Abstract
Nuclear lamins are integral components of the nuclear envelope and are important for the regulation of many aspects of nuclear function, including gene transcription and DNA replication. During interphase, the lamins form an intranuclear intermediate filament network that must be disassembled and reassembled when cells divide. Little is known about factors regulating this assembly/disassembly cycle. Using in vitro nuclear assembly and lamin assembly assays, we have identified a role for the nuclear transport factor importin α in the regulation of lamin assembly. Exogenous importin α inhibited nuclear lamin assembly in Xenopus interphase egg nuclear assembly assays. Fractionation of the egg extract used for nuclear assembly identified a high molecular weight complex containing the major egg lamin, XLB3, importin α, and importin β. This complex could be dissociated by RanGTP or a competing nuclear localization sequence, indicating that lamin assembly is Ran- and importin α-dependent in the egg extract. We show that the addition of importin α to purified lamin B3 prevents the assembly of lamins in solution. Lamin assembly assays show that importin α prevents the self-association of lamins required to assemble lamin filaments into the typical paracrystals formed in vitro. These results suggest a role for importin α in regulating lamin assembly and possibly modulating the interactions of lamins with lamin-binding proteins.
The nuclear envelope (NE)3 segregates the cytoplasm from the nucleus of eukaryotic cells and regulates macromolecular traffic between these two compartments (1). The NE is an asymmetric organelle composed of a double bilayer membrane and two major protein complexes, the nuclear pore complexes (NPCs) and the nuclear lamina. NPCs punctuate the membranes at points of membrane fusion, forming the channels through which regulated macromolecular traffic occurs. The NPCs and membranes are associated with and supported by the lamina, which is composed mainly of a filamentous meshwork of type V intermediate filament proteins, the nuclear lamins, and dozens of associated proteins (2). Lamins are also found in lesser amounts throughout the nucleoplasm (3, 4).
Nuclear lamins are the products of three genes: one gene encoding two A-type lamins and two genes encoding B-type lamins. The two A-type lamins, lamins A and C, are the result of alternative splicing of a single transcript of the LMNA gene (5). The two B-type lamins, lamins B1 and B2, and lamin A are farnesylated at their C termini. Although lamins B1 and B2 retain this modification for their lifetimes, mature lamin A is formed following a proteolytic cleavage to remove the lipid moiety and the C-terminal 15 amino acid residues (6). Both A- and B-type lamins are developmentally regulated, with B-type lamins expressed in all cells from the egg to terminally differentiated adult cells, whereas A-type lamins are expressed only at or after gastrulation (7) and then only in a subset of cell types. Both A- and B-type lamins are involved in the regulation of transcription and replication and in the maintenance of the shape, size, and mechanical stability of the nucleus (2).
Lamins are unique among the intermediate filament protein family in that they are exclusively nuclear in their localization throughout interphase but are exposed to the cytoplasmic environment of the cell during mitosis. All lamins have classical nuclear localization sequences (NLS) and are predicted to be transported into the nucleus by importin α/β (8). At the onset of mitosis, the NE breaks down, the membranes are redistributed into the endoplasmic reticulum, and the lamina and NPCs are disassembled by a mechanism involving protein phosphorylation (9, 10). At late anaphase, lamins begin to reassemble on the surface of the segregated chromosomes with membranes and NPCs (11–13). After enclosure of the chromatin by the nuclear envelope at telophase, newly synthesized lamins are transported through NPCs and continue to be incorporated into the NE as the interphase nucleus grows (13). Although the lamins are incorporated into higher order structures in the nucleus and interact with many other proteins, little is known about the mechanism(s) regulating their assembly in cells or the regulation of their interactions with other proteins.
A single Xenopus laevis egg contains all of the components required for the assembly of ∼4000 nuclei dispersed in its cytoplasm. Extracts of these eggs have been used extensively to determine the temporal order of nuclear assembly. In recent years, some mechanistic details of nuclear membrane and NPC assembly have been delineated (14). However, the role of nuclear lamin assembly in NE formation has remained somewhat controversial. Amphibian eggs express a unique lamin, lamin B3 (XLB3), with expression restricted to eggs and early embryos (22). Early experiments suggested that assembly of the NE in egg extracts did not require co-assembly of lamins into a lamina structure (15, 16). In such a mechanism lamins would only be incorporated late in the process after the formation of functional NPCs and the initiation of nuclear protein import. However, other studies have shown that fragments of XLB3 that inhibit lamin polymerization block NE assembly at the early membrane fusion events, suggesting that lamin assembly is required early in the process of NE formation (17, 18). Surprisingly, little is known about the physical state of the XLB3 that is stored in the egg for nuclear assembly during early embryogenesis.
The assembly of a NE in egg extracts is regulated by the small nuclear GTPase Ran (14). Nucleoporins and membranes in the egg extract are bound by importin β or α, which prevents their assembly into NPCs or nuclear membranes. RanGTP acts as a molecular switch by binding to importin β and releasing the bound components in a spatially and temporally regulated process. Because RanGTP is generated at the surface of chromatin by the chromatin-bound Ran guanine nucleotide exchange factor RCC1, NE assembly can only occur at the chromatin surface. We initiated a series of experiments to determine whether nuclear lamin assembly in egg extracts is also regulated by Ran and the importins. The experiments described here demonstrate that importin α is involved not only in lamina assembly but also in maintaining the solubility of the lamins prior to assembly.
