SUMMARY
The frontal eye field (FEF) participates in selecting the location of behaviorally relevant stimuli for guiding attention and eye movements. We simultaneously recorded local field potentials (LFPs) and spiking activity in the FEF of monkeys performing memory-guided saccade and covert visual search tasks. We compared visual latencies and the time course of spatially selective responses in LFPs and spiking activity. Consistent with the view that LFPs represent synaptic input, visual responses appeared first in the LFPs followed by visual responses in the spiking activity. However, spatially selective activity identifying the location of the target in the visual search array appeared in the spikes about 30 ms before it appeared in the LFPs. Because LFPs reflect dendritic input and spikes measure neuronal output in a local brain region, this temporal relationship suggests that spatial selection necessary for attention and eye movements is computed locally in FEF from non-spatially selective inputs.
Keywords: vision, attention, monkey, physiology, evoked potentials, action potentials
INTRODUCTION
Visual spatial selection describes the process that guides visual attention (Serences and Yantis, 2006) and selectively couples perception to action (Allport, 1987). Understanding the time course of this process is key to understanding the neural computations that underlie it. Typically, this question has been addressed by analyzing event-related brain potentials (ERPs) recorded from scalp electrodes in humans (Hillyard and Anllo-Vento, 1998; Luck et al., 2000) and neuronal spiking activity in behaving primates (Schall and Thompson, 1999). In visual search studies, in which subjects are required to discriminate a target among distractors, human ERPs (Luck and Hillyard, 1994) and single units recorded in primate frontal eye field (FEF) (Sato et al., 2001; Thompson et al., 1996), lateral intraparietal area (Ipata et al., 2006; Thomas and Pare, 2007) and superior colliculus (McPeek and Keller, 2002) exhibit an initial period of non-selective activation followed by a discrimination process that identifies the location of the target in the search array.
Local field potentials (LFPs) are electrical potentials recorded with an electrode positioned in the brain. The LFP signal represents the summed synaptic activity occurring near the tip of the electrode. It is a combined measure of local processing and synaptic inputs from other brain regions regardless of whether or not spikes are generated (Chen et al., 2007; Cruikshank et al., 2002; Juergens et al., 1999; Kaur et al., 2004; Kreiman et al., 2006; Logothetis and Wandell, 2004; Mitzdorf, 1985; Mitzdorf, 1987; Nielsen et al., 2006). In contrast, spiking activity represents the results of local neural processing and is the output signal from the neurons near the tip of the electrode. Although both LFPs and spiking activity have been used to measure the time course of spatial attention processes, the relationship between these neurophysiological signals is still unclear. Analysis of concurrently recorded LFP and spiking activity can shed light on how sensory representations in dendritic input are transformed into cognitive signals (Kreiman et al., 2006; Nielsen et al., 2006).
The FEF is a brain area in monkeys and humans that participates in the visual spatial selection process (Awh et al., 2006; Pessoa et al., 2003; Schall and Thompson, 1999; Serences and Yantis, 2007). The spatial selection process localizes behaviorally important objects in a complex visual scene and is necessary for guiding visual attention and goal directed behaviors. In a previous report we showed that spiking activity in monkey FEF reflects the locus of spatial attention during covert visual search tasks in the absence of eye movements (Thompson et al., 2005b). During the collection of these neuronal spiking data, LFPs were also recorded simultaneously from the same electrodes. The goals of this study were to determine whether LFP responses were spatially selective, and if so, to compare the time course and spatial tuning of the spatially selective signals in neuronal spiking activity and LFP responses.
We found that in the covert visual search task both the LFPs and the spiking activity exhibited initial non-selective visual responses that evolved into significant spatial tuning in the time period before the monkeys’ behavioral report. The directional tuning of the spatially selective responses in the visual search task matched the directional tuning of the visually evoked responses to a single visual stimulus in the memory-guided saccade task. Although the initial visual responses appeared first in the LFP signals in both tasks, the spatially selective responses in the visual search task appeared first in the spiking activity. These results suggest that during visual search, spatial selectivity is generated in FEF from non-spatially selective inputs. Some of these results have appeared in abstract form (Monosov et al., 2006).
RESULTS
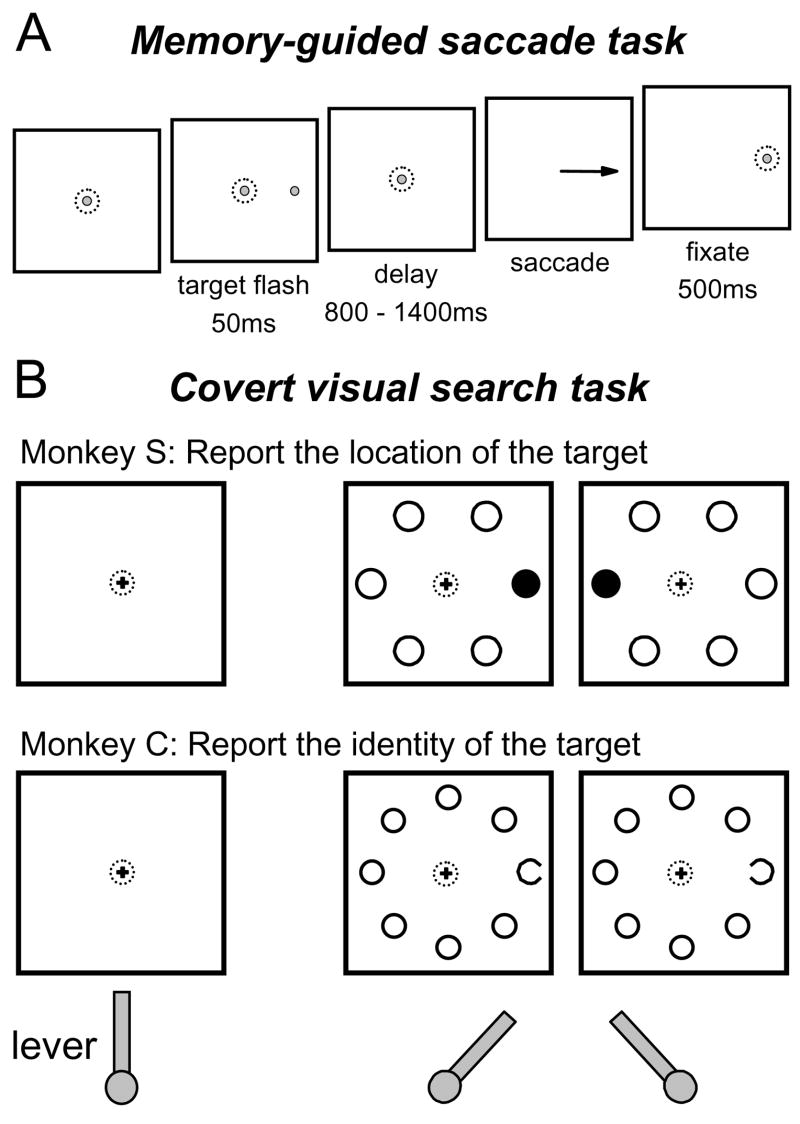
Spiking activity and LFP responses were recorded concurrently on single electrodes inserted into the FEF of two monkeys in 43 separate recording sessions. The monkeys performed a memory-guided saccade task (Figure 1A) and one of two covert visual search tasks (Figure 1B). In the covert visual search tasks the monkeys made a manual lever turn as the behavioral report. Monkey S was required to report the location of the singleton target in the search array (20 recording sites) and monkey C was required to report the orientation of the C among Os in the search array (23 recording sites). Single neuron activity recorded with this task was described previously (Thompson et al., 2005b). For this study we combined the activity from simultaneously recorded single neurons into a single representation of spiking activity at each recording site. The primary aim of this study was to compare the times that a spatially selective response first appeared in the LFPs and spikes in the covert visual search task. We refer to this time as the selection time. For the data collected at a recording site to be included in the study, there must have been measurable visual response onset latencies in both the LFPs and spikes, and a measurable selection time in the visual search task for either the LFP response or the spiking activity. In addition, the visual response latencies and selection times must have occurred before the average reaction time of the session. Over all sessions, lever turn reaction times averaged 284 ms for monkey S and 297 ms for monkey C.
