Abstract
DNase II enzymes are highly conserved proteins that are required for the degradation of DNA within phagolysosomes. Engulfment of apoptotic cells and/or bacteria by phagocytic cells requires the function of DNase II to completely destroy ingested DNA. Mutation of the dnase II gene results in an increase of undegraded apoptotic DNA within phagocytic cells in mice and nematodes. Additionally, reduction of DNase II enzymatic activity in Drosophila melanogaster has been shown to lead to increased accumulation of DNA in the ovaries. Due to the importance of DNA clearance during infection, we hypothesized that a severe reduction of DNase II activity would result in diminished immune function and viability. To test this hypothesis, we knocked down DNase II activity in flies using RNAi. As expected, expression of a dnase II-RNAi construct in flies resulted in a dramatic reduction of DNase II activity and a significant decrease in total hemocyte numbers. Furthermore, infection of dnase II-RNAi flies with Gram negative or positive bacteria resulted in a severe reduction in fly viability. These results confirm that DNase II and the ability to clear macromolecular DNA is essential for maintaining proper immune function in Drosophila.
Keywords: DNase II, Drosophila, Innate immunity, Hemocytes, Fruit fly, Immunodeficiency, Microbial infection
1. Introduction
DNase II enzymes belong to a unique family of nucleases whose main function is to degrade DNA within phagolysosomes [1,2]. Unlike most nucleases, these enzymes do not require divalent cations and are most active at an acidic pH [1,2]. Consistent with a generalized role in DNA degradation and an acidic pH optima, DNase II enzymes exhibit a ubiquitous tissue distribution and are localized within lysosomes [1,3]. While DNase II enzymes can degrade DNA substrates to completion, they also exhibit cleavage preferences that are site- and species-specific [1,4-6].
Early studies in the nematode Caenorhabditis elegans were the first to suggest that DNase II enzymes were required for engulfment-mediated DNA degradation [7,8]. Mutation of the C. elegans dnase II homologue, nuc-1, resulted in persistent dead-cell nuclei within neighboring phagocytic cells as well as accumulation of DNA within the gut and ovaries [7,8]. Interestingly, targeted mutation of the dnase II gene in the germline of mice resulted in perinatal lethality presumably due to loss definitive erythropoiesis [9,10]. Subsequent analyses of these mutant mice revealed that dnase II-deficient macrophages accumulate ingested DNA and overexpress β-interferon (INFβ) resulting in embryonic lethality [11,12].
As in C. elegans, early biochemical studies in Drosophila melanogaster demonstrated the presence of an acid endonuclease activity in crude animal extracts [13]. Mutants deficient in an acidic nuclease were subsequently generated and partially characterized [14,15], but the gene encoding this enzyme was not identified. Our database queries using the C. elegans NUC-1 protein sequence identified a highly homologous open reading frame in D. melanogaster (CG7780) which we subsequently demonstrated to encode the fly homologue of DNase II/NUC-1 [6]. The fly nuclease was found not only to be highly homologous in sequence to the other DNase II family members, particularly in the catalytic domain, but also in its enzymatic activity and requirements [6].
During the process of programmed cell death, DNA degradation is initiated by the action of caspase-activated DNase (CAD) [16]. Although CAD is clearly essential for the initiation of inter-nucleosomal DNA cleavage, this activity is apparently dispensable as CAD-deficient mice do not exhibit phenotypic abnormalities [17]. In these mutant mice, removal of apoptotic cell DNA was apparently efficiently mediated by phagocytosis and the action of DNase II [17]. As would be expected, CAD-deficient flies exhibit a loss of nucleosomal DNA fragmentation, while flies deficient in DNase II (DNase IIlo mutants) exhibited an enhanced fragmentation phenotype (due to the action of CAD [1,18]). Since flies deficient in both enzymes did not contain fragmented/degraded DNA [18], it would appear that CAD is required to generate the nucleosomal fragments while DNase II is required to completely degrade such fragments. DNase II-deficient flies were generated by introducing a dnase II-RNAi construct into the germline of flies (see Fig. 1) in an attempt to determine its biological function. As described herein, our results demonstrate that flies deficient in DNase II are highly susceptible to bacterial infection due, at least in part, to the loss of phagocytic function.