EXPERIMENTAL PROCEDURES
Expression and Purification of Protein—Full-length lamin B3 (XLB3) expressed in NovaBlue (DE3) cells (Novagen, San Diego, CA) was purified from inclusion bodies as described (19). The partially purified lamins were further purified by size exclusion chromatography on a Superdex 200 10/300 GL column in 8 m urea. XLB3 with a mutated NLS (413KKRK to 413ASSK), XLB3ΔNLS, was obtained by site-directed mutagenesis using a QuikChange II site-directed mutagenesis kit (Stratagene, Cedar Creek, TX).
Xenopus importin α (20) was expressed as either an S-peptide fusion protein from pET30a (Novagen) or a maltose-binding protein (MBP) fusion from pMal-c2X (New England Biolabs, Ipswich, MA) at 15 °C for 16 h in 2XYT medium supplemented with 25 mm phosphate buffer and 2 mm MgSO4. Bacteria expressing S-tagged importin α were lysed in 50 mm Tris, pH 8.0; 200 mm NaCl; 5% glycerol; 10 μm EDTA; 1 mm dithiothreitol; 0.1 mm phenylmethylsulfonyl fluoride; 1 μg/ml each aprotinin, leupeptin, and pepstatin; and 0.5 mg/ml lysozyme for 1 h at 4 °C. The lysates were brought to 0.5 m NaCl and centrifuged at 30,000 rpm in a Type 35 rotor for 90 min at 4 °C in a Beckman L-90K ultracentrifuge. The S-tagged protein was purified on a 1-ml HiTrap chelating column (GE Healthcare) charged with Ni2+ and eluted in the loading buffer containing 400 mm imidazole. The eluted protein was further purified on a Mono Q column with a 50–600 mm NaCl gradient in 50 mm Hepes, pH 7.3. MBP-expressing or MBP-importin α expressing cells were lysed in 20 mm Hepes, pH 7.4; 200 mm NaCl; 5 mm EDTA; 1 mm dithiothreitol; 0.1 mm phenylmethylsulfonyl fluoride; and 1 μg/ml each aprotinin, leupeptin, and pepstatin in four freeze/thaw cycles in a dry ice/methanol bath. This was followed by ultracentrifugation of the lysates as per the preparation of S-importin α. The supernatant was loaded on a 10-ml column of amylose resin (New England Biolabs) and washed with lysis buffer. The bound MBP or MBP-importin α was eluted with 10 mm maltose in MBP lysis buffer. The eluted protein was further purified on a Mono Q column as described above for S-importin α. Purified MBP or importin α was dialyzed into egg buffer (10 mm Hepes, pH 7.4, 50 mm KCl, 2.5 mm MgCl2, 250 mm sucrose, and 2 mm dithiothreitol), aliquoted, and frozen in liquid nitrogen prior to storage at -80 °C.
RanQ69L (21) was prepared by site-directed mutagenesis of the wild-type Ran cDNA in pQE31 (Qiagen) using a QuikChange XL II site-directed mutagenesis kit. RanQ69L was expressed in NovaBlue (DE3) cells grown in 2XYT medium with 4 mm MgSO4 and purified on a HiTrap chelating column charged with Zn2+. The column was eluted with 250 mm histidine in 20 mm Hepes, pH 7.0, 0.5 m NaCl, and 1 mm MgCl2. The purified Ran was charged with GTP or GDP as described elsewhere (43). RanGDP and RanGTP were separated by cation exchange chromatography on a Mono S HR5/5 column (GE Healthcare) at pH 6.5 in 50 mm MES buffer. GST-SV40NLS-EGFP was prepared as described (17).
Nuclear Assembly Assays in Xenopus Egg Extract—Nuclear assembly assays were performed using Xenopus interphase egg extracts under standard conditions as described (17, 18). The centrifugal disruption of dejellied and washed eggs at 10,000 × g yields an extract containing all of the components, including membranes and cytosolic factors, necessary to form a complete NE around demembranated sperm chromatin. Twenty microliters of this egg extract was first incubated on ice for 30 min with purified MBP or MBP-importin α added to a final concentration of 10 μm. After incubation, an ATP-regenerating system mix was added along with 40,000 demembranated sperm heads for a final reaction volume of 25 μl. The mixture was incubated at 23 °C for 75 min, and nuclear assembly was stopped by dilution with 500 μl of egg buffer (see above). The diluted assembly assay was overlaid on a cushion of 30% sucrose in egg buffer and centrifuged onto poly-l-lysine-coated coverslips at 2,000 × g for 10 min. The supernatants were removed, and the coverslips were fixed in 2% paraformaldehyde in egg buffer for 10 min. The coverslips were washed three times with egg buffer containing 0.1% Triton X-100 and blocked for 10 min in egg buffer containing 2% normal goat serum. To detect lamins and nucleoporins, the coverslips were incubated with a rabbit polyclonal antiserum to full-length purified XLB3 (Cocalico) and MAb414 (Covance, Princeton, NJ) to nucleoporins. The primary antibodies used were detected with Alexa488 anti-rabbit and Alexa568 anti-mouse. Images were obtained using a Zeiss Axiovision microscope equipped with a 100× 1.4 NA Plan Apochromat objective.