Figure 1. The tasks.
(A) The memory-guided saccade task. After the monkey fixated on a central spot, a peripheral stimulus identical to the fixation spot was flashed for 50 ms randomly at one of the six or eight locations matching the stimulus locations in the covert visual search task. After a delay, the fixation spot disappeared, and the monkey was rewarded for making a saccade to the remembered target location.
(B) The covert visual search tasks. After the monkey grasped the lever in the vertical position, a small fixation cross appeared. After fixating the central cross, a search array appeared in which one of the stimuli was different. Monkey S was rewarded for turning the lever in the same direction as a different-colored stimulus in relation to the fixation cross. Monkey C was rewarded for turning the lever in the same direction as the gap in the C target stimulus regardless of its location in the search array. The depiction of the lever at the bottom shows the correct behavioral responses for the example trials shown in the search displays.
There were strong correlations between the directional tuning of the spatially selective responses in the LFPs and spikes within and across the visual search task and the memory-guided saccade task, which is consistent with a functional relationship between the LFPs and spikes (see Figure 8 below). But first we describe the results of the time course analysis which is blind to the preferred target directions of the two signals.
Figure 8. Comparisons of spatial tuning in spiking activity and LFP responses recorded in the memory-guided saccade and covert visual search tasks.
(A–D) The distributions of the differences in the preferred target directions measured from the spiking activity and LFP responses for the recording sites that exhibited significant spatial tuning in the spiking activity. LFP responses exhibited significant spatial tuning at all 43 recording sites in both the memory-guided saccade and covert visual search tasks. Angle differences can range from −180° to +180°. All of the distributions are peaked near 0° (Rayleigh test; p < 0.001) A circular correlation analysis (Mardia and Jupp, 2000) showed that the preferred target directions are significantly correlated between:
(A) LFPs and spikes recorded in the memory-guided saccade task (N = 42; p < 10−9).
(B) LFPs and spikes recorded in the covert visual search tasks (N = 38; p = 0.001).
(C) Spikes recorded in the memory-guided saccade task and spikes recorded in the covert visual search tasks (N = 37; p < 10−8).
(D) LFPs recorded in the memory-guided saccade task and LFPs recorded in the covert visual search tasks (N = 43; p = 0.001).
(E) The distribution of tuning widths of the LFP (open bars) and spike (filled bars) responses in the memory-guided saccade task. Tuning width was defined as the standard deviation (Tϕ) parameter of the best fit Gaussian curves. The average response field width is 38.7 ± 3.7° for spiking activity, and 64.0 ± 3.0° for LFP responses; and the two distributions differ significantly (paired t-test; p < 10−7).
(F) The distribution of tuning widths of the LFP and spike responses in the covert visual search tasks. The average response field width is 26.5 ± 2.6° for spiking activity, and 44.7 ± 3.3° for LFP responses (paired t-test; p < 10−4).
Visual response latencies and spatial selection times of LFPs and spikes
The spiking activity and LFP signals recorded simultaneously at each recording site were analyzed using the same methods to obtain the visual response onset latencies and the time of spatial selection measured from the time of search array presentation. Figures 2, 3 and 4 illustrate the analysis applied to the data collected from a single recording site in monkey S (see EXPERIMENTAL PROCEDURES for details). Briefly, selection time was defined as the first time following visual stimulus presentation that the response differed significantly across target locations based on an ANOVA at each millisecond (Figures 2 and 3). In the memory-guided saccade task, spikes and LFPs exhibited initial responses that differed across target location. Therefore, for the memory-guided saccade task, selection time measures the initial visual response latency to a single stimulus. In the visual search task, however, a visual stimulus appears at all location on every trial and the initial responses of spikes and LFPs did not vary with target position. Selection time in the visual search task, therefore, measures the first time that the response differentiates the target stimulus from the distractors. To get a measure of the visual response latency in the visual search task, we defined the visual latency as the first time following the visual search array presentation that the combined response across all trials differed from baseline (Figure 4).
Figure 2. Spatial tuning analysis of spiking activity recorded at the same site as the LFP shown in Figure 3.
(A) Spike density functions, derived from a filter resembling an EPSP, are plotted above tick marks representing times of action potentials for three representative trials.
(B) The average target-aligned spiking activity at each target location from the memory-guided saccade task (gray) and the covert visual search task (black). The box-whisker plot in each panel indicates the median, quartiles and range of reaction times in the covert visual search task. The neuron’s preferred target direction (60°) corresponds to the filled circle in the search array at the center.
(C) The superimposed average activity for each target position from the memory-guided saccade task (left) and the visual search task (right). The thick line represents the average activity on trials when the target was at the preferred spatial location.
(D) The p-value (ANOVA) at each millisecond in the memory-guided saccade task (left) and in the visual search task (right) that estimates the probability that the spiking activity did not vary across target locations. The black triangle at the bottom of the plot marks the selection time (vertical dotted line: memory-guided = 70 ms, visual search = 128 ms;) which was defined as the first millisecond that the p-value crossed p = 0.05 (horizontal dotted line), only if it continued past p = 0 001 and p < 0.05 for more than 20 of the next 25 milliseconds.
(E) The spatially selective response measured from 50 – 300 ms following the target flash in the memory-guided saccade task (left), and from 100 – 300 ms following the time of search array presentation (right) as a function of target direction. The time ranges for measuring spatial tuning are indicated by black bars in (C). The parameters of the best fit Gaussian curve from the memory guided saccade task (left) are B = 40.26 sp/s, R = 36.54 sp/s, Φ = 64.42°, and Tϕ = 38.25°; and from the covert visual search task (right) are B = 45.75 sp/s, R = 55.36 sp/s, Φ = 63.22°, and Tϕ = 45.02°.
Figure 3. Spatial tuning analysis of the LFP response recorded at the same site as the spiking activity shown in Figure 2. Conventions are the same as in Figure 2.
(A) The LFP responses on three representative visual search trials.
(B) The average target-aligned LFP response in the memory-guided saccade task (gray) and in the covert visual search task (black) sorted by target location
(C) The superimposed average LFP response for each target position from the memory guided saccade task (left) and the covert visual search task (right).
(D) The p-value (ANOVA) at each millisecond that estimates the probability that the LFP response did not vary across target locations. The selection time of the LFP response at this recording site is 69 ms for the memory-guided saccade task and 142 ms for the visual search task.
(E) The spatially selective response measured from 100 – 200 ms following the target flash in the memory-guided saccade task (left), and from 180 – 300 ms following the time of search array presentation (right) as a function of target direction. The time interval used for determining the spatial tuning of the LFP response was the interval that exhibited the most variability in the ANOVA analysis shown in D (see Supplemental Data and Figure S1). The parameters of the best fit Gaussian curve from the memory guided saccade task (left) are B = −4.59, R = −33.14, Φ = 43.05°, and Tϕ = 59.24°; and from the covert visual search task (right) are B = 19.59, R = −16.33, Φ = 64.05°, and Tϕ = 62.91°.
Figure 4. Visual response latency analysis of the spike (left) and LFP (right) responses recorded during the covert visual search task. The activity is from the same recording session as shown in Figures 2 and 3.

(A) The average spike density function constructed by convolving each spike with a kernel that resembles an EPSP and averaging across all trials.