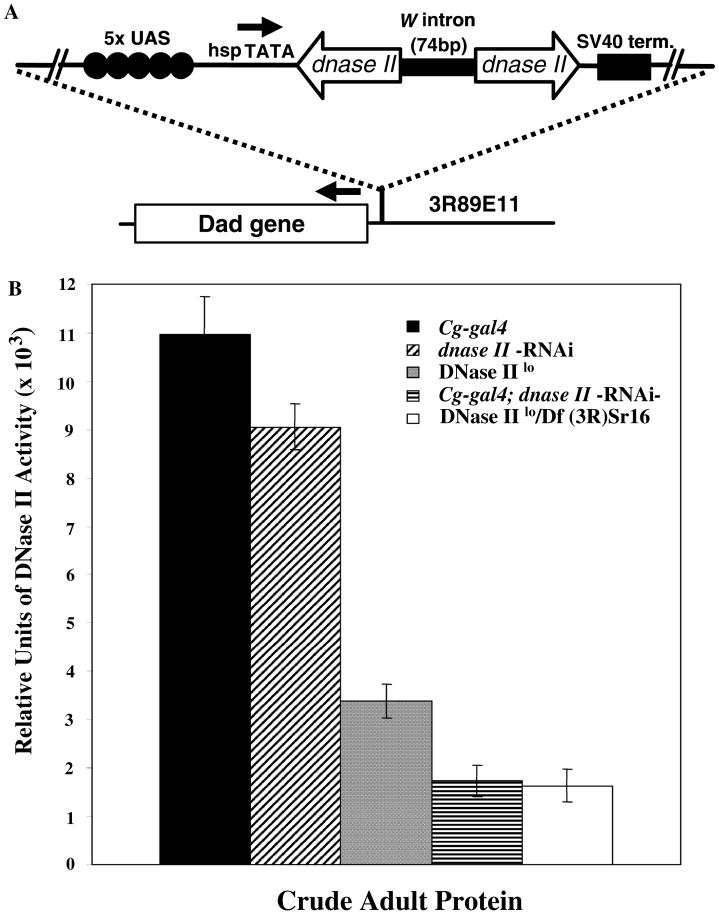
Fig. 1.
Generation of dnase II-RNAi transgenic fly lines and analysis of DNase II activity in these animals. (A) An RNAi vector (pWIZ) was modified to contain two inverted copies of the fly dnase II gene (from nt position 39-639). The site of integration was determined to be upstream of the Dad gene on chromosome 3 (3R89E11). The site of integration was verified by inverse PCR and sequencing of the region adjacent to the RNAi transposon. Arrows indicate direction of transcription from either the tandem GAL4 binding sites (5× UAS, upstream activation sequences) or the Dad promoter region. (B) DNase II enzyme activity in crude extracts derived from the various fly lines used in this study determined by a radial diffusion assay (see Section 2.4). The activity of the Cg-gal4 and the dnase II-RNAi lines was compared to Cg-gal4; dnase II-RNAi homozygous flies that resulted from the cross between the first two lines. In addition, extracts derived from homozygous DNase IIlo and heterozygous DNase IIlo/Df(3R)Sr16 (see text for details) mutant lines were also tested alongside the RNAi lines for comparison purposes. The relative units of activity from crude extracts (3 mg per sample) were determined as described in Section 2.4 and represent the average and standard deviation of the mean of three assays performed at the same time.
2. Materials and methods
2.1. Drosophila stocks
The GAL4 driver lines used in this study were C564-gal4 (w[1118]; P{w[+mW.hs] = GawB}c564) and Cg-gal4 (w[1118]; P{w[+mC] = Cg-gal4.A}2) and these were obtained from the Blomington fly stock center. In the C564-gal4 line, the GAL4 transcriptional activator is expressed in the larval brain, leg disc, fat body, gut and salivary glands, adult male accessory gland, seminal vesicle, ejaculatory duct, testis sheath, gut, and cyst cells [19,20]. In the collagen type IV-specific enhancer-promoter Cg-gal4 line, GAL4 is predominantly expressed in the fat body, anterior-most lobes of the lymph gland and hemocytes but apparently not in lamellocytes [21]. Additional lines that were obtained from the fly stock center were the following: yw, DNase IIlo, Df(3R)Sr16 or w[*]:Df(3R)Sr16, h[1] red[1] ca[1]/Tm3, P{ry[+T7.2] = ftz/lacC}SC1, and Sb[1] ry[RK]. W1118 and the chromosome balancer-marker lines were generously provided by Dr. Kyung-An Han (Penn State University). Additionally, the following crosses and lines were generated for this study: yw; 2; DNase IIlo, Df(3R)Sr16, h[1] red[1] ca[1]/DNase IIlo, C564-gal4; dnase II-RNAi and Cg-gal4; dnase II-RNAi. All fly lines were maintained under standard conditions at 25 °C.
2.2. RNAi vector construction and microinjection
Two identical 601 bp fragments (bp 39-639) of the Drosophila dnase II gene were generated by PCR amplification with the following primer sets: 5′-CTAGCTAGCGGCGATCTCTGTGCTTCGT-3′ (NheI-DNase II), 5′-GCTCTAGACAGGCTCGGGAACAGCTC-3′ (XbaI-DNase II), 5′-GACTAGTGCGATCTCTGTGCTTCGT-3′ (SpeI-DNase II), and 5′-GAAGATCTCAGGCTCGGGAACAGCTC-3′ (BglII-DNase II). The amplified fragments were purified and cloned into the pGEM-T vector (Promega. Madison, WI) and excised from the vector with either NheI/XbaI or SpeI/BglII restriction enzymes, respectively. The two fragments were then subcloned into the pWiz plasmid to generate an inverted stem-loop structure (see Fig. 1; [22]). Microinjection on W1118 embryos was followed by standard P-element mediated germ line transformation [23].
