Abstract
Regional variations in the electrophysiological properties of myocytes across the left ventricular wall play an important role in both the normal physiology of the heart and the genesis of arrhythmias. To investigate the possible contributions of calcium channels to transmural electrical heterogeneity, whole-cell patch-clamp recordings were made from isolated canine epicardial and endocardial left ventricular myocytes. Two major differences in Ca2+ channel properties were found between epi- and endocardial cells. First, the L-type Ca2+ current was larger in endocardial than in epicardial myocytes. The average peak current density at +10 mV in endocardial myocytes was 3.4 ± 0.2 pA pF−1, and was 45 % higher than that in epicardium (2.3 ± 0.1 pA pF−1). The kinetic properties of the L-type current in epi- and endocardial cells were not significantly different. Second, a low-threshold, rapidly activating and inactivating Ca2+ current that resembled the T-type current was present in all endocardial myocytes but was small or absent in epicardial myocytes. This T-like current had an average peak density of 0.5 pA pF−1 at −40 mV in endocardial cells. In most endocardial cells the T-like Ca2+ current comprised two components: a Ni2+-sensitive T-type current and a tetrodotoxin-sensitive Ca2+ current. We conclude that there are considerable regional variations in the density and properties of Ca2+ channels across the canine left ventricular wall. These variations may contribute to the overall transmural electrical heterogeneity.
Cardiac myocytes differ with respect to action potential duration and configuration across the thickness of the left ventricular myocardium. In the hearts of a number of mammalian species including dog, human, guinea-pig, cat, pig and rat (Litovsky & Antzelevitch, 1988; Kimura et al. 1990; Clark et al. 1993; Nabauer et al. 1996; Rodriguez-Sinovas, 1997; Bryant et al. 1998), the action potential of epicardial myocytes is shorter in duration compared with that of endocardial cells. In addition, in most of these species the epicardial, but not endocardial, action potential has a prominent phase 1 repolarization and a characteristic spike-and-dome morphology. The transmural electrical gradient generated as a result of the difference in action potential duration is responsible for the upright T wave in the electrocardiogram (Burgess, 1979). Alteration of the transmural electrical dispersion under clinical pathological conditions is thought to provide the substrate for the development of re-entrant arrhythmias (Antzelevitch & Fish, 2001).
Regional variations in the density and properties of ionic and exchanger currents underlie the differences in action potential waveform and duration across the ventricle. In particular, a significantly higher density and faster recovery-from-inactivation kinetics of the transient outward current (Ito) in epicardium compared with that in endocardial myocytes has been described in a number of species including human, and appears to be an important contributing factor (Furukawa et al. 1990; Fedida & Giles, 1991; Liu et al. 1993; Wettwer et al. 1994; Nabauer et al. 1996; Rodriguez-Sinovas, 1997). Recently it has been shown that, in canine and human hearts, the expression level and properties of Ito are regulated by an accessory subunits known as the Kv Channel Interacting Protein 2 (KChIP2), and that a gradient in KChIP2 gene expression across the left ventricular wall is the primary determinant of the transmural Ito gradient (Rosati et al. 2001).
One group of ion channels that are critical for cardiac function as well as the electrophysiological properties of myocytes are the Ca2+ channels. Two major types of Ca2+ channels, the L-type and the T-type, are expressed in the heart. The high-threshold L-type channels are ubiquitous in cardiac myocytes and provide the Ca2+ influx that triggers excitation-contraction coupling in the heart. The L-type current also contributes importantly to the configuration of the cardiac action potential by generating the plateau phase. The T-type Ca2+ channel is characterized by its low threshold of activation and rapid inactivation kinetics. It is expressed at relatively high levels in the rabbit sino-atrial node cells and probably contributes to the pacemaker activity of these cells (Hagiwara et al. 1988). Its presence in ventricular myocytes has been described in the hearts of dog and guinea-pig (Mitra & Morad, 1986; Tseng & Boyden, 1989; Balke et al. 1992), although here its amplitude is only a fraction of that of the L-type current, and its function is poorly understood. Besides the above two major types of Ca2+ currents, a third type, the tetrodotoxin-sensitive Ca2+ current (ICa(TTX)) was described in human atrial and rat and guinea-pig ventricular myocytes (Lemaire et al. 1995; Aggarwal et al. 1997; Cole et al. 1997). This current is believed to be the result of Ca2+ conduction by a distinct subpopulation of Na+ channels (Chen-Izu et al. 2001). Like the T-type current, the function of ICa(TTX) is not clear. Despite extensive past studies on cardiac Ca2+ channels, a complete description of the Ca2+ channel properties in various regions across the left ventricle is lacking, and the possible contribution of Ca2+ currents to the transmural electrical heterogeneity is unknown. In this paper, we investigate the distribution and properties of Ca2+ channels in epicardial and endocardial myocytes in canine left ventricle.
