Abstract
The adaptor complex 3 (AP-3) targets membrane proteins from endosomes to lysosomes, lysosome-related organelles and synaptic vesicles. Phosphatidylinositol-4-kinase type II α (PI4KIIα) is one of several proteins possessing catalytic domains that regulate AP-3–dependent sorting. Here we present evidence that PI4KIIα uniquely behaves both as a membrane protein cargo as well as an enzymatic regulator of adaptor function. In fact, AP-3 and PI4KIIα form a complex that requires a dileucine-sorting motif present in PI4KIIα. Mutagenesis of either the PI4KIIα-sorting motif or its kinase-active site indicates that both are necessary to interact with AP-3 and properly localize PI4KIIα to LAMP-1–positive endosomes. Similarly, both the kinase activity and the sorting signal present in PI4KIIα are necessary to rescue endosomal PI4KIIα siRNA-induced mutant phenotypes. We propose a mechanism whereby adaptors use canonical sorting motifs to selectively recruit a regulatory enzymatic activity to restricted membrane domains.
INTRODUCTION
Organelles exchange components via vesicle carriers whose composition is defined by cytosolic coats. Among these coats, four heterotetrameric adaptor complexes (AP-1 to AP-4) act as scaffolds recruiting enzymatic and nonenzymatic machineries necessary for the generation of vesicles of unique composition (Bonifacino and Traub, 2003; Bonifacino and Glick, 2004; Robinson, 2004). For example, the adaptor complex AP-3 functions to produce vesicles that traffic selected membrane proteins from endosomes to lysosomes, lysosome-related organelles, or synaptic vesicles (Di Pietro and Dell'Angelica, 2005; Danglot and Galli, 2007; Newell-Litwa et al., 2007). It is generally believed that membrane proteins packed into vesicles (cargoes) and enzymes required for vesicle formation (e.g., the GTPase ARF1) are separate molecular entities that play distinct roles in vesicle biogenesis. However, adaptors bring cargoes and these enzymatic regulators together at the time of vesicle generation.
Adaptor function can be deconstructed in at least two steps. First, adaptors must be recruited to restricted membrane domains in specific organelles. Second, once on membranes, adaptors must then recognize and concentrate membrane proteins containing sorting determinants such as tyrosine or dileucine-based sorting motifs (Bonifacino and Glick, 2004). Both processes are tightly regulated and coordinated by proteins possessing catalytic activities. Among those, GTPases of the ARF family and lipid-modifying enzymes regulate adaptor recruitment to membranes and can influence adaptor interaction with cargo (Bonifacino and Glick, 2004; De Matteis and Godi, 2004; Honing et al., 2005; Di Paolo and De Camilli, 2006). As an example, PtdIns(4,5)P2 at the plasma membrane and PtdIns(4)P at the trans-Golgi network play crucial roles for the selective recruitment of AP-2 and AP-1, respectively (Godi et al., 1999; Krauss et al., 2003; Wang et al., 2003; Di Paolo et al., 2004; Bairstow et al., 2006; Nakano-Kobayashi et al., 2007). In these examples, GTP-bound forms of ARF GTPases regulate the activities of either phosphatidylinositol 4-kinase type IIIβ present in the Golgi apparatus where it generates PtdIns(4)P (Godi et al., 1999) or phosphatidylinositol 5-kinase type Iγ, which catalyzes PtdIns(4,5)P2 production at the plasma membrane (Godi et al., 1999; Krauss et al., 2003; Di Paolo et al., 2004; Bairstow et al., 2006; Nakano-Kobayashi et al., 2007).
The functional integration of the enzymatic activity of GTPases and lipid kinases occurs because adaptors provide a scaffold to bind PI lipids, GTPases, and lipid kinases as well as membrane proteins bearing sorting signals. Binding of this diverse group of molecules to adaptors modifies the function of adaptors themselves and/or the activity of lipid kinases. For example, PI lipids regulate the affinity of adaptors for tyrosine-based sorting motifs (Honing et al., 2005), whereas the interaction of adaptors and lipid kinases increases the enzymatic activity of the latter (Krauss et al., 2006). In contrast with many regulatory enzymes, cargoes are membrane-bound proteins. Moreover, the function performed by cargoes and regulatory kinases are separated to distinct molecules recruited to adaptors. Here we present a new paradigm in which a lipid kinase, phosphatidylinositol 4-kinase type IIα (PI4KIIα), associated with membranes via palmitoylation (Barylko et al., 2001), behaves as both a cargo and enzymatic regulator of AP-3. PI4KIIα contains a kinase domain and a dileucine-sorting motif. This dileucine-sorting motif is identical to the motif found in the AP-3 cargo tyrosinase (Honing et al., 1998; Blagoveshchenskaya et al., 1999; Theos et al., 2005). We demonstrate that both the kinase domain and the sorting motif are required to facilitate the function of the adaptor complex AP-3, indicating that PI4KIIα behaves both as a membrane protein cargo as well as an enzymatic regulator of AP-3 function. Our results indicate that direct recruitment of enzymatic regulators of adaptor function, in this case PI4KIIα, could operate in a positive feedback mechanism for further adaptor recruitment.
MATERIALS AND METHODS
Antibodies and Reagents
The following antibodies were used: early endosome antigen 1 (EEA1), anti-syntaxin 8, anti-Vti1b, and clathrin heavy chain, (BD Biosciences, Franklin Lakes, NJ); anti-transferrin receptor (H68.4; Zymed Laboratories, South San Francisco, CA); and anti-hemagglutinin (HA; 12CA5; Roche Molecular Biochemicals, Indianapolis, IN). Anti-clathrin heavy-chain mouse mAb (X22) was from Calbiochem (La Jolla, CA). Anti-GFP mAb 3E6 was from Molecular Probes (Eugene, OR). Anti-SV2 (10H4), anti-δ (SA4), and anti-LAMP-1 (1DCB and H4A3) were from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA). anti-μ3 and -β3 AP-3 polyclonal antibodies have already been described (Salem et al., 1998; Faundez and Kelly, 2000; Salazar et al., 2004). KF4 mAb against AP-3 δ was a gift from Andrew Peden (Department of Clinical Biochemistry, University of Cambridge, London, England). Mouse anti-VAMP-7 (Advani et al., 1999) and affinity-purified polyclonal antibodies against phosphatidylinositol-4-kinase type IIα were reported previously (Guo et al., 2003).
Cell Culture
PC12 cells and stably transfected PC12 ZnT3 clone 4 cell lines were cultured as described previously (Salazar et al., 2004). HEK293T cells were maintained in DMEM + 10% FBS + 100 U/ml penicillin and 100 μg/ml streptomycin (Mediatech, Herndon, VA).
Plasmids and Transfections
HA- and green fluorescent protein (GFP)-tagged human wild-type (WT) and kinase inactive PI4KII type IIα constructs were gifts from Tamas Balla (Section on Molecular Signal Transduction, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD; Balla et al., 2002). GST-tagged rat PI4KIIα was a gift from Pietro de Camilli (Department of Cell Biology, Yale University, New Haven, CT; Guo et al., 2003). All mutants were created by QuickChange mutagenesis kit (Stratagene, La Jolla, CA) except the rat L60A L61A mutant created by synthesizing (IDT Technologies, Coralville, IA) an oligo corresponding to PI4KIIα base pairs 79–193 with the sequence 5′CACTTTCCACAAGTACCAGGAGGAGCAGTAGCAGCAGCAGGATCAGGACCATCACCACCATGTTCTCCAGGACATGATCGTGAACGACAACCAGCAGCAGATCGAGCC-3′. The reverse complement oligo was also synthesized, and the two oligos were annealed and ligated into the SacI and SmaI sites in rat PI4KII. WT and L60A L61A mutant rat PI4KIIα was then subcloned into pEGFP-C2 using the EcoRI and SalI restriction sites. PC12 cells were transfected as described previously (Salazar et al., 2005). HEK293T cells were seeded in six-well plates and transfected with 200 ng of plasmid DNA in 1 ml of OPTI-MEM media (Invitrogen, Carlsbad, CA). After 4 h the medium was replaced with 2 ml DMEM + 10% FBS, and cells were assayed the following day.
RNA Interference
Double-stranded RNA oligos corresponding to nucleotides 888–908 of human PI4K type IIα (NM_018425; Wang et al., 2003) and siCONTROL nontargeting small interfering RNA (siRNA) 2 (5′-UAAGGCUAUGAAGAGAUAC-3′) control siRNA oligos were obtained from Dharmacon (Lafayette, CO). HEK293T cells, 5 × 105, were seeded in six-well plates and transfected on day 2 with 100 nmol of siRNA in 1 ml OPTI-MEM. After 4 h, 1 ml of DMEM + 10% FBS was added to each well. On day 3 the medium was replaced with 2 ml DMEM + 10% FBS. On day 4 the cells were trypsinized with 1 ml of 0.05% trypsin in PBS for 1 min and then 1 ml of DMEM + 10% FBS was added to each well. After 2 h the cells were washed and transfected again with siRNA as described above. On day 5 the media was replaced as above, and cells were either left alone or passaged in order to seed coverslips. On day 6 the cells were either left alone or transfected with plasmid DNA as described above, and cells were processed for immunoblot and immunofluorescence on day 7.