(B) The p-value (paired t-test) at each millisecond that estimates the probability that the spiking activity is equal to the baseline activity (measured from −50 to 0 ms). The visual response latency was defined as the first time that the p-value crossed p = 0.01 (horizontal dotted lines), only if it continued past p = 0 001 and p < 0.01 for more than 20 of the next 25 milliseconds. The spiking visual response latency in the covert visual search task at this recording site is 63 ms (black triangles and vertical dotted lines).
(C) The average LFP signal across all trials.
(D) The p-value (paired t-test) at each millisecond that estimates the probability that the LFP signal is equal to the baseline signal (measured from −50 to 0 ms). The LFP visual response latency is 53 ms.
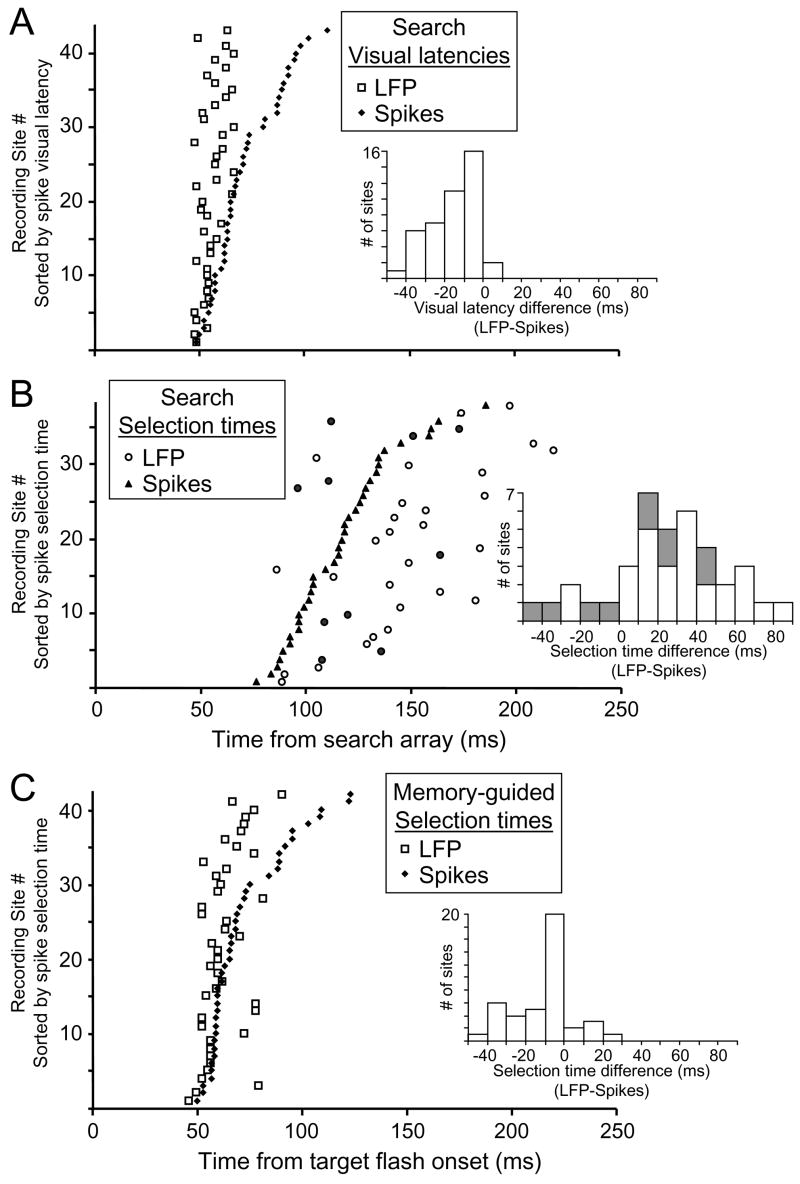
Even though visual response latencies were measured using different visual stimuli and measurement methods in the memory-guided saccade and visual search tasks, the temporal relationship between initial visual response latencies measured in LFPs and spikes was the same across the two tasks. The initial visual response occurred earlier in the LFPs than in the spikes. For the memory-guided saccade task, the average ± SE selection time was 63.4 ± 3.2 ms for LFPs, and 72.8 ± 4.3 ms for spikes (paired t-test: p < 0.001). For the visual search task, the average ± SE onset latency was 56.5 ± 2.4 ms for LFPs, and 71.8 ± 4.0 ms for spikes (p < 0.001). There were also strong correlations between the selection times obtained from the memory-guided saccade task and the visual response onset latencies obtained from the visual search task at each recording site (LFPs: r = 0.48, p = 0.001; spikes: r = 0.78, p < 0.001). Because we were interested comparing visual onset times to spatial selection times in visual search, in this study we will focus mostly on results obtained in the visual search tasks.
Cumulative distributions of onset latencies and selection times measured in the visual search task are shown separately for the two monkeys in Figure 5A and B. Visual response latencies were obtained for the spiking activity and the LFP response from all 43 recording sites. For spiking activity, the average ± SE onset latency was 68.4 ± 3.5 ms for monkey S, and 74.7 ± 3.3 ms for monkey C. For the LFP response, the average ± SE onset latency was 53.6 ± 1.0 ms for monkey S, and 59.0 ± 0.9 ms for monkey C. An ANOVA that factored the monkey and response measure revealed a significant difference in response latencies between the 2 monkeys (p = 0.02), and between spiking activity and LFP response (p < 0.001) with no interaction between monkey and activity measure (p = 0.86).
Figure 5. Population results from the covert visual search tasks shown separately for the two monkeys.

(A) Cumulative distributions of visual response latencies and spatial selection times for all recording sites in monkeys S performing the ‘location’ version of the covert visual search task. The average ± SE times from left to right were 53.6 ± 1.0 ms for LFP visual latencies (thin dotted line; median = 53.5), 68.4 ± 3.5 ms for spike visual latencies (thin solid line; median = 65), 124.6 ± 5.1 ms for spike selection times (thick solid line; median = 119), and 155.2 ± 6.3 ms for LFP selection times (thick dotted line; median = 152.5).
(B) The same as (A) but for monkey C performing the ‘identity’ version of the covert visual search task. The average ± SE times from left to right were 59.0 ± 0.9 ms for LFP visual latencies (median = 59 ms), 74.7 ± 3.3 ms for spike visual latencies (median = 72 ms), 113.0 ± 6.2 ms for spike selection times (median = 102.5 ms), and 133.3 ± 7.1 ms for LFP selection times (median = 129 ms).
(C and D) The percentage of recording sites showing significant modulation at each millisecond following the presentation of the search array in the monkey S (C), and monkey C (D). The plots were smoothed using a running window of 5 ms for easier viewing. The line types correspond to those in A and B.
Selection times in the visual search task were obtained for spiking activity from 38 (88.4%) recording sites and for the LFP response from all 43 recording sites. For spiking activity, the average selection time was 124.6 ± 5.1 ms for monkey S, and 113.0 ± 6.2 ms for monkey C. For the LFP response, the average selection time was 155.2 ± 6.3 ms for monkey S, and 133.3 ± 7.1 ms for monkey C. An ANOVA revealed a significant difference in the selection times in the visual search task between the 2 monkeys (p = 0.01), and between spiking activity and LFP response (p < 0.001) with no interaction between monkey and the activity measure (p = 0.43).
The differences in visual response latencies and selection times between the two monkeys may be due to individual differences or to the different visual stimuli used in two different visual search tasks in the two monkeys. It has previously been shown that a search for a gap in a C among Os is very easy (Treisman and Gormican, 1988) and the visual system may be able to resolve a single gap in a circle faster than it can resolve a color difference in a search array. Nevertheless, the important result is the absence of significant interaction between monkeys performing different visual search tasks and the measured timing differences between LFPs and spikes. This means that in spite of the individual differences, the temporal relationships between LFPs and spikes were the same in the two monkeys.