METHODS
Preparation of isolated canine ventricular myocytes
Adult dogs of either sex and 15–20 kg in body weight were killed with an intravenous injection of sodium pentobarbital at a concentration of 80 mg (kg body weight)−1. Myocytes from the epicardium and endocardium of the left ventricle were dissociated using a trituration method as previously described (Gintant et al. 1985). Epicardial or endocardial ventricular chunks of 2 mm thickness were removed from each layer using a scalpel. After recovery for 0.5 h in Ca2+-free Tyrode solution at 37 °C, the tissue chunks were subjected to successive digestion and trituration in an oxygenated K+-reversed solution. The K+-reversed solution contained (mm): KCl 140, KH2CO3 8, KH2PO4 0.4, MgCl2 22, glucose 10, taurine 25, β-OH-butyric acid 5, Na-pyruvate 5, albumin (Sigma, 1.6–4.0 mg ml−1), pH 7.0. Tissue was digested at 37 °C with K+-reversed solution containing 0.8–1.2 mg ml−1 collagenase (Boehringer Manheim Type D) followed by trituration with plain K+-reversed solution. Live cells were obtained after the second and third collagenase digests and after trituration in the absence of collagenase. Isolated myocytes were first stored in KB solution containing (mm): KCl 83, K2HPO4 30, MgSO4 5, Na-pyruvic acid 5, β-OH butyric acid (Na+ salt) 5, taurine 20, creatine 5, glucose 10, EGTA 0.5, Hepes 5, and Na2ATP 5 (pH 7.2) for 30 min at room temperature, and then transferred to a Medium 199 (GIBCO, Gaithersburg, MD, USA) based culture medium containing: carnitine 2 mm, creatine 5 mm, taurine 5 mm, BSA (bovine serum albumin) 2 mg ml−1, penicillin streptomycin 100 u ml−1, and insulin 1 μg ml−1 (Ellingsen et al. 1993). Cells were then plated on laminin-coated cover-slips at a density of approximately 5 × 104 cells cm−2 and allowed to adhere for 10 min at 37 °C. Dead myocytes did not adhere to the cover-slips and were rinsed off with additional medium. Myocytes were stored at 37 °C in the culture medium for up to 24 h. Alternatively, myocytes were stored in KB solution after isolation at 4 °C without culturing. No differences in myocyte appearance or Ca2+ channel properties were found between myocytes stored in the two methods. Myocytes were studied 1–24 h after dissociation. No change in Ca2+ channel properties was found within this period.
Electrophysiological recordings
Isolated ventricular myocytes were maintained at 29 °C and perfused with Tyrode solution containing (mm): NaCl 140, KCl 5.4, MgCl2 1, CaCl2 1.8, Hepes 5, and glucose 10 (pH 7.4). Ca2+ currents were recorded using the whole-cell patch-clamp technique with an Axopatch-1B amplifier. To eliminate contamination from other ionic and exchange currents, the perfusion solution was switched to a Na+- and K+-free solution that contained (mm): TEA-Cl 137, CsCl 5.4, CaCl2 2, MgCl2 1, Hepes 5, glucose 10, and 4-aminopyridine 3 (pH 7.4). Glass pipettes were filled with solution containing (mm): Caesium aspartate 115, CsCl 20, EGTA 11, Hepes 10, MgCl2 2.5, and Mg-ATP 2 (pH 7.2), and had a resistance of 1.5–2.5 MΩ. After the membrane was ruptured, cells were clamped at −60 mV for 10 min to allow dialysis of the intracellular solution and stabilization of the Ca2+ currents before measurement of Ca2+ currents began. All drugs were purchased from Sigma (St Louis, MO, USA). Data collection and analysis were performed using pCLAMP software (Axon Instruments, Union City, CA, USA).
Data analysis
Group data are presented as means ±s.e.m. Statistical tests of drug effects were performed using Student's unpaired two-tailed t tests unless otherwise stated. A t value giving P < 0.02 was considered to be significant.
