus laevis oocytes, either alone or together with KChIP1, and the K+ currents were recorded using the whole-oocyte voltage-clamp and patch-clamp methods. KChIP1 similarly remodels gating of both channels. At positive voltages, KChIP1 slows the early phase of the development of macroscopic inactivation. By contrast, the late phase is accelerated, which allows complete inactivation in < 500 ms. Thus, superimposed traces from control and KChIP1-remodelled currents crossover. KChIP1 also accelerates closed-state inactivation and recovery from inactivation (3- to 5-fold change). The latter effect is dominating and, consequently, the prepulse inactivation curves exhibit depolarizing shifts (ΔV = 4–12 mV). More favourable closed-state inactivation may also contribute to the overall faster inactivation at positive voltages because Kv4 channels significantly inactivate from the preopen closed state. KChIP1 favours this pathway further by accelerating channel closing. The peak G-V curves are modestly leftward shifted in the presence of KChIP1, but the apparent ‘threshold’ voltage of current activation remains unaltered. Single Kv4.1 channels exhibited multiple conductance levels that ranged between 1.8 and 5.6 pS in the absence of KChIP1 and between 1.9 and 5.3 pS in its presence. Thus, changes in unitary conductance do not contribute to current upregulation by KChIP1. An allosteric kinetic model explains the kinetic changes by assuming that KChIP1 mainly impairs open-state inactivation, favours channel closing and lowers the energy barrier of closed-state inactivation.
Kv4 channels (members of the Shal family of K+ channels) are key components of the neuronal somatodendritic A-type K+ current and the transient K+ currents expressed in the heart (Pak et al. 1991; Serodio et al. 1994, 1996; Dixon et al. 1996; Johns et al. 1997; Song et al. 1998; Shibata et al. 2000; Greenstein et al. 2000; Guo et al. 2000; Malin & Nerbonne, 2000). In the nervous system, Kv4 channels prevent backpropagating action potentials, help to establish slow repetitive spike firing and contribute to spike repolarization and signal amplification (Connor & Stevens, 1971; Connor, 1978; Hoffman et al. 1997; Schoppa & Westbrook, 1999; Shibata et al. 2000). In the heart, on the other hand, these channels mainly help to shape the repolarizing phase of the action potential (Nerbonne, 2001; Oudit et al. 2001). All the physiological actions of these channels depend critically on the kinetics and voltage-dependence of inactivation gating. Earlier studies found that functional expression and inactivation of Kv4 channels are modulated by factors encoded by the small-molecular-weight mRNA from brain (Chabala et al. 1993; Serodio et al. 1994, 1996). More recently, some of these factors were identified as members of a family of small-molecular-weight calcium-binding proteins, which were dubbed KChIPs (Kv-Channel-Interacting-Proteins) (An et al. 2000). These proteins are related to known calcium-binding proteins, including frequenin, recoverin and calsenilin-DREAM (a transcriptional factor) (Pawlowski et al. 1996). KChIP1, KChIP2 and KChIP3 interact specifically with Kv4 channels in situ, upregulate current expression and modulate various aspects of inactivation gating (An et al. 2000; Bähring et al. 2001b). Previous observations showed that KChIPs slow rapid inactivation and accelerate the recovery from inactivation. Fast recovery from inactivation is a characteristic feature of native A-type K+ currents mediated by Kv4 channels (Song et al. 1998).
Recent reports (Jerng & Covarrubias, 1997; Jerng et al. 1999; Bähring et al. 2001a; Beck & Covarrubias, 2001) have demonstrated that Kv4 channels probably inactivate by mechanisms that differ from those better known in Shaker K+ channels (i.e. N-type and C-type inactivation; Yellen, 1998). These studies suggest that Kv4 channels undergo significant closed-state inactivation over a wide range of relevant voltages. Although upon depolarizations to positive voltages the channels might initially inactivate from the open state, the final slow pathway of inactivation probably involves channel closing and subsequent inactivation from the preopen closed state (Jerng et al. 1999; Beck & Covarrubias, 2001). The early fast phase of inactivation is mediated by the cytoplasmic N-terminal domain, probably in conjunction with proximal regions of the cytoplasmic C-terminal domain (Jerng & Covarrubias, 1997) and the slower and final phase of inactivation involves components of the internal vestibule of the pore (Jerng et al. 1999). These hypotheses constitute the main premises of the current study.
Here, we applied voltage-clamp and patch-clamp recording methods and a previously proposed model of inactivation gating (see above) to investigate the mechanism of action of KChIP1 on Kv4.1 and Kv4.3 channels expressed in Xenopus oocytes. Although these channels exhibit distinct inactivation when expressed alone, when co-expressed with KChIP1, Kv4.1 and Kv4.3 currents are nearly indistinguishable. The main hypothesis under test in this study is that KChIP1 remodels inactivation gating of Kv4 channels by modifying activation and inactivation transitions near the open state, which has a significant impact upon inactivation from the preopen closed state. Kinetic analysis revealed that KChIP1 slows fast inactivation from the open state and facilitates closed-state inactivation. Additionally, KChIP1 favours inactivation from the preopen closed state by accelerating channel closing. These observations can be modelled by assuming an allosteric kinetic scheme of K+ channel gating (Beck & Covarrubias, 2001). Overall, the results underscore the significance of closed state inactivation in Kv4 channels at all relevant voltages and are consistent with the presence of coupled internal gates that control channel closing and both fast and slow inactivation. A preliminary report of this study was previously published in abstract form (Beck et al. 2001).
METHODS
Molecular biology and cRNA microinjection
Wild-type mouse Kv4.1 was maintained in pBluescript II KS (Strategene, La Jolla, CA, USA). Kv4.3 cDNA (from rat) was kindly provided by Dr J. Nerbonne (Washington University, St Louis, MO, USA) and was maintained in pBK-CMV (Strategene). KChIP1 was maintained in a pBluescript vector, pBJ/KSM (kindly provided by W. J. Joiner, Yale University). Capped cRNA for expression in Xenopus laevis oocytes was produced by in vitro transcription using the Message Machine Kit (Ambion, Austin, TX, USA). Female Xenopus laevis were obtained from Nasco (Fort Atkinson, WI, USA). Oocytes were collected according to the guidelines of the Institutional Animal Care and Use Committee of Thomas Jefferson University, under anaesthesia (immersion in 0.2 % 3-aminobenzoic acid ethyl ester (Sigma, St Louis, MO, USA) for about 30 min), from frogs that were humanely killed after the final oocyte collection. Before injection, oocytes were defolliculated by digestion with collagenase (2 mg ml−1, Boehringer-Mannheim, Indianapolis, IN, USA) in calcium-free external solution (see below). The Kv4 and KChIP1 cRNAs were mixed (1:4 molar ratio) and immediately injected (total RNA injected ∼2–100 ng per oocyte) into defolliculated oocytes using a Nanoject microinjector (Drummond, Broomall, PA, USA). Currents were recorded 1–7 days postinjection.