XLB3 Polymerization Assays—The methods employed to assess polymerization of lamins in vitro were as described previously. XLB3 paracrystal formation was assessed by negative stain electron microscopy as published elsewhere (19). To determine the effects of importin α on paracrystal formation, purified MBP-importin α was added to the purified XLB3 at a 5-fold molar excess in high pH buffer and immediately dialyzed against low pH buffer. After dialysis for 2 h, the assembled paracrystals were absorbed to Formvar/carbon-coated grids and negatively stained with 1% uranyl acetate. The paracrystals were observed using a Jeol JEM-1200EX electron microscope.
The XLB3 pelleting assay was performed as described (19) with the exception that the assembly buffer was maintained at pH 7.3, rather than pH 6.6, as the binding of importin α to XLB3 is greatly reduced at the lower pH (data not shown). Supernatant and pellet fractions were resolved by SDS-PAGE and staining with Coomassie Brilliant Blue G-250 (Fisher). The densities of the stained XLB3 bands were measured using a Kodak IS440CF Image Station and Kodak molecular imaging software. The linearity of protein detection on immunoblots was determined from a standard curve prepared from serial dilutions of purified recombinant XLB3.
Gel Filtration Chromatography of Egg Cytosol—Aliquots of Xenopus egg interphase extract were separated into membrane and cytosolic fractions by centrifugation at 200,000 × g for 90 min at 4 °C in a Beckman TLA100.3 rotor followed by filtration of the supernatant through a 0.2-μm Nanosep microfiltration spin filter (Pall Life Sciences, Ann Arbor, MI). Five hundred microliters of the demembranated cytosol was separated on a Superose 6 gel filtration chromatography column (GE Healthcare) equilibrated in egg buffer (see above) at 4 °C. One-milliliter fractions were collected, and 50 μl of each fraction was separated by SDS-PAGE and transferred to nitrocellulose. Replicate immunoblots were probed with a monoclonal anti-XLB3 antibody (L6-5D5 provided by Reimer Stick, Institute for Cell Biology, University of Bremen, Bremen, Germany) (22), rabbit polyclonal anti-importin β antibodies (BD Transduction Laboratories), or a rabbit polyclonal antiserum prepared against Xenopus importin α and anti-rabbit or anti-mouse horseradish peroxidase-labeled secondary antibodies (Pierce). The proteins were detected with SuperSignal West-Pico chemiluminescence detection reagent (Pierce) and Kodak XAR-5 film. The developed films were imaged with a Kodak IS440CF Image Station. Size standards for the Superose 6 column were separated under conditions identical to fractionation of the egg cytosol.
Immunoadsorption of Importin α-XLB3 Complexes—Rabbit antiserum directed against importin α or pre-immune serum was adsorbed to protein A-Trisacryl beads (Pierce) and washed with phosphate-buffered saline. The bound antibodies were cross-linked to the beads with 20 mm dimethylpimelimidate in 0.2 m sodium borate, pH 9.0, for 30 min. The antibody-coupled beads were washed consecutively in 0.2 m ethanolamine, pH 8.0, phosphate-buffered saline, 0.1 m citric acid, pH 3.0, and finally phosphate-buffered saline. Aliquots of the Superose 6 column fractions identified as containing XLB3 by immunoblotting were diluted into egg buffer (see above) containing 0.01% Tween 20 and incubated with antibody beads for 1 h at 4 °C. The beads were then washed five times with egg buffer, and the bound proteins were eluted in SDS-PAGE sample buffer. The eluted proteins were separated by SDS-PAGE and transferred to nitrocellulose. The membrane was probed with the polyclonal anti-XLB3 antibody and detected with horseradish peroxidase-conjugated rabbit IgG TrueBlot secondary antibody (eBiosciences, San Diego, CA) as described above. The amount of input XLB3 adsorbed by the anti-importin α antibodies was determined by comparison of the band intensities from the immunoblot with a standard curve prepared from dilutions of the input material.
RanGTP Release Assay—Aliquots of the fractions from the Superose 6 column identified as containing XLB3 by immunoblotting were incubated with 5 μm RanQ69L-GTP, RanQ69L-GDP, or GST-SV40NLS-EGFP for 90 min on ice. After centrifugation at 16,100 × g for 20 min at 4 °C, the supernatants were carefully removed, and the pellets were washed with egg buffer and centrifuged as before. The resulting pellets were solubilized in SDS-PAGE sample buffer, and equivalent amounts of soluble and insoluble fractions were separated by SDS-PAGE. The XLB3 in each fraction was detected by immunoblotting with anti-XLB3 polyclonal antiserum. Image collection and band quantification were performed on a Kodak IS440CF Image Station.