To summarize the results across the population, we plotted the percentage of recording sites showing significant modulation at each millisecond following the presentation of the search array separately for monkey S (Figure 5C) and monkey C (Figure 5D). These continuous measures of significant modulation across the population are another way to visualize the timing differences across LFPs and spikes and validate the results obtained from the calculations of initial visual response onset latencies and spatial selection times. For both monkeys, significant visual responses are evident in the LFPs before the spikes and significant spatially selective responses are evident in the spikes before the LFPs. Because the relationships between spiking activity and LFP responses were the same for both monkeys, the data from the two monkeys are combined in the following analyses.
We compared the response latencies and selection times measured from the spiking activity and LFP responses recorded simultaneously at individual recording sites during the visual search task (Figure 6A and 6B). Significant positive correlations between spiking activity and LFP responses for onset latencies (r = 0.46; p = 0.002), and for selection times (r = 0.51, p = 0.001) support the claim that spiking activity and LFP responses are related. Spiking activity and LFP response onset latencies for each recording site are plotted in Figure 6A, and selection times are plotted in Figure 6B. In both plots, the times from each site are sorted according to the time measured in the spiking activity and a histogram shows the distribution of differences between the times obtained from the LFPs and spikes. For nearly all (41/43 = 95%) of the recording sites, the measured response onset latency was earlier in the LFP response than in the spiking activity. On average, the LFP visual response began 15.3 ± 2.2 ms earlier than the spike visual response. The visual latencies of the LFP responses varied less than the spike responses. As a consequence, the difference between visual onset latency measured in the spikes and in the LFP increased with increasing spike response latency. Nevertheless, even the recording sites with the earliest spike responses had LFP response latencies that were significantly earlier. For the quartile of recording sites with the earliest spike visual response (range: 48 – 60 ms), the LFP visual response began on average 2.3 ± 0.7 ms earlier than the spike visual response (paired t-test: p = 0.01). The earlier initial visual onsets in the LFP signal is consistent with the expected result that feedforward visual inputs in postsynaptic potentials precedes the visual evoked spiking activity (Schroeder et al., 1998).
Figure 6. Population results from the covert visual search task at each recording site combined across the two monkeys.
(A) Visual response latencies in the covert visual search task of the LFP responses (open squares) and spikes (filled diamonds) at each recording site sorted by the visual response latency of the spikes. LFP and spike visual response latencies were obtained from all 43 recording sites. The histogram shows the distribution of LFP visual response latency relative to spike visual response latency obtained across all recording sites (LFP – spikes; mean = −15 ± 2.2 ms). Similar results were obtained from the selection times measured in the memory-guided saccade task (see Figure 6C).
(B) Selection times in the covert visual search task of the LFP responses (open and filled circles) and spikes (filled triangles) at each recording site sorted by the selection time of the spikes. LFP and spike selection times were obtained from 38 recording sites. The histogram shows the distribution of LFP selection time relative to spike selection time obtained across all recording sites (LFP – spikes; mean = 24.7 ± 5.0 ms). The filled circles in the scatter plot and filled bars in the histogram represent the 10 recording sites that the spatial tuning of the LFP and spikes differed by more than 40° of visual angle (see Fig. 8B).
(C) Selection times in the memory-guided saccade task measured from the LFP responses (open squares) and spikes (filled diamonds) at each recording site sorted by the selection time of the spikes (N = 42). The histogram shows the distribution of LFP selection time relative to spike selection time across all recording sites (LFP – spikes; mean = −9.9 ± 2.5 ms). Compare to results in Figure 6A.
Selection times in the visual search tasks were obtained from all 43 sites for the LFP response and from 38 sites for spiking activity. For the 38 recording sites with selection times from both measures, selection times occurred later in the LFP response than in the spiking activity for 84% (32/38) of the recording sites and differed, on average, by 24.7 ± 5.0 ms (Figure 6B). However, at 10 recording sites, the spatial tuning of the LFP response and spiking activity differed by more than 40° of visual angle (see Figure 8B); these are indicated in Figure 6B by the filled circles in the scatter plot and shaded bars in the histogram. It is possible that at these recording sites the LFP response and spiking activity were less related to each other than at the sites in which the spatial tuning of the two signals corresponds. When these 10 sessions were removed from the analysis, the selection times occurred later in the LFP response than in the spiking activity at 93% (26/28) of the recording sites and differed, on average, by 31.5 ± 5.1 ms.
For comparison we also compared the selection times for LFPs and spiking activity in the memory-guided saccade task (Figure 6C). Selection time in the memory-guided saccade task measures visual response latency because it identifies the first time that the responses differed across target locations for a single visual stimulus presented alone. It corresponds to the visual response latency measured in the covert visual search task, and across the recording sites the two measures were strongly correlated for both spikes (Pearson’s r = 0.78, p < 0.001) and LFPs (r = 0.60, p < 0 001). Just like the visual response latencies measured in the visual search task (Figure 6A), the selection times measured in the memory-guided saccade task were earlier (9.9 ± 2.5 ms) for LFPs than for spikes (Figure 6C). The similarity in the results across the tasks and analysis methods adds to our confidence in the accuracy of our timing measurements (also see Supplemental Data).
Relationship of LFP visual response latency to selection times
Studies have shown that the earliest visual response latencies of LFPs recorded in dorsal stream areas of visual cortex are in cortical layer 4 which corresponds to the feedforward projection of visual inputs (Chen et al., 2007; Schroeder et al., 1998). We hypothesized that if the inputs to FEF from visual cortex were spatially selective, they would be evident first at the recording sites with the earliest LFP visual response latencies. Therefore, we examined whether LFP visual response latencies were related to times of spatial selection (Figure 7). It should be noted that this analysis does not establish the cortical layer of the recording sites, but it is motivated by the assumption that recording sites in FEF with earlier visually evoked LFP activity are functionally closer to the feedforward visual input from visual cortex. Surprisingly, this was not the case. To visualize the data we plotted how the spike visual response latencies and selection times, and LFP selection times changed with increasing LFP visual response latency (Figure 7). For statistical analysis, the recording sites were divided into two groups based on LFP visual response latency measured in the visual search task. The sites with LFP visual response latencies between 48 and 55 ms were assigned to the ‘early’ group (N = 22), and sites with latencies between 56 and 67 ms to the ‘late’ group (N=21). The large symbols in Figure 7 indicate the average ± SE of each group. The spike visual response latencies differed significantly across the ‘early’ (65.0 ± 3.3 ms) and ‘late’ groups (79.0 ± 2.9 ms) (t-test: p = 0.003). This is consistent with the result that LFP and spike visual latencies were positively correlated. LFP response selection times did not differ significantly between the ‘early’ (145.4 ± 6.9 ms) and ‘late’ (141.5 ± 7.6 ms) groups (p = 0.7). For the spiking activity, the selection times of the ‘early’ (109.5 ± 4.7 ms) and ‘late’ (126.3 ± 6.6 ms) groups were marginally different (p = 0.04). The surprising result was that the recording sites with the earliest LFP visual response latencies, and therefore functionally closest to with the feedforward visual input to FEF, exhibited the earliest spike selection times and the latest LFP selection times. The difference between spike and LFP selection times in the ‘early’ group was highly significant (paired t-test: p < 10−5). For the ‘late’ group, the difference between the LFP and spike selection times did not reach statistical significance (p = 0.07). We also divided the recording sessions into ‘early’ and ‘late’ groups based on the ‘selection times’ measured in the data collected from the memory-guided saccade task which were recorded in a separate block of trials in each session. Note that ‘selection time’ for the memory-guided saccade data is determined using the exact same analysis method as for visual search data but actually measures the visual onset latency to a single visual stimulus. The results were statistically identical to those shown in Figure 7.