RESULTS
Comparison of Ca2+ currents (ICa) in canine epi- and endocardial ventricular myocytes
To examine and compare calcium channel properties in epicardial and endocardial myocytes of the left ventricle, recordings were made from isolated ventricular myocytes from 14 dogs using the whole-cell voltage-clamp. Figure 1 shows typical responses of an epicardial and an endocardial cell to a series of depolarizing steps from a holding potential of −80 mV. In both types of cells the elicited currents were dominated by a large, rapidly activating and slowly inactivating L-type ICa (Fig. 1A). However, two major differences can be readily observed between the two cell types. First, in addition to the L-type current, a low-threshold, rapidly activating and inactivating current was present in all endocardial cells (Fig. 1B, arrow). This current was most prominent in the voltage range of −50 and −30 mV. It resembled the T-type calcium current, and will be referred to as the T-like current hereafter. By contrast, in most epicardial cells little or no detectable inward current was observed at voltages more negative than −20 mV, at which potential a slow L-type current was activated (Fig. 1B). Secondly, the L-type current density was typically larger in endocardial than in epicardial myocytes. These two differences are reflected in the current-voltage relationships of ICa for epi- and endocardial cells (Fig. 1C). We compared the voltage dependence of the L-type ICa in epicardial and endocardial myocytes by normalizing the I−V relations in Fig. 1C to the peak currents recorded at +10 mV (Fig. 1D). Excluding the voltage range in which the T-like current contributes (−50 mV to −20 mV) the two I−V relations superimpose.
Figure 1. Recordings of Ca2+ currents from epicardial (EPI, top) and endocardial (ENDO, bottom) myocytes isolated from canine left ventricle.

A, the currents were activated by depolarizing steps ranging from −70 to +20 mV in 10 mV increments, from a holding potential of −80 mV at a frequency of 0.1 Hz. B, low-threshold current traces of the same recordings shown in A. Currents in response to voltage steps from −70 to −20 mV are shown, and the arrow points to the low-threshold Ca2+ current in endocardial cells. C, average peak current-voltage relationships of whole-cell Ca2+ currents in epicardial (n = 15) and endocardial cells (n = 13). D, the I−V relations in C are normalized to their peak values at +10 mV and plotted together. Error bars are ±s.e.m.
The peak density of the L-type ICa measured at +10 mV for all epicardial (n = 20) and endocardial myocytes (n = 21) that were studied are plotted along with their respective average current densities in Fig. 2. The data showed a large degree of dispersion; nevertheless, the separation of the two groups is apparent. The average current density for endocardial myocytes was 3.4 ± 0.2 pA pF−1, which was 45 % higher than that for epicardium (2.3 ± 0.1 pA pF−1, P < 0.0001).
Figure 2. Comparison of the L-type Ca2+ current density in epicardial and endocardial cells.

Open symbols, L-type Ca2+ current densities for individual epicardial (EPI, n = 20) and endocardial (ENDO, n = 22) cells measured at +10 mV. Filled symbols, average current densities ±s.e.m.
Characterization of the T-like current
The low-threshold T-like current in endocardial cells could be separated from the L-type current using voltage-clamp protocols with different holding potentials (Fig. 3). Depolarizing steps from a holding potential of −80 mV first activated the T-like current at voltages more negative than −20 mV, and then both the T-like current and the much slower L-type current at voltages of −20 mV or above (Fig. 3A, left). The T-like current was completely eliminated when the holding potential was −50 mV, leaving only the L-type current (Fig. 3A, right). Subtraction of the two sets of current traces revealed the T-like current at various voltages (Fig. 3B). Due to the presence of the T-like current, the current-voltage relationships measured from holding potentials of −80 and −50 mV are markedly different in endocardial cells (Fig. 3C). The ‘hump’ between −60 and −20 mV that is apparent in the former is lacking in the latter. At voltages positive to 0 mV the two curves are almost superimposable. Figure 3D shows the average current-voltage relationship of the T-like current. The activation threshold is around −50 mV and the current peaks between −40 and −30 mV. The T-like current diminishes at potentials positive to 0 mV, suggesting that the peak L-type current measured at +10 mV in endocardial cells is not contaminated by the T-like current.
Figure 3. Low-threshold T-like Ca2+ current in endocardial ventricular myocytes.

A, current traces in response to depolarizing test steps from a holding potential of −80 mV (left) and −50 mV (right). Numbers to the left of the current traces indicate the potential of the test steps. Subtraction of the traces in the right column (Vhold=−50 mV) from those in the left column (Vhold=−80 mV) yielded the difference currents, which are shown in B. C, current-voltage relationships of the whole-cell Ca2+ current for the same cell as in A, measured from holding potentials of −80 and −50 mV. D, current-voltage relationship of the T-like Ca2+ current in endocardial cells. Data points are average current densities from 11 cells, and are shown as ±s.e.m.