RESULTS
Importin α Inhibits Lamina Assembly in Vitro—Earlier studies describing the effect of the depletion of importin α from Caenorhabditis elegans embryos by RNA interference demonstrated a requirement for the germ line-intrinsic importin α IMA-2 in NE assembly during embryogenesis (23, 24). Subsequently, it was also shown that NE assembly could be inhibited in Xenopus egg extract nuclear assembly assays either by the depletion of importin α or by the addition of an excess of importin α or bovine serum albumin-NLS (25). Using dominant negative fragments of XLB3, we have shown that lamin incorporation into the nascent NE is an essential step in nuclear assembly (17, 18). Because XLB3 contains a classical NLS that binds importin α (26), we have determined whether importin α also regulates lamin assembly in egg extracts. When demembranated sperm chromatin was added to an egg extract supplemented with an energy-regenerating system, a complete NE formed around the decondensed chromatin within 75 min (Fig. 1, a–c). In control assays, purified MBP was added to 10 μm and incubated for 30 min prior to the addition of sperm chromatin and an ATP-regenerating system. Under these conditions, XLB3 is concentrated at the nuclear periphery and is also present throughout the nucleoplasm in assembled nuclei. The NEs assembled under these conditions contain NPCs as detected with an antibody to a family of glycosylated nucleoporins, MAb414. To test the effect of exogenous importin α on lamin assembly, we added an MBP fusion of importin α to 10 μm in egg extract for 30 min prior to the addition of the sperm chromatin. Following an additional 75-min incubation at 23 °C, the preparations were fixed and stained for immunofluorescence. The addition of excess importin α to the egg extract had dramatic effects on nuclear assembly. In the presence of importin α, the sperm chromatin did not decondense, and a NE did not form around the sperm chromatin (Fig. 1, d–f). Instead, distinct patches of XLB3, nucleoporins, and membranes (data not shown) were seen on the surface of the chromatin with little overlap of the XLB3 and nucleoporin staining patterns, suggesting that lamin assembly and nuclear membrane/NPC assembly take place simultaneously on the chromatin surface during nuclear assembly. Therefore, exogenous importin α inhibits both nuclear membrane formation and assembly of the lamina.
FIGURE 1.
Inhibition of nuclear assembly by importin α. Interphase egg extracts were pre-incubated with 10 μm MBP (a–c) or with 10 μm importin α (Impα; d–f) for 30 min prior to the introduction of demembranated sperm chromatin. After a 75-min incubation at 23 °C, the assembled nuclei were centrifuged onto poly-l-lysine-coated coverslips, fixed, and processed for immunofluorescence. XLB3 was detected with a polyclonal antiserum to XLB3, and nucleoporins (Nup) were detected with MAb414. Images were collected on a Zeiss Axiovision microscope. Optimal exposure times were determined for the control assembly reactions in a–c and used to image assembly reactions with importin α in d and e. In f, the brightness and contrast were adjusted to the same levels as in a–c to more clearly show the protein localization. All images were processed using Zeiss AxiovisionLE version 4.2 software. The scale bar on each panel = 10 μm.
Lamin B3 and Importin α Form a High Molecular Weight Complex in Xenopus Egg Cytosol—Although nuclear assembly in Xenopus egg extracts and in live cells has been well studied, little is known about the mechanisms responsible for regulating the assembly of the nuclear lamins during the formation of the lamina. Although phosphorylation and dephosphorylation of lamins are important for lamina assembly and disassembly (27), little is known about the physical form of disassembled lamins in cells. In Xenopus egg extracts, ∼95% of XLB3 is not associated with membranes and cannot be pelleted by high speed centrifugation (28).4 We analyzed the physical state of this unassembled XLB3 by size exclusion chromatography on a Superose 6 column and immunoblotting to detect XLB3 and importins (Fig. 2A). All of the detectable XLB3 in egg cytosol was found in a discrete peak with an estimated molecular weight of >700,000. The majority of both soluble importin α and importin β also separated on the column as high molecular weight species, including in the fractions containing XLB3.
FIGURE 2.
XLB3 is present in a large complex with importin α. A, demembranated egg cytosol was fractionated by gel filtration chromatography. Duplicate immunoblots were probed with antibodies to XLB3, importin α (Impα), and importin β (Impβ). The performance of the Superose 6 column was determined by separation of purified standard proteins in the same buffer as the egg cytosol. v, void volume; tg, thyroglobulin (Mr 669,000); f, ferritin (Mr 440,000). B, aliquots of the peak fraction of XLB3 were immunoadsorbed with immobilized antibodies to importin α. Pre-immune serum was used as the control. The bound proteins were analyzed by immunoblotting with antibodies to XLB3. The same amount of XLB3 added to the immunoadsorption was loaded in the first lane (Input). C, two independent immunadsorptions were performed as in B, and the amount of XLB3 adsorbed was quantified as described under “Experimental Procedures.” The error bars indicate average deviation.
As XLB3 has a classical NLS that binds to importin α, we wanted to determine whether XLB3 was in a complex with importins in the high molecular weight fraction obtained from egg cytosol. Therefore, we carried out immunoadsorptions from the high molecular weight gel filtration fractions with immobilized anti-importin α followed by immunoblotting with anti-XLB3 (Fig. 2B). Approximately 40% of the XLB3 in the fraction was co-adsorbed with importin α (Fig. 2C), demonstrating that both proteins are components of a high molecular weight complex in egg cytosol. The inability to co-adsorb all of the XLB3 with importin α may be evidence that other large complexes of XLB3, such as lamin oligomers, are also present in the egg cytosol. Alternatively, some of the importin α-XLB3 complexes may not be accessible to the antibody used to immunoadsorb the complex. Given the large number of potential cargoes for importin α in egg cytosol, it is likely that the high molecular weight fractions containing XLB3 and importin α also contain other importin-cargo complexes.