2.3. Ligase-mediated inverse PCR
To determine the insertion site of the RNAi construct, we used an inverse PCR method [24,25]. Genomic DNA was isolated from 50 flies using a modification of the method described by Ballinger and Benzer [26]. Twenty microliters of DNA (isolated from 10 flies) was digested with Sau3AI for 3 h at 37 °C in a 50 μl reaction volume. A ligation reaction was then performed in a 50 μl volume using half the digested DNA. The ligated samples were used as templates for PCR with the following primers: p-31(p-element): 5′-CGACGGGACCACCTTATGTTATTTCATCATG-3′, pWiz-F1: 5′-TAGAGCCAGATATGCGAGCAC-3′and pWiz-R1: 5′-GTCCGTGGGGTTTGAATTAAC-3′. Two different size products were amplified with p-31/pWiz-R1 (∼0.8 Kb) and p-31/pWiz-F1 (∼0.9 Kb). After cloning the amplified fragments into the pGEM-T vector, the inserts were sequenced using the SequiTherm EXCEL™ II DNA sequencing Kit-LC (Epicentre technologies, Madison, WI).
2.4. Detection of DNase II enzymatic activity
Adult fly crude protein extracts were obtained from animals crushed with a micro-pestle in 50 mM sodium acetate buffer (pH 5.0) and assayed using radial diffusion assay as previously described [6]. After obtaining a digital image of the extent of DNA digestion by DNase II, the diameter and intensity (pixels) of area of digestion was measured using the SigmaScan densitometry program (Systat Software Inc., Richmond, CA).
2.5. Generation of recombinant protein and anti-dDNase II antisera
A fragment encoding for a portion of the carboxyl-terminus of DNase II was amplified using the following primers: 5′-CATATGATGCTCTGCGTCACACTGAA-3′ and 5′-GGATCCCTATTCCTGTTGGGCGCACT-3′. The amplified fragment of 638 bp was subcloned into the NheI/BamHI sites of pET15b vector (Novagen, San Diego, CA). After protein induction with 1 mM IPTG for 7 h, recombinant protein was separated using SDS-PAGE gel and the induced ∼27 KDa protein was extracted by electroelution [27]. The purified protein was subsequently used to immunize rabbits by standard immunization procedures (Proteintech Co. Chicago, IL).
2.6. Quantitative real-time PCR
Total RNA was isolated from 3rd instar larvae using Trizol reagent (Invitrogene, Carlsbad, CA). RNA (2 μg) from each fly line was used for cDNA synthesis with oligo (dT)15 primer (Promega, Madison, WI). The first strand cDNA synthesis was performed by Titan One Tube RT-PCR System (Roche Applied Science, Indianapolis, IN). A dilution (1/10) of the synthesized material was used as a template for PCR. Real-time PCR was performed with iQ™ SYBR Green Supermix using a Bio-Rad iCycler (Bio-Rad, Richmond, CA). The following primers were used to amplify the actin 79B, dnase II, and Dad genes: for actin 79B: 5′-CCACGCCATCCTTCGTCTA-3′and 5′-GCACAGCTTCTCCTTGATGTC-3′; for dnase II: 5′-GCTGTTTGGCAAGAGTGGA-3′ and 5′-CGCAGCTATTCGGTAAGTTG-3′; and for Dad: 5′-ACTTGACGTATTGCCACGAGA-3′ and 5′-GAAAGGCGAAAAAGTCCGATA-3′. Reaction parameters were 2 min at 95 °C followed by 45 cycles of 10 s at 95 °C, 15 s at 62 °C and 20 s at 72 °C. Results were analyzed using the 2-ΔΔCt method [28,29] and a standard mathematical model [30].
2.7. Microorganisms and survival tests
Escherichia coli DH5α-GFP and the Micrococcus luteus strains used in this study were provided by Drs. David Schneider (Stanford University, USA) and Bruno Lemaitre (CNRS-CGM, France), respectively. A total of 100 flies (two days old) were injected with bacteria using a sharpened tungsten needle (0.2 mm diameter) previously dipped into a highly concentrated bacterial culture (OD = 1. 3-1.5). After infection, flies were transferred to a new vial and maintained at 29 °C. The few flies that died within two hours post-infection were not included in the analysis as they probably died from physical trauma.