The T-like current was ubiquitous in all endocardial cells studied with an average current density of 0.5 ± 0.04 pA pF−1 at −40 mV (n = 20). By contrast, the same current was present in only 4 out of 20 epicardial cells, and the average current density at −40 mV was 0.03 ± 0.02 pA pF−1 (n = 20). Even for those epicardial cells where a T-like current was present, the average current density was 0.14 ± 0.04 pA pF−1 (n = 4), and was over 70 % smaller than that for endocardium (P < 0.01).
Steady-state inactivation properties of the Ca2+ currents
The separation of the L-type and T-like currents in endocardial cells using different holding potentials was possible because of the large difference in their steady-state inactivation properties. A standard protocol for testing steady-state inactivation properties was used, and representative recordings of the endocardial L-type and T-like currents are shown in Fig. 4A and B. The midpoint of the steady-state inactivation curve for the endocardial L-type current was −23.9 ± 0.9 mV (n = 4), and was 40 mV more positive than that for the T-like current (−64.6 ± 1.8 mV, n = 5). In addition, the T-like current had a steeper slope than did the L-type ICa (slope factor: 4.8 ± 0.1 and 5.5 ± 0.3 mV, respectively; P < 0.02). The L-type ICa in epicardial cells had steady-state inactivation characteristics similar to those of the endocardial L-type ICa (Fig. 4C, midpoint −22.9 ± 0.7 mV, slope factor 5.8 ± 0.4 mV, n = 6).
Figure 4. Comparison of the steady-state inactivation properties of the L-type current (A) and the T-like current (B), both recorded from endocardial myocytes.

Membrane potential was clamped at various conditioning voltages (ranging from −90 to −40 mV for the T-like current and from −90 to +10 mV for the L-type current) for 1 s, and was then stepped to the testing potential (−40 mV for the T-like current and 0 mV for the L-type current). Conditioning potentials are indicated for selected traces. C, steady-state inactivation curves for the endocardial T-like current, endocardial L-type current and epicardial L-type current. Data points are averages from five, four and six cells, respectively, and were fitted with the equation G/Gmax= 1/(1 + exp((V−Vh)/kh)), where the midpoint for steady-state inactivation Vh=−64.6, −23.9 and −22.9 mV and the slope factor kh= 4.8, 5.5 and 5.8 mV for the endocardial T-like current, endocardial L-type current and epicardial L-type current, respectively. Error bars are ±s.e.m.
Two components of the T-like current
Due to the large conductance of the cardiac Na+ channels, even the presence of a trace amount of extracellular Na+ can result in a low-threshold inward current. Therefore, we examined the dependence of the T-like current in endocardial cells on extracellular Ca2+. Removing Ca2+ from the perfusion solution completely eliminated the T-like current (Fig. 5A), suggesting that the current is carried by Ca2+ and minimally contaminated by other currents. We further explored the nature of the current using pharmacological tools. Two types of low-threshold Ca2+ currents have been previously described in cardiac myocytes: a Ni2+-sensitive T-type current (Mitra & Morad, 1986; Hagiwara et al. 1988; Tseng & Boyden, 1989; Balke et al. 1992), and a tetrodotoxin (TTX)-sensitive Ca2+ current (ICa(TTX), Lemaire et al. 1995; Aggarwal et al. 1997; Cole et al. 1997). We found that in a typical canine endocardial myocyte, the T-like ICa was partially blocked by 50 μm Ni2+, and the remaining current was blocked by Ni2+ plus 20 μm TTX (Fig. 5B, top). Conversely, the T-like ICa was only partially blocked by 20 μm TTX, and application of 50 μm Ni2+ in addition to TTX blocked the remainder of the current (Fig. 5B, bottom). There was much variability in the percentage of current that was Ni2+-sensitive or TTX-sensitive, ranging from being nearly completely Ni2+-sensitive to completely TTX-sensitive. The average blockade of the total current by each drug in the eight cells studied was around 50 %. An example of the dose-response curves for the blockade of the T-like ICa by TTX and subsequently by Ni2+ in the presence of 30 μm TTX is shown in Fig. 5C. It clearly demonstrates the separation of the total current into a TTX- and a Ni2+-sensitive component. The approximate estimations of KD values for TTX and Ni2+ blockade of the T-like ICa are 1.0 ± 0.1 μm (n = 3) and 9.6 μm (n = 2), respectively. Thus, the T-like current in endocardial cells seems to comprise two components: ICa(TTX) and a T-type ICa.
Figure 5. Pharmacological properties of the endocardial T-like current.