Effect of Importin α on XLB3 Assembly in Vitro—The inhibition of lamin assembly in the nuclear assembly assay and the presence of a high molecular weight complex containing XLB3 and importins in egg cytosol suggested to us that importins may negatively regulate lamin polymerization. Complexes between importins α and β can be dissociated by the binding of RanGTP to importin β, resulting in a decreased affinity of importin α for the NLS by an autoinhibitory mechanism (29). In vivo, this mechanism is responsible for the release of transported proteins into the nuclear interior. Because XLB3 is in a complex with the importins, the addition of RanGTP to the fractions containing XLB3 and importins should release some of the bound XLB3 if the assembly of lamins is regulated by Ran. XLB3 is only moderately soluble under conditions of neutral pH and physiological salt concentration (19), so we expected to observe an increase in insoluble XLB3 upon the addition of RanGTP to the complex. Aliquots of the Superose 6 column fractions containing the XLB3-importin complex were incubated with RanGDP or RanGTP (Fig. 3). Following a 90-min incubation, ∼10–20% of the XLB3 could be pelleted at 16,000 × g when RanGTP, but not RanGDP, was mixed with the XLB3 complex (compare P lanes). This demonstrates that the solubility of XLB3 in egg cytoplasm is at least partially Ran-dependent and that importin α binding to XLB3 maintains lamin solubility.
FIGURE 3.
Release of XLB3 from importin complex by RanGTP. The pooled XLB3 peak fractions from the gel filtration chromatography in Fig. 2 were incubated with 5 μm concentrations of the indicated proteins to release XLB3. After incubation, the soluble and insoluble XLB3 were separated by centrifugation as described under “Experimental Procedures.” The soluble (S) and pelleted (P) fractions were separated by SDS-PAGE and immunoblotted with antibodies to XLB3. Band intensity was determined with a Kodak 440CF Image Station.
If the solubility of XLB3 is maintained by the binding of importin α to its NLS, the addition of a synthetic protein import substrate containing the SV40 large T antigen NLS should act as a competitive inhibitor of XLB3 binding. This inhibition should result in an increase in the amount of insoluble XLB3. Addition of a fusion protein containing a single NLS was sufficient to displace ∼10–20% of the XLB3 from importin α and increase the pelletable fraction of XLB3 (Fig. 3, NLS lanes).
Purified lamins have limited solubility under physiological conditions, spontaneously forming aggregates or more ordered structures such as head-to-tail dimers or paracrystals (19, 30). Lamin solubility in the presence of importin α was measured in a pelleting assay using bacterially expressed and purified XLB3 and importin α (18). Purified XLB3 in 8 m urea was renatured by stepwise dialysis into a high pH buffer of moderate ionic strength in which it is soluble. Dilution of soluble XLB3 into a low salt buffer at pH 6.6 causes the XLB3 to aggregate into a form that can be pelleted at 16,000 × g. To determine whether purified importin α can maintain the solubility of XLB3 under these conditions, we added a 5-fold molar excess of purified importin α to XLB3 in the high pH buffer and then further diluted the mixture into a buffer at pH 7.3 to promote aggregation. We chose the higher pH rather than the more commonly used pH of 6.6 because importin α binds XLB3 weakly at the lower pH (data not shown).
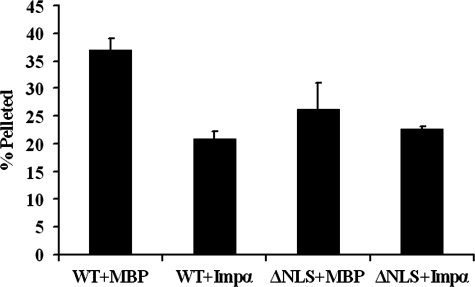
Under these conditions, with MBP added as a control, ∼35–40% of the XLB3 was pelletable (Fig. 4, WT+MBP bar). However, the addition of a 5-fold molar excess of importin α reduced the amount of pelletable XLB3 by almost half (Fig. 4, WT+Impα bar). Also, importin α had no effect on the solubility of XLB3 in which three residues of the NLS were changed so that XLB3 no longer binds importin α (Fig. 4, compare ΔNLS+MBP and ΔNLS+Impα bars) (26). At the concentration of importin α added, XLB3 pelleting was not completely inhibited because the self-interaction of XLB3 is in competition with the XLB3-importin α interaction. Once the lamin aggregates were formed, further incubation of the aggregate with importin α did not solubilize the XLB3 (data not shown). In these experiments, we also observed that XLB3 with a mutated NLS was more soluble than the wild-type lamin (Fig. 4, compare the percentage of protein pelleted in WT+MBP and ΔNLS+MBP bars). This was also repeatedly observed during purification of the XLB3 proteins from bacteria, as XLB3ΔNLS could be prepared as a more concentrated protein solution than wild-type XLB3 without the lamin precipitating (data not shown).
FIGURE 4.
Importin α prevents XLB3 aggregation in vitro. Bacterially expressed XLB3 or XLB3ΔNLS was dialyzed under buffer conditions that promote aggregation of lamins. Importin α (Impα) or purified MBP was added at a 5-fold molar excess over the concentration of XLB3 to inhibit aggregation. After incubation, the mixture was separated into soluble and insoluble (pellet) fractions by centrifugation. The soluble and pellet fractions were separated by SDS-PAGE, and the amount of XLB3 in each fraction was determined by Coomassie Brilliant Blue G-250 staining and quantification using a Kodak Image Station as described under “Experimental Procedures.” The amount of pelletable XLB3 is expressed as a percentage of the total XLB3 added. The error bars indicate average deviation of data from duplicate experiments. WT, wild-type.