Dithiobis(succinimidylpropionate) Cross-linking of Intact Cells and Immunoprecipitation of Cross-linked Protein Complexes
To assess low-affinity interactions between AP-3 and cargoes, we performed cross-linking in whole cells with DSP [dithiobis(succinimidylpropionate)]. Cross-linking by DSP can be reverted by reducing agents allowing the identification of individual proteins in SDS-PAGE. Cross-linked complexes were immunoprecipitated with AP-3 antibodies, cross-linking was reversed by reducing agents and precipitated proteins were analyzed by SDS-PAGE and immunoblot. Briefly, 5 × 105 cells were plated in six-well plates and on the day of confluence were placed on ice, rinsed twice with PBS, and incubated with 1 mM DSP (Pierce, Rockford, IL) or vehicle diluted in PBS for 2 h on ice. Tris, pH 7.4, was added to 25 mM and incubated for 15 min to quench the DSP reaction. The cells were then rinsed twice with PBS and lysed in buffer A (150 mM NaCl, 10 mM HEPES, 1 mM EGTA, and 0.1 mM MgCl2, pH 7.4) + 0.5% TX-100 by incubation for 30 min on ice. The cells were then scraped from the dish, and cell homogenates were centrifuged at 16,100 × g for 10 min. The supernatant was recovered, diluted to 1 mg/ml in 0.5 ml of buffer A + 0.5% TX-100, applied to Dynal magnetic beads (Invitrogen) coated with antibody, and incubated overnight at 4°C. The beads were then washed 4 times with buffer A + 0.1% TX-100, eluted by 5 min of incubation at 75°C with SDS-PAGE sample buffer, and immunoprecipitated material was analyzed by SDS-PAGE and immunoblot.
To quantify the extent of PI4KIIα association with AP-3 in the presence of DSP, HA-tagged versions of WT PI4KIIα were used as a reference point to which compare mutant PI4KIIα versions. The percent of association was estimated as [(AP-3–bound mutant PI4KIIα-HA/Mutant PI4KIIα-HA expression) × (AP-3–bound PI4KIIα-HA/PI4KIIα-HA expression)−1] × 100.
Membrane and cytosol fractions were prepared after cross-linking as described above. HEK293 cells were washed in PBS and homogenized in buffer A containing Complete antiprotease cocktail, by 18 passages in a cell cracker. Homogenate was sedimented at 27,000 × g for 40 min to generate a P1 membrane fraction and a S1 supernatant. Cytosol-free of membranes was obtained by centrifugation of S1 at 210,000 × g for 30 min in a Beckman Coulter TLA120.2 rotor. Membrane fractions and cytosol were dissolved in buffer A 0.5% Triton X-100 (TX-100) during 30 min on ice. Cell fractions were clarified by centrifugation, and Triton-soluble supernatants were immunoprecipitated as described above.
AP-3 Immunoaffinity Chromatography and In Vitro Binding Assay
Magnetic beads coated with anti AP-3 δ antibodies or control beads, either coated with an irrelevant antibody (SV2) or lacking antibodies, were preincubated overnight with 500 μg of rat brain cytosol diluted in buffer A + 3% BSA + 0.1% TX-100, washed five times with the same buffer, and then incubated with 125 nM glutathione S-transferase (GST) or GST-PI4KIIα diluted in the same buffer for 2 h at 4°C. The beads were then washed four times with the same buffer and then once with buffer A + 0.1% TX-100. The beads were eluted by incubating with SDS-PAGE sample buffer at 75°C for 5 min, and bound material was analyzed by SDS-PAGE and immunoblot. The sequence of the peptide corresponding to the epitope recognized by the anti-δ SA4 mAb was AQQVDIVTEEMPENALPSDEDDKDPNDPYRA corresponding to the amino acids 680–710 of human δ adaptin (AAD03777; GI:1923266).
Microscopy
Immunofluorescence was performed as described previously (Salazar et al., 2004). All cells were seeded on coverslips coated with Matrigel (BD Biosciences). Images were acquired either by confocal microscopy (detailed in Salazar et al., 2004) or with a scientific-grade cooled charge-coupled device (Cool-Snap HQ with ORCA-ER chip) on a multiwavelength, wide-field, three-dimensional microscopy system (Intelligent Imaging Innovations, Denver, CO), based on a 200M inverted microscope using a 63× NA 1.4 lens (Carl Zeiss, Thornwood, NY). Immunofluorescent samples were imaged at room temperature using a Sedat filter set (Chroma Technology, Rockingham, VT), in successive 0.20-μm focal planes. Out-of-focus light was removed with a constrained iterative deconvolution algorithm (Swedlow et al., 1997). Images were processed and analyzed using Metamorph software Version 3.0 (Universal Imaging, West Chester, PA). All images were thresholded to similar levels. Fluorescent signal colocalization or overlapping was determined as follows. For each cell, an optical section obtained through the nuclear equator was analyzed. Pixels containing both fluorescent signals were considered colocalized or overlapped. This value was expressed as a percent of the total number of pixels positive for just one fluorochrome. Endosomal colocalization was quantified by creating a region of interest surrounding a single enlarged endosome, and only the pixels included in that region of interest were measured for colocalization. Penetrance of siRNA phenotypes was determined by counting the number of cells displaying or not displaying a given phenotype and dividing the number of cells displaying the phenotype by the number of total cells, yielding the percentage of cells displaying the phenotype.
Statistical Analysis
All data are expressed as average ± SE. Experimental conditions were compared with the one-way ANOVA followed by Student-Newman-Keuls Multiple Comparison as a post hoc test using Synergy KaleidaGraph v3.6.2 (Reading, PA).
RESULTS
PI4KIIα Colocalizes Specifically with AP-3 and AP-3 Cargoes on the Limiting Membrane of Early Endosomes
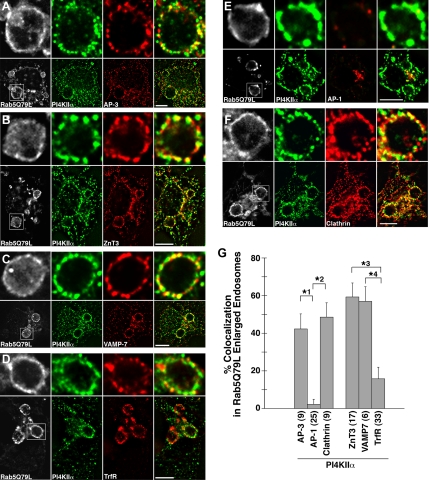
PI4KIIα specifically colocalizes with AP-3 and AP-3 cargoes in neuronal and nonneuronal cells (Salazar et al., 2005, 2006) and is present in AP-3–derived microvesicles and synaptic vesicles (Guo et al., 2003; Salazar et al., 2005; Takamori et al., 2006). The localization of PI4KIIα to these compartments requires AP-3 (Salazar et al., 2005, 2006), suggesting that PI4KIIα is targeted by interacting with AP-3. If the kinase is indeed targeted by AP-3, a prediction arising from this hypothesis is that PI4KIIα, AP-3, and other AP-3 cargo molecules should colocalize on the limiting membrane of early endosomes, a donor compartment where AP-3 vesicles are formed (Faundez et al., 1998; Peden et al., 2004). We tested this hypothesis using high-resolution deconvolution immunofluorescence microscopy of PC12 cells transfected with constitutively active Rab5a (Rab5Q79L, Figure 1). Rab5Q79L causes the formation of enlarged early endosomes (Stenmark et al., 1994) that allow the visualization of presumptive sorting microdomains on their limiting membrane at the light microscopy level (Raiborg et al., 2002, 2006).
Figure 1.
PI4KIIα colocalizes with AP-3 and AP-3 cargoes in discrete domains of endosomes. GFP-Rab5 Q79L was expressed in PC12 cells to visualize the limiting membrane of single enlarged endosomes. Cells were then fixed and stained for PI4KIIα and AP-3 (A), ZnT3 (B), VAMP-7 (C), transferrin receptor (TrfR, D), AP-1 (E), or clathrin (F). Images were pseudocolored. The limiting membrane of endosomes was visualized by GFP fluorescence (gray). PI4KIIα was visualized with Alexa 647 secondary antibodies (green channel), and all other markers were visualized with Alexa 555 secondary antibodies (red channel). The percentage of endosomal PI4KIIα colocalizing with the various markers was quantified using Metamorph analysis (G). In G numbers in parentheses represent the number of endosomes quantified and collected in four independent experiments. Scale bars, 5 μm. * p < 0.0001.