Figure 7. The relationship of selection time in LFPs and spikes to LFP visual response latency.

The symbols representing the different times are the same as in Fig. 6. The visual response latencies and selection times across all recording sites are sorted by increasing LFP visual response latency. Each of the data points plot the average for a group of eight sorted recording sites. Consecutive data points represent the average of eight recording sites after shifting the averaging window by one. The statistical comparisons are shown at the top (large symbols). The averages ± SE of the response latencies and selection times are plotted after dividing the recording sites into 2 groups based on LFP visual response latency. The recording sites with LFP visual response latencies between 48–55 ms were assigned to the ‘early’ group (N = 22; LFP visual response latencies = 51.7 ± 0.5 ms; spike visual response latencies = 65.0 ± 3.3 ms; spike selection times = 109.5 ± 4.7 ms; LFP selection times = 145.4 ± 6.9 ms), and recording sites with visual response latencies between 56–67 ms to the ‘late’ group (N=21; LFP visual response latencies = 61.5 ± 0.8 ms; spike visual response latencies = 79.0 ± 2.9 ms; spike selection times = 126.3 ± 6.6 ms; LFP selection times = 141.5 ± 7.6 ms).
Comparison of directional tuning
The variation of spatially selective LFP and spiking responses with target direction in the memory-guided saccade and visual search tasks was characterized with Gaussian functions (Figures 2E and 3E). The spatial parameters of the best-fit Gaussian curves provide estimates of the preferred direction and spatial extent of the LFP and spiking response fields. Details of the spatial tuning analysis are provided in the EXPERIMENTAL PROCEDURES. There were no differences in the directional tuning measures between the two monkeys. The preferred direction was provided by the optimum direction (Φ) parameter. The preferred tuning directions of the spiking activity and LFP responses in the memory-guided saccade and visual search tasks were compared by taking the angle difference between the two measures. Angle differences can range from −180° to +180°. Figure 8 shows the distributions of angle differences between the preferred target directions of the LFPs and spikes for the memory-guided saccade and visual search tasks (Figures 8A and B), and between the preferred target directions obtained from the memory-guide saccade and visual search tasks for spikes and LFPs (Figures 8C and D). All the distributions are peaked near 0° (Rayleigh test; p < 0.001). An analysis that measures the correlation between two circular variables (Mardia and Jupp, 2000) showed that there were strong correlations between the preferred directions obtained from LFPs and spikes in the memory-guided saccade task (Figure 8A; p < 10−9) and the visual search task (Figure 8B; p = 0.001). There were also strong correlations between the preferred directions obtained across the two tasks for both spikes (Figure 8C; p < 10−8) and LFPs (Figure 8D, p = 0.001). In summary, there were overall strong correlations between the directional tuning of the LFP and spike response fields across the memory-guided saccade task in which a visual stimulus is presented alone and the covert visual search tasks in which the target must be identified among distractors.
The tuning widths of the LFP and spike response fields were estimated by the standard deviation (Tϕ) parameter of the best fit Gaussian curves. The distributions of tuning widths (in polar angle coordinates) for the single visual stimulus in the memory-guided saccade task and for the target among distractors in the search tasks are shown in Figures 8E and 8F, respectively. For the memory guided saccade task, the average response field width is 38.7 ± 3.7° for spiking activity, and 64.0 ± 3.0° for LFP responses. For the visual search tasks, the average response field width is 26.5 ± 2.6° for spiking activity, and 44.7 ± 3.3° for LFP responses. The results of an ANOVA showed that LFP tuning widths were significantly larger than spike tuning widths (p < 0.001), and the tuning widths of responses in the memory-guided saccade task were significantly larger than in the visual search task (p < 0.001). When converted to visual field angles according to the law of cosines, the width of receptive fields for a single visual stimulus alone averaged 6.5 ± 0.4° for spikes and 10.4 ± 0.4° for LFPs; and receptive fields in the visual search task averaged 4.6 ± 0.4° for spikes and 7.4 ± 0.5° for LFPs. The sizes of receptive fields of the spiking activity and the narrower spatial tuning in the visual search task than for single targets are similar to previous reports that used comparable methods (Schall et al., 1995a; Schall et al., 2004).
DISCUSSION
We show for the first time that LFPs in FEF exhibit visually evoked responses that are spatially selective; they identify the location of a target presented alone in a memory-guided saccade task and identify the location of a behaviorally important stimulus during covert visual search in the absence of eye movements. We compared the LFP responses to the single unit activity recorded concurrently on the same electrodes (Thompson et al., 2005b). In the covert visual search task, both the LFPs and spikes exhibited a short latency non-spatially selective visual response followed by a selective response that identified the location of the behaviorally relevant stimulus that instructed the monkey to manually turn a lever to the left or right. The spatial selectivity for the behaviorally relevant target in the visual search task appeared in the spiking activity before the LFP response. This result is especially intriguing because it suggests that a cognitive representation identifying the location of behaviorally important visual stimuli is computed in the FEF from non-spatially selective inputs (Thompson and Bichot, 2005; Thompson et al., 2005a).
The spatial tuning for target location was consistent across tasks and across LFPs and spikes at each recording site, but was generally broader in the LFP signal than in the spikes. Previous spike vs. LFP comparisons either used full field visual stimulation (e.g. Chen et al., 2007; Logothetis et al. 2001) or placed visual stimuli based on the spatial extent of the spike receptive fields (Fries et al., 2001; Liu and Newsome, 2006; Pesaran et al., 2002). We are not aware of any study that compared the spatial extent of visual responses of LFPs to that of spikes recorded on the same electrode. But the broader spatial tuning in LFPs than in the spikes is consistent with the view that LFPs reflect synaptic activity over a larger area of cortex than is reflected in the spiking output of a few localized neurons (Kreiman et al., 2006; Liu and Newsome, 2006; Logothetis et al., 2007; Logothetis and Wandell, 2004; Mitzdorf, 1985; Mitzdorf, 1987). Nevertheless, the overall strong correlations of spatial tuning between the LFP responses and spiking activity when a target was presented alone and when presented among distractors indicate that the LFP and spike signals originate from the same region of FEF.
In a recent study, Buschman and Miller (2007) compared the time course of spatially selective spiking activity recorded simultaneously in FEF and the lateral intraparietal area (LIP), an area that is interconnected with FEF, in monkeys performing visual search tasks. Their results suggest that spatial attention signals appear first in the FEF during top-down attention and first in LIP during bottom-up attention. The implication is that visually driven attention signals flow from LIP to FEF and cognitively driven attention signals flow from FEF to LIP. Although simultaneous spike recordings can be used to compare signals in interconnected areas, this experimental method does not address whether or how different brain areas influence each other or how synaptic inputs are transformed into spiking outputs in a given area. In addition, the results of Buschman and Miller (2007) have been called into question mainly due the difficulty in knowing whether the neurons recorded in LIP and FEF in that study were those that received input from or influenced activity in the other brain area (Schall et al., 2007). The combined LFP-spike analysis described in this study may be able to address some of these unresolved issues.
