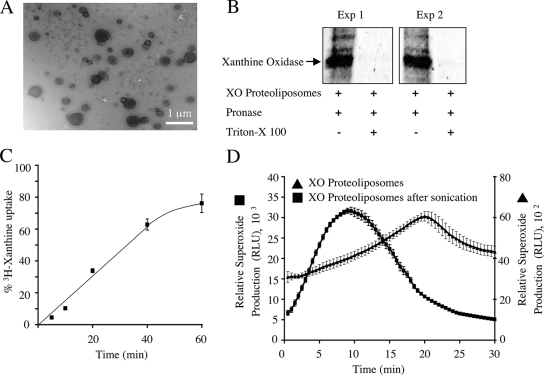
FIG. 5.
Proteoliposome model for generating intraluminal ·O2− anions. MCF-7 cells were treated with IL-1β (5 ng/ml), and peak endosomal fractions were concentrated by centrifugation at 100,000 × g for 2 h. Reconstituted proteoliposomes were generated with encapsulated xanthine oxidase. (A) Electron micrograph of xanthine oxidase-loaded proteoliposome. (B) To evaluate encapsulation of xanthine oxidase, the proteoliposomes were incubated in PBS with pronase or in PBS with pronase plus Triton X-100 (0.5%) at 37°C for 30 min. The samples were then separated by SDS-PAGE and analyzed by Western blotting using an anti-xanthine oxidase antibody. (C) The ability of xanthine to enter the proteoliposomes was investigated by incubating the vesicles with [3H]xanthine. Xanthine taken up by proteoliposomes was separated from free xanthine by column chromatography, and the radioactivity associated with the vesicles was measured (values are means and standard errors; n = 3). The time-dependent accumulation of [3H]xanthine within proteoliposomes is expressed as a percentage of maximal loading, as determined by direct encapsulation of [3H]xanthine into proteoliposomes at the time of their synthesis. (D) The ability of xanthine oxidase-loaded proteoliposomes to generate ·O2− in the presence of extravesicular xanthine was determined using lucigenin-based chemiluminescence. Both native and sonicated proteoliposomes were evaluated for ·O2− production (values are means and standard errors of the means; n = 3). RLU, relative light units.