Electrophysiology
To record whole-oocyte currents, the two-electrode voltage-clamp method was applied as described previously (Jerng & Covarrubias, 1997; Beck et al. 1998). The bath solution for these experiments had the composition of the external solution described below. Patch-clamp recording was conducted as described previously (Chabala et al. 1993; Jerng et al. 1999; Beck & Covarrubias, 2001) using an Axopatch 200A or 200B amplifier (Axon Instruments, Foster City, CA, USA). Patch pipettes were constructed from Corning glass 7052 or 7056 (Warner Instrument Corp., Hamden, CT, USA). For the recording of fast currents (e.g. tail current relaxations) and single channel currents the pipettes (∼0.5–1 M Ω and 5–10 M Ω in the bath solution, respectively) were coated with Sylgard elastomer (Dow Corning Co. Midland, MI, USA). The pipette solution (external) contained (mm): 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, 5 Hepes (pH 7.4, adjusted with NaOH). From macroscopic currents, the passive leak current was subtracted off-line assuming a linear leak current (for whole-oocyte currents) or, alternatively (for tail currents), both the passive leak current and the capacitive transients were subtracted on-line using a P/4 procedure. The passive leak current and the capacitive transients from single channel records were removed by subtracting blank sweeps (no unitary currents) from active traces. The recordings were filtered at 0.5–8 kHz (−3 dB, 8-pole Bessel filter; Frequency Devices, Haverhill, MA, USA) and digitized at 2–40 kHz. All experiments were recorded at room temperature (23 ± 1 °C).
Data acquisition, analysis and model simulations
A Pentium class computer interfaced to a 12-bit A/D converter (Digidata 1200 using Clampex 8.0; Axon Instruments, Foster City, CA, USA) controlled the voltage-clamp protocols and data acquisition. Data analysis was conducted using Clampfit 8.1 (Axon Instruments) and Origin 6.0 (Microcal Software Inc., Northhampton, MA, USA). Current relaxations and other time-dependent processes were described assuming a simple exponential function or the sum of exponential terms (Jerng & Covarrubias, 1997). The mean amplitude of the unitary currents was determined by fitting the sum of Gaussian terms to all-point histograms; the single channel conductance was also estimated by applying voltage ramp protocols to evoke the unitary currents (−100 to +100 mV; 0.38 mV ms−1; see Fig. 9 legend). Results throughout the manuscript are expressed as means ± s.e. Model simulations were conducted by determining the initial equilibrium probabilities of occupying a set of states and the characteristic differential equations of the model. For a particular pulse protocol and set of rate constants, this system of equations was solved numerically using SCoP 3.51w (Simulation Resources, Berrien Springs, MI, USA). The simulations were evaluated qualitatively (Fig. 11). The initial parameter values of the model (Fig. 10) were obtained from Beck & Covarrubias (2001) and were further adjusted until the same parameter set generated simulations that generally agreed with the main observations derived from the macroscopic currents (Figs 1–8).
Figure 9. Amplitude analysis of Kv4.1 single channel currents.
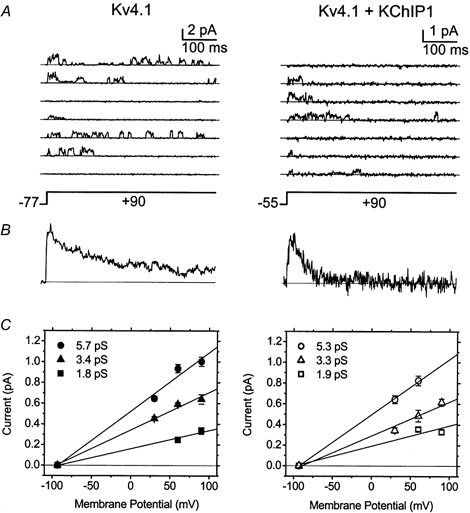
A, single channel currents evoked by a step depolarization to +90 mV from the indicated holding potentials. The interpulse interval was 1–2 s. The holding potential was adjusted to suppress the total current and allow the recording of unitary currents. The traces shown are 2 sets of 3 and 4 consecutive sweeps. The continuous straight line across the traces indicates the zero current level (closed level). Recordings were low-pass filtered at 2 kHz (8-pole Bessel filter) and digitized at 10 kHz. For display and analysis the records were digitally filtered at 500 Hz. B, ensemble average currents (90 traces from the experiments shown in A). Note that the kinetics of these currents correspond to those of the macroscopic Kv4.1 currents (e.g. Fig. 1). Clearly, in the presence of KChIP1 the channels undergo faster inactivation. C, single-channel current-voltage relations. The mean amplitudes were derived by fitting a sum of Gaussian terms (2–4) to all-point histograms generated from the experiments shown above (n = 7–27 selected traces at the indicated membrane potentials). The continuous lines are linear regressions constrained to cross the x-axis at the estimated reversal potential (Fig. 8 legend; −93 to −95 mV). The derived slope conductances are indicated on the graphs. In the presence of KChIP1 the large conductance (∼5 pS) was not generally observed. Similar results were obtained from voltage ramp protocols.
Figure 11. Observed and simulated Kv4.3 currents.

A, observed currents replotted from Fig. 1 at intervals of 20 mV (−50, −30, −10, +10, +30 and +50 mV). Normalized and superimposed traces depict control Kv4.3 currents (thin lines) and KChIP1-remodelled Kv4.3 currents (thick lines). B, simulated currents. These simulations assume Scheme 2 and the parameter values from Table 1. The set of parameter values used here was constrained to simultaneously account for the following properties: voltage dependence of activation and inactivation, time-to-peak, recovery from inactivation, the development of inactivation at negative voltages and the overall time course of the tail currents at hyperpolarized voltages. The parameter values (rate constants in s−1) that are changed to account for the effects of KChIP1 on inactivation gating are shown along with the corresponding family of simulated currents.
Figure 10. Kinetic model of Kv4 gating (Scheme 2).

A, Kv4 gating in the absence of KChIP1. This model is based on previously developed models of Kv4 gating (Jerng et al. 1999; Beck & Covarrubias, 2001; Bähring et al. 2001a). B, Kv4 gating in the presence of KChIP1. Thicker arrows represent transitions favoured by KChIP1. A dashed arrow between O and I5 indicates a less favourable inactivation from the open state. The modelled currents are shown in Fig. 11, assuming the parameter values from Table 1. A relatively small allosteric factor (f = 0.3) allows for a significant coupling between voltage-dependent activation and inactivation from closed states (i.e. inactivation is more likely to occur from late closed states in the activation pathway).
Figure 1. Outward currents mediated by Kv4.1 and Kv4.3 channels.

A and B, from a holding potential of −100 mV, control Kv4.1 or Kv4.3 currents were evoked by step depolarizations from −80 to +50 mV, in 10 mV intervals. The interpulse interval was 5 s. Note that Kv4.3 currents exhibit a larger fast phase in the development of inactivation. C and D, currents mediated by Kv4.1 or Kv4.3 channels coexpressed with KChIP1. Note that KChIP1 renders the Kv4.1 and Kv4.3 currents nearly indistinguishable. E and F, Kv4.1 and Kv4.3 currents at 0 and +50 mV (from panels A-D) were scaled and superimposed to compare the development of inactivation. Thin line, control; thick line, coexpressed with KChIP1. The inset shows the first 200 ms of the corresponding traces. Note the crossover of the currents at +50 mV.
Figure 8. Voltage dependence of prepulse inactivation and activation parameters.