A, the T-like current in an endocardial cell recorded in the presence of 2 mm and 0 mm extracellular Ca2+. B, blockade of the T-like current by Ni2+, TTX and Ni2+ plus TTX. The test potential was −40 mV and the holding potential was −80 mV for both A and B. C, dose-dependent blockade of the T-like current in an endocardial cell by TTX and then by Ni2+ in the presence of 30 μm TTX. Data points were fitted with the Hill equation with KD= 1.0 and 11.1 μm and maximum blockade of 70 and 30 %, respectively, for the two curves.
The L-type ICa had the pharmacological properties of a classic L-type current. It was also strongly dependent on extracellular Ca2+, and was blocked by 1 μm nitrendipine (data not shown).
Other aspects of Ca2+ channel kinetic properties
The time course of inactivation of the L-type and the T-like channels can be described by a single exponential decay (Fig. 6A). Inactivation of the T-like channel was rapid, and the rate of inactivation was strongly voltage-dependent (Fig. 6B). The time constants for inactivation were 23.3 ± 2.4, 9.2 ± 0.7 and 7.2 ± 0.7 ms at −50, −30 and −20 mV, respectively (n = 9). Although it is possible that the two components of the T-like current have different inactivation rates, the overall amplitude of the T-like current was relatively small, and below the amplitude necessary for reliable determination of any potential differences in the inactivation rates of the two components. The inactivation rates of the L-type channels were generally similar for epi- and endocardial cells, and were slower compared with those of the T-like current (Fig. 6B).
Figure 6. Inactivation properties of the L-type and T-like Ca2+ currents.

A, inactivation of the L-type current measured at 0 mV and of the T-like current measured at −30 mV. Current traces were fitted with a single exponential function. B, time constant of inactivation of the endocardial T-like current, endocardial L-type current and epicardial L-type current at various voltages. Data points are averages from nine, eight and eight cells, respectively. Error bars are ±s.e.m.
The kinetics of recovery from inactivation of the ICa channels were studied using a standard double-pulse protocol, and representative results for the endocardial L-type and the T-like currents are shown in Fig. 7A and C. Recovery of the T-like current was relatively slow at −80 mV, and the time course of recovery followed a single exponential function with a time constant of 150 ± 11 ms (n = 5, Fig. 7D). By comparison, the L-type channels had faster recovery kinetics. The time constants at −80 mV for epi- and endocardial cells were 43 ± 2 and 38 ± 3 ms, respectively (n = 4 for both groups, P > 0.2, Fig. 7B).
Figure 7. Recovery kinetics of the ventricular Ca2+ currents.
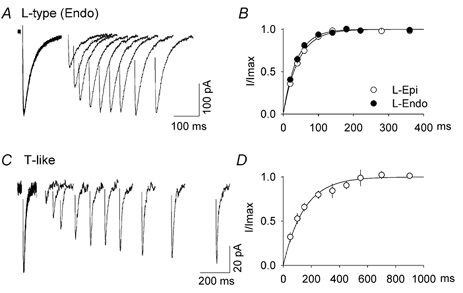
Recovery from inactivation of the L-type and T-like currents recorded from endocardial cells are shown in A and C, respectively. The first depolarizing step (to −40 mV for 100 ms for the T-like current and to +10 mV for 200 ms for the L-type current) completely inactivated the current. The recovery potential was −80 mV, followed by a second identical step at various time intervals. The time courses of recovery at −80 mV for the L-type current in epi- and endocardial cells are shown in B, and that for the T-like current in D. Data points are averages from four cells for all groups, and were fitted with the equation I/Imax= 1 − exp(−t/τ), where τ= 150, 38 and 43 ms for the endocardial T-like, endocardial L-type and epicardial L-type currents, respectively. Error bars are ±s.e.m.
DISCUSSION
It is well known that the electrical properties of ventricular cardiac myocytes are not homogeneous. The action potential in endocardium is longer than that in epicardium and does not possess a rapid repolarization phase following the initial upstroke that is characteristic of epicardium (Litovsky & Antzelevitch, 1988; Kimura et al. 1990; Nabauer et al. 1996; Rodriguez-Sinovas, 1997). Previous studies have shown that there is a larger Na+–K+ pump current density in epicardium than endocardium (Gao et al. 1998), and in the absence of other compensatory changes this should result in a lower internal Na+ and Ca2+ concentration. It was these initial studies that prompted us to investigate possible differences in the ion channels governing calcium entry. In the studies presented above we demonstrated a larger L-type calcium current density in endocardial than epicardial myocytes and the presence of a T-like calcium current in endocardial but not in epicardial myocytes in canine left ventricle. The gradient in the L-type calcium current is consistent with the other known functional differences since the larger inward current density should help generate the longer endocardial action potential.