Combined analysis of LFP and spiking activity can provide information about computations that cannot be obtained when these signals are considered separately (Kreiman et al., 2006; Nielsen et al., 2006). In the cerebral cortex, there is strong evidence that the LFP is a mass signal that is primarily influenced by the excitatory postsynaptic potentials of dendrites (Chen et al., 2007; Cruikshank et al., 2002; Juergens et al., 1999; Kaur et al., 2004; Kreiman et al., 2006; Logothetis and Wandell, 2004; Mitzdorf, 1985; Mitzdorf, 1987; Nielsen et al., 2006), and thus reflects inputs from other brain regions as well as local neural processes mediated by interneurons. Spiking activity reflects local processing and the long range outputs of neurons to other brain regions. Simultaneous LFP and spike recordings provide a way to compare the dendritic input to the spiking output, which is required to understand the transformation of neural signals from one processing stage to the next. In general, brain areas where cognitive functions are computed should show response modulations in the spiking activity of single units before they appear in the LFP. Whereas, the brain areas that receive this information from other areas should show response modulations first in the LFP, or simultaneously in the LFP and spiking activity (Nielsen et al., 2006). In this study, we specifically examined the transformation of a non-selective visual representation of items in a search array into a cognitive signal that identifies the location of the behaviorally relevant target stimulus.
The FEF is an important site of convergence in the visual system (Jouve et al., 1998; Schall, 1997; Schall et al., 1995b; Vezoli et al., 2004). The FEF receives retinotopically organized input from dorsal stream visual areas MT, MST, and LIP; ventral stream visual areas V4, TEO, and TE; and from the supplementary eye field and prefrontal areas 46 and 12. The dorsal stream innervation is most likely responsible for the fast non-selective initial visual responses we measured in the LFP and spikes (Bisley et al., 2004; Chen et al., 2007; Pouget et al., 2005; Schmolesky et al., 1998). The latencies of the initial visually-evoked LFP and spike responses were correlated, appearing in the LFP signals about 15 ms before the spikes in the visual search tasks, and about 10 ms before the spikes in the memory-guided saccade task. At the recording sites with the earliest spike latencies, the LFP latency was about 2 ms earlier. The earlier visually-evoked modulation in the LFP than in the spike response is consistent with studies in visual cortex (Logothetis et al., 2001; Schroeder et al., 1998), and with the hypothesis that the LFP signal reflects synaptic input and indicates that the initial visual response was relayed to the FEF from other brain areas.
The reverse temporal relationship was found in the visual search data when we compared the time course of spatial selectivity in the LFP response and spiking activity. Following the initial non-selective visual response, a spatially selective signal identifying the location of the search array target emerged first in the spiking activity, and then in the LFP signal about 30 ms later. The earlier spatially selective signal in the spiking activity suggests that the representation of the location of the behaviorally relevant target stimulus is computed within the FEF rather than relayed from other brain areas.
The alternative interpretation is that some modulations in synaptic activity cannot be detected in event related brain potentials using the methods we employed in this study. It is possible that FEF generates the strong spatially selective spiking signals by amplifying weak differences in the synaptic inputs. Although the exact nature of the input signals to FEF is currently unknown, they must contain information about the visual stimuli, and differences between them. Our results suggest that computations in FEF convert these differences into a strong categorical representation identifying the target location, regardless of the visual feature that differentiates the target from distractors. Consistent with this view, in our study we used two different classes of visual features, color and shape, and we obtained the same results.
The recording sites with the earliest LFP visual response latencies tended to have the earliest spatial selection times in the spiking activity. In dorsal stream visual areas of monkey cortex, LFPs recorded in lamina 4 have the shortest visual response latencies due to feedforward input from lower areas (Chen et al., 2007; Schroeder et al., 1998). We therefore made the reasonable assumption that the FEF recording sites with the earliest LFP visual response latencies were functionally closer to the feedforward inputs. Although we cannot identify the cortical layers we were recording from, the results depicted in Fig. 7 suggest that spatial selectivity in FEF originates first in neurons near the feedforward input and then is distributed to the functionally more distant regions in FEF via local connections or feedback from other areas. Consistent with this view, at the recording sites with the latest LFP visual response latencies, the selection times measured in the LFP and spikes did not differ significantly. This is the first evidence for such functional architecture and further studies are clearly needed test this hypothesis.
Our results suggest that spatial selectivity during a pop-out covert visual task is generated in FEF from non-spatially selective inputs. A few studies have examined the relationships between LFP and spiking responses in other areas. In area MT, for example, Liu and Newsome (2006) found that tuning for motion direction and speed in LFP responses are highly correlated with that of spike activity. In inferotemporal (IT) cortex, Krieman et al. (2006) showed a simultaneous time course of object selectivity in LFP responses and spiking activity. A study by Nielsen et al. (2006) showed that spikes and LFPs in IT exhibited learned object selectivity and the modulation of LFP responses, but not spiking activity, grew stronger from posterior to anterior IT. Because LFP modulation reflects the synaptic input, they concluded that learned object selectivity was encoded first in posterior IT and then transmitted to anterior IT. Only one study, conducted in area V4, has compared the spatial selection process measured in LFPs and spikes during visual search (Bichot et al., 2005). In that study, spatially selective responses appeared in the LFP and spikes at the same time. Although it was not specifically addressed in that study, the simultaneous modulation in LFP and spikes suggests that the spatial selectivity was present in the inputs.
The combined analysis of LFPs and spikes promises to provide useful information for understanding computations in the brain. Also, LFPs recorded in monkeys can be an important link between monkey single unit data and human EEG and imaging data (Logothetis and Wandell, 2004; Woodman et al., 2007). For example, the spatially selective LFP response we report could be related to the attention-related modulations observed in human EEG recordings during visual search (Luck and Hillyard, 1994). Single units, LFPs and EEG recordings provide high temporal resolution. It is more difficult, however, to localize the source of the computations reflected in EEG recordings than in the other two signals. EEGs recorded from scalp electrodes reflect the post-synaptic potentials summed over a large region of the brain that could include many areas that are related to spatial vision. The FEF is just one of the potential sources of the spatially selective signals necessary for spatial attention (Pessoa et al., 2003; Serences and Yantis, 2006). Further work is needed to determine the relationships between LFPs and spikes within and between the many regions of the brain involved in spatial attention.
EXPERIMENTAL PROCEDURES
Data collection
The data were collected from two male monkeys (Macaca mulatta) weighing 8 kg (monkey S) and 6.5 kg (monkey C). All surgical and experimental protocols were approved by the National Eye Institute Animal Care and Use Committee and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
The surgical procedures, behavioral control, and visual stimulation techniques have been described previously (Thompson et al., 2005b). The single unit spiking activity analyzed in this study is the same as in the previous study (Thompson et al., 2005b). Often two or three units were recorded simultaneously on one electrode and sorted offline. For this study, all the single units recorded at each site were combined to represent the overall spiking activity at each recording site.
The local field potentials (LFPs) were recorded simultaneously on the same glass insulated tungsten electrodes as the spikes using a Plexon data acquisition system (Plexon Inc.). The impedance of the head-stage was 40 MΩ at 1 kHz. Electrode impedance ranged from 0.5 to 1.5 MΩ. A stainless steel guide tube resting on the surface of the dura served as the reference. The signals were amplified and filtered between 154 Hz and 8.8 kHz to obtain spike data. LFP signals were digitized and sampled at 1 kHz after filtering the electrode signal between 3 Hz and 88 Hz. Analog eye position and lever position signals were digitized and sampled at 1 kHz. A test of the effects of the LFP signal filtering is provided in the Supplemental Data (see Figure S2). This test showed that signal distortions from the data acquisition system did not affect the results.
Behavioral tasks
At each recording site monkeys performed a memory-guided saccade task (Figure 1A) and one of two visual search tasks (Figure 1B) in separate blocks of trials as described in a previous report (Thompson et al., 2005b). In the memory-guided saccade task, after the monkey fixated on a 0.3° diameter gray spot for 400–800 ms, an identical gray spot was flashed for 50 ms at one of six or eight isoeccentric peripheral target locations spaced equally around the central fixation spot. The eccentricity was adjusted so that at least one of the stimulus locations was inside the receptive field of the neuron being recorded. The eccentricities of the stimuli ranged between 8° and 12° across recording sessions depending on receptive field location. Monkeys were required to maintain fixation on the central fixation spot for a random period ranging from 800 to 1400 ms. After the fixation spot disappeared, the monkeys were rewarded for making a saccade to the remembered target location.