A and B, relation between the prepulse potential and the normalized peak current (I/Imax). The voltage protocol in these experiments was as described in the legend to Fig. 7, except that the duration of the prepulse was fixed and the prepulse voltage was varied. The prepulse length was 12 s and 15 s for Kv4.1 in the absence and presence of KChIP1, respectively, and 20 s for Kv4.3, both in the absence and presence of KChIP1. Filled and open symbols represent the mean values of the normalized peak current (n = 3–6) in the absence and presence of KChIP1, respectively. The continuous lines are the best-fit first-order Boltzmann functions (plus a constant). The best-fit parameters (Vm = midpoint potential, k = slope factor; and NI = non-inactivating fraction) are shown in the graphs. C and D, peak G-V curves. The corresponding peak conductance-voltage relations (Gp-Vt) derived from currents evoked by the pulse protocol described in Fig. 1 legend. The peak chord conductance (Gp) was calculated from the following relation: Gp= Ip/(Vt - Vr), where Ip is the peak current, Vt is the test potential and Vr is the reversal potential of the current (−93 to −95 mV, as determined from the instantaneous current-voltage relation derived from the corresponding tail current experiments). The instantaneous current-voltage relations are linear over the range of voltages within which the channels activate (not shown). Each symbol represents the mean value of 5–13 experiments. The continuous lines represent the best-fit 4th order Boltzmann functions (Smith-Maxwell et al. 1998). The parameters of these fits are given in the graph (Vm is the midpoint voltage for the activation of 1 subunit and k is the corresponding slope factor). The data are shown normalized to the maximum estimated peak conductance (Gp,Max). E and F, voltage dependence of time-to-peak. Currents were evoked by the pulse protocol described in Fig. 1 legend.
RESULTS
Differential effects of KChIP1 on fast and slow inactivation of Kv4 channels
To investigate the modulatory changes induced by KChIP1, the macroscopic inactivation of outward K+ currents was examined at various membrane potentials in oocytes expressing Kv4.1 or Kv4.3 alone (control) and oocytes coexpressing KChIP1 and either Kv4.1 or Kv4.3. Upon depolarization, control currents exhibited characteristic rapid activation and slower inactivation (Fig. 1A and B). At positive voltages, the kinetics of inactivation were complex and included fast and slow phases. Typically, depolarizations > 1 s were necessary to nearly reach the zero-current level. In the presence of KChIP1, the development of inactivation underwent a significant transformation. Specifically, the early phase of the current decayed more slowly (this was especially apparent from Kv4.3 currents) and inactivation was nearly complete within ∼500 ms. Although the waveforms of the Kv4.1 and Kv4.3 control currents differed significantly (Fig. 1A and B), expressed with KChIP1 they became remarkably similar (Fig. 1C and D). Superimposed and normalized control and KChIP1-remodelled currents at 0 and +50 mV clearly showed that the late phase of the decay appeared faster while the early phase (at +50 mV) was slower. As a result of these changes, the control and KChIP1-remodelled currents evoked at +50 mV characteristically cross over (Fig. 1E and F). Thus, it can be hypothesized that KChIP1 exerts differential and opposite effects on the fast and slow processes of inactivation, allowing faster and virtually complete macroscopic inactivation with an apparently slowed initial phase.
To test this hypothesis and examine the changes described above more quantitatively, the development of inactivation was described assuming a sum of exponential terms, and the best-fit parameters (time constants and fractional amplitudes) were plotted against membrane potential (−10 to +70 mV; Fig. 2 and Fig. 3 for Kv4.1 and Kv4.3, respectively). In agreement with earlier reports, control Kv4.1 currents were well described assuming the sum of three exponential terms and a constant (Jerng & Covarrubias, 1997; Beck & Covarrubias, 2001). Between −10 and +70 mV, the fast time constant decreased (from 31 ± 3 to 17 ± 0.5 ms), the intermediate one decreased slightly (from 88 ± 6 to 75 ± 4 ms) and the slow one increased (from 221 ± 22 to 276 ± 25 ms). All exponential components contributed significantly to the fitted decay (20–40 %; Fig. 2B) and their corresponding fractional amplitudes exhibited little voltage dependence (the fast and slow components decreased and increased slightly, respectively). Over the voltage range studied, the non-inactivating fraction of the current increased significantly with depolarization (from 0.007 ± 0.001 to 0.03 ± 0.01), but was always < 3 % of the fitted decay (Fig. 2B). In contrast, Kv4.1 currents coexpressed with KChIP1 could be well described by the sum of two exponential terms plus a constant (Fig. 2C and D and legend). Notably, KChIP1 eliminated the robust slow component of the control Kv4.1 current decay. Furthermore, the remaining components exhibited novel properties. The fast time constant (∼40 ms) displayed little or no voltage dependence, while the slow one decreased sharply between −10 and +10 mV (280 ± 37 and 88 ± 6 ms) and its fractional contribution increased from ∼0.03 to ∼0.4 over the same voltage range. The fractional amplitude of this component continued to increase moderately with depolarization, reaching 0.8 at +50 mV (Fig. 2D), and the non-inactivating fraction of the current remained small, increasing slightly between 0 and +50 mV (from 0.006 ± 0.001 to 0.013 ± 0.004). Thus, at voltages >+30 mV the decay of the Kv4.1 currents remodelled by KChIP1 were dominated by one time constant (∼80 ms) that was ∼3.5-fold faster than the slow time constant of the control Kv4.1 currents (Fig. 2). These results suggested that KChIP1 favoured inactivation of Kv4.1 channels by eliminating the slowest process of current decay and, simultaneously, allowed a nearly monophasic decay by slowing the fast process of inactivation.
Figure 2. Kinetic analysis of the development of macroscopic Kv4.1 inactivation.
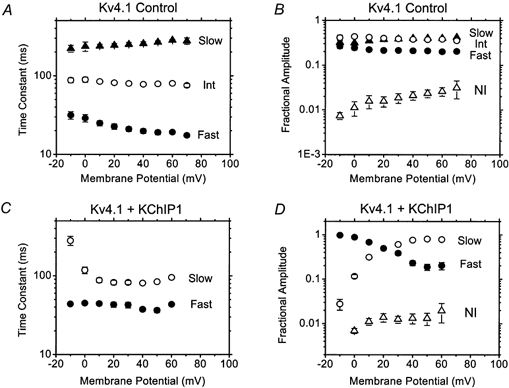
A, voltage dependence of the time constants derived from fitting a sum of 3 exponential terms plus a constant to the development of macroscopic inactivation in the absence of KChIP1; •,τFAST, ○, τINT and ▴, τSLOW (Jerng & Covarrubias, 1997). B, corresponding fractional amplitudes of the exponential terms (e.g. fraction AFAST = AFAST / (AFAST + AINT + ASLOW + ANI)). ▵, the fractional amplitude of the constant term, NI. C, voltage dependence of the time constants derived from fitting a sum of 2 exponential terms plus a constant to the development of macroscopic inactivation in the presence of KChIP1; •, τFAST and ○, τSLOW. In the latter case, a close examination of the residuals from the fits (comparing 1, 2 or 3 exponential terms; Jerng & Covarrubias, 1997) showed that the most significantly improved fits are obtained when upgrading from 1 to 2 exponential terms. In contrast with the control currents, no appreciably improved fits were obtained when 3 exponential terms were assumed to describe the decay of the currents expressed in the presence of KChIP1. D, corresponding fractional amplitudes of the exponential terms (see B). ▵, the fractional amplitude of the constant term, NI. Note that, in the presence of KChIP1, a new slow time constant dominates the development of inactivation at voltages > +30 mV. Each symbol represents the mean value of 3–6 experiments. Here and in subsequent figures, error bars are not apparent when they are not larger than the symbol.
Figure 3. Kinetic analysis of the development of macroscopic Kv4.3 inactivation.

Panels and symbols are as described in Fig. 2. Note that as a result of the remodelling by KChIP1 C and D in Figs 2 (Kv4.1) and 3 (Kv4.3) are very similar. Each symbol represents the mean value of 3–10 experiments.