At acidic pH in low salt buffers, lamins will assemble into paracrystalline structures that can be observed by negative staining and electron microscopy (Fig. 5, XLB3 panel). Negatively stained lamin paracrystals show a 24.5-nm axial repeat in thick bundles (19). When we added a 5-fold molar excess of importin α to XLB3 under conditions in which paracrystals form, paracrystal formation was inhibited. However, we did observe branching structures on the grid that had a fibrous appearance in places (Fig. 5, XLB3+Impα panels). This material possibly represents some of the pelletable material seen in the presence of importin α in the pelleting assay in Fig. 4. Also, as described above for the pelleting assay, the addition of importin α to pre-formed paracrystals did not solubilize any XLB3 (data not shown). As a control, we formed paracrystals from the purified XLB3 with a mutated NLS used in the pelleting assay above. The NLS mutant XLB3 formed paracrystals in the presence of a 5-fold molar excess of importin α (Fig. 5, XLB3ΔNLS and XLB3ΔNLS+Impα panels) indicating that binding of the importin to XLB3 inhibits higher order assembly of the lamins. The paracrystals formed from the XLB3 with a mutated NLS were always thinner than those formed from the WT lamin yet retained the 24.5-nm axial repeat spacing. This suggests that the NLS region of XLB3 may be involved in the formation of higher order lamin structures.
FIGURE 5.
Importin α inhibits formation of XLB3 paracrystals. Paracrystals of XLB3 were formed as described under “Experimental Procedures” and visualized by electron microscopy after negative staining. Importin α (Impα) was added in a 5-fold molar excess to inhibit paracrystal formation. For all micrographs, the magnification is ×25,000, and the scale bar = 200 nm. WT, wild-type.
DISCUSSION
Although the lamins have been studied since the 1970s, little is known about the mechanisms regulating their assembly in cells. Lamins are type V intermediate filament proteins consisting of a small N-terminal head segment, a long α-helical rod domain, and a non-α-helical C-terminal domain. The only structured portion of the C-terminal domain is an Ig fold (31, 32). Previous studies from our laboratory demonstrated the importance of the C terminus and Ig fold of XLB3 in lamin polymerization during the early stages of NE assembly in Xenopus interphase egg extracts (17, 18). The lamin Ig fold is a critical motif in the self-association of lamins in vitro and in vivo and likely constitutes part of the domain involved in the interaction of lamins with their many binding partners, as most of these bind to the C-terminal domain (33). Notably, the NLS in both A- and B-type lamins is located only a few residues upstream of the Ig fold. The proximity of the NLS to the Ig fold suggests that the binding of importin α to lamins is likely to affect the binding of other lamin-binding proteins in addition to the inhibition of lamin self-association demonstrated here. At least 18 related diseases known as laminopathies have been shown to be due to mutations in the LMNA gene that produce mature proteins with truncations or point mutations (2). The Ig fold in particular is a hot spot for mutations causing Emery-Dreifuss muscular dystrophy, familial partial lipodystrophy, dilated cardiomyopathy, limb girdle muscular dystrophy, mandibuloacral dysplasia, and Hutchinson-Gilford progeria syndrome (17). These mutated lamins may have altered assembly characteristics and defective interactions with their specific binding partners, including importin α, that could be responsible for the etiology of the disease.
Importins are important regulators of multiple cellular functions. Importin β has interphase roles in nuclear import as a motor adaptor for microtubule transport and as a chaperone for highly charged proteins. In contrast, centrosome dynamics, mitotic spindle assembly, nuclear membrane formation, and nuclear pore complex assembly are negatively regulated by importin β (34). The adaptor proteins for importin β, the importin α family, also play important negative regulatory roles in nuclear pore complex, nuclear membrane, and mitotic spindle assembly (23–25, 35). Evidence for the involvement of importins in lamin assembly was first suggested by knockdown experiments in the nematode C. elegans (23). Specific depletion of one of the three importin α proteins expressed in C. elegans, IMA-2, led to the production of embryos with defective NEs. Embryos depleted of IMA-2 could not form a complete NE around their chromosomes, and lamina assembly was inhibited. Depletion of the single lamin in C. elegans, LMN-1, gave a strikingly similar phenotype, suggesting that some of the effects of IMA-2 depletion may involve a direct effect on lamin assembly during formation of the NE (36). Depletion or addition of importin α from interphase Xenopus egg extracts also demonstrated a role for importin α in NE assembly (25). The removal of importin α from the egg extract could co-deplete components of the NE, and this is likely the mechanism for inhibition in those experiments. It is apparent from earlier studies and the work presented here that multiple aspects of nuclear assembly are disrupted when the concentration of importin α is experimentally manipulated (23, 25). Although the interruption of an early step in the assembly process may prevent the initiation or completion of downstream events, it is likely that importin α is an important regulator of several of these events. The concentrations of importin α and XLB3 in egg cytosol have been estimated to be 3 μm and 70 nm, respectively (20, 28). Therefore, importin α is in large excess over XLB3 in the cytosol and is likely to be bound to multiple cargoes. The results presented here indicate that, in addition to inhibition of other nuclear structures, assembly of a XLB3 polymer network is also disrupted by the addition of an excess of importin α. XLB3 has been shown previously to be required for the early steps of NE assembly in egg extracts (17, 18).