PC12 cells expressing GFP-Rab5Q79L were fixed and stained for PI4KIIα, AP-3, clathrin, and two well-defined AP-3 cargo molecules, ZnT3 (Salazar et al., 2004, 2005) and VAMP-7 (Martinez-Arca et al., 2003; Salazar et al., 2006; Scheuber et al., 2006). Approximately 50% of the PI4KIIα on the membrane of these enlarged endosomes was found to coexist with AP-3 (Figure 1A) and the AP-3 cargoes ZnT3 and VAMP-7 (Figure 1, B and C) as well as clathrin (Figure 1F) in discrete microdomains. The extent of colocalization between PI4KIIα and either AP-3, ZnT3, or VAMP7-TI are in agreement with those obtained from quantification of whole cell colocalization in untransfected PC12 cells and mouse skin fibroblasts (Salazar et al., 2005, 2006). To test if this colocalization was specific to AP-3 and AP-3 cargoes, we stained enlarged endosomes with antibodies to the adaptor AP-1 and transferrin receptor (TrfR, Figure 1, D and E), a transmembrane protein that traffics independently of AP-3 (Dell'Angelica et al., 1999; Janvier and Bonifacino, 2005). We observed only minimal amounts of AP-1 on enlarged endosomes (Figure 1G), and a perinuclear pool of AP-1 that partially colocalized with PI4KIIα (Figure 1E). Furthermore, the colocalization between PI4KIIα and TrfR on these endosomes was 2.7-fold lower than that of PI4KIIα and AP-3, and 3.8-fold lower than that of PI4KIIα and ZnT3 (Figure 1G). These results indicate that PI4KIIα, AP-3, AP-3 cargoes, and clathrin are specifically enriched in early endosome microdomains that are mostly devoid of TrfR and AP-1.
PI4-Kinase Interacts with AP-3 In Vitro and In Vivo
The enrichment of PI4KIIα and AP-3 in endosomal microdomains, combined with the observations that AP-3 is required to target PI4KIIα to postendocytic compartments (Salazar et al., 2005) suggest that the kinase and AP-3 could interact. To test this hypothesis, we developed an in vitro binding assay using affinity-purified AP-3 complexes and purified recombinant PI4KIIα. AP-3 complexes were isolated from rat brain cytosol by immunoaffinity chromatography using magnetic beads decorated with a mAb directed against the ear domain of the δ subunit of AP-3 (Figure 2A, KF4, lanes 9 and 10). Beads decorated with AP-3 complexes were washed and challenged with purified GST (Figure 2A, lane 9) or GST-tagged PI4KIIα (Figure 2A, lane 10). In contrast with GST, which failed to bind to AP-3–containing beads (Figure 2A, lane 9), GST-PI4KIIα specifically bound to beads carrying immobilized AP-3 complexes (Figure 2A, lane 10). Furthermore, GST-PI4KIIα did not bind to beads lacking antibodies (Figure 2A, lanes 4 and 5) or to beads coated with an irrelevant antibody directed against SV2, a multispanning membrane protein (Bajjalieh et al., 1992; Figure 2A, lanes 6 and 7). These results were recapitulated using a different mAb whose antigenic peptide epitope in the δ subunit of AP-3 has been identified (anti-δ SA4, Figure 2B, δ). To further test the selectivity of the AP-3/PI4KIIα interaction, we performed competition experiments using a synthetic peptide corresponding to the epitope recognized by anti-δ SA4 mAb. Inclusion of the peptide during the AP-3 immunoprecipitation completely out-competed the interaction between the δ antibody and AP-3 and prevented the subsequent binding of GST-PI4KIIα to beads (Figure 2B, lanes 9 and 10). These in vitro data provide evidence of an interaction between AP-3 and PI4KIIα.
Figure 2.
Purified PI4KIIα and AP-3 interact in vitro. (A) AP-3 was purified from rat brain cytosol by immunoaffinity chromatography using a mAb (KF4) to the δ subunit of AP-3. The cytosol preincubated beads were washed extensively (see Material and Methods) and incubated with 125 nM purified GST-PI4KIIα (lanes 4–8 and 10) or 125 nM GST alone (lane 9). Bound GST-PI4KIIα was detected by SDS-PAGE and immunoblot using antibodies to the β3 subunit of AP-3 and GST. GST alone fails to bind to beads coated with AP-3 (lane 9). Control immunoprecipitations using beads without antibody (lanes 4 and 5), irrelevant antibody (SV2, lanes 6 and 7), or absence of cytosol (lane 8) display only background levels of bound GST-PI4KIIα, whereas beads coated with purified AP-3 display significant GST-PI4KIIα binding. GST and GST-PI4KIIα inputs (lanes 1 and 2) are 15% and cytosol input is 2.5%. (B) Purification, binding of AP-3 and GST-PI4KIIα, and detection of bound GST-PI4KIIα was performed as in A, except that the mAb used for AP-3 immunoaffinity purification was δ (anti-δ SA4 monoclonal). A peptide corresponding to the epitope recognized by the δ antibody was used to elute bound AP-3 (lanes 9 and 10). Inclusion of the peptide during AP-3 immunoaffinity purification out-competed the interaction between the antibody and AP-3 (lanes 9 and 10). As in A, GST-PI4KIIα failed to bind to uncoated beads (lane 4), beads coated with irrelevant antibody (lane 5, SV2), or to beads coated with δ antibody but lacking AP-3 (lane 6), and GST alone does not bind AP-3 (lane 7).
We hypothesized that a sorting motif in PI4KIIα was required for the interaction between the kinase and AP-3. This hypothesis was founded on the observation that sorting motifs interact directly with AP-1 or AP-3 (Janvier et al., 2003; Doray et al., 2007). Computational analysis of rat PI4KIIα amino acid sequence revealed the presence of three putative dileucine motifs that conform to the canonical dileucine sorting signal {DER}XXXL{LVI}: 3ETSPLV8, 56ERQPLL61, and 378EIKDLI383; the latter contained in the kinase domain of PI4KIIα. We focused on putative sorting signals present outside the kinase domain. Among these, 56ERQPLL61 was conserved in all vertebrate PI4KIIα isoforms. The conservation of 56ERQPLL61 suggests that this motif may play a central role in PI4KIIα interaction with AP-3 and targeting. In fact, this motif is identical to the AP-3–interacting motif (ERQPLL) found in tyrosinase, a well-characterized membrane protein that depends on AP-3 for sorting (Honing et al., 1998; Blagoveshchenskaya et al., 1999; Huizing et al., 2001; Theos et al., 2005). To test this hypothesis, we first developed a strategy to analyze adaptor-cargo interactions on membranes in vivo using whole-cell cross-linking. We chose DSP, a homobifunctional cell-permeable cross-linker with a 12-Å spacer arm that contains a disulfide bond that allows the cleavage of cross-linked products (Lomant and Fairbanks, 1976; Alloza et al., 2004; Xiang et al., 2004). Adaptor complexes bound to cargoes were immunoprecipitated, and their molecular composition was analyzed by reducing SDS-PAGE and immunoblot. Immunoprecipitation of AP-3 from cells treated with DSP resulted in coprecipitation of PI4KIIα (Figure 3A, lane 8). The complex between AP-3 and PI4KIIα was observed even though DSP treatment decreased the immunoreactivity of the AP-3 δ subunit, as revealed by the decreased precipitation of AP-3 subunits (Figure 3A, μ3 and β3 blots, compare lanes 7 and 8). Control immunoprecipitations using beads without antibody (Figure 3A, lanes 3 and 4) or immunoprecipitation of transferrin receptor (TrfR, Figure 3A, lanes 5 and 6) failed to coprecipitate PI4KIIα, indicating a specific interaction between PI4KIIα and AP-3. We predicted that AP-3 cargo proteins colocalizing with AP-3 and PI4KIIα in Rab5Q79L-enlarged endosomes (Figure 1) would also be found cross-linked to PI4KIIα-AP-3 complexes. In fact, PI4KIIα was present in a complex containing AP-3 subunits, the AP-3 cargo ZnT3, and clathrin (Figure 3B, lanes 5 and 6). Similarly, PI4KIIα-AP-3 and clathrin were immunoprecipitated as a complex from cross-linked PC12 cells using X22 clathrin antibodies (data not shown). The presence of PI4KIIα and ZnT3 in AP-3 cross-linked complexes is selective because minimal or undetectable levels of PI4KIIα or ZnT3, respectively, were found in cross-linked AP-1 complexes immunoprecipitated with γ adaptin antibodies (Figure 3B, compare lanes 2 and 6). The efficient cross-linking of clathrin to AP-1 excludes the possibility that AP-1 and its interactors are refractory to DSP treatment. These data demonstrate that PI4KIIα can be cross-linked and selectively coimmunoprecipitated with AP-3. Furthermore, these findings are consistent with a model in which PI4KIIα behaves as a cargo that binds to AP-3.
Figure 3.
PI4KIIα coimmunoprecipitates with AP-3 in vivo. PC12 (A and B) or HEK293 (C) cells were treated with the cell-permeable cross-linker DSP (even lanes) or DMSO vehicle (odd lanes) and immunoprecipitations using empty beads (A, lanes 3 and 4; B, lanes 3 and 4; C, lanes 5–8), beads decorated with AP-3 δ antibodies (A, lanes 7 and 8; B, lanes 5 and 6; C, lanes 9–12), beads decorated with transferrin receptor antibodies (TrfR. A, lanes 5 and 6), or beads decorated with AP-1 γ antibodies (B, lanes 1 and 2). Immunocomplexes were resolved by SDS-PAGE and analyzed by immunoblot using antibodies to PI4KIIα, AP-3 subunits (δ, μ3 and β3), transferrin receptor (TrfR), clathrin, ZnT3, AP-1 γ adaptin, and GFP. PI4KIIα specifically coprecipitated with AP-3, clathrin, and ZnT3 but not with transferring receptor or AP-1 after DSP cross-linking. HEK293 cells (C) expressing WT or dileucine mutant (L60A L61A) PI4KIIα tagged with GFP were processed for immunoprecipitation as described. AP-3 coprecipitates WT GFP-PI4KIIα but does not coprecipitate the dileucine mutant. Input lanes correspond to 10–15%.