The decay of Kv4.3 control currents was also well described by assuming the sum of three exponential terms and the magnitudes of the fast and intermediate time constants were very similar to those observed for Kv4.1 currents (Fig. 3A). The slow time constant was slower, but only differed by < 2-fold. However, in contrast to the decay of Kv4.1 currents, the fast component (which decreased slightly with voltage) dominated at all test voltages (> 50 %, from −10 to +70 mV), causing the decay of the Kv4.3 currents to appear faster than that of Kv4.1 currents (Fig. 1A and B). The fractional amplitudes of the slow and intermediate components decreased and increased with depolarization, respectively (Fig. 2B) and the non-inactivating fraction of the Kv4.3 current was small but modestly larger than that of the Kv4.1 current, ranging between 0.04 and 0.06 (with no apparent voltage dependence; Fig. 2B). Most remarkably, however, KChIP1 rendered the kinetic parameters from Kv4.1 and Kv4.3 outward currents almost indistinguishable (Figs 2C and D and 3C and D). The development of Kv4.3 inactivation remodelled by KChIP1 exhibited a slow time constant that decreased sharply between 0 and +20 mV (598 ± 124 and 121 ± 23, respectively) and the corresponding fractional amplitude increased with depolarization from 0.03 ± 0.006 to 0.84 ± 0.04 between 0 and +50 mV. The dominating time constant at voltages > +30 mV was ∼90 ms. Overall, the data were consistent with the idea that KChIP1 differentially influenced the fast and slow processes of Kv4 inactivation, allowing an almost exponential current decay and virtually complete inactivation in ∼500 ms.
KChIP1 sets the rate of recovery from inactivation in Kv4 channels
Previous studies have shown that KChIPs accelerate the recovery from inactivation of Kv4 channels (An et al. 2000; Bähring et al. 2001b). Figure 4 illustrates the effect on Kv4.1 and Kv4.3 channels of applying a double-pulse protocol, showing their time course of recovery from inactivation at −100 mV together with the best-fit exponential curves. In both cases, KChIP1 decreased the time constant by between 2.5- and 4-fold (5-fold at −80 mV; not shown), setting its value at 40–50 ms for Kv4.1 and Kv4.3 (Fig. 4). It is interesting, however, that in spite of faster recovery from inactivation, the previous section demonstrated that Kv4 outward currents expressed in the presence of KChIP1 still exhibited almost complete inactivation. Moreover, KChIP1 also slowed fast inactivation probably occurring from the open state (Fig. 2 and Fig. 3). These data suggest that, as previously proposed, other pathways play a significant role in determining inactivation gating of Kv4 channels (see Discussion). To test this hypothesis, subsequent experiments investigated whether KChIP1 might influence inactivation from a preopen closed state (i.e. inactivation coupled to channel closing) by examining channel closing and closed-state inactivation.
Figure 4. Kinetic analysis of recovery from inactivation.

A-D, Kv4.1 and Kv4.3 currents evoked by a double pulse protocol to investigate the kinetics of recovery from inactivation. The first pulse (from −100 mV to +50 mV) allows activation and complete inactivation of the evoked current and the second shorter pulse (+50 mV) tests the amount of current recovered after an increasingly prolonged interpulse interval (at −100 mV). The interval between episodes was 5 s. E and F, the time course of recovery from inactivation. The data points are normalized peak currents evoked as explained above. The normalized value is the result of dividing the test peak currents, I, by the corresponding control peak current, Imax. Each symbol represents the mean value of 3–5 experiments. Continuous line, the best-fit exponential. The derived time constants are shown in the graph.
KChIP1 accelerates closing of Kv4 channels
Weakly voltage-dependent channel closing in Kv4 channels probably contributes to closed-state inactivation near the open state (Beck & Covarrubias, 2001). To study the effect of KChIP1 on this interaction, Kv4.1 and Kv4.3 tail current relaxations were examined at hyperpolarized and moderately depolarized membrane potentials (−140 to −50 mV; Fig. 5 and Fig. 6). An earlier study reported that Kv4 tail currents were well described by assuming the sum of two exponential terms (Beck & Covarrubias, 2001). Fast and weakly voltage-dependent channel closing appeared to underlie the main component of the tail current at hyperpolarized voltages. The process(es) underlying the second component were less clear, but may have included closing from other open states (Fig. 9) and/or reopening of a fraction of channels that inactivated before eliciting the tail current (see Fig. 5 legend, for pulse protocol and Beck & Covarrubias, 2001). Tail current relaxations from Kv4.1 and Kv4.3 were similar and exhibited weak voltage dependence (Fig. 5 and Fig. 6). For Kv4.1, the fast time constants were dominant at hyperpolarized voltages (−140 to −110 mV; Fig. 5D) and ranged from 1 to 2.5 ms between −140 and −50 mV (where the estimated equivalent electronic charge, z, ∼ 0.3 e0; see Fig. 5 legend). The corresponding slow time constants ranged from 5 to 20 ms (z ∼ 0.6 e0). Note that the voltage dependence of the slow time constant tended to level off at hyperpolarized voltages, which suggested the presence of a process with little or no voltage dependence. Only one time constant could be resolved from control Kv4.3 tail currents at hyperpolarized voltages (Fig. 6C and D), but two time constants were clearly resolved at more positive voltages (from −80 to −50 mV).
Figure 5. Kinetic analysis of Kv4.1 tail current deactivation.

A and B, tail currents in the absence and presence of KChIP1, respectively. From a holding potential of −100 mV, a 5 ms pulse to +50 mV was delivered first to activate a significant outward current and a subsequent membrane repolarization evoked the tail current to examine the deactivation kinetics (the tested membrane potentials are indicated in C). The interval between episodes was 5 s. The outward current evoked by the pulse to +50 mV has been clipped to emphasize visualization of the inward tail currents. C, voltage dependence of the fast and slow time constants extracted from double exponential fits to the tail current relaxations. Filled and open symbols represent the time constants in the absence and presence of KChIP1, respectively. The continuous lines describe the voltage dependence of the time constants assuming a simple exponential function (τFAST) or an exponential term plus a constant (τSLOW). From these fits, the estimated equivalent electronic charges are: 0.3 e0 (τFAST) and 0.6 e0 (τSLOW) in the absence of KChIP1 and 0.4 e0 (τFAST) and 1.0 e0 (τSLOW) in the presence of KChIP1. The constant term (voltage-independent) is 3 ms and 1 ms in the absence and presence of KChIP1, respectively. D, voltage dependence of the amplitude ratios (AFAST /ASLOW) extracted from double exponential fits to the tail current relaxations. Filled and open symbols represent the amplitude ratios in the absence and presence of KChIP1, respectively. The symbols in all panels represent the mean values of 4–5 experiments. Note that the fast time constant dominates at hyperpolarized voltages and that, especially in this voltage range (−110 to −140 mV), both time constants of deactivation at are reduced in the presence of KChIP1.
Figure 6. Kinetic analysis of Kv4.3 tail current deactivation.

Pulse protocol, panels and symbols are as described in Fig. 5. Note that in the absence of KChIP1 a single exponential function was sufficient to describe the tail currents at hyperpolarized voltages (−110 to −140 mV), but 2 exponential terms are needed to describe them at voltages above the reversal potential (∼ −95 mV). The continuous lines describe the voltage dependence of the time constants assuming a simple exponential function (τFAST and τSLOW in the absence of KChIP1 and τFAST in the presence of KChIP1) or an exponential term plus a constant (τSLOW in the presence of KChIP1). The equivalent electronic charges estimated from these fits are: 0.4 e0 (τFAST and τSLOW in the absence of KChIP1) and 0.3 e0 (τFAST) and 0.8 e0 (τSLOW) in the presence of KChIP1. The constant term (voltage-independent) is 3 ms (τSLOW in the presence of KChIP1). The symbols in all panels represent the mean values of 3–4 experiments. Note that in the presence of KChIP1 a new fast time constant dominates the relaxation of the tail current at hyperpolarized voltages.