2.8. Hemocyte collection and immunodetection
Hemocytes were collected by cutting the larval cuticle with fine forceps under stereomicroscope [31,32]. The number of cells per milliliter was estimated by counting them on a hemocytometer. At least 10 larvae were counted per individual strain. For in situ detection of DNase II protein in larval hemocytes, cells were first fixed with IntraPrep reagent 1 for 15 min and permeabilized with reagent 2 for 5 min (IntraPrep Kit, Beckman Coulter, Miami, FL). Fixed cells were blocked with PBS containing 5% normal goat serum (NGS) for 2 h at 4 °C and incubated with anti-dDNase II antibodies (1:500). After washing with PBS containing 1% NGS, the fixed cells where incubated with FITC-conjugated goat anti-rabbit IgG (1:500: Santa Cruz Biotech, Santa Cruz, CA). As controls for non-specific binding, pre-immune rabbit serum was used instead of the anti-DNase II antisera. Stained cells and tissues were visualized by standard fluorescence microscopy (Axiovert 200, Zeiss). For Western blot analysis, a single 3rd instar larvae from each line was homogenized in 24 μl protease inhibitor buffer (one tablet of Complete Mini [Roche Diagnostics, Indianapolis, IN] dissolved in 1.5 ml water). The crude extract was mixed with 6 μl 5× SDS sample buffer and separated by electrophoresis on a 12% SDS-PAGE gel and transferred to 0.2 μm nitrocellulose membrane (MSI, Westboro, MA). The membrane was blocked with TBST buffer containing 2% non-fat dry milk for 1 h and then incubated overnight at 4 °C with anti-dDNase II anti-sera (1:2000). After washing three times for 10 min with TBST buffer, HRP-conjugated goat anti-rabbit IgG (1:3000; Pierce, Rockford, IL) was added to detect the specific complex. The signal was subsequently visualized using SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL).
3. Results
3.1. Use of RNA interference to reduce DNase II activity in flies
In order to determine the function of DNase II in D. melanogaster, RNA interference (RNAi) [33,34] was utilized to significantly reduce DNase II activity. Fly embryos were injected with a vector carrying two inverted copies of the dnase II gene separated by the White gene intron engineered within the pWiz transformation vector [22]; see Fig. 1A. Flies carrying a copy of the RNAi construct (referred to as dnase II-RNAi) were selected by expression of the White gene (red eye color; [22]). In order to deplete DNase II in hemocytes, flies carrying the dnase II-RNAi construct were crossed with the Collagen-gal4 (Cg-gal4) driver line that expresses high levels of the GAL4 transcriptional activator in hemocytes and the fat body [21,35]. As shown in Fig. 1B, DNase II enzymatic activity was diminished by approximately 6-fold in adult flies expressing dnase II-RNAi construct, while extracts obtained from Cg-gal4 and dnase II-RNAi controls exhibited high levels of DNase II activity. Comparison between RNAi expressing flies and those that contain a point mutation within the DNase II gene (DNase IIlo), which significantly reduces enzymatic activity [6], revealed that extracts derived from DNase IIlo flies exhibit higher levels (∼1.8×) of activity than those detected in dnase II-RNAi expressing flies (Fig. 1B). Interestingly, the fold reduction of DNase II activity detected in Cg-gal4; dnase II-RNAi flies was similar to that detected in DNase IIlo/Df(3R)Sr16 heterozygous flies, which carry a DNase IIlo allele and an extended chromosomal deletion of the dnase II locus on the other allele [6].
3.2. Cg-gal4; dnase II-RNAi flies have reduced levels of DNase II protein and mRNA
To assess the reduction in DNase II protein in larvae expressing dnase II dsRNA, polyclonal antibodies were generated against the carboxy-terminal portion of a Drosophila DNase II recombinant protein (see Section 2 for details). As expected, Western blot analysis of crude extracts with the anti-Drosophila DNase II (dDNase) antibodies confirmed that the loss of enzymatic activity was due to a severe reduction of dDNase II protein in Cg-gal4; dnase II-RNAi larvae as compared to controls (Fig. 2A). To confirm that the reduction in dDNase II protein was due to the degradation of dnase II mRNA, real-time PCR was utilized to determine the fold decrease in the dnase II message in flies expressing the RNAi construct. As shown in Fig. 2B, dnase II-RNAi expressing flies exhibited an approximate 5-fold lower level of dnase II message than controls. Furthermore, the expression pattern of the actin (79B) and Dad genes in the Cg-gal4; dnase II-RNAi line was found to be within the normal range of expression when compared to the appropriate controls (Fig. 2B).
Fig. 2.