PI4KIIα binding to AP-3 could be mediated by the conserved 56ERQPLL61 dileucine motif in PI4KIIα. To test this prediction, we mutated the critical leucine residues (Bonifacino and Traub, 2003) in this motif to alanines and expressed WT and dileucine mutant (L60A L61A) GFP-tagged rat PI4KIIα in HEK293 cells (Figure 3C) and PC12 cells (data not shown). Cells were cross-linked with DSP, and immunoprecipitations were performed using lysates from each of these cell lines. Immunoprecipitation of AP-3 coprecipitated GFP-PI4KIIα (Figure 3C, lane 10), whereas no detectable dileucine mutant GFP-PI4KIIα coprecipitated with AP-3 (Figure 3C, compare lanes 10 and 12). Similar results were obtained using WT and dileucine mutant HA-tagged PI4KIIα (see Figure 9). Control immunoprecipitations using beads without antibody failed to coprecipitate all but background levels of the kinase (Figure 3C, lanes 5–8). These immunoprecipitations were repeated using PC12 cells and yielded similar results (data not shown). Collectively our results indicate that the 56ERQPLL61 motif is necessary for the PI4KIIα/AP-3 interaction in vivo.
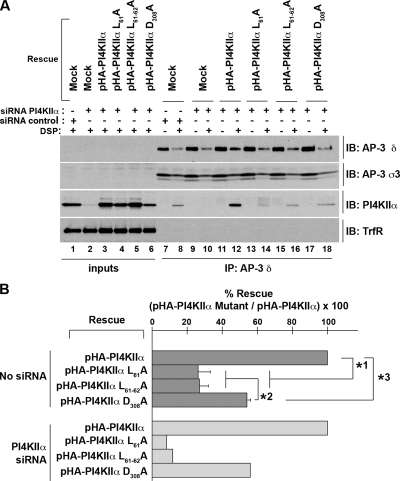
Figure 9.
PI4KIIα kinase activity and dileucine-sorting motif are required to interact with AP-3. (A) HEK293 cells were treated with control siRNA (lanes 1, 7, and 8) or with PI4KIIα siRNA (lanes 2–6 and 9–18). siRNA-treated cells were transfected with PI4KIIα-HA WT (lanes 3, 11, and 12) or PI4KIIα-HA carrying mutations in its sorting motif (L61A, lanes 4, 13, and 14; L61-62A, lanes 5, 15, and 16) or kinase active site (D308A, lanes 6, 17, and 18). Cells were treated with the cell-permeable cross-linker DSP (even lanes) or DMSO vehicle (odd lanes), and cross-linked complexes were immunoprecipitated using beads decorated with AP-3 δ SA-4 antibodies. Immunocomplexes were resolved by SDS-PAGE and analyzed by immunoblot using antibodies to PI4KIIα, AP-3 subunits (δ and ς3) and transferrin receptor (TrfR). Inputs (lanes 1–6) correspond to a 10%. (B) Light gray bars, the quantification of the experiment depicted in A. Dark gray bars, the average of three independent experiments identical to A except that HEK293 cells were not siRNA treated. Note that all quantifications were normalized to the expression level of the recombinant PI4KIIα (input lanes). p values“ *1 and *3 < 0.0001, *2 < 0.003.
PI4-Kinase siRNA Knockdown Selectively Redistributes AP-3 Complexes from Membranes to Cytosol
If cargo possesses catalytic activity capable of selectively regulating adaptor recruitment to membranes, then depletion of such a molecule should lead to adaptor redistribution to cytosolic compartments. To test this hypothesis, we performed siRNA-mediated PI4KIIα knockdown in HEK293 cells (Figure 4A, compare lanes 2 and 3, and Figure 9A, compare lanes 1 and 2). We analyzed AP-3 and -1 adaptor membrane localization both by confocal immunofluorescence microscopy (Figure 4, B and C) as well as subcellular fractionation of control or PI4KIIα siRNA-treated cells (Figure 4D). As previously reported by us in other cell types (see Figure 1; Salazar et al., 2005, 2006), PI4KIIα preferentially colocalizes with AP-3, mostly in the perinuclear area, but minimally with AP-1 complexes (data not shown and Figure 5C, ■). Consistent with these findings, PI4KIIα down-regulation selectively affected AP-3 but not AP-1 adaptor subcellular localization (Figure 4, compare B and C). Reduced PI4KIIα levels induced a redistribution of AP-3 from a perinuclear cap into a disperse pattern throughout the cytoplasm. To confirm these observations, we performed subcellular fractionation of HEK293 cells either treated with control or PI4KIIα siRNA oligonucleotides (Figure 4D). siRNA-treated cells were incubated in the absence (Figure 4D, odd lanes) or presence of DSP (Figure 4D, even lanes), as previously described (Figure 3), homogenized, and fractionated into cytosol and membrane fractions. Detergent-solubilized subcellular fractions were immunoprecipitated with antibodies against AP-3 δ or AP-1 γ adaptins. Cytosolic fractions were mostly devoid of contaminant membrane proteins as determined by the absence of PI4KIIα (Figure 4D, compare lanes 9 and 11) and TrfR (not shown). Antibodies against AP-3 δ adaptin effectively precipitated PI4KIIα and associated clathrin from membranes isolated from control siRNA and cross-linker–treated cells (Figure 4D, compare lanes 3 and 4, top panels). However, under identical conditions, AP-1 γ adaptin antibodies failed to precipitate detectable PI4KIIα. Despite this, clathrin coprecipitating with AP-1 was readily detectable (Figure 4D, compare lanes 3 and 4, bottom panels). PI4KIIα down-regulation (Figure 4D, lanes 5–8) drastically decreased the amount of AP-3 and associated clathrin present in cross-linked membrane fractions (Figure 4D, compare lanes 4 and 8, top panels). We observed a similar reduction of AP-3 on non-cross-linked membranes isolated from PI4KIIα-deficient cells (Figure 4D, compare lanes 3 and 7, top panels). Concomitantly, AP-3 content increased in the soluble fraction of PI4KIIα siRNA-treated cells (Figure 4D, compare lanes 2 and 6, top panels). In contrast, neither the membrane content of AP-1 and coprecipitated clathrin (Figure 4D, compare lanes 4 and 8, bottom panels) nor the soluble AP-1 content (Figure 4D, compare lanes 2 and 6, bottom panels), were modified by the decreased levels of PI4KIIα. These results demonstrate that PI4KIIα stabilizes AP-3 on membranes and suggest that the adaptor complex AP-1 is regulated by a lipid kinase other than PI4KIIα.
Figure 4.
PI4KIIα siRNA selectively affects the subcellular distribution of AP-3 but not AP-1. (A) Western blot analysis of control and PI4KIIα siRNA-treated HEK293 cells. PI4KIIα siRNA treatment significantly reduced PI4KIIα levels. Actin blot indicates equivalent protein loads. Control and PI4KIIα siRNA-treated HEK293 cells were stained with δ AP-3 (B) or γ AP-1 (C) adaptin antibodies together with PI4KIIα antibodies. PI4KIIα down-regulation selectively affects AP-3. Scale bars, 10 μm. (D) Control (lanes 1–4) and PI4KIIα siRNA-treated HEK293 cells (lanes 5–8) were treated with the cell-permeable cross-linker DSP (even lanes) or DMSO vehicle (odd lanes) and fractionated into cytosol (lanes 1, 2, 5, and 6) and membrane (lanes 3, 4, 7, and 8) fractions by ultracentrifugation. Cell fractions were dissolved in detergent and immunoprecipitated using beads decorated with AP-3 δ antibodies (lanes 1–8, top panels) or beads decorated with AP-1 γ antibodies (lanes 1–8, bottom panels). Immunocomplexes were resolved by SDS-PAGE and analyzed by immunoblot using antibodies to PI4KIIα, AP-3 δ adaptin (δ), AP-1 γ adaptin (γ), and clathrin. PI4KIIα specifically coprecipitated with AP-3 and clathrin from membrane fractions after DSP cross-linking. PI4KIIα down-regulation selectively decreases AP-3 content in membranes (compare lanes 4 and 8). Input lanes correspond to 10–15% of either cytosol (lanes 9 and 10) or membrane (lanes 11 and 12) extracts. N = 2.
Figure 5.
PI4KIIα kinase activity and sorting motif are required for proper PI4KIIα localization with AP-3. Human wild-type (WT), kinase-inactive (D308A), or dileucine mutant (L61–62A) PI4KIIα-HA were expressed in HEK293 cells, and cells were stained with δ AP-3 or γ AP-1 adaptin antibodies together with HA epitope antibodies. WT PI4KIIα-HA colocalized significantly with AP-3 (A) and minimally with AP-1 (B). (C) Depicts quantification of colocalization of AP-1 and -3 with endogenous kinase (■) or expressed HA-tagged kinase (▩) using Metamorph. Numbers in parentheses depict the number of cell analyzed. Scale bars, 10 μm.