KChIP1 accelerated Kv4.1 and Kv4.3 tail current relaxations, especially at hyperpolarized voltages (Fig. 5B and Fig. 6B, open symbols). KChIP1 reduced Kv4.1 fast and slow time constants (between −140 and −110 mV) by 2-and 3-fold, respectively (Fig. 5C). Because this effect was more significant on the slow time constant at hyperpolarized voltages, it appeared to be slightly more voltage-dependent (z ∼ 1 e0). At all examined voltages, Kv4.3 tail currents with KChIP1 were clearly biexponential (like those from Kv4.1) with a novel dominant fast time constant at hyperpolarized voltages (which was faster than the corresponding single time constant of the control Kv4.3 currents; Fig. 6C and D). These results were consistent with more favourable channel closing when Kv4.1 and Kv4.3 channels are coexpressed with KChIP1. Thus, because channel closing was accelerated by KChIP1 and was nearly voltage-independent, KChIP1-remodelled channels were more likely to visit the preopen inactivation-permissive closed state upon a prolonged membrane depolarization, and preferentially inactivate from such a state. KChIP1, however, may also have a more direct effect on inactivation from closed states.
KChIP1 accelerates Kv4 inactivation from the preopen closed state
Both Kv4.1 and Kv4.3 channels exhibited perceptible current activation at voltages more depolarized than −45 mV (Fig. 8). To examine inactivation from closed states, the development of inactivation was investigated at −50 mV, at which the channels do not open significantly. In a series of consecutive recording episodes, a variable −50 mV prepulse (which started at t = 0 ms and increased in duration with every episode) preceded a test pulse of constant duration to +50 mV (see Fig. 7 legend). Thus, as the prepulse duration increased, the peak currents evoked by the test pulse decreased (Fig. 7A and B). The envelope of these currents delineated the development of inactivation at −50 mV, which at this voltage was well described as an exponential decay (Kv4.1: τ = 415 ± 41 ms, n = 4; Kv4.3: τ = 3053 ± 85 ms, n = 4). Currents remodelled by KChIP1 clearly inactivated faster at −50 mV and the development of inactivation was also well described as an exponential decay (Kv4.1 + KChIP1: τ = 167 ± 7 ms, n = 4; Kv4.3 + KChIP1: τ = 1107 ± 27 ms, n = 5). Thus, KChIP1 favoured closed-state inactivation near the open state by accelerating weakly voltage-dependent channel closing and inactivation from the preopen closed state. Such a mechanism could therefore contribute to the earlier completion of inactivation of the macroscopic Kv4 currents at positive voltages (Figs 1–3), while the rapid phase of inactivation (probably from the open state) was actually slowed and recovery from inactivation was accelerated (Figs 2–4).
Figure 7. Kinetic analysis of the development of inactivation at −50 mV.

To examine the development of closed state inactivation, outward currents were evoked by a 250 ms step depolarization to +50 mV from an increasingly prolonged prepulse to −50 mV (the corresponding control traces were evoked from a holding potential of −100 mV). This voltage activates < 3 % of the total conductance (Fig. 9). The interval between episodes was ≥ 5 s. A and B, time courses of closed-state inactivation at −50 mV. The data points are normalized peak currents evoked as explained above. The normalized values resulted from dividing the peak currents, I, evoked from −50 mV by the peak control current, Imax. Each symbol represents the mean value of 4–5 experiments. The continuous lines are the best-fit exponential curves. For Kv4.1 the derived time constants are 408 ms and 166 ms in the absence and presence of KChIP1, respectively. For Kv4.3 the derived time constants are 2.9 s and 1.1 s in the absence and presence of KChIP1, respectively.
Kv4 channels coexpressed with KChIP1 exhibit depolarized prepulse inactivation curves
Upon a prolonged depolarization, closed-state inactivation is the preferred pathway of inactivation in Kv4 channels (Beck & Covarrubias, 2001). Thus, upon hyperpolarization, Kv4 channels rapidly recover from inactivation by a direct return to closed states in the activation pathway. The midpoint voltage of prepulse inactivation (Vm) corresponds to the membrane potential at which there is a kinetic balance between the development of inactivation and recovery from inactivation. If KChIP1 preferentially favoured the development of closed-state inactivation or the recovery from inactivation, the prepulse inactivation curve might exhibit a leftward or rightward shift, respectively (or no apparent effect when the effects were equivalent). In agreement with a preferential effect on Kv4.1 recovery from inactivation (Fig. 4 and Fig. 7), the prepulse inactivation curve with KChIP1 was significantly shifted to the right and slightly steeper (Fig. 8A; Vm (control) = −69.5 ± 0.9 mV and k (control) = 5.4 ± 0.05 mV e-fold−1, n = 4; Vm (KChIP1) = −57.8 ± 0.4 mV and k (KChIP1) = 3.7 ± 0.04 mV e-fold−1, n = 7). A similar but more modest effect of KChIP1 was found on Kv4.3 prepulse inactivation (Fig. 8B; Vm (control) = −62.5 ± 0.9 mV and k (control) = 3.7 ± 0.2 mV e-fold−1, n = 3; Vm (KChIP1) = −58.5 ± 0.5 mV and k (KChIP1) = 3.7 ± 0.04 mV e-fold−1; n = 5), which was consistent with a smaller effect of KChIP1 on Kv4.3 recovery from inactivation. Again, it was noteworthy that Kv4.1 and Kv4.3 prepulse inactivation curves became indistinguishable in the presence of KChIP1. These changes further underscored the significant remodelling of Kv4 closed state inactivation by KChIP1.
KChIP1 does not significantly alter Kv4 current activation
Activation gating of intact Kv4 channels is difficult to study because the peak currents are strongly influenced by rapid inactivation. Nevertheless, as a relative measurement, the peak conductance-voltage relation (peak G-V curve; see Fig. 8 legend) was examined to investigate whether depolarizing shifts described above could also be the result of altered voltage-dependent activation. Contrary to the rightward shifts introduced by KChIP1 on prepulse inactivation (Fig. 8), the peak G-V curves of Kv4.1 or Kv4.3 with KChIP1 appeared shifted to the left (Fig. 8C and D) but, regardless of KChIP1, the conductance was perceptibly activated at about the same membrane potential (−45 to −40 mV). Thus, it was unlikely that the rightward shifted prepulse inactivation curves observed with KChIP1 were a consequence of altered voltage-dependent activation. The apparent leftward shifts in the peak G-V curves could be the result of slowed rapid inactivation (Fig. 2 and Fig. 3), which might involve open-state inactivation. Consistent with minimal effects on activation gating, the time-to-peak was unaffected or only slightly decreased by KChIP1 (Fig. 8E and F). Additional biophysical analyses outside the scope of this study (gating currents and kinetic analysis of single channel currents) are necessary to investigate the effects of KChIP1 on voltage-dependent activation of Kv4 channels more directly.
Does KChIP1 enhance current expression by increasing the single channel current?