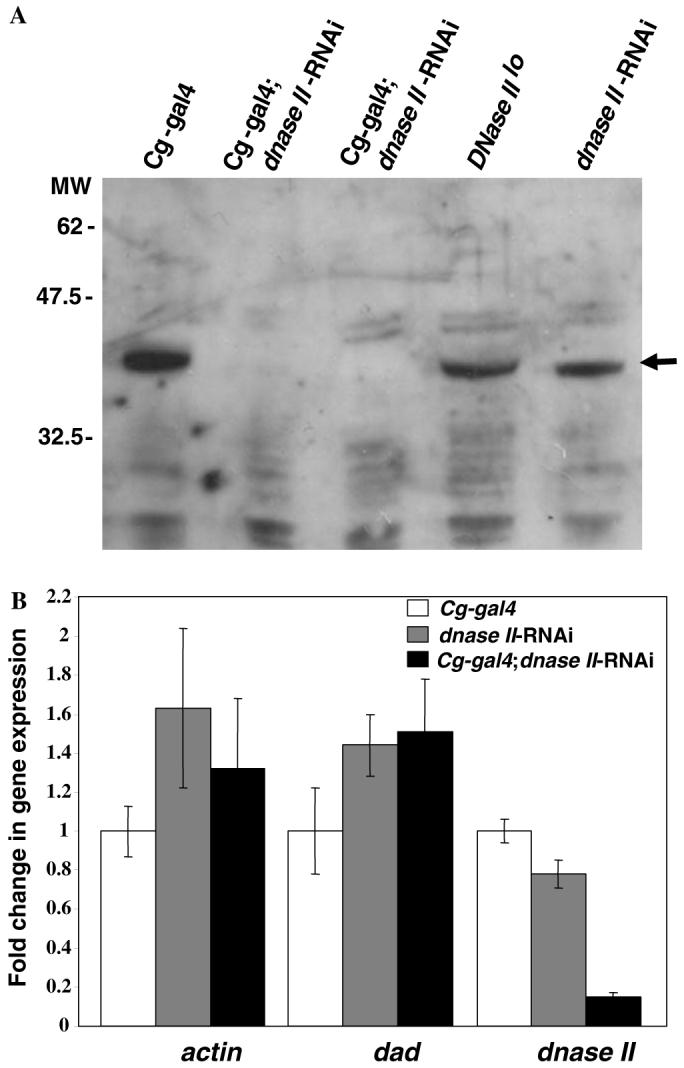
Analysis of DNase II protein and message. (A) Western blot analysis of protein extracts derived from control (Cg-gal4, dnase II-RNAi, DNase IIlo lines), and Cg-gal4; dnase II-RNAi larvae. Arrow indicates the position of the specific protein detected with anti-DNase II antibodies (see Section 2). The relative position of molecular weight (MW) marker proteins is shown on left side in KDa. (B) Expression analysis of the dnase II, actin and dad genes in control (Cg-gal4 and dnase II-RNAi) and Cg-gal4; dnase II-RNAi larvae. Bars represent standard deviations of the mean from three independent assays.
3.3. Bacterial infection causes a dramatic reduction in viability in DNase II-deficient flies
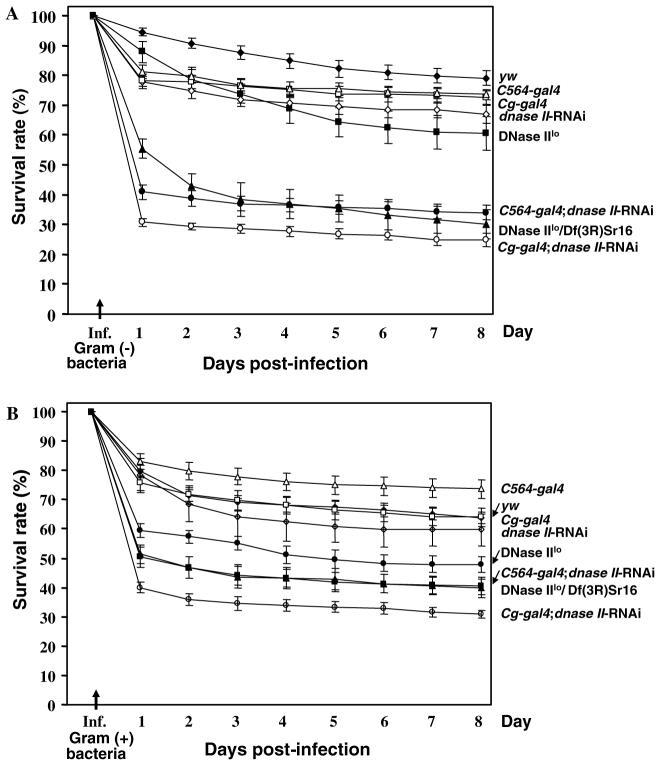
To explore the impact of DNase II deficiency on immune function, flies expressing dnase II-RNAi were examined for increased susceptibility to bacterial infection. Upon challenge with either Gram(+) or Gram(-) bacteria, the Cg-gal4 and dnase II-RNAi lines were found to be similar to the yw control line, with a modest 15-30% reduction in viability by eight days post-infection (Fig. 3). However, viability was significantly reduced in flies deficient in DNase II activity and this reduction correlated with DNase II activity levels (see Fig. 1B). For example, homozygous DNase IIlo flies [6] were only partially affected by infection with Gram(+) [55%] or Gram(-) [35%] bacteria. However, loss in viability was more pronounced in DNase IIlo/Df(3R)Sr16 heterozygous flies, which contain similar levels of nuclease activity as the RNAi expressing lines (see Fig. 1B). Interestingly, the loss of viability in these heterozygous dnase II-deficient flies closely resembled the loss viability observed in the C564-gal4; dnase II-RNAi line which express GAL4 in a variety of tissues including the fat body and hemocytes (see Fig. 3). However, the most dramatic loss of viability was observed in flies expressing dnase II dsRNA with the Cg-gal4 driver. By 8 days post-infection, the viability of Cg-gal4; DNase II RNAi flies was reduced 70 and 65% with Gram(-) (Fig. 3A) and Gram(+) bacteria, respectively (Fig. 3B). Interestingly, the most precipitous drop in viability, >60%, was observed within one day of infection (Figs. 3A and B).