The T-like calcium current in endocardial cells was about half blocked by either TTX or Ni2+, and completely eliminated by both. These results suggest that two populations of low-threshold calcium channels, ICa,T and ICa(TTX), co-exist in canine endocardial myocytes. A similar result has been described in guinea-pig ventricular myocytes (Heubach et al. 2000). The functional relevance of the T-like calcium current in endocardial cells is more obscure. Possibly the earlier entry of calcium provided by this current triggers the release of a larger fraction of calcium stores, leading to a stronger contraction. Measurements of intracellular Ca2+ concentration during action potentials in endocardial and epicardial cells would be the first step towards testing this hypothesis.
The L- and T-type calcium currents in canine heart have been studied previously. Tseng & Boyden (1989) investigated the properties of Purkinje and ventricular myocytes in canine heart. They found a larger L-type calcium current in ventricular myocytes than Purkinje myocytes, consistent with a larger role of ventricular myocytes in the contractile process. A T-type calcium current was observed in all Purkinje myocytes with a peak density of 60 % of the L-type calcium current, while it was present in only 2/3 of ventricular myocytes with a density of 20 % of the L-type calcium current in that preparation. Their dissociation procedure (which was a modification of the one used by Powell & Twist, 1976) was likely to sample endocardial myocytes at a higher rate than those from epicardium. Our results provide additional insight into the gradient in calcium current in the ventricle. We found the L-type calcium current to be larger in endocardial than epicardial myocytes and the T-like calcium current to be present in all endocardial myocytes with a peak density of about 15 % of the L-type current, while it was present in only 20 % of epicardial myocytes with a peak density of 6 % of the L-type current. Thus taken together the L-type calcium current is highest in endocardium and declines when one proceeds more proximal or more distal in the ventricular conduction pathway, while the prevalence of the T-type current declines as one proceeds more distal than the endocardium, and the density progressively declines from Purkinje to endocardium to epicardium. It will be important in future studies to add midmyocardial cells to complete the description of the calcium current gradient across the ventricular wall.
Recently another study reported the absence of a transmural gradient in L-type calcium currents across the wall of the canine left ventricle (Li et al. 2002). Although we are uncertain as to the reasons for this discrepancy, a number of experimental differences exist between the two studies. First, in the dissociation, we removed chunks of tissue from the region of interest and then exposed to enzymes, while a perfusion method was used in Li et al.'s study. Second, the recording temperature was 29 °C in our studies and 22 °C in theirs. Finally, it is worth pointing out that differences also exist between our previous studies of the transmural properties of Ito (Rosati et al. 2001) and those of other investigators (Liu et al. 1993), and the results of Li et al. In our studies and those of Liu et al. a 6–7-fold higher current level for Ito in canine epicardium than in endocardium was described, while Li et al. report a difference of only about a factor of two between these same two tissues. Therefore, the transmural electrical gradient, at least as measured by the gradient in Ito, seems less steep in the study by Li et al. Further experiments will be required to determine the reasons for these apparently inconsistent results.
Our previous studies (Yu et al. 2000) have demonstrated that the transmural gradient in Ito electrical properties can be reversed by incubating endocardial myocytes with an angiotensin type 1 receptor blocker (losartan) and epicardial myocytes with angiotensin II. At that time it was not possible to test the generality of this regulatory mechanism because only Ito was demonstrated to have a definable gradient in the canine heart. Similar future studies on the L- and T-like calcium current should tell us whether this regulation is channel-specific or represents a more general mechanism for maintaining the transmural gradient in electrical properties.
Although the density of the L-type current differed in endocardial and epicardial myocytes, gating properties of the current, including the I−V relation (Fig. 1D), the voltage dependence of inactivation (Fig. 4C), the kinetics of inactivation (Fig. 6B), and the recovery from inactivation (Fig. 7D) are all nearly identical, suggesting that the L-type channels in epicardium and endocardium are of the same type. The molecular understanding of the L-type calcium channel is complex and still being explicitly determined. For example in young adult rat heart age 4–6 weeks, the L-type channel is composed of α1c, β and α2δ subunits. In this system, expression of recombinant β subunits alone enhanced calcium channel current density, suggesting that β subunits may be limiting in expression of calcium channels (Wei et al. 2000). Thus one hypothesis among many for the transmural gradient in canine heart is an elevated concentration of β subunits in endocardium. This hypothesis is attractive because the gating properties of the L-type calcium current do not differ in endocardial and epicardial myocytes. Investigation of this possibility must await a detailed molecular characterization of the L-type calcium channel in the canine heart.