One of the most significant effects of KChIP1 is to upregulate Kv4 currents in heterologous expression systems (An et al. 2000; Bähring et al. 2001b). KChIP1 in this study increased the expression of Kv4.1 and Kv4.3 by 30-fold and 9-fold, respectively (at +50 mV the peak currents were: 0.5 ± 0.06 (n = 10) and 16 ± 1.7 μA (n = 11) from Kv4.1 and Kv4.1+KChIP1, respectively and 1.4 ± 0.09 (n = 13) and 12 ± 0.7 μA (n = 13), from Kv4.3 and Kv4.3+KChIP1, respectively). In part, such a change could be the result of increasing the single channel conductance. Voltage steps to various voltages (+30, +60 and +90 mV) evoked unitary currents in cell-attached patches expressing Kv4.1 channels (Fig. 9). In agreement with the development of inactivation, single channel fluctuations were frequently observed near the onset of the depolarization and reopenings might occur throughout the pulse before reaching a longer lived inactivated state (Fig. 9A). These reopenings were, however, less frequent in the presence of KChIP1, which suggests more favourable inactivation. The ensemble average currents confirmed an overall faster development of inactivation upon remodelling by KChIP1 (Fig. 9). Sometimes no openings were observed during the depolarization as the channel became prematurely inactivated from closed states in the activation pathway. Although the kinetic complexity of the single channel records prevented an accurate estimation of the number of channels in the patch, at least three conducting levels were consistently observed in patches expressing Kv4.1 alone (or Kv4.3, not shown). Although occasionally these levels occurred in isolation, they were more evident during bursts of single channel activity. The estimated unitary amplitudes from all-point histograms were 5.6, 3.6 and 1.8 pS (Fig. 9). The unitary conductances from Kv4.1 channels coexpressed with KChIP1 were similar (5.3, 3.3 and 1.9 pS) but the large conducting level appeared absent at +90 mV. Similar results were obtained from unitary currents evoked by voltage ramp protocols (from −100 to +100 mV; not shown). Thus, upregulation of the Kv4.1 current by KChIP1 does not involve an altered single channel conductance.
DISCUSSION
KChIPs and Kv4 channels are likely components of the rapidly inactivating K+ channels that mediate A-type K+ currents (IKA) and transient outward currents (ITO) in brain and heart, respectively (An et al. 2000; Li & Adelman, 2000). This study investigated how KChIP1 remodels inactivation gating of Kv4.1 and Kv4.3 channels expressed in Xenopus oocytes and examined whether a change in single channel conductance contributes to the KChIP1-induced upregulation of the Kv4 currents.
Streamlining Kv4 currents by KChIP1
A detailed kinetic analysis of the macroscopic currents expressed alone or along with KChIP1 demonstrated an apparently complex set of changes induced by KChIP1 that most significantly remodelled inactivation gating of Kv4.1 and Kv4.3 channels rendering them functionally alike. In both cases, the channels exhibit a complex development of macroscopic inactivation in the absence of KChIP1, which became streamlined in its presence. Thus, a dominant time constant (80–100 ms, between +30 to +70 mV) accounts for ≥ 80 % of the current decay in the presence of KChIP1 (Fig. 2 and Fig. 3). Consequently, the remodelled currents decay almost exponentially and exhibit an overall faster and nearly complete inactivation. However, because KChIP1 also slows the early phase of macroscopic inactivation, the control and KChIP1-remodelled currents characteristically cross over (Fig. 1).
KChIP1 also accelerates the recoveries from inactivation of Kv4.1 and Kv4.3 at hyperpolarized voltages by 4-fold and 2.5-fold, respectively (Fig. 4). This result appeared paradoxical if the channels were to inactivate mainly from the open state (O → IO in Scheme 1), a transition that is actually slowed by KChIP1. The simplified Scheme 1 shows two possible inactivation pathways that might occur near the open state in Kv4 channels (Jerng et al. 1999).
Scheme 1.

The depicted transitions are assumed to exhibit little or no voltage dependence (see below for a more detailed analysis assuming an expanded state diagram). If increasing IO → O accelerates the recovery from inactivation and O → IO is slowed, a significant increase in the level of the outward current at steady state must also be observed (e.g. Holmgren et al. 1996). Contrary to this prediction, KChIP1 clearly favoured nearly complete inactivation of the outward currents along with an accelerated recovery from inactivation (Figs 1–4). Thus, as suggested in previous studies (Jerng et al. 1999; Beck & Covarrubias, 2001; Bähring et al. 2001a) it is more likely that Kv4 channels inactivate preferentially from a preopen closed state (C → IC). Supporting this hypothesis, the data demonstrate that KChIP1 favours channel closing and the development of inactivation at negative voltages (before the channels open significantly) in both Kv4.1 and Kv4.3 channels (Figs 5–7). At more positive voltages, the combination of both changes favours inactivation from the preopen closed state (O → C → IC). Because, in the presence of KChIP1, inactivation from the open state has probably become slower and channel closing is more favourable, streamlined outward currents mainly reflect closed-state inactivation, which is the dominant rate-limiting process. Accordingly, channels mainly recover from IC, which upon a long depolarization (> 500 ms) is the most stable state, both in the absence and presence of KChIP1.
A viable mechanism underlying the remodelling of Kv4 gating by KChIP1
Scheme 2 was assumed to examine more quantitatively possible mechanisms that might account for the remodelling of Kv4 inactivation gating by KChIP1 (Fig. 10 and Table 1). This model was previously proposed to explain Kv4 inactivation and its modulation by other cytoplasmic factors (Beck & Covarrubias, 2001). Here, transitions between closed-states are strongly voltage-dependent (z = 9.8 e0, the total equivalent electronic charge of the activation pathway) and the opening transition is only weakly voltage dependent (z = 0.25 e0) (Bezanilla, 2000). The open state in Scheme 2 may represent an aggregate of several open states because single channel recording suggested the presence of at least three conducting levels (Fig. 9). All other transitions involving inactivation are voltage independent. In the absence of KChIP1, Kv4.3 channels exhibit significant inactivation from the open state at positive voltages (O ⇋ I5 ⇋ I6). However, inactivation from C4 is the preferred pathway of inactivation upon a prolonged depolarization because it is assumed that I5 and I6 are relatively unstable and that channel closing is significant at all relevant voltages (O ⇋ C4 ⇋ I4). Thus, at positive voltages, I4 is the most populated state at steady state and that is where the channels recover from upon repolarization. Assuming the parameters listed in Table 1, Scheme 2 closely models the macroscopic properties of Kv4.3 (see Methods and Fig. 11) and Kv4.1 (Beck & Covarrubias, 2001) currents. Based on the observations summarized above, four rate constants are changed to explain the macroscopic properties of the KChIP1-remodelled currents (including the characteristic crossover effect; see Fig. 11 and Table 1): (1) inactivation from the open state (kOI) is slowed 10- to 60-fold; (2) weakly voltage-dependent channel closing is 2-fold faster; (3) inactivation from closed state (kCI) is ∼2-fold faster; and (4) recovery from closed-state inactivation (kIC) is 2.6-fold faster. Thus, KChIP1 might remodel Kv4 gating by impairing open-state inactivation, favouring channel closing and reducing the energy barrier of closed state inactivation.
Table 1.
Rate constants for Scheme 2
| Parameter (s−1) | Kv4.3 | Kv4.3 + KChIP1 |
|---|---|---|
| α(V = 0) | 300 | 300 |
| β(V = 0) | 2.9 | 2.9 |
| kCO(V = 0) | 100 | 100 |
| kOC(V = 0) | 300 | 600 |
| kCI | 7 | 15 |
| kIC | 0.08 | 0.21 |
| kOI | 60 | 1 |
| kIO | 8 | 8 |
| k56 | 5 | 5 |
| k65 | 4 | 4 |
In the presence of KChIP1, the opposite voltage dependencies of the fractional amplitudes corresponding to the fast and slow exponential terms of the development of inactivation demonstrates that macroscopic inactivation becomes slower with membrane depolarization (Fig. 2D and Fig. 3D). As our model proposes, this behaviour can be accounted for by assuming preferable inactivation from the preopen closed state and a weakly voltage dependent opening equilibrium (Beck et al. 2001). As the membrane is depolarized the opening step is modestly facilitated (involving a small charge movement of ∼0.3 e0). Because such a relation shifts the opening equilibrium away from the preopen inactivation permissive closed state, the observed development of inactivation appears slower at more positive potentials.