In the covert visual search tasks, monkeys initiated a trial by grasping a lever and holding it in a vertical position. Once the lever was within 10° of vertical, a small central yellow fixation cross (0.3°) appeared. After fixating the cross for 400 to 800 ms, a search array appeared that was made up of a target randomly placed at one of the locations used in the memory-guided saccade task and distractors at the remaining locations. Each of the search array stimuli subtended 1.5° of visual angle. The monkeys were rewarded for maintaining fixation on the central cross and making the correct lever turn (> 15° from vertical) within 2 s after search array presentation; in practice, the monkeys nearly always turned the lever to the physical limit of 35° from vertical. If the monkey broke fixation on the central cross, released the lever, or made an incorrect lever turn the trial was aborted immediately. The reward was given after a correct lever turn; however, the search array remained on for an additional 250–500 ms to probe for latent saccade plans. The monkeys did not tend to make saccades to the target of the search array after obtaining the reward (Thompson et al., 2005b).
Monkey S was trained to report the location of the color singleton target of the search array (Figure. 1B, upper). The stimuli were isoluminant green and red discs. The target could be either green or red, but within a block of trials the color of the target and distractors did not change. The singleton target appeared randomly at one of six stimulus locations, three to the left and three to the right of the fixation cross. A correct response was a lever turn to the left or right corresponding to the location of the target stimulus relative to the fixation cross.
Monkey C was trained to report the orientation of a C among O distractors (Figure 1B, lower). The stimuli were gray rings with one of them having a 0.5° gap randomly on the left or right. The C target appeared randomly at one of eight locations positioned around the fixation cross. A correct response was a lever turn to the left or right corresponding to the location of the gap in the C target regardless of its location in the search array.
Data analysis
The LFP signal is a continuous measure of brain activity. A comparable measure of spiking activity was obtained by convolving each spike with a function that resembles an EPSP (Thompson et al., 1996). With this method, each spike exerts influence only forward in time and represents the post-synaptic consequences of spiking activity. The resulting spike density function reflects the onset of spiking activity at a 1 millisecond time resolution and is comparable to the onset of activity measured in the LFP signal. Examples of the EPSP spike density functions are shown in Figure 2A. Below we describe the analytical methods used to determine the time course of visual activation and spatial selection, and characterize the spatial tuning of spiking activity and LFP responses recorded during the memory-guided saccade and covert visual search tasks.
Selection time
The time course of spatial selectivity in the LFP and spiking activity was determined with an analysis of variance (ANOVA) at each millisecond following the target flash in the memory-guided saccade task and the presentation of the search array in the visual search tasks (Figures 2 and 3). The running ANOVA estimated the probability (p) at each millisecond that the response did not vary across target locations. Figures 2 and 3 illustrate the time course analysis for the spiking activity (Figure 2) and the LFP response (Figure 3) recorded concurrently at a single site. The selection times of the spiking activity and the LFP response were determined separately and were defined as the first millisecond that the p-value dropped below the 0.05 level only if it continued past the 0.001 level and remained below the 0.05 level for more than 20 of the next 25 milliseconds. To obtain the earliest possible selection times, a threshold of p = 0.05 was used. However, a threshold of p = 0 01 did not alter the temporal relationship between the selection times of the LFP and spiking activity. Again, the important point is that the same statistical analysis and threshold was used to determine selection times in the LFP and spiking activity in the memory-guided saccade task and in the visual search tasks. In Figures 2D and 3D, p-values obtained from the running ANOVA are plotted as a function of time on a log axis from 1 to 10−10 for spikes (Figure 2D) and LFPs (Figure 3D) recorded concurrently at a single site during the memory-guided saccade task (left) and the visual search task (right).
It is important to note that selection time measured in the memory-guided saccade task is qualitatively different from that measured in the visual search task. In the memory-guided saccade task a single target stimulus is presented alone and evokes a different initial response across target locations. Therefore, selection time in the memory-guided saccade task corresponds to the initial visual response latency to a single visual stimulus. In the visual search task, however, selection time measures the first time that the responses to the target of the search array are different from the responses to the distractors. As previously shown for spiking activity (Thompson et al., 1996), and now we demonstrate for LFPs, the initial visually evoked responses in FEF during visual search do not distinguish the target from the distractors. Therefore we used a different method to determine visual response latency in the visual search task.
Visual response latency during visual search
Figure 4 illustrates how we measured the initial visual response latencies of the spiking activity and the LFP response recorded simultaneously during the visual search task. A paired t-test was performed across all correct trials comparing the average activity during the 50 ms preceding the appearance of the search array on each trial to the activity at each millisecond following the appearance of the search array. Reliable results were obtained when the visual response latency was defined as the first time that the p-value dropped below the 0.01 level only if it continued past the 0.001 level and remained below the 0.01 level for more than 20 of the next 25 milliseconds. When the p-value threshold was 0.05 the results were about the same except that the results from a few of the recording sites were obviously false. Therefore a more strict threshold of p = 0.01 was used to determine visual response latency than was used to determine selection time (above). The important point is that the same threshold was used for determining visual response latencies in the LFP and spiking activity recording during the visual search task.
Spatial tuning
To describe the variation in the spiking and LFP responses with the location of the singleton target, the response averaged over a time interval was fit with a Gaussian function of the form
where activation (A) as a function of meridional direction (ϕ) depends on the baseline response (B), peak response (R), optimum direction (Φ), and tuning width (Tϕ). Previous reports have shown that this function effectively characterizes the spatial pattern of FEF spiking activity (Bruce and Goldberg, 1985; Schall et al., 1995a; Schall et al., 2004).
The best fit Gaussian curve was obtained for the average activity measured over a time range following visual stimulus presentation. For spiking activity, the time range was from 50 ms to 300 ms for the memory-guided saccade task, and from 100 ms to 300 ms for the visual search task. These time intervals were used because it encompassed the period of spatial selectivity observed across the data (Thompson et al., 2005b). For the LFP response in the memory-guided saccade task, the time range was from 100 ms to 200 ms because this interval encompassed a strong spatially selective negative-going deflection observed across all the LFP recordings (see Figure 3C, left panel). For the LFP response in the visual search task, it was necessary to determine the appropriate time interval individually for the different recording sites. This is because a spatially selective response could emerge in a positive or in a negative difference in the LFP signal. Therefore, to determine the spatial tuning of the LFP signal, we made the reasonable assumption that the preferred direction was in the visual hemifield contralateral to the brain hemisphere in which the LFP signals were recorded. In some of the LFP recordings, spatial tuning was evident in positive tuning during one time interval and in negative tuning during another time interval that was separated by a non-selective period during which time the polarity of the spatial tuning switched (see Figure S1). The time interval we used for determining the directional tuning was the interval that exhibited the strongest spatial selectivity in the running ANOVA analysis described above because it was most reliable. In the Supplemental Data we show that the spatial tuning during the two time intervals were essentially the same across the population (see Figure S1). For the recording site shown in Figure 3, the strongest spatial selectivity was in the interval between 180 and 300 milliseconds (Figures 3C and D), and during this interval the preferred direction corresponded to the most negative LFP signal (Figure 3E).