PI4KIIα Kinase Activity and Sorting Motif Are Required for PI4KIIα Endosomal Localization
The hypothesis that PI4KIIα is an enzymatic cargo/regulator of a transport pathway predicts that mutants in its sorting motif or kinase domain should similarly affect PI4KIIα endosomal distribution, generate similar endosomal phenotypes, and similarly affect PI4KIIα interaction with AP-3. To explore these predictions, we ablated the PI4KIIα-sorting motif (56ERQPLL61 in rat or 57ERQPLL62 in human) and its kinase activity by site-directed mutagenesis. PI4KIIα mutants lacking the first 92 amino acids, a domain where the dileucine motif resides, display WT levels of kinase activity when expressed in HEK293 cells (Barylko et al., 2002). Because the ERQPLL sorting motif and kinase activity reside in independent PI4KIIα domains, we used this to our advantage.
We first sought to determine if PI4KIIα enzymatic activity and/or sorting motif would be required to properly localize PI4KIIα with the adaptor complex AP-3 and endocytic compartments. Human PI4KIIα tagged with HA as well as mutants in the kinase catalytic aspartate (D308A; Barylko et al., 2002) or its dileucine-sorting motif (L61-62A) were expressed in HEK293 cells and analyzed by immunofluorescence. Transfected cells were double-labeled with antibodies against AP-3 δ or AP-1 γ adaptins (Figure 5) with one of the following antibodies against: the HA epitope (Figures 5 and 6) as well as antigens from either early stages of the endocytic route (TrfR) or late endosome/lysosomes (LAMP-1, Figure 6). Half of WT PI4KIIα-HA containing organelles were also positive for AP-3 (Figures 5,A and C, ▩, 52.6 ± 2.8, n = 13, p < 0.0001). Importantly, the extent of colocalization between AP-3 and PI4KIIα-HA was significantly reduced to 28 and 36% in PI4KIIα-HA mutants lacking either its sorting motif (Figure 5, A and C, L61-62A PI4KIIα-HA) or its kinase activity (Figure 5, A and C, D308A PI4KIIα-HA, p = 0.0002), respectively. In contrast, AP-1 colocalized poorly with either endogenous (Figure 5C, nontransfected, NT, ■) or exogenously expressed WT PI4KIIα-HA (Figure 5B-C, PI4KIIα-HA, ▩). Furthermore, ablation of the PI4KIIα-HA–sorting motif minimally affected PI4KIIα-HA colocalization with AP-1 (Figure 5, B and C, L61-62A PI4KIIα-HA). Curiously, and in stark contrast with AP-3, the colocalization of the kinase-deficient D308A PI4KIIα-HA mutant with AP-1 increased twofold when compared with WT PI4KIIα-HA (Figure 5, B and C, D308A PI4KIIα-HA, p < 0.0001). These results support the hypothesis that PI4KIIα preferentially associates with AP-3–positive compartments by a mechanism that requires PI4KIIα enzymatic activity and sorting motif.
Figure 6.
PI4KIIα kinase activity and sorting motif are required for proper PI4KIIα localization. Human wild-type (WT), kinase-inactive (D308A), or dileucine mutant (L61-62A) PI4KIIα-HA were expressed in HEK293 cells, and cells were stained with HA antibodies plus either LAMP1 or transferring receptor (TrfR) antibodies. WT PI4KIIα-HA colocalized significantly with LAMP-1 and minimally with TfR, whereas D308A and L61-62A PI4KIIα-HA displayed the opposite pattern, colocalizing significantly with TfR and minimally with LAMP-1. Scale bars, 5 μm.
The decreased colocalization of mutant forms of PI4KIIα-HA with AP-3 occurred concomitantly with a change of the kinase subcellular localization along the endocytic route. Wild-type PI4KIIα-HA colocalized with LAMP-1 and minimally with TrfR (Figure 6). These findings recapitulate the subcellular distribution of endogenous PI4KIIα in neuronal and nonneuronal cell types (Salazar et al., 2005, 2006; Minogue et al., 2006). In contrast, D308A PI4KIIα-HA and L61-62A PI4KIIα-HA displayed identical phenotypes characterized by colocalization with TrfR, yet minimal colocalization with LAMP-1 (Figure 6). These results demonstrate that both PI4KIIα kinase activity and dileucine-based sorting information are required to target the kinase to its proper subcellular localization.
PI4KIIα Kinase Activity and Sorting Motif Are Required to Rescue PI4KIIα Loss-of-Function Endocytic Mutant Phenotypes
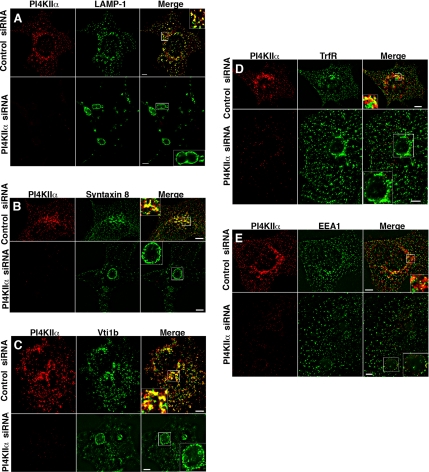
PI4KIIα lacking either its kinase activity or its sorting motif display a similarly abnormal subcellular distribution. This observation is consistent with a model wherein PI4KIIα behaves both as a cargo and a regulator of a transport route. To further test this model, we determined whether kinase activity and the 57ERQPLL62 motif are required to rescue PI4KIIα loss-of-function endosomal mutant phenotypes. The cellular levels of PI4KIIα were reduced in HEK293 cells using siRNA. Immunofluorescence microscopy was performed to assess hypomorphic phenotypes on AP-3 cargoes and membrane proteins found in the transition between early and late endosome/lysosomes. Significant reduction of PI4KIIα by siRNA was observed both by immunoblot (Figures 4A, lane 3, and 9A, lane 2) and immunofluorescence (Figure 7, A–E). Immunostaining for the AP-3 cargo LAMP-1 in PI4KIIα siRNA-treated cells revealed a dramatic impact on LAMP-1–positive organelles. In contrast to control siRNA-treated cells in which LAMP-1 displays punctate localization throughout the cytoplasm (Figure 7A), PI4KIIα siRNA treatment induced a phenotype characterized by 1) the formation of large (1–7 μm) circular organelles that contained LAMP-1 and 2) a reduction in the punctate pattern of LAMP-1 seen in control siRNA-treated cells (Figure 7A). Quantification of the enlarged organelle phenotype revealed a penetrance of ∼5% in control siRNA-treated cells. In contrast, ∼50% of PI4KIIα siRNA-treated cells displayed this phenotype (44.5 ± 8.1%, n = 3, Figure 8). We analyzed whether membrane proteins that reside in the interface between early and late endosomes were also accumulated on similar large circular organelles in PI4KIIα siRNA-treated cells. We focused on syntaxin 8 and Vti1b, Q-t-SNAREs involved in fusion steps between early and late endosomes (Mullock et al., 2000; Ward et al., 2000; Bogdanovic et al., 2002; Pryor et al., 2004). Much like LAMP-1, these SNAREs accumulated in enlarged endosomes. Furthermore, there was a reduction of puncta positive for Vti1b and syntaxin 8 in the cytoplasm in PI4KIIα siRNA-treated cells (Figure 7, B and C). TrfR and to a much lesser extent EEA1 were also observed on enlarged endosomal compartments yet without altering TrfR or EEA1-positive puncta in the cytoplasm (Figure 7, D and E). Enlarged organelles were negative for TGN46, a marker for the trans-Golgi network (data not shown). Together these results suggest that down-regulation of PI4KIIα results in mis-targeting of an AP-3 cargo (LAMP-1) and alters the distribution of Q-t-SNARES (syntaxin 8 and Vti1b) involved in early to late endosomal fusion events.
Figure 7.
PI4KIIα siRNA results in enlarged endocytic compartments and perturbs the targeting of AP-3 cargo and endosomal SNAREs. HEK293 cells were treated with control or PI4KIIα siRNA and stained for PI4KIIα plus either LAMP-1 (A), syntaxin 8 (B), Vti1b (C), transferrin receptor (TfrR, D), or early endosomal antigen 1 (EEA1, E). Scale bars, 5 μm.
Figure 8.
Quantification of PI4KIIα siRNA-induced enlarged endocytic compartments. Quantification of the penetrance of PI4KIIα knockdown-induced enlarged organelles immunoreactive for LAMP-1. Data are expressed as the percentage of cells displaying enlarged organelles immunoreactive for LAMP-1. For rescue experiments, cells were transfected with the rescue plasmid 24 h before processing for immunofluorescence. The total number of cells analyzed (Σ) was collected in three independent experiments. p values: *1 = 0.0003, *2 = 0.01, *3 = 0.007, *4 = 0.001.