The mechanisms described above cannot explain the significant upregulation of the Kv4 currents expressed in the presence of KChIP1 (see Results; An et al. 2000; Bähring et al. 2001b). The modelled kinetic changes alone actually reduce the peak current by almost 2-fold. Also, KChIP1 did not significantly affect the mean amplitudes of the unitary currents (Fig. 10). Therefore, improved surface expression is more likely to account for the enhanced Kv4 current levels observed with KChIP1 (K. Rhodes and M. Bowlby, unpublished observations). It is thus clear that KChIP1 plays two separate roles: (1) favouring surface expression of Kv4 channels and (2) remodelling Kv4 inactivation gating.
Putative interactions between KChIP1 and the inactivation machinery of Kv4 channels
Earlier work on the molecular mechanisms of Kv4 inactivation has suggested that the cytoplasmic N- and C-terminal domains of the Kv4 α-subunit and components of the internal vestibule of the pore (the distal region of the S6 segment and the internal S4-S5 loop) contribute to fast and slow processes of inactivation, respectively (Jerng & Covarrubias, 1997; Jerng et al. 1999). Because KChIPs directly interact with the distal N-terminal region of Kv4 channels (Bähring et al. 2001b), they might directly hinder fast inactivation, which involves the N-terminal region. Figure 12 demonstrates a working hypothesis that conceptually explains the molecular basis of Kv4 inactivation gating and its remodelling by KChIP1. Basically, in the absence of KChIP1 the proximal C-termini of the tetramer (green ribbons, Fig. 12A) hold the N-termini (dark red ribbons tethered to the T1 domain; see Fig. 12 legend) near the internal mouth of the pore (Jerng & Covarrubias, 1997), where they might readily occlude it upon channel opening. Io is, however, not stable. Thus, the channel reopens and closes to undergo preferential inactivation from the preopen closed state, which involves a conformational change at the internal vestibule of the pore (V-type inactivation; Jerng et al. 1999). Calcium-bound KChIP1 molecules (hexagons with red dots, see Fig. 12B) hold the Kv4 α N-termini away from the internal mouth and thereby remove the steric effects that these regions might exert on the internal gating machinery of the channel. Consequently, channel closing and V-type inactivation are more favourable. Recovery from inactivation is also favoured because the internal machinery controlling slow inactivation is less constrained when KChIP1 immobilizes the Kv4 α N-termini (effectively, the energy barrier of inactivation gating is reduced). If KChIPs are integrally associated with Kv4 channels in native tissues (An et al. 2000), it is also tantalizing to hypothesize that the main function of the Kv4 α N-terminus is to act as the binding site of KChIPs rather than to play a significant direct role in fast inactivation. This is in contrast to fast N-type inactivation mediated by the distal N-terminus of certain Kv1 and Kv3 channels and that of the Kv β1-subunit, which plug the pore by a deep direct interaction with the hydrophobic lining of the conduction pathway (Zhou et al. 2001).
Figure 12. Conceptual state diagram of Kv4 inactivation gating.
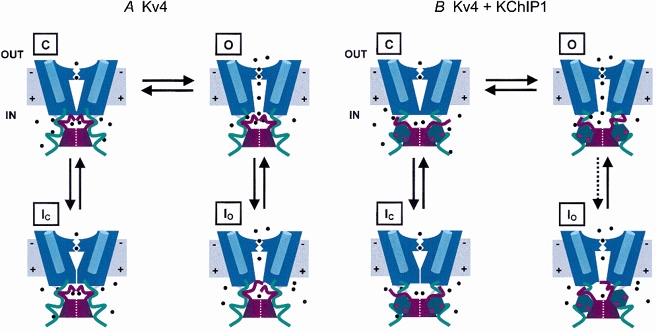
A, inactivation gating near the open state in the absence of KChIP1. Only 2 opposing subunits of the K+ channel tetramer are represented. The N-terminal tetramerization T1 domain is represented as a 2 part dark red block at the base of the channel. In the closed state, C, the channel is activated but still closed. The positive charges in the voltage sensor (blue cylinders) have moved outward (the membrane is depolarized). This closed state precedes the opening of the pore and is inactivation permissive. Note the opening of an internal gate when the channel opens (O). Ions (black dots) enter the channel through lateral internal windows (Miller, 2000) and cross the membrane through the K+-selective pore (i.e. mediating an outward current under physiological conditions). The following 2 inactivated states are represented: Io (from the open state) and Ic (from the preopen closed state). Note that the proximal region of the cytoplasmic C-terminal domains of the tetramer (green ribbons) holds the distal N-termini in place (dark red ribbons tethered to the T1 domain). The N-terminus readily occludes the inner mouth of the conduction pathway when the channel opens. Additionally, the channel inactivates from the preopen inactivation permissive closed state (C). Note a conformational change at the internal mouth of the pore, which partly collapses its internal vestibule. B, inactivation gating near the open state in the presence of KChIP1. Calcium-bound KChIPs (hexagons with red dots) interact with the distal N-terminal moieties. This interaction immobilizes the channel's N-termini, hindering inactivation from the open state (dotted arrow). Consequently, by immobilizing the channel's N-termini away from the internal mouth of the pore KChIP1 favours channel closing and the conformational changes that mediate closed-state inactivation. This inactivation pathway contributes significantly to the development of inactivation at positive voltages because the opening step of Kv4 channels is not strongly forward-biased (Table 1). Effectively, KChIP1 lowers the energy barrier of closed state inactivation by reducing the steric effects of the channel's N-termini.
Physiological implications
The colocalization and direct association of Kv4 α-subunits and KChIPs in brain and heart (An et al. 2000; Bähring et al. 2001b; Rosati et al. 2001) suggest that these auxiliary subunits are integral components of major A-type K+ currents in those tissues (IKA and ITO). KChIPs are close relatives of well-known small-molecular-weight calcium-binding proteins, which bear highly conserved EF-hand motifs (An et al. 2000; Pawlowski et al. 1996). Given the important signalling roles of cytosolic calcium, future studies should examine its contribution to the remodelling of Kv4 currents by KChIPs. Earlier results suggested that the EF-hand motifs characteristic of these proteins are required for remodelling of inactivation gating (An et al. 2000).
Because KChIP1 renders the inactivation properties of Kv4.1 and Kv4.3 currents almost redundant, Kv4 currents probably evolved to operate similarly, using inactivation from the preopen closed state as the main pathway of inactivation. Preferential closed-state inactivation at all relevant membrane potentials (which contributes to a low open probability) and a low single channel conductance might allow Kv4 channels to exert moderating effects on membrane excitability. For instance, helping to control the rate of slow repetitive spike firing, dampening backpropagating action potentials and shaping the early repolarization phase of the cardiac action potential. These functions require a modest opposition to excitation that might not abort firing or terminate the action potential prematurely.
Acknowledgments
We thank Andrew Graber and Thanawath Harris for harvesting high-quality Xenopus laevis oocytes. We also thank Drs Richard Horn and Michael O'Leary for critically reading earlier versions of the manuscript. This work was supported by NIH research grant NS32337 to M.C. E.B. was supported by NIH training grant AA07463. This work constitutes part of E. J. Beck's doctoral thesis.