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the NIH, National Eye Institute. We thank P. Pouget, J. D. Schall, D. L. Sheinberg, E. Bromberg-Martin, R. E. Mruczek, and B. Anderson for helpful discussions and valuable comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allport A. Selection for action: Some behavioral and neurophysiological considerations of attention and action. In: Heuer H, Sanders AF, editors. Perspectives on Perception and Action. Hillsdale, NJ: Lawrence Erlbaum Associates; 1987. pp. 395–419. [Google Scholar]
- Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: links, causes and implications for spatial attention. Trends Cogn Sci. 2006;10:124–130. doi: 10.1016/j.tics.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Rossi AF, Desimone R. Parallel and serial neural mechanisms for visual search in macaque area V4. Science. 2005;308:529–534. doi: 10.1126/science.1109676. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Krishna BS, Goldberg ME. A rapid and precise on-response in posterior parietal cortex. J Neurosci. 2004;24:1833–1838. doi: 10.1523/JNEUROSCI.5007-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields: I. Single neurons discharging before saccades. J Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Chen CM, Lakatos P, Shah AS, Mehta AD, Givre SJ, Javitt DC, Schroeder CE. Functional Anatomy and Interaction of Fast and Slow Visual Pathways in Macaque Monkeys. Cereb Cortex. 2007;17:1561–1569. doi: 10.1093/cercor/bhl067. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol. 2002;87:361–384. doi: 10.1152/jn.00549.2001. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc Natl Acad Sci U S A. 1998;95:781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipata AE, Gee AL, Goldberg ME, Bisley JW. Activity in the lateral intraparietal area predicts the goal and latency of saccades in a free-viewing visual search task. J Neurosci. 2006;26:3656–3661. doi: 10.1523/JNEUROSCI.5074-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouve B, Rosenstiehl P, Imbert M. A mathematical approach to the connectivity between the cortical visual areas of the macaque monkey. Cereb Cortex. 1998;8:28–39. doi: 10.1093/cercor/8.1.28. [DOI] [PubMed] [Google Scholar]
- Juergens E, Guettler A, Eckhorn R. Visual stimulation elicits locked and induced gamma oscillations in monkey intracortical- and EEG-potentials, but not in human EEG. Exp Brain Res. 1999;129:247–259. doi: 10.1007/s002210050895. [DOI] [PubMed] [Google Scholar]
- Kaur S, Lazar R, Metherate R. Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. J Neurophysiol. 2004;91:2551–2567. doi: 10.1152/jn.01121.2003. [DOI] [PubMed] [Google Scholar]
- Kreiman G, Hung CP, Kraskov A, Quiroga RQ, Poggio T, DiCarlo JJ. Object selectivity of local field potentials and spikes in the macaque inferior temporal cortex. Neuron. 2006;49:433–445. doi: 10.1016/j.neuron.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Liu J, Newsome WT. Local field potential in cortical area MT: stimulus tuning and behavioral correlations. J Neurosci. 2006;26:7779–7790. doi: 10.1523/JNEUROSCI.5052-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Kayser C, Oeltermann A. In vivo measurement of cortical impedance spectrum in monkeys: implications for signal propagation. Neuron. 2007;55:809–823. doi: 10.1016/j.neuron.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Woodman GF, Vogel EK. Event-related potential studies of attention. Trends Cogn Sci. 2000;4:432–440. doi: 10.1016/s1364-6613(00)01545-x. [DOI] [PubMed] [Google Scholar]
- Mardia KV, Jupp PE. Directional Statistics. Chichester: John Wiley & Sons Ltd; 2000. [Google Scholar]
- McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol. 2002;88:2019–2034. doi: 10.1152/jn.2002.88.4.2019. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U. Properties of the evoked potential generators: current source-density analysis of visually evoked potentials in the cat cortex. Int J Neurosci. 1987;33:33–59. doi: 10.3109/00207458708985928. [DOI] [PubMed] [Google Scholar]
- Monosov IE, Trageser JC, Thompson KG. A comparison of the spatial tuning and time course of attention-related signals in the primate frontal eye field. Soc Neurosci Abstr. 2006;32:439.415. [Google Scholar]
- Nielsen KJ, Logothetis NK, Rainer G. Dissociation between local field potentials and spiking activity in macaque inferior temporal cortex reveals diagnosticity-based encoding of complex objects. J Neurosci. 2006;26:9639–9645. doi: 10.1523/JNEUROSCI.2273-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nature Neuroscience. 2002;5:805–811. doi: 10.1038/nn890. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: From modulation of sensory processing to top-down control. J Neurosci. 2003;23:3990–3998. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget P, Emeric EE, Stuphorn V, Reis K, Schall JD. Chronometry of visual responses in frontal eye field, supplementary eye field, and anterior cingulate cortex. J Neurophysiol. 2005;94:2086–2092. doi: 10.1152/jn.01097.2004. [DOI] [PubMed] [Google Scholar]
- Sato T, Murthy A, Thompson KG, Schall JD. Search efficiency but not response interference affects visual selection in frontal eye field. Neuron. 2001;30:583–591. doi: 10.1016/s0896-6273(01)00304-x. [DOI] [PubMed] [Google Scholar]
- Schall JD. Visuomotor areas of the frontal lobe. In: Rockland K, Kaas JH, Peters A, editors. Cerebral Cortex. New York: Plenum Press; 1997. pp. 527–638. [Google Scholar]
- Schall JD, Hanes DP, Thompson KG, King DJ. Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. J Neurosci. 1995a;15:6905–6918. doi: 10.1523/JNEUROSCI.15-10-06905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: Convergence and segregation of processing streams. J Neurosci. 1995b;15:4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Pare M, Woodman GF. Comment on “Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices”. Science. 2007;318:44. doi: 10.1126/science.1144865. [DOI] [PubMed] [Google Scholar]
- Schall JD, Sato TR, Thompson KG, Vaughn AA, Juan CH. Effects of search efficiency on surround suppression during visual selection in frontal eye field. J Neurophysiol. 2004;91:2765–2769. doi: 10.1152/jn.00780.2003. [DOI] [PubMed] [Google Scholar]
- Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Ann Rev Neurosci. 1999;22:241–259. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, Leventhal AG. Signal timing across the macaque visual system. J Neurophysiol. 1998;79:3272–3278. doi: 10.1152/jn.1998.79.6.3272. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Mehta AD, Givre SJ. A spatiotemporal profile of visual system activation revealed by current source density analysis in the awake macaque. Cereb Cortex. 1998;8:575–592. doi: 10.1093/cercor/8.7.575. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10:38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Spatially Selective Representations of Voluntary and Stimulus-Driven Attentional Priority in Human Occipital, Parietal, and Frontal Cortex. Cereb Cortex. 2007;17:284–293. doi: 10.1093/cercor/bhj146. [DOI] [PubMed] [Google Scholar]
- Thomas NW, Pare M. Temporal Processing of Saccade Targets in Parietal Cortex Area LIP During Visual Search. J Neurophysiol. 2007;97:942–947. doi: 10.1152/jn.00413.2006. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP. A visual salience map in the primate frontal eye field. Prog Brain Res. 2005;147:251–262. doi: 10.1016/S0079-6123(04)47019-8. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP, Sato TR. Frontal eye field activity before visual search errors reveals the integration of bottom-up and top-down salience. J Neurophysiol. 2005a;93:337–351. doi: 10.1152/jn.00330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J Neurosci. 2005b;25:9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol. 1996;76:4040–4055. doi: 10.1152/jn.1996.76.6.4040. [DOI] [PubMed] [Google Scholar]
- Treisman A, Gormican S. Feature analysis in early vision: evidence from search asymmetries. Psychol Rev. 1988;95:15–48. doi: 10.1037/0033-295x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Vezoli J, Falchier A, Jouve B, Knoblauch K, Young M, Kennedy H. Quantitative analysis of connectivity in the visual cortex: extracting function from structure. Neuroscientist. 2004;10:476–482. doi: 10.1177/1073858404268478. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Kang MS, Rossi AF, Schall JD. Nonhuman primate event-related potentials indexing covert shifts of attention. Proc Natl Acad Sci U S A. 2007;104:15111–15116. doi: 10.1073/pnas.0703477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.