In summary, we have presented evidence demonstrating for the first time, a gradient in L- and T-like calcium currents across the canine left ventricular wall. How this gradient is generated and the role these calcium current differences play in generating the observed dispersion of action potential durations awaits future investigation.
Acknowledgments
We gratefully acknowledge Ms Joan Zuckerman for her skillful technical assistance and Drs Michael Rosen and David McKinnon for comments on the manuscript. This work was supported by a Scientist Development Grant from the American Heart Association to H.S.W. and grants HL20558, HL28958 and HL67101from the NHLBI to I.S.C.
REFERENCES
- Aggarwal R, Shorofsky SR, Goldman L, Balke CW. Tetrodotoxin-blockable calcium currents in rat ventricular myocytes; a third type of cardiac cell sodium current. J Physiol. 1997;505:353–369. doi: 10.1111/j.1469-7793.1997.353bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C, Fish J. Electrical heterogeneity within the ventricular wall. Basic Res Cardiol. 2001;96:517–527. doi: 10.1007/s003950170002. [DOI] [PubMed] [Google Scholar]
- Balke CW, Rose WC, Marban E, Wier WG. Macroscopic and unitary properties of physiological ion flux through T-type Ca2+ channels in guinea-pig heart cells. J Physiol. 1992;456:247–265. doi: 10.1113/jphysiol.1992.sp019335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant SM, Wan X, Shipsey SJ, Hart G. Regional differences in the delayed rectifier current (IKr and IKs) contribute to the differences in action potential duration in basal left ventricular myocytes in guinea-pig. Cardiovasc Res. 1998;40:322–331. doi: 10.1016/s0008-6363(98)00133-3. [DOI] [PubMed] [Google Scholar]
- Burgess MJ. Relation of ventricular repolarization to electrocardiographic T wave-form and arrhythmia vulnerability. Am J Physiol. 1979;236:H391–402. doi: 10.1152/ajpheart.1979.236.3.H391. [DOI] [PubMed] [Google Scholar]
- Chen-Izu Y, Sha Q, Shorofsky SR, Robinson SW, Wier WG, Goldman L, Balke CW. ICa(TTX) channels are distinct from those generating the classical cardiac Na+ current. Biophys J. 2001;81:2647–2659. doi: 10.1016/s0006-3495(01)75908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RB, Bouchard RA, Salinas-Stefanon E, Sanchez-Chapula J, Giles WR. Heterogeneity of action potential waveforms and potassium currents in rat ventricle. Cardiovasc Res. 1993;27:1795–1799. doi: 10.1093/cvr/27.10.1795. [DOI] [PubMed] [Google Scholar]
- Cole WC, Chartier D, Martin M, Leblanc N. Ca2+ permeation through Na+ channels in guinea pig ventricular myocytes. Am J Physiol. 1997;273:H128–137. doi: 10.1152/ajpheart.1997.273.1.H128. [DOI] [PubMed] [Google Scholar]
- Ellingsen O, Davidoff AJ, Prasad SK, Berger HJ, Springhorn JP, Marsh JD, Kelly RA, Smith TW. Adult rat ventricular myocytes cultured in defined medium: phenotype and electromechanical function. Am J Physiol. 1993;265:H747–754. doi: 10.1152/ajpheart.1993.265.2.H747. [DOI] [PubMed] [Google Scholar]
- Fedida D, Giles WR. Regional variations in action-potentials and transient outward current in myocytes isolated from rabbit left-ventricle. J Physiol. 1991;442:191–209. doi: 10.1113/jphysiol.1991.sp018789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Myerburg RJ, Furukawa N, Bassett AL, Kimura S. Differences in transient outward currents of feline endocardial and epicardial myocytes. Circ Res. 1990;67:1287–1291. doi: 10.1161/01.res.67.5.1287. [DOI] [PubMed] [Google Scholar]
- Gao J, Cohen IS, Mathias RT, Baldo GJ. Na+ and Ca2+ transport in epicardial and endocardial ventricular myocytes. Biophys J. 1998;74:A160. [Google Scholar]
