Abstract
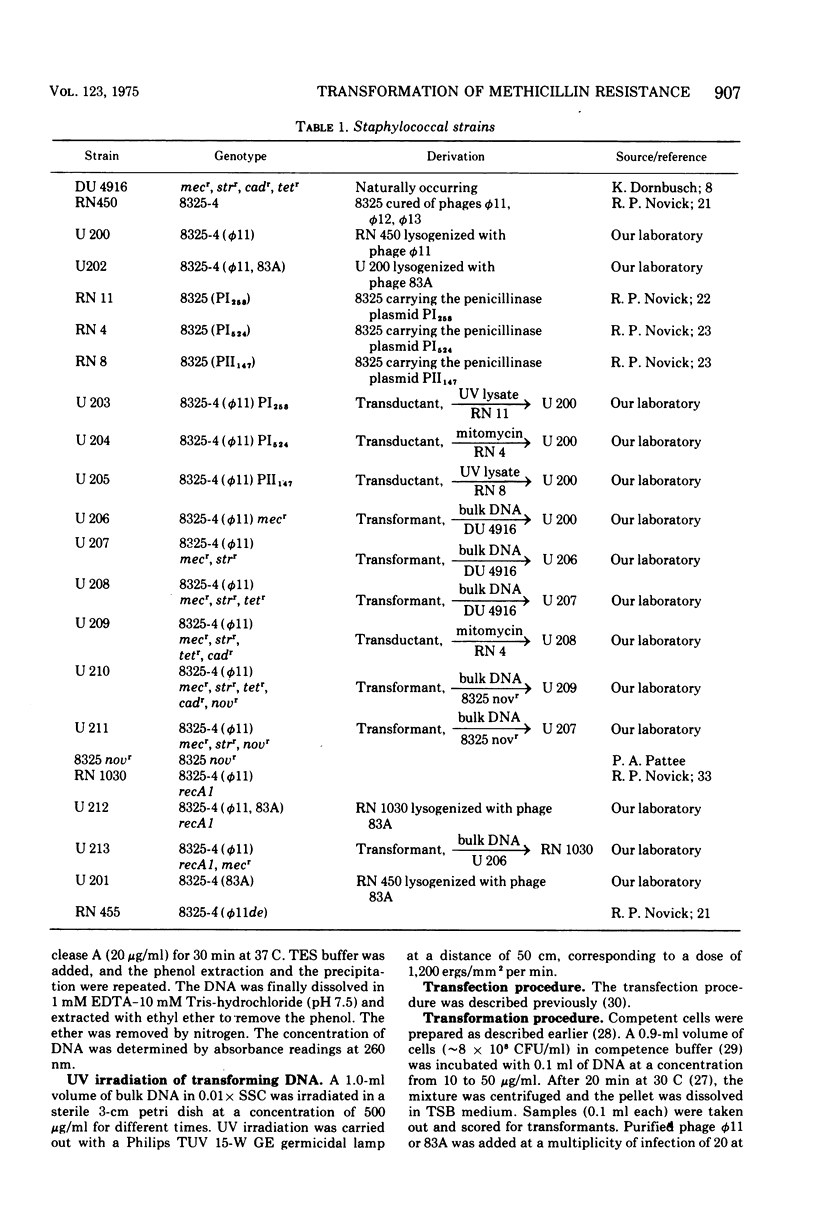
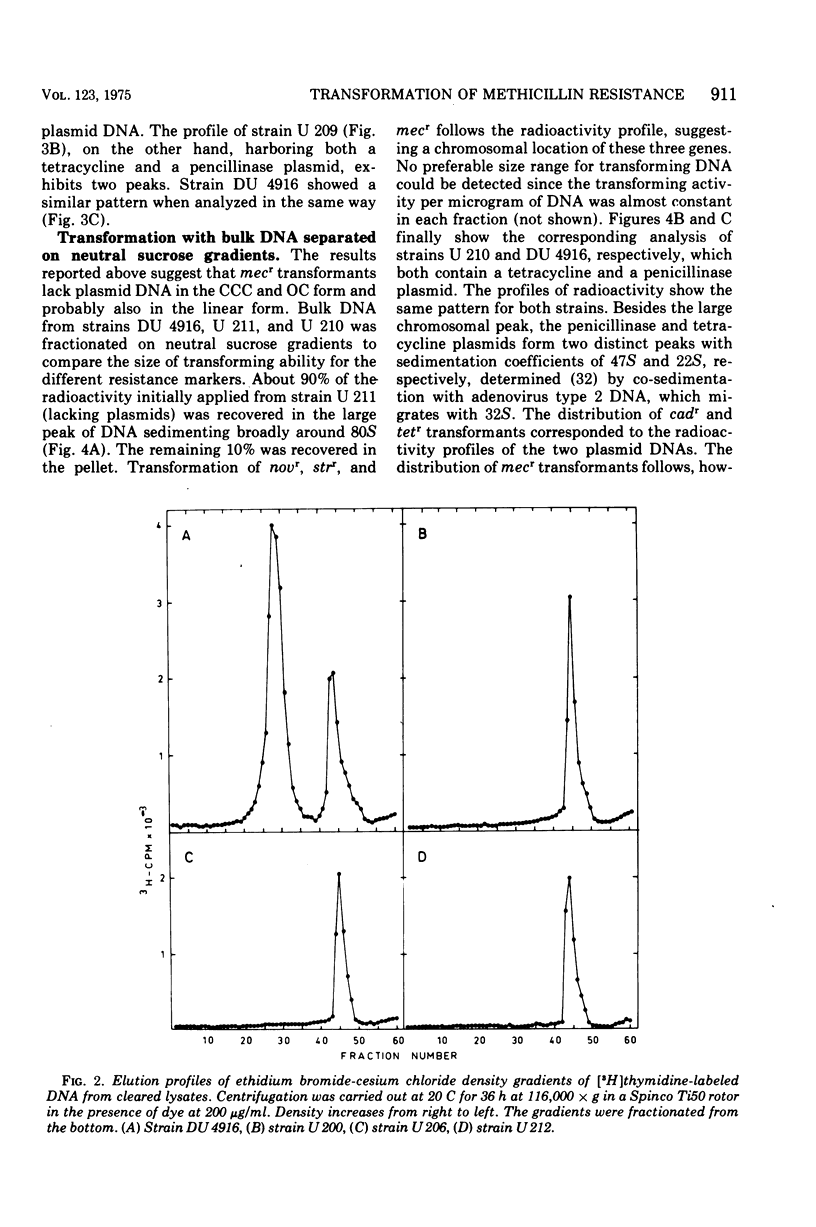
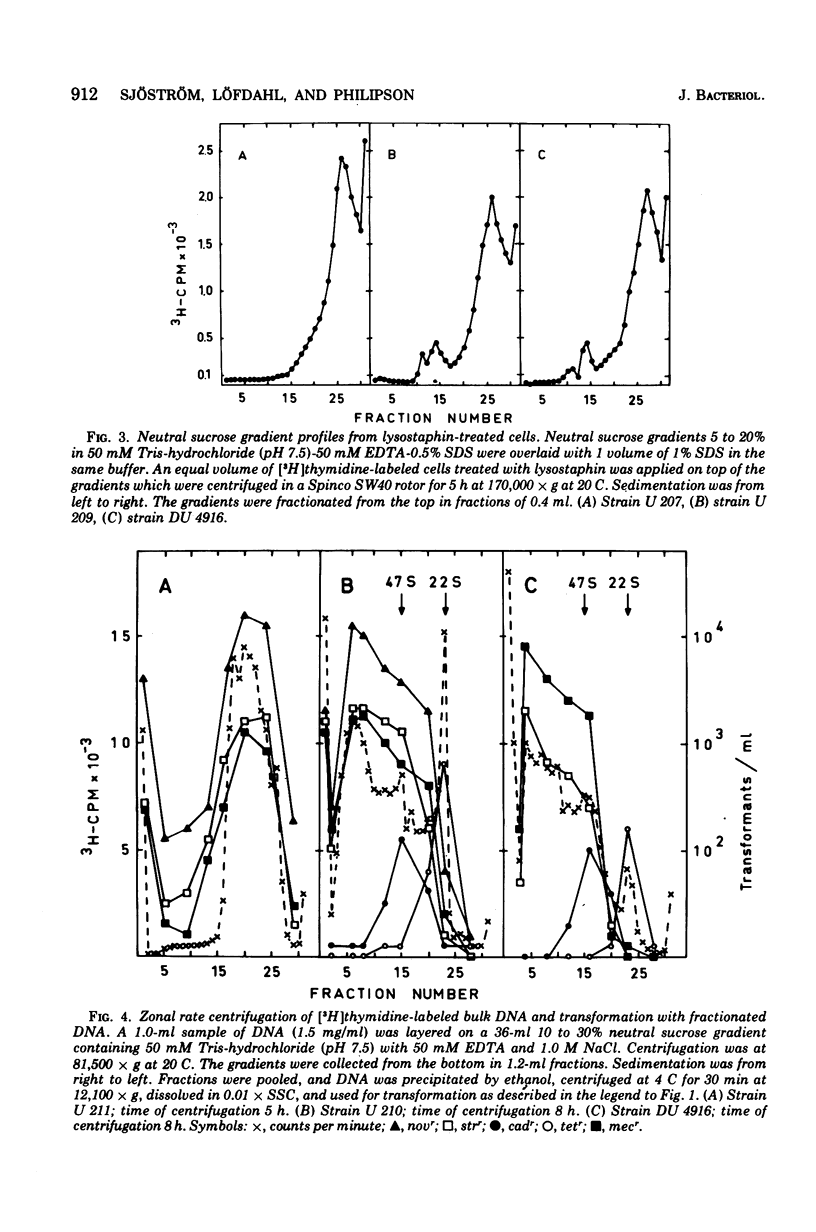
The localization of the gene(s) mediating methicillin (mecr) in Staphylococcus aureus was determined by transformation with deoxyribonucleic acid (DNA) from a natural mecr strain (DU 4916) and transformation obtained with DNA from this strain. Streptomycin resistance genes (strr) and novobiocin resistance genes (novr) were used concurrently as representatives for chromosomal genes; penicillinase (PI254) and tetracycline plasmids were used as examples of medium- and small-size extrachromosomal genes, respectively. Superinfection of the lysogenic recipients with the competence-inducing phage phi11 or 83A enhanced transformation for all markers. Phenotypic expression of cadmium (cadr), tetracycline (tetr), or methicillin resistance (mecr) did not appear to require a host recombination system since a recA1 mutant could serve as the recipient provided it was superinfected with a competence-inducing phage. There was, furthermore, no requirement for preexisting plasmids for phenotypic expression. Ultraviolet irradiation of transforming DNA enhanced at low doses the transformation frequency for chromosomal genes strr and novr but not for mecr, cadr, or tetr. The gene(s) for mecr was transformed with chromosomal DNA after sodium dodecyl sulfate-sodium chloride extraction and after neutral sucrose gradient centrifugation of bulk DNA from wild-type strain DU 4916 and the transformats. No cavalently closed circular DNA or open circular DNA carrying the methicillin resistance gene(s) could be detected in the wild type or the transformants either by ethidium bromide-cesium chloride gradient centrifugation or by zonal rate centrifugation of cells directly lysed on top of the gradients. The mecr gene(s) is thus probably of chromosomal nature but possibly under recombinational control of