We utilized the PI4KIIα siRNA-induced LAMP-1 enlarged organelle phenotype to test whether PI4KIIα kinase activity and its sorting motif were required to rescue this phenotype (Figure 8). For this purpose, we used a sequential protocol of PI4KIIα siRNA followed by re-expression of WT and mutant versions of human HA-tagged PI4KIIα driven from a CMV promoter. This allowed us to express HA-PI4KIIα without the need to create siRNA-resistant PI4KIIα constructs (see Figure 9). Reexpression of WT PI4KIIα-HA reduced the penetrance of the enlarged endosome phenotype from 44.5 ± 8.1% (n = 3, Figure 8) to 24.7 ± 2% (n = 3, Figure 8). This partial rescue may be related to the expression levels of HA-PI4KIIα, because overexpression of the kinase also induced enlarged endosomal vesicles (Balla et al., 2002). In contrast, re-expression of the kinase-inactive D308A PI4KIIα-HA (49.5 ± 2.5%, n = 3, Figure 8) or the L61-62A PI4KIIα-HA mutant lacking its sorting motif (60.1 ± 4%, n = 3, Figure 8) failed to rescue the enlarged endosome phenotype, indicating a requirement for both PI4KIIα kinase activity and the dileucine-sorting motif for proper PI4KIIα function.
The phenotypic similarities between PI4KIIα mutants lacking kinase activity or AP-3–sorting information in their colocalization with AP-3, their subcellular localization, as well as their inability to rescue PI4KIIα loss-of-function could be ascribed to a similar failure of both PI4KIIα mutants to form complexes with AP-3 on membranes. We explored this hypothesis by performing cross-linking and AP-3 immunoprecipitations using HEK293 cells expressing either WT kinase, kinase-inactive D308A, or the dileucine mutants L61A or L61-62A PI4KIIα-HA (Figure 9). To exclude the contribution of endogenous PI4KIIα, similar experiments were performed in which the levels of endogenous PI4KIIα were reduced using siRNA. In each case, comparable results were obtained (Figure 9, A and B). PI4KIIα siRNA effectively and selectively abrogated the expression of PI4KIIα (Figure 9A, compare lanes 1 and 2). We used PI4KIIα siRNA-treated cells and rescued with PI4KIIα-HA as a reference (Figure 9A, lanes 3, 11, and 12) to assess the consequences of mutations either in the dileucine-sorting motif (Figure 9A, lanes 4, 5, and 13–16) or the kinase domain (Figure 9A, lanes 6, 17, and 18). Wild-type or mutant PI4KIIα-HA were expressed to levels similar to those found in control siRNA-treated cells (Figure 9A, compare lanes 1 and 3–6). As previously described (Figure 3C) mutants lacking either one or two leucines of the 57ERQPLL62-sorting motif (Figure 9A, lanes 13–16) failed to interact with AP-3. In fact, only ∼25% of dileucine mutant PI4KIIα-HA formed a cross-linkable complex with AP-3 in non-siRNA-treated HEK293 cells (Figure 9B, dark gray bars, n = 3). Similarly, only ∼10% of the dileucine mutant PI4KIIα-HA formed a cross-linkable complex with AP-3 in PI4KIIα-siRNA–treated cells (Figure 9B, light gray bars, n = 1). The D308A PI4KIIα-HA mutant lacking kinase activity formed a cross-linkable complex with AP-3, with half of the efficiency displayed by WT PI4KIIα-HA (Figure 9A, lanes 17 and 18). The association of D308A PI4KIIα-HA with AP-3 was identical whether cross-linking and AP-3 immunoprecipitations were performed in nontreated (54.1 ± 2.1%, n = 3, Figure 9B) or PI4KIIα-siRNA-treated cells (56%, n = 1, Figure 9, A and B). These results indicate that PI4KIIα kinase activity and the dileucine-sorting motif facilitate PI4KIIα interaction with AP-3 on membranes. Moreover, the decreased interaction of PI4KIIα mutants with AP-3 correlates with the inability of these mutants to rescue PI4KIIα hypomorph endosome phenotypes. Because the ablation of the PI4KIIα dileucine motif produces more pronounced phenotypes that those observed in kinase deficient PI4KIIα, these results suggest that the dileucine motif acts upstream of the kinase activity in facilitating adaptor recruitment.
DISCUSSION
Here we demonstrate a selective interaction between the adaptor complex AP-3 and the membrane-anchored lipid kinase PI4KIIα. This interaction requires a dileucine motif present in PI4KIIα, which is necessary for the proper endosomal targeting of this protein. The PI4KIIα sorting sequence (ERQPLL) is identical to the motif found in tyrosinase, a membrane protein targeted from endosomes to melanosomes by virtue of its binding to AP-3 (Honing et al., 1998; Blagoveshchenskaya et al., 1999; Theos et al., 2005). Our data support the hypothesis that PI4KIIα is an AP-3 cargo protein. Consistent with this model are the observations that PI4KIIα is enriched in AP-3–containing vesicles and that AP-3 is required to properly localize PI4KIIα both in neuronal and nonneuronal cells (Salazar et al., 2005, 2006). In addition, our work demonstrates a requirement for PI4KIIα kinase activity in regulating the delivery of AP-3–dependent cargoes to lysosomal compartments. PI4KIIα mutants that selectively abrogate its kinase activity or its sorting information display similar endosomal mutant phenotypes, comparably impair their capacity to rescue PI4KIIα loss-of-function phenotypes and alter PI4KIIα binding to AP-3 either completely or partially. These data support a model whereby PI4KIIα possesses two domains required for localization to late endosomal–lysosomal compartments via an AP-3–dependent mechanism.
PI4KIIα acts as a cargo that encodes in its primary sequence a catalytic activity capable of regulating its own sorting mechanism. This behavior seems unique among enzymatic activities regulating adaptor function such as GTPases (Bonifacino and Glick, 2004), chaperones (Sousa and Lafer, 2006), protein kinases (Conner and Schmid, 2003), lipid kinases (Di Paolo and De Camilli, 2006), and lipid phosphatases (Di Paolo and De Camilli, 2006). In contrast with PI4KIIα, these regulatory components are recruited from cytosol to membranes at different stages of vesicle biogenesis through interactions with adaptors or other vesicle biogenesis components. For example, phosphatidylinositol 4-kinase type IIIβ and phosphatidylinositol kinase type I γ are recruited to membranes by activation of GTPases and/or interactions with the adaptors (Godi et al., 1999; Krauss et al., 2003; Di Paolo et al., 2004; Bairstow et al., 2006; Nakano-Kobayashi et al., 2007). Importantly, these enzymes do not become enriched in the vesicles that they help to assemble (Blondeau et al., 2004; Borner et al., 2006; Takamori et al., 2006).
Phosphatidylinositol kinase type I γ exemplifies a regulatory sorting mechanism through interactions with the adaptor complexes AP-2 and -1 (Ling et al., 2007). Phosphatidylinositol kinase type I γ associates with AP-1 via a YXXΦ motif present in this kinase. However, the membrane protein whose sorting is regulated by phosphatidylinositol kinase type I γ and AP-1 is E-cadherin. This cell adhesion protein is sorted because it binds phosphatidylinositol kinase type I γ rather than AP-1 (Ling et al., 2007). Thus, type I γ kinase has been proposed to act as a scaffold bridging AP-1 and E-cadherin (Ling et al., 2007). In contrast, our data demonstrate a novel mechanism in which a cargo encodes a self-contained regulatory enzymatic function. However, the PI4KIIα-AP-3 interaction does not preclude that PI4KIIα could act as a scaffold for other cargo and/or regulatory proteins.
PI4KIIα possesses two reported subcellular localizations. In two reports PI4KIIα was observed in the Golgi complex (Wang et al., 2003, 2007). In other studies, PI4KIIα was found to preferentially localize to endocytic compartments or postendocytic vesicles (Balla et al., 2002; Guo et al., 2003; Salazar et al., 2005, 2006; Balla and Balla, 2006; Minogue et al., 2006; Takamori et al., 2006; Xu et al., 2006), and only minimal PI4KIIα colocalized with Golgi markers or the adaptor complex AP-1 (Balla et al., 2002; Salazar et al., 2005, 2006). Similarly, here we demonstrate that PI4KIIα is present on the limiting membranes of Rab5Q79L-enlarged rat neuroendocrine endosomes where it colocalizes with AP-3 and the AP-3–interacting molecules clathrin (Dell'Angelica et al., 1998), ZnT3 (Salazar et al., 2004), and VAMP7-TI (Martinez-Arca et al., 2003) but not with AP-1. PI4KIIα endosomal localization is further supported by: 1) PI4KIIα down-regulation selectively affects recruitment of an endosomal adaptor, AP-3 (Faundez et al., 1998; Peden et al., 2004), to membranes without altering the subcellular distribution of the Golgi localized AP-1 adaptor complex (Bonifacino and Traub, 2003; Robinson, 2004). 2) PI4KIIα mutants lacking dileucine sorting information accumulate in TrfR-positive endosomes and 3) PI4KIIα siRNA-induced engorged organelles abnormally accumulate LAMP-1, a well-established AP-3 cargo. Similarly, enlarged organelles containing TrfR or the SNAREs syntaxin 8 or Vti1b can be found in PI4KIIα siRNA-treated cells. However, enlarged endosomes lack detectable levels of the trans-Golgi network marker TGN46, suggesting that these abnormal organelles are unlikely to result from a fusion of endosomes and the trans-Golgi network. These findings do not preclude that some PI4KIIα may be present in the Golgi complex (Wang et al., 2003, 2007).