- Gintant GA, Datyner NB, Cohen IS. Gating of delayed rectification in acutely isolated canine cardiac Purkinje myocytes. Evidence for a single voltage-gated conductance. Biophys J. 1985;48:1059–1064. doi: 10.1016/S0006-3495(85)83869-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N, Irisawa H, Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heubach JF, Kohler A, Wettwer E, Ravens U. T-Type and tetrodotoxin-sensitive Ca2+ currents coexist in guinea pig ventricular myocytes and are both blocked by mibefradil. Circ Res. 2000;86:628–635. doi: 10.1161/01.res.86.6.628. [DOI] [PubMed] [Google Scholar]
- Kimura S, Bassett AL, Furukawa T, Cuevas J, Myerburg RJ. Electrophysiological properties and responses to simulated ischemia in cat ventricular myocytes of endocardial and epicardial origin. Circ Res. 1990;66:469–477. doi: 10.1161/01.res.66.2.469. [DOI] [PubMed] [Google Scholar]
- Lemaire S, Piot C, Seguin J, Nargeot J, Richard S. Tetrodotoxin-sensitive Ca2+ and Ba2+ currents in human atrial cells. Receptors Channels. 1995;3:71–81. [PubMed] [Google Scholar]
- Li GR, Lau CP, Ducharme A, Tardif JC, Nattel S. Transmural action potential and ionic current remodeling in ventricles of failing canine hearts. Am J Physiol Heart Circ Physiol. 2002;283:H1031–1041. doi: 10.1152/ajpheart.00105.2002. [DOI] [PubMed] [Google Scholar]
- Litovsky SH, Antzelevitch C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res. 1988;62:116–126. doi: 10.1161/01.res.62.1.116. [DOI] [PubMed] [Google Scholar]
- Liu DW, Gintant GA, Antzelevitch C. Ionic bases for electrophysiological distinctions among epicardial, mid-myocardial, and endocardial myocytes from the free wall of the canine left-ventricle. Circ Res. 1993;72:671–687. doi: 10.1161/01.res.72.3.671. [DOI] [PubMed] [Google Scholar]
- Mitra R, Morad M. Two types of calcium channels in guinea pig ventricular myocytes. Proc Natl Acad Sci U S A. 1986;83:5340–5344. doi: 10.1073/pnas.83.14.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabauer M, Beuckelmann DJ, Uberfuhr P, Steinbeck G. Regional differences in current density and rate-dependent properties of the transient outward current in subepicardial and subendocardial myocytes of human left ventricle. Circulation. 1996;93:168–177. doi: 10.1161/01.cir.93.1.168. [DOI] [PubMed] [Google Scholar]
- Powell T, Twist VW. A rapid technique for the isolation and purification of acult cardiac cells having respiratory control and tolerance to calcium. Biochem Biophys Res Commun. 1976;72:327–333. doi: 10.1016/0006-291x(76)90997-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sinovas A, Cinca J, Tapias A, Armadans L, Tresanchez M, Soler-Soler J. Lack of evidence of M-cells in porcine left ventricular myocardium. Cardiovasc Res. 1997;33:307–313. doi: 10.1016/s0008-6363(96)00205-2. [DOI] [PubMed] [Google Scholar]
- Rosati B, Pan Z, Lypen S, Wang HS, Cohen I, Dixon JE, McKinnon D. Regulation of KChIP2 potassium channel beta subunit gene expression underlies the gradient of transient outward current in canine and human ventricle. J Physiol. 2001;533:119–125. doi: 10.1111/j.1469-7793.2001.0119b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng GN, Boyden PA. Multiple types of Ca2+ currents in single canine Purkinje cells. Circ Res. 1989;65:1735–1750. doi: 10.1161/01.res.65.6.1735. [DOI] [PubMed] [Google Scholar]
- Wei SK, Colecraft HM, Demaria CD, Peterson BZ, Zhang R, Kohout TA, Rogers TB, Yue DT. Ca2+ channel modulation by recombinant auxillary beta subunits expressed in young adult heart cells. Circ Res. 2000;86:175–184. doi: 10.1161/01.res.86.2.175. [DOI] [PubMed] [Google Scholar]
- Wettwer E, Amos GJ, Posival H, Ravens U. Transient outward current in human ventricular myocytes of subepicardial and subendocardial origin. Circ Res. 1994;75:473–482. doi: 10.1161/01.res.75.3.473. [DOI] [PubMed] [Google Scholar]
- Yu H, Gao J, Wang HS, Wymore R, Steinberg S, McKinnon D, Rosen MR, Cohen IS. Effects of the rennin-angiotensin system on the current Ito in epicardial and endocardial ventricular myocytes from the canine heart. Circ Res. 2000;86:1062–1068. doi: 10.1161/01.res.86.10.1062. [DOI] [PubMed] [Google Scholar]