phage genes, since transfer of mecr is independent of the recA1 gene(s) but can be accomplished in this strain after superinfection with a competence-inducing phage. Ultraviolet light inactivation of transforming DNA shows first-order kinetics for mecr transformability similar to that observed for both transfecting and plasmid DNA.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- Asheshov E. H. Chromosomal location of the genetic elements controlling penicillinase production in a strain of Staphylococcus aureus. Nature. 1966 May 21;210(5038):804–806. doi: 10.1038/210804a0. [DOI] [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M. Effect of the prophage and penicillinase plasmid of the recipient strain upon the transduction and the stability of methicillin resistance in Staphylococcus aureus. J Bacteriol. 1973 Nov;116(2):803–811. doi: 10.1128/jb.116.2.803-811.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M. Transduction of Methicillin Resistance in Staphylococcus aureus Dependent on an Unusual Specificity of the Recipient Strain. J Bacteriol. 1970 Dec;104(3):1158–1167. doi: 10.1128/jb.104.3.1158-1167.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbusch K., Hallander H. O., Löfquist F. Extrachromosomal control of methicillin resistance and toxin production in Staphylococcus aureus. J Bacteriol. 1969 May;98(2):351–358. doi: 10.1128/jb.98.2.351-358.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbusch K., Hallander H. O. Transduction of penicillinase production and methicillin resistance-enterotoxin B production in strains of Staphylococcus aureus. J Gen Microbiol. 1973 May;76(1):1–11. doi: 10.1099/00221287-76-1-1. [DOI] [PubMed] [Google Scholar]