Enlarged endosomes induced by PI4KIIα knockdown could result from enhanced accruement of membrane components due to defective PI4KIIα-dependent vesicle formation. These vesicles could participate in forward transport between early and late stages of the endocytic route. Alternatively, vesicles formed by a PI4KIIα-dependent mechanism could participate in endosome maturation (Rink et al., 2005) by retrieval of components. Vesicles formed by a PI4KIIα-dependent process could deliver SNAREs, among other membrane proteins, either in forward or retrograde (retrieval) transport processes. This hypothesis is attractive because AP-3 deficiency (Salazar et al., 2006), defects in the BLOC-1 complex that interacts with AP-3 (Di Pietro et al., 2006; Salazar et al., 2006), and PI4KIIα deficiency (data shown here) perturb the subcellular localization or cellular levels of endosomal SNARE proteins. Moreover, syntaxin 8 and Vti1b, two SNAREs found in the PI4KIIα-knockdown–induced enlarged endosomes, form a SNARE fusogenic complex with VAMP-7-TI (Mullock et al., 2000; Ward et al., 2000; Bogdanovic et al., 2002; Pryor et al., 2004), a SNARE that binds to and is targeted by AP-3 (Martinez-Arca et al., 2003; Salazar et al., 2006).
We utilized the high transfection and siRNA efficiency of HEK293 cells to demonstrate that knockdown of PI4KIIα levels by siRNA results in aberrant morphology of compartments of the endosomal system and abnormal accumulation of LAMP1 or TrfR in enlarged structures. This phenotype could be partially rescued by transiently transfecting PI4KIIα after knockdown of the endogenous PI4KIIα. In contrast, PI4KIIα either lacking its kinase activity or its ERQPLL dileucine motif failed to rescue the phenotype, indicating a binary requirement in restoring the targeting of LAMP-1 and the morphology of these endocytic organelles. Despite the fact that PI4KIIα mutants lacking either their ERQPLL motif or kinase activity display endocytic phenotypes of similar magnitude and penetrance (Figures 6–8), these two mutants differ in the extent to which they bind to AP-3 (Figure 9). PI4KIIα lacking a dileucine motif abrogates AP-3 binding by 80–90%. In contrast, PI4KIIα lacking kinase activity binds AP-3 with half efficiency. These findings confirm that the kinase activity is in part required to recruit AP-3 to membranes and suggest that the dileucine motif acts upstream of the kinase activity in facilitating adaptor recruitment. We propose a model in which initial interactions between AP-3 and the PI4KIIα dileucine motif result in concentration of PI4KIIα in microdomains on the limiting membrane of endosomes. This concentrated “patch” of PI4KIIα would then result in a localized patch of kinase activity, resulting in a microdomain enriched in PtdIns(4)P. PtdIns(4)P-enriched microdomains could function in a positive feedback loop to recruit additional AP-3 complexes and/or other machineries involved in either the formation and/or the targeting of AP-3 vesicles. Without kinase activity and the production of a PtdIns(4)P-enriched microdomain, PI4KIIα would transiently interact with AP-3 via the dileucine motif, but fail to recruit the additional AP-3 complexes and/or additional components required to form and/or target vesicles. Our work and this model suggest a novel mechanism in which adaptors utilize canonical sorting motifs to recruit membrane-anchored enzymes that regulate adaptor function.
ACKNOWLEDGMENTS
We thank Drs. Win Sale, Andrew Kowalczyk, Erica Werner, and the Faundez lab members for helpful discussions and suggestions. This work was supported by grants from the National Institutes of Health to V.F. (NS42599 and GM 077569).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-12-1239) on February 6, 2008.
REFERENCES
- Advani R. J., Yang B., Prekeris R., Lee K. C., Klumperman J., Scheller R. H. VAMP-7 mediates vesicular transport from endosomes to lysosomes. J. Cell Biol. 1999;146:765–776. doi: 10.1083/jcb.146.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloza I., Martens E., Hawthorne S., Vandenbroeck K. Cross-linking approach to affinity capture of protein complexes from chaotrope-solubilized cell lysates. Anal. Biochem. 2004;324:137–142. doi: 10.1016/j.ab.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Bairstow S. F., Ling K., Su X., Firestone A. J., Carbonara C., Anderson R. A. Type Igamma661 phosphatidylinositol phosphate kinase directly interacts with AP2 and regulates endocytosis. J. Biol. Chem. 2006;281:20632–20642. doi: 10.1074/jbc.M601465200. [DOI] [PubMed] [Google Scholar]
- Bajjalieh S. M., Peterson K., Shinghal R., Scheller R. H. SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science. 1992;257:1271–1273. doi: 10.1126/science.1519064. [DOI] [PubMed] [Google Scholar]
- Balla A., Balla T. Phosphatidylinositol 4-kinases: old enzymes with emerging functions. Trends Cell Biol. 2006;16:351–361. doi: 10.1016/j.tcb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Balla A., Tuymetova G., Barshishat M., Geiszt M., Balla T. Characterization of type II phosphatidylinositol 4-kinase isoforms reveals association of the enzymes with endosomal vesicular compartments. J. Biol. Chem. 2002;277:20041–20050. doi: 10.1074/jbc.M111807200. [DOI] [PubMed] [Google Scholar]
- Barylko B., Gerber S. H., Binns D. D., Grichine N., Khvotchev M., Sudhof T. C., Albanesi J. P. A novel family of phosphatidylinositol 4-kinases conserved from yeast to humans. J. Biol. Chem. 2001;276:7705–7708. doi: 10.1074/jbc.C000861200. [DOI] [PubMed] [Google Scholar]
- Barylko B., Wlodarski P., Binns D. D., Gerber S. H., Earnest S., Sudhof T. C., Grichine N., Albanesi J. P. Analysis of the catalytic domain of phosphatidylinositol 4-kinase type II. J. Biol. Chem. 2002;277:44366–44375. doi: 10.1074/jbc.M203241200. [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskaya A. D., Hewitt E. W., Cutler D. F. Di-leucine signals mediate targeting of tyrosinase and synaptotagmin to synaptic-like microvesicles within PC12 cells. Mol. Biol. Cell. 1999;10:3979–3990. doi: 10.1091/mbc.10.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondeau F., et al. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc. Natl. Acad. Sci. USA. 2004;101:3833–3838. doi: 10.1073/pnas.0308186101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanovic A., Bennett N., Kieffer S., Louwagie M., Morio T., Garin J., Satre M., Bruckert F. Syntaxin 7, syntaxin 8, Vti1 and VAMP7 (vesicle-associated membrane protein 7) form an active SNARE complex for early macropinocytic compartment fusion in Dictyostelium discoideum. Biochem. J. 2002;368:29–39. doi: 10.1042/BJ20020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S., Glick B. S. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Traub L. M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Borner G. H., Harbour M., Hester S., Lilley K. S., Robinson M. S. Comparative proteomics of clathrin-coated vesicles. J. Cell Biol. 2006;175:571–578. doi: 10.1083/jcb.200607164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner S. D., Schmid S. L. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Danglot L., Galli T. What is the function of neuronal AP-3? Biol. Cell. 2007;99:349–361. doi: 10.1042/BC20070029. [DOI] [PubMed] [Google Scholar]
- De Matteis M. A., Godi A. PI-loting membrane traffic. Nat. Cell Biol. 2004;6:487–492. doi: 10.1038/ncb0604-487. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica E. C., Klumperman J., Stoorvogel W., Bonifacino J. S. Association of the AP-3 adaptor complex with clathrin. Science. 1998;280:431–434. doi: 10.1126/science.280.5362.431. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica E. C., Shotelersuk V., Aguilar R. C., Gahl W. A., Bonifacino J. S. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the beta 3A subunit of the AP-3 adaptor. Mol. Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- Di Paolo G., De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Di Paolo G., Moskowitz H. S., Gipson K., Wenk M. R., Voronov S., Obayashi M., Flavell R., Fitzsimonds R. M., Ryan T. A., De Camilli P. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431:415–422. doi: 10.1038/nature02896. [DOI] [PubMed] [Google Scholar]
- Di Pietro S. M., Dell'Angelica E. C. The cell biology of Hermansky-Pudlak syndrome: recent advances. Traffic. 2005;6:525–533. doi: 10.1111/j.1600-0854.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- Di Pietro S. M., Falcon-Perez J. M., Tenza D., Setty S. R., Marks M. S., Raposo G., Dell'angelica E. C. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol. Biol. Cell. 2006;17:4027–4038. doi: 10.1091/mbc.E06-05-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doray B., Lee I., Knisely J., Bu G., Kornfeld S. The γ/ς1 and α/ς2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol. Biol. Cell. 2007;18:1887–1896. doi: 10.1091/mbc.E07-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faundez V., Horng J. T., Kelly R. B. A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell. 1998;93:423–432. doi: 10.1016/s0092-8674(00)81170-8. [DOI] [PubMed] [Google Scholar]