REFERENCES
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- Bähring R, Boland LM, Varghese A, Gebauer M, Pongs O. Kinetic analysis of open- and closed-state inactivation transitions in human Kv4. 2 A-type potassium channels. Journal of Physiology. 2001a;535:65–81. doi: 10.1111/j.1469-7793.2001.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähring R, Dannenberg J, Peters HC, Leicher T, Pongs O, Isbrandt D. Conserved Kv4 N-terminal domain critical for effects of Kv channel-interacting protein 2. 2 on channel expression and gating. Journal of Biological Chemistry. 2001b;29:23888–23894. doi: 10.1074/jbc.M101320200. [DOI] [PubMed] [Google Scholar]
- Beck EJ, Bowlby MR, An WF, Rhodes KJ, Covarrubias M. Modulation of Kv4 inactivation gating by a calcium binding protein KChIP-1. Biophysical Journal. 2001;80:439A. [Google Scholar]
- Beck EJ, Covarrubias M. Kv4 channels exhibit modulation of closed-state inactivation in inside-out patches. Biophysical Journal. 2001;81:867–883. doi: 10.1016/S0006-3495(01)75747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck EJ, Sorensen RG, Slater SJ, Covarrubias M. Interactions between multiple phosphorylation sites in the inactivation particle of a K+ channel: Insights into the molecular mechanism of protein kinase C action. Journal of General Physiology. 1998;112:71–84. doi: 10.1085/jgp.112.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiological Reviews. 2000;80:555–592. doi: 10.1152/physrev.2000.80.2.555. [DOI] [PubMed] [Google Scholar]
- Connor JA. Slow repetitive activity from fast conductance changes in neurons. Federation Proceedings. 1978;37:2139–2145. [PubMed] [Google Scholar]
- Connor JA, Stevens CF. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. Journal of Physiology. 1971;213:21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JE, Shi W, Wang HS, Mcdonald C, Yu H, Wymore RS, Cohen IS, Mckinnon D. Role of the Kv4. 3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circulation Research. 1996;79:659–668. doi: 10.1161/01.res.79.4.659. [DOI] [PubMed] [Google Scholar]
- Greenstein JL, Wu R, Po S, Tomaselli GF, Winslow RL. Role of the calcium-independent transient outward current I(to1) in shaping action potential morphology and duration. Circulation Research. 2000;87:1026–1033. doi: 10.1161/01.res.87.11.1026. [DOI] [PubMed] [Google Scholar]
- Guo W, Li H, London B, Nerbonne JM. Functional consequences of elimination of i(to,f) and i(to,s): early afterdepolarizations atrioventricular block, and ventricular arrhythmias in mice lacking Kv1. 4 and expressing a dominant-negative Kv4 alpha subunit. Circulation Research. 2000;87:73–79. doi: 10.1161/01.res.87.1.73. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Holmgren M, Jurman ME, Yellen G. N-type inactivation and the S4-S5 region of the Shaker K+ channel. Journal of General Physiology. 1996;108:195–206. doi: 10.1085/jgp.108.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng HH, Covarrubias M. K+ channel inactivation mediated by the concerted action of the cytoplasmic N- and C-terminal domains. Biophysical Journal. 1997;72:163–174. doi: 10.1016/S0006-3495(97)78655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng HH, Shahidullah M, Covarrubias M. Inactivation gating of Kv4 potassium channels: molecular interactions involving the inner vestibule of the pore. Journal of General Physiology. 1999;113:641–660. doi: 10.1085/jgp.113.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DC, Nuss HB, Marban E. Suppression of neuronal and cardiac transient outward currents by viral gene transfer of dominant-negative Kv4. 2 constructs. Journal of Biological Chemistry. 1997;27231:598–603. doi: 10.1074/jbc.272.50.31598. [DOI] [PubMed] [Google Scholar]
- Li M, Adelman JP. ChIPping away at potassium channel regulation. Nature Neuroscience. 2000;202:202–204. doi: 10.1038/72898. [DOI] [PubMed] [Google Scholar]
- Malin SA, Nerbonne JM. Elimination of the fast transient in superior cervical ganglion neurons with expression of Kv4. 2W362F: molecular dissection of IA. Journal of Neuroscience. 2000;20:5191–5199. doi: 10.1523/JNEUROSCI.20-14-05191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. An overview of the potassium channel family. Genome Biology. 2000;1:00041–00045. doi: 10.1186/gb-2000-1-4-reviews0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerbonne JM. Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium. Journal of Physiology. 2000;525:285–298. doi: 10.1111/j.1469-7793.2000.t01-1-00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit G, Kassiri Z, Sah R, Ramirez RJ, Zobel C, Backx PH. The molecular physiology of the cardiac transient outward potassium current (I(to)) in normal and diseased myocardium. Journal of Molecular and Cellular Cardiology. 2001;33:851–872. doi: 10.1006/jmcc.2001.1376. [DOI] [PubMed] [Google Scholar]
- Pak MD, Baker K, Covarrubias M, Butler A, Ratcliffe A, Salkoff L. mShal, a subfamily of A-type K+ channel cloned from mammalian brain. Proceedings of the National Academy of Sciences of the USA. 1991;88:4386–4390. doi: 10.1073/pnas.88.10.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski K, Bierzynski A, Godzik A. Structural diversity in a family of homologous proteins. Journal of Molecular Biology. 1996;258:349–366. doi: 10.1006/jmbi.1996.0255. [DOI] [PubMed] [Google Scholar]
- Rosati B, Pan Z, Lypen S, Wang HS, Cohen I, Dixon JE, Mckinnon D. Regulation of KChIP2 potassium channel β subunit gene expression underlies the gradient of transient outward current in canine and human ventricle. Journal of Physiology. 2001;533:119–125. doi: 10.1111/j.1469-7793.2001.0119b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, Westbrook GL. Regulation of synaptic timing in the olfactory bulb by an A-type potassium current. Nature Neuroscience. 1999;2:1106–1113. doi: 10.1038/16033. [DOI] [PubMed] [Google Scholar]
- Serodio P, Kentros C, Rudy B. Identification of molecular components of A-type channels activating at subthreshold potentials. Journal of Neurophysiology. 1994;72:1516–1529. doi: 10.1152/jn.1994.72.4.1516. [DOI] [PubMed] [Google Scholar]
- Serodio P, Vega-saenz DM, Rudy B. Cloning of a novel component of A-type K+ channels operating at subthreshold potentials with unique expression in heart and brain. Journal of Neurophysiology. 1996;75:2174–2179. doi: 10.1152/jn.1996.75.5.2174. [DOI] [PubMed] [Google Scholar]
- Shibata R, Nakahira K, Shibasaki K, Wakazono Y, Imoto K, Ikenaka K. A-type K+ current mediated by the Kv4 channel regulates the generation of action potential in developing cerebellar granule cells. Journal of Neuroscience. 2000;20:4145–4155. doi: 10.1523/JNEUROSCI.20-11-04145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Maxwell CJ, Ledwell JL, Aldrich RW. Uncharged S4 residues and cooperativity in voltage-dependent potassium channel activation. Journal of General Physiology. 1998;111:421–439. doi: 10.1085/jgp.111.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai ST, Surmeier DJ. Somatodendritic depolarization-activated potassium current in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4. 2 and Kv4.1 subunits. Journal of Neuroscience. 1998;18:3124–3137. doi: 10.1523/JNEUROSCI.18-09-03124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. The moving parts of voltage-gated ion channels. Quarterly Reviews of Biophysics. 1998;31:239–295. doi: 10.1017/s0033583598003448. [DOI] [PubMed] [Google Scholar]
- Zhou M, Morais-Cabral JH, Mac Mann S, Kinnon R. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature. 2001;411:657–661. doi: 10.1038/35079500. [DOI] [PubMed] [Google Scholar]