- Ephrati-Elizur E., Yosuv D., Shmueli E., Horowitz A. Thymineless death in Bacillus subtilis: correlation between cell lysis and deoxyribonucleic acid breakdown. J Bacteriol. 1974 Jul;119(1):36–43. doi: 10.1128/jb.119.1.36-43.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi-Taylor H., Sicard A. M., Kamen R. Genetic Recombination in DNA-Induced Transformation of Pneumococcus. I. the Problem of Relative Efficiency of Transforming Factors. Genetics. 1965 Mar;51(3):455–475. doi: 10.1093/genetics/51.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Jacob A. E. Transposition of ampicillin resistance from RP4 to other replicons. Mol Gen Genet. 1974;132(1):31–40. doi: 10.1007/BF00268228. [DOI] [PubMed] [Google Scholar]
- Hewitt J. H., Parker M. T. Sensitivity of penicillinase-forming strains of Staphylococcus aureus and of their penicillinase-negative variants to cephaloridine, cephalothin, methicillin, and benzylpenicillin. J Clin Pathol. 1968 Jan;21(1):75–84. doi: 10.1136/jcp.21.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Genetic control in methicillin-resistant strains of Staphylococcus aureus. J Med Microbiol. 1972 Nov;5(4):497–508. doi: 10.1099/00222615-5-4-497. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Grinsted J. Genetic analysis of methicillin-resistant strains of Staphylococcus aureus; evidence for their evolution from a single clone. J Med Microbiol. 1973 Nov;6(4):511–526. doi: 10.1099/00222615-6-4-511. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Staphylococcus aureus strain DU4916--an atypical methicillin-resistant isolate? J Gen Microbiol. 1974 Sep;84(1):1–10. doi: 10.1099/00221287-84-1-1. [DOI] [PubMed] [Google Scholar]
- Meisner L. F., Chuprevich T. W., Johnson C. B., Inhorn S. L., Carter J. J. Banding of human chromosomes with caesium chloride. Lancet. 1973 Jan 13;1(7794):100–101. doi: 10.1016/s0140-6736(73)90494-7. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P., RICHMOND M. H. NATURE AND INTERACTIONS OF THE GENETIC ELEMENTS GOVERNING PENICILLINASE SYNTHESIS IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1965 Aug;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Parker M. T., Hewitt J. H. Methicillin resistance in Staphylococcus aureus. Lancet. 1970 Apr 18;1(7651):800–804. doi: 10.1016/s0140-6736(70)92408-6. [DOI] [PubMed] [Google Scholar]