- Faundez V., Kelly R. B. The AP-3 complex required for endosomal synaptic vesicle biogenesis is associated with a casein kinase ialpha-like isoform. Mol. Biol. Cell. 2000;11:2591–2604. doi: 10.1091/mbc.11.8.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godi A., Pertile P., Meyers R., Marra P., Di Tullio G., Iurisci C., Luini A., Corda D., De Matteis M. A. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex [see comments] Nat. Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- Guo J., Wenk M. R., Pellegrini L., Onofri F., Benfenati F., De Camilli P. Phosphatidylinositol 4-kinase type IIalpha is responsible for the phosphatidylinositol 4-kinase activity associated with synaptic vesicles. Proc. Natl. Acad. Sci. USA. 2003;100:3995–4000. doi: 10.1073/pnas.0230488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honing S., Ricotta D., Krauss M., Spate K., Spolaore B., Motley A., Robinson M., Robinson C., Haucke V., Owen D. J. Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol. Cell. 2005;18:519–531. doi: 10.1016/j.molcel.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Honing S., Sandoval I. V., von Figura K. A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP-3. EMBO J. 1998;17:1304–1314. doi: 10.1093/emboj/17.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizing M., Sarangarajan R., Strovel E., Zhao Y., Gahl W. A., Boissy R. E. AP-3 mediates tyrosinase but not TRP-1 trafficking in human melanocytes. Mol. Biol. Cell. 2001;12:2075–2085. doi: 10.1091/mbc.12.7.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier K., Bonifacino J. S. Role of the endocytic machinery in the sorting of lysosome-associated membrane proteins. Mol. Biol. Cell. 2005;16:4231–4242. doi: 10.1091/mbc.E05-03-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier K., Kato Y., Boehm M., Rose J. R., Martina J. A., Kim B. Y., Venkatesan S., Bonifacino J. S. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J. Cell Biol. 2003;163:1281–1290. doi: 10.1083/jcb.200307157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss M., Kinuta M., Wenk M. R., De Camilli P., Takei K., Haucke V. ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Igamma. J. Cell Biol. 2003;162:113–124. doi: 10.1083/jcb.200301006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss M., Kukhtina V., Pechstein A., Haucke V. Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2mu-cargo complexes. Proc. Natl. Acad. Sci. USA. 2006;103:11934–11939. doi: 10.1073/pnas.0510306103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling K., Bairstow S. F., Carbonara C., Turbin D. A., Huntsman D. G., Anderson R. A. Type Igamma phosphatidylinositol phosphate kinase modulates adherens junction and E-cadherin trafficking via a direct interaction with micro1B adaptin. J. Cell Biol. 2007;176:343–353. doi: 10.1083/jcb.200606023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomant A. J., Fairbanks G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate) J. Mol. Biol. 1976;104:243–261. doi: 10.1016/0022-2836(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Martinez-Arca S., et al. A dual mechanism controlling the localization and function of exocytic v-SNAREs. Proc. Natl. Acad. Sci. USA. 2003;100:9011–9016. doi: 10.1073/pnas.1431910100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minogue S., Waugh M. G., De Matteis M. A., Stephens D. J., Berditchevski F., Hsuan J. J. Phosphatidylinositol 4-kinase is required for endosomal trafficking and degradation of the EGF receptor. J. Cell Sci. 2006;119:571–581. doi: 10.1242/jcs.02752. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., et al. Syntaxin 7 is localized to late endosome compartments, associates with Vamp 8, and is required for late endosome-lysosome fusion. Mol. Biol. Cell. 2000;11:3137–3153. doi: 10.1091/mbc.11.9.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano-Kobayashi A., Yamazaki M., Unoki T., Hongu T., Murata C., Taguchi R., Katada T., Frohman M. A., Yokozeki T., Kanaho Y. Role of activation of PIP5Kgamma661 by AP-2 complex in synaptic vesicle endocytosis. EMBO J. 2007;26:1105–1116. doi: 10.1038/sj.emboj.7601573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell-Litwa K., Seong E., Burmeister M., Faundez V. Neuronal and non-neuronal functions of the AP-3 sorting machinery. J. Cell Sci. 2007;120:531–541. doi: 10.1242/jcs.03365. [DOI] [PubMed] [Google Scholar]
- Peden A. A., Oorschot V., Hesser B. A., Austin C. D., Scheller R. H., Klumperman J. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J. Cell Biol. 2004;164:1065–1076. doi: 10.1083/jcb.200311064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor P. R., Mullock B. M., Bright N. A., Lindsay M. R., Gray S. R., Richardson S. C., Stewart A., James D. E., Piper R. C., Luzio J. P. Combinatorial SNARE complexes with VAMP7 or VAMP8 define different late endocytic fusion events. EMBO Rep. 2004;5:590–595. doi: 10.1038/sj.embor.7400150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg C., Bache K. G., Gillooly D. J., Madshus I. H., Stang E., Stenmark H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 2002;4:394–398. doi: 10.1038/ncb791. [DOI] [PubMed] [Google Scholar]
- Raiborg C., Wesche J., Malerod L., Stenmark H. Flat clathrin coats on endosomes mediate degradative protein sorting by scaffolding Hrs in dynamic microdomains. J. Cell Sci. 2006;119:2414–2424. doi: 10.1242/jcs.02978. [DOI] [PubMed] [Google Scholar]
- Rink J., Ghigo E., Kalaidzidis Y., Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Robinson M. S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Salazar G., et al. BLOC-1 complex deficiency alters the targeting of adaptor protein complex-3 cargoes. Mol. Biol. Cell. 2006;17:4014–4026. doi: 10.1091/mbc.E06-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar G., Craige B., Wainer B. H., Guo J., De Camilli P., Faundez V. Phosphatidylinositol-4-kinase type II α is a component of adaptor protein-3-derived vesicles. Mol. Biol. Cell. 2005;16:3692–3704. doi: 10.1091/mbc.E05-01-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar G., Love R., Werner E., Doucette M. M., Cheng S., Levey A., Faundez V. The zinc transporter ZnT3 interacts with AP-3 and it is targeted to a distinct synaptic vesicle subpopulation. Mol. Biol. Cell. 2004;15:575–587. doi: 10.1091/mbc.E03-06-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem N., Faundez V., Horng J. T., Kelly R. B. A v-SNARE participates in synaptic vesicle formation mediated by the AP3 adaptor complex. Nat. Neurosci. 1998;1:551–556. doi: 10.1038/2787. [DOI] [PubMed] [Google Scholar]
- Scheuber A., Rudge R., Danglot L., Raposo G., Binz T., Poncer J. C., Galli T. Loss of AP-3 function affects spontaneous and evoked release at hippocampal mossy fiber synapses. Proc. Natl. Acad. Sci. USA. 2006;103:16562–16567. doi: 10.1073/pnas.0603511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa R., Lafer E. M. Keep the traffic moving: mechanism of the Hsp70 motor. Traffic. 2006;7:1596–1603. doi: 10.1111/j.1600-0854.2006.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., Parton R. G., Steele-Mortimer O., Lutcke A., Gruenberg J., Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedlow J. R., Sedat J. W., Agard D. A. Deconvolution in optical microscopy. In: Jansson P. A., editor. Deconvolution of Images and Spectra. San Diego: Academic Press; 1997. pp. 284–307. [Google Scholar]
- Takamori S., et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Theos A. C., et al. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol. Biol. Cell. 2005;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Sun H. Q., Macia E., Kirchhausen T., Watson H., Bonifacino J. S., Yin H. L. PI4P promotes the recruitment of the GGA adaptor proteins to the trans-Golgi network and regulates their recognition of the ubiquitin sorting signal. Mol. Biol. Cell. 2007;18:2646–2655. doi: 10.1091/mbc.E06-10-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. J., Wang J., Sun H. Q., Martinez M., Sun Y. X., Macia E., Kirchhausen T., Albanesi J. P., Roth M. G., Yin H. L. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114:299–310. doi: 10.1016/s0092-8674(03)00603-2. [DOI] [PubMed] [Google Scholar]
- Ward D. M., Pevsner J., Scullion M. A., Vaughn M., Kaplan J. Syntaxin 7 and VAMP-7 are soluble N-ethylmaleimide-sensitive factor attachment protein receptors required for late endosome-lysosome and homotypic lysosome fusion in alveolar macrophages. Mol. Biol. Cell. 2000;11:2327–2333. doi: 10.1091/mbc.11.7.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C. C., Mezey E., Chen M., Key S., Ma L., Brownstein M. J. Using DSP, a reversible cross-linker, to fix tissue sections for immunostaining, microdissection and expression profiling. Nucleic Acids Res. 2004;32:e185. doi: 10.1093/nar/gnh185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Huang G., Kandror K. V. Phosphatidylinositol 4-kinase type IIalpha is targeted specifically to cellugyrin-positive glucose transporter 4 vesicles. Mol. Endocrinol. 2006;20:2890–2897. doi: 10.1210/me.2006-0193. [DOI] [PubMed] [Google Scholar]