Importin α maintains XLB3 in a soluble form in the Xenopus egg. The release of XLB3 from importin α may represent a cooperative process regulating the temporal sequence of filament assembly as has been demonstrated for other importincargo interactions (37). Specifically, importin α inhibition of lamin-lamin interactions may be required to modulate the stepwise higher order assembly required for the controlled formation of the filamentous lamina, which takes place at the surface of chromosomes in the early stages of NE assembly during the anaphase/telophase transition (13).
The failure of RanGTP or a NLS to cause all of the XLB3 to become insoluble after incubation with the importin-XLB3 complex could be due to a variety of factors. First, XLB3 binds importin α in the absence of importin β (26) and, under the conditions of the experiment, the binding is probably sufficient to maintain XLB3 solubility. Second, XLB3 is relatively soluble in the buffers used in the experiments (see the pelleting assay in Fig. 4), with only about 40% of the bacterially expressed XLB3 becoming insoluble at pH 7.3. Phosphorylation of the XLB3 in the egg cytosol may also explain the lamin solubility. Earlier studies have shown the importance of phosphorylation of lamins in the assembly and disassembly of the lamina (10, 38, 39). We do not know the phosphorylation state of the native XLB3 in the importin α-XLB3 complex isolated from the egg extract. Phosphorylated XLB3 may not only be less likely to assemble into higher order structures but also have increased affinity for importin α. Phosphorylation of sites around the NLS of lamins has been reported to be important for their nuclear import (38, 40). The modification of NLS motifs by phosphorylation is an important mechanism for increasing the interaction of NLS-containing proteins with importins (41, 42).
Importin α may also assist in the incorporation of nascent lamins into the existing lamin network during interphase. Rather than simply releasing newly transported lamins into the nucleoplasm, the cooperative release of lamins from the importin, coupled with incorporation of the lamin into a filament, may drive the dissociation of the complex. Such a mechanism would be useful to prevent the inappropriate aggregation of lamins outside of the lamina. This would be particularly important during mitosis, when the lamins are depolymerized and mixed in the cytoplasm. The high molecular weight complex we observe in the egg cytosol suggests that lamins may be held in a tetrameric or oligomeric assembly state when the lamina is depolymerized. A more detailed analysis of the complex should add a great deal to our knowledge of the mechanisms for lamin assembly and disassembly in cells.
The ability of importin α to inhibit the self-interaction of lamins in our pelleting and paracrystal assembly assays suggests a role for the importins in regulating lamin assembly and thereby function. Lamins are found distributed throughout the nucleoplasm as well as in the nuclear envelope (4). Although the function of these nucleoplasmic lamins remains unresolved, it has been suggested that these more soluble lamins are directly involved in the organization of transcription and replication (4). Importin α binding to the lamins may regulate the interaction of other lamin-binding proteins with the lamins either in the nucleoplasm or at the lamina. This could be an important mechanism for the regulation of many lamin-associated activities in the cell.
Acknowledgments
We thank Dale Shumaker for reading of the manuscript and advice.
This work was supported by grants from the Ellison Foundation and NCI, National Institutes of Health Grant 5RO1 CA031760–25 (to R. D. G.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: NE, nuclear envelope; NPC, nuclear pore complex; NLS, nuclear localization sequence(s); MBP, maltose-binding protein; MES, 4-morpholineethanesulfonic acid; GST, glutathione S-transferase; EGFP, enhanced green fluorescent protein.
S. A. Adam, K. Sengupta, and R. D. Goldman, unpublished results.
References
- 1.Burke, B. (1990) Curr. Opin. Cell Biol. 2 514-520 [DOI] [PubMed] [Google Scholar]
- 2.Mattout, A., Dechat, T., Adam, S. A., Goldman, R. D., and Gruenbaum, Y. (2006) Curr. Opin. Cell Biol. 18 335-341 [DOI] [PubMed] [Google Scholar]
- 3.Dorner, D., Gotzmann, J., and Foisner, R. (2007) FEBS J. 274 1362-1373 [DOI] [PubMed] [Google Scholar]
- 4.Shumaker, D. K., Kuczmarski, E. R., and Goldman, R. D. (2003) Curr. Opin. Cell Biol. 15 358-366 [DOI] [PubMed] [Google Scholar]
- 5.Nakajima, N., and Abe, K. (1995) FEBS Lett. 365 108-114 [DOI] [PubMed] [Google Scholar]
- 6.Sinensky, M., Fantle, K., Trujillo, M., McLain, T., Kupfer, A., and Dalton, M. (1994) J. Cell Sci. 107 61-67 [DOI] [PubMed] [Google Scholar]