- RITZ H. L., BALDWIN J. N. Transduction of capacity to produce staphylococcal penicillinase. Proc Soc Exp Biol Med. 1961 Jul;107:678–680. doi: 10.3181/00379727-107-26726. [DOI] [PubMed] [Google Scholar]
- Rudin L., Sjöström J. E., Lindberg M., Philipson L. Factors affecting competence for transformation in Staphylococcus aureus. J Bacteriol. 1974 Apr;118(1):155–164. doi: 10.1128/jb.118.1.155-164.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Competence for transfection in Staphylococcus aureus. J Bacteriol. 1973 Feb;113(2):576–585. doi: 10.1128/jb.113.2.576-585.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Transfection of Staphylococcus aureus with bacteriophage deoxyribonucleic acid. J Bacteriol. 1972 Jan;109(1):285–291. doi: 10.1128/jb.109.1.285-291.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Philipson L. Role of the phi 11 phage genome in competence of Staphylococcus aureus. J Bacteriol. 1974 Jul;119(1):19–32. doi: 10.1128/jb.119.1.19-32.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler P. W., Sweeney H. M., Cohen S. Absence of circular plasmid deoxyribonucleic acid attributable to a genetic determinant for methicillin resistance in Staphylococcus aureus. J Bacteriol. 1973 Nov;116(2):771–777. doi: 10.1128/jb.116.2.771-777.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman L., Goering R. V., Novick R. P. Genetic control of chromosomal and plasmid recombination in Staphylococcus aureus. Genetics. 1974 Apr;76(4):681–702. doi: 10.1093/genetics/76.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Salihy S. M., James A. M. Loss of methicillin-resistance from resistant strains of Staphylococcus aureus. Lancet. 1972 Aug 12;2(7772):331–332. doi: 10.1016/s0140-6736(72)92937-6. [DOI] [PubMed] [Google Scholar]