Fig. 3.
Septic injury of DNase II-deficient flies results in severe reduction of viability. Injection of Gram negative (E. coli; A) or positive (M. luteus; B) bacteria led to a precipitous drop in viability after infection (arrow) but only in DNase II-deficient lines. Expression of the dnase II-RNAi construct by means of the potent transcriptional activator GAL4 was accomplished by crossing this line with the C564-gal4 or the Cg-gal-4 fly lines (see Section 2.1 for details). Infection of control flies that were either not defective in DNase II expression (yw wild-type control) or did not express the RNAi construct (C564-gal4, Cg-gal4, [UAS] dnase II-RNAi) were also subjected to bacterial infection. Additionally, the previously characterized DNase II-deficient lines, DNaseIIlo and a DNaseIIlo/Df(3R)Sr16, were also subjected to infection. Results are presented as the percentage of flies surviving infection.
3.4. Effects of dnase II-RNAi expression on dDNase II levels and hemocyte numbers
As can be seen in Fig. 4, immunofluorescence microscopy revealed that dDNase II is localized within lysosomes of hemocytes but almost completely absent from dnase II-RNAi expressing cells (see arrows, Figs. 4C and D). Apart from the loss of DNase II protein, one interesting outcome of this analysis was the noticeable reduction in the total number of hemocytes in DNase II-deficient flies. DNase IIlo flies contained approximately 37% less hemocytes, in comparison to control flies (yw; Fig. 4E). Furthermore, hemocyte numbers were also significantly reduced in all dnase II-RNAi expressing fly lines with Cg-gal4; dnase II-RNAi and C564-gal4; dnase II-RNAi flies containing ∼46 and 19% lower numbers than controls, respectively. Although it would appear that the dnase II-RNAi control line was also depressed in hemocyte numbers, when compared to the appropriate background control line (W1118), the number of hemocytes in this line was found to be similar to the control (data not shown). As demonstrated above, DNase II deficiency leads not only to reduced viability after bacterial infection but also a reduction of the cells needed to combat the infection.
Fig. 4.
Detection of DNase II within hemocytes and determination of total hemocyte numbers. Detection of DNase II within hemocytes with anti-dDNase II antibodies revealed an intra-organelle localization in control hemocytes (Cg-gal4; A-B). A similar pattern was detected by staining with LysoTracker Red (Molecular Probes, Carlsbad, CA; data not shown) indicating that like other family members, dDNase II is localized within lysosomes. Little if any (see arrows) signal was detected in Cg-gal4; dnase II-RNAi hemocytes (C-D). (A) and (C) show the merged DIC and fluorescent images while (B) and (D) show only the fluorescent (FITC) signal detected with the anti-dDNase II antibodies, (E) Hemocyte counts from control (yw, Cg-gal4, C564-gal4, and dnase II-RNAi) and dnase II-RNAi expressing (Cg-gal4; dnase II-RNAi and C564-gal4; dnase II-RNAi) larvae.