- 7.Rober, R. A., Weber, K., and Osborn, M. (1989) Development (Camb.) 105 365-378 [DOI] [PubMed] [Google Scholar]
- 8.Loewinger, L., and McKeon, F. (1988) EMBO J. 7 2301-2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dessev, G., Iovcheva-Dessev, C., Bischoff, J. R., Beach, D., and Goldman, R. (1991) J. Cell Biol. 112 523-533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerace, L., and Blobel, G. (1980) Cell 19 277-287 [DOI] [PubMed] [Google Scholar]
- 11.Foisner, R. (2003) Sci. World J. 3 1-20 [Google Scholar]
- 12.Ellenberg, J., and Lippincott-Schwartz, J. (1999) Methods 19 362-372 [DOI] [PubMed] [Google Scholar]
- 13.Moir, R. D., Yoon, M., Khuon, S., and Goldman, R. D. (2000) J. Cell Biol. 151 1155-1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke, P. R., and Zhang, C. (2004) Symp. Soc. Exp. Biol. 56 193-204 [PubMed] [Google Scholar]
- 15.Newport, J. W., Wilson, K. L., and Dunphy, W. G. (1990) J. Cell Biol. 111 2247-2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins, H., Holman, T., Lyon, C., Lane, B., Stick, R., and Hutchison, C. (1993) J. Cell Sci. 106 275-285 [DOI] [PubMed] [Google Scholar]
- 17.Shumaker, D. K., Lopez-Soler, R. I., Adam, S. A., Herrmann, H., Moir, R. D., Spann, T. P., and Goldman, R. D. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 15494-15499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Soler, R. I., Moir, R. D., Spann, T. P., Stick, R., and Goldman, R. D. (2001) J. Cell Biol. 154 61-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moir, R. D., Donaldson, A. D., and Stewart, M. (1991) J. Cell Sci. 99 363-372 [DOI] [PubMed] [Google Scholar]
- 20.Gorlich, D., Prehn, S., Laskey, R. A., and Hartmann, E. (1994) Cell 79 767-778 [DOI] [PubMed] [Google Scholar]
- 21.Dickmanns, A., Bischoff, F. R., Marshallsay, C., Luhrmann, R., Ponstingl, H., and Fanning, E. (1996) J. Cell Sci. 109 1449-1457 [DOI] [PubMed] [Google Scholar]
- 22.Stick, R. (1988) EMBO J. 7 3189-3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geles, K. G., Johnson, J. J., Jong, S., and Adam, S. A. (2002) Mol. Biol. Cell 13 3138-3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Askjaer, P., Galy, V., Hannak, E., and Mattaj, I. W. (2002) Mol. Biol. Cell 13 4355-4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hachet, V., Kocher, T., Wilm, M., and Mattaj, I. W. (2004) EMBO J. 23 1526-1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai, M. Y., Wang, S., Heidinger, J. M., Shumaker, D. K., Adam, S. A., Goldman, R. D., and Zheng, Y. (2006) Science 311 1887-1893 [DOI] [PubMed] [Google Scholar]
- 27.Nigg, E. A. (1992) Semin. Cell Biol. 3 245-253 [DOI] [PubMed] [Google Scholar]
- 28.Lourim, D., and Krohne, G. (1998) J. Cell Sci. 111 3675-3686 [DOI] [PubMed] [Google Scholar]
- 29.Kobe, B. (1999) Nat. Struct. Biol. 6 388-397 [DOI] [PubMed] [Google Scholar]
- 30.Aebi, U., Cohn, J., Buhle, L., and Gerace, L. (1986) Nature 323 560-564 [DOI] [PubMed] [Google Scholar]
- 31.Krimm, I., Ostlund, C., Gilquin, B., Couprie, J., Hossenlopp, P., Mornon, J. P., Bonne, G., Courvalin, J. C., Worman, H. J., and Zinn-Justin, S. (2002) Structure 10 811-823 [DOI] [PubMed] [Google Scholar]
- 32.Dhe-Paganon, S., Werner, E. D., Chi, Y. I., and Shoelson, S. E. (2002) J. Biol. Chem. 277 17381-17384 [DOI] [PubMed] [Google Scholar]
- 33.Zastrow, M. S., Vlcek, S., and Wilson, K. L. (2004) J. Cell Sci. 117 979-987 [DOI] [PubMed] [Google Scholar]
- 34.Harel, A., and Forbes, D. J. (2004) Mol. Cell 16 319-330 [DOI] [PubMed] [Google Scholar]
- 35.Quimby, B. B., and Dasso, M. (2003) Curr. Opin. Cell Biol. 15 338-344 [DOI] [PubMed] [Google Scholar]
- 36.Liu, J., Rolef Ben-Shahar, T., Riemer, D., Treinin, M., Spann, P., Weber, K., Fire, A., and Gruenbaum, Y. (2000) Mol. Biol. Cell 11 3937-3947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pemberton, L. F., Rosenblum, J. S., and Blobel, G. (1999) J. Cell Biol. 145 1407-1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haas, M., and Jost, E. (1993) Eur. J. Cell Biol. 62 237-247 [PubMed] [Google Scholar]
- 39.Eggert, M., Radomski, N., Linder, D., Tripier, D., Traub, P., and Jost, E. (1993) Eur. J. Biochem. 213 659-671 [DOI] [PubMed] [Google Scholar]
- 40.Leukel, M., and Jost, E. (1995) Eur. J. Cell Biol. 68 133-142 [PubMed] [Google Scholar]
- 41.Hubner, S., Xiao, C. Y., and Jans, D. A. (1997) J. Biol. Chem. 272 17191-17195 [DOI] [PubMed] [Google Scholar]
- 42.Fontes, M. R., Teh, T., Toth, G., John, A., Pavo, I., Jans, D. A., and Kobe, B. (2003) Biochem. J. 375 339-349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chi, N. C., Adam, E. J., Visser, G. D., and Adam, S. A. (1996) J. Cell Biol. 135 559-569 [DOI] [PMC free article] [PubMed] [Google Scholar]