4. Discussion
Since we were unable to generate dnase II-null mutant flies by chemical (EMS) mutagenesis possibly due to a lethal effect of the mutation, we opted to reduce the expression of this gene by RNAi. As shown in Fig. 1A, the dnase II-RNAi construct was introduced immediately upstream of the Dad (Daughters against Decapentaplegic [Dpp]) gene. Dad is a distant member of the SMAD family of proteins and shares sequence similarity with Drosophila Mad (Mothers against dpp) protein which is required for Dpp-mediated signaling [36]. Expression of Dad is activated by Dpp and overexpression of DAD leads to a feedback inhibition of Dpp [36]. Real-time PCR analysis of Dad mRNA expression revealed that transposon insertion did not significantly affect expression of the Dad gene (Fig. 2B). Based on this result and the fact that flies deficient in DNase II [DNase IIlo/Df(3R)Sr16] are also highly susceptible to infection (Fig. 3), it is very unlikely that the site of integration near the Dad gene had anything to do with the immunodeficiency detected in dnase II-RNAi expressing lines. Furthermore, a defect in Dad gene expression in the RNAi lines would have been detected by defects in wing development and this was not observed.
Drosophila has an effective immune system comprised of both humoral and cellular responses [37,38]. While the humoral response is mediated by induction of antimicrobial peptides, the cellular response is mediated by hemocytes which are mainly composed of macrophage-like cells called plasmatocytes and crystal cells [35,37,38]. The lymph gland is the major site of hemocyte differentiation and the ensuing mature hemocytes compose a significant proportion of the hemolymph [39]. The hemolymph, which in essence is the insect’s circulatory system [39], is the major site for pathogen encounter and clearance. As in most organisms with an immune response, both arms of the fly immune system act cooperatively in the neutralization and removal of invading pathogens. Disruption of either the cellular or humoral response lowers the insect’s ability to counteract invading pathogens. Interestingly, inhibition of the ability of plasmatocytes to engulf bacteria by the prior application of polystyrene beads into the body cavity results in a significant loss of viability in flies carrying a mutation in the humoral response [40]. Interestingly the negative effect mediated by bead accumulation in plasmatocytes resembled the deleterious effect of accumulation of apoptotic nuclei in macrophages of dnase II-/- mice [10]. Due to this resemblance, we predicted that the loss of DNase II activity would result in the accumulation of DNA within phagocytic cells with a concomitant loss of immune function. Interestingly, a recent analysis of hemocytes isolated from DNase IIlo and dnase II-RNAi expressing lines (and controls) did not reveal an accumulation of extranuclear undegraded DNA in these cells (data not shown). It is therefore likely that the low levels of nuclease activity present in DNase II-deficient lines is sufficient to degrade ingested DNA.
It has been recently reported that reduction of DNase II activity in dnase II hypomorphic DNase IIlo mutant flies resulted in an increased expression of the antibacterial genes, diptericin and attacin A, and this activation was further increased in flies deficient for both the dDNase II and dCAD activities [18]. Although the mechanism for enhanced antimicrobial peptide production was not elucidated, it was suggested that this enhancement might be triggered by an increase in undegraded DNA in a manner analogous to the immunostimulatory effects of bacterial CpG DNA [18]. Although an increase in antimicrobial peptide production would be expected to partially compensate for the loss of hemocyte function, this was clearly not the case. This perplexing result is reminiscent of the embryonic lethality observed in dnase II-knockout mice which is caused by an unexpected overexpression of INF-β by macrophages [10-12]. Apart from antimicrobial peptide induction, it has been demonstrated that dDNase II itself is significantly induced by bacterial and fungal infection [41,42]. Since DNase II enzymes can be secreted into the extracellular environment [1], overexpression and secretion of dDNase II may enhance the degradation of released bacterial DNA not yet been cleared by phagocytes. Thus, a deficiency in this enzyme may not only affect how hemocytes cope with large quantities of ingested bacterial DNA but it may also result in accumulation of undegraded DNA in the hemolymph. How a defect in bacterial DNA degradation leads to a severely impaired immune system is an important question that needs to be addressed in the near future.
Acknowledgments
The authors thank Drs. David Schneider (Stanford University, USA) and Bruno Lemaitre (CNRS-CGM, France) for the bacterial strains and Dr. Kyung-An Han (Penn State University) for several of the fly lines used in this study. We also thank Dr. Max Shpak (UTEP) and members of our research group for critically reading this manuscript. This work was supported by MBRS-SCORE (S06 GM8012-35) subproject grant to R.J.A and an institutional NIH RCMI Grant 2G12RR08124.
References
- [1].Evans CJ, Aguilera RJ. DNase II: genes, enzymes and function. Gene. 2003;322(115):1–15. doi: 10.1016/j.gene.2003.08.022. [DOI] [PubMed] [Google Scholar]
- [2].MacLea KS, Krieser RJ, Eastman A. A family history of deoxyribonuclease II: surprises from Trichinella spiralis and Burkholderia pseudomallei. Gene. 2003;305:1–12. doi: 10.1016/s0378-1119(02)01233-7. [DOI] [PubMed] [Google Scholar]
- [3].Krieser RJ, Eastman A. Deoxyribonuclease II: structure and chromosomal localization of the murine gene, and comparison with the genomic structure of the human and three C. elegans homologs. Gene. 2000;252:155–162. doi: 10.1016/s0378-1119(00)00209-2. [DOI] [PubMed] [Google Scholar]
- [4].Lyon CJ, Miranda GA, Piao JS, Aguilera RJ. Characterization of an endonuclease activity which preferentially cleaves the G-rich immunoglobulin switch repeat sequences. Mol. Immunol. 1996;33:157–169. doi: 10.1016/0161-5890(95)00125-5. [DOI] [PubMed] [Google Scholar]
- [5].Lyon CJ, Evans CJ, Bill BR, Otsuka AJ, Aguilera RJ. The C. elegans apoptotic nuclease NUC-1 is related in sequence and activity to mammalian DNase II. Gene. 2000;252:147–154. doi: 10.1016/s0378-1119(00)00213-4. [DOI] [PubMed] [Google Scholar]
- [6].Evans CJ, Merriam JR, Aguilera RJ. Drosophila acid DNase is a homolog of mammalian DNase II. Gene. 2002;295:61–70. doi: 10.1016/s0378-1119(02)00819-3. [DOI] [PubMed] [Google Scholar]
- [7].Sulston JE. Post-embryonic development in the ventral cord of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1976;275:287–297. doi: 10.1098/rstb.1976.0084. [DOI] [PubMed] [Google Scholar]
- [8].Hedgecock EM, Sulston JE, Thomson JN. Mutations affecting programmed cell deaths in the nematode Caenorhabditis elegans. Science. 1983;220:1277–1279. doi: 10.1126/science.6857247. [DOI] [PubMed] [Google Scholar]
- [9].Krieser RJ, MacLea KS, Longnecker DS, Fields JL, Fiering S, Eastman A. Deoxyribonuclease II alpha is required during the phagocytic phase of apoptosis and its loss causes perinatal lethality. Cell Death Differ. 2002;9:956–962. doi: 10.1038/sj.cdd.4401056. [DOI] [PubMed] [Google Scholar]
- [10].Kawane K, Fukuyama H, Kondoh G, Takeda J, Ohsawa Y, Uchiyama Y, Nagata S. Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science. 2001;292:1546–1549. doi: 10.1126/science.292.5521.1546. [DOI] [PubMed] [Google Scholar]
- [11].Yoshida H, Okabe Y, Kawane K, Fukuyama H, Nagata S. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat. Immunol. 2005;6:49–56. doi: 10.1038/ni1146. [DOI] [PubMed] [Google Scholar]
- [12].Okabe Y, Kawane K, Akira S, Taniguchi T, Nagata S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J. Exp. Med. 2005;202:1333–1339. doi: 10.1084/jem.20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Boyd JB, Mitchell HK. Identification of deoxyribonucleases in polyacrylamide gel following their separation by disk electrophoresis. Anal. Biochem. 1965;13:28–42. [Google Scholar]
- [14].Detwiler CR. A Genetic and Developmental Analysis of DNase-1, an Acid Deoxyribonuclease in Drosophila melanogaster. Cornell University; Ithica, NY: 1979. Dissertation. [DOI] [PubMed] [Google Scholar]
- [15].Grell EH. Genetics of some deoxyribonucleases of Drosophila melanogaster. Genetics. 1976;83:s28–s29. [Google Scholar]
- [16].Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- [17].McIlroy D, Tanaka M, Sakahira H, Fukuyama H, Suzuki M, Yamamura K, Ohsawa Y, Uchiyama Y, Nagata S. An auxiliary mode of apoptotic DNA fragmentation provided by phagocytes. Genes Dev. 2000;14:549–558. [PMC free article] [PubMed] [Google Scholar]
- [18].Mukae N, Yokoyama H, Yokokura T, Sakoyama Y, Nagata S. Activation of the innate immunity in Drosophila by endogenous chromosomal DNA that escaped apoptotic degradation. Genes Dev. 2002;16:2662–2671. doi: 10.1101/gad.1022802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Harrison DA, Binari R, Nahreini TS, Gilman M, Perrimon N. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 1995;14:2857–2865. doi: 10.1002/j.1460-2075.1995.tb07285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hrdlicka L, Gibson M, Kiger A, Micchelli C, Schober M, Schock F, Perrimon N. Analysis of twenty-four Gal4 lines in Drosophila melanogaster. Genesis. 2002;34:51–57. doi: 10.1002/gene.10125. [DOI] [PubMed] [Google Scholar]
- [21].Asha H, Nagy I, Kovacs G, Stetson D, Ando I, Dearolf CR. Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics. 2003;163:203–215. doi: 10.1093/genetics/163.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee YS, Carthew RW. Making a better RNAi vector for Drosophila: use of intron spacers. Methods. 2003;30:322–329. doi: 10.1016/s1046-2023(03)00051-3. [DOI] [PubMed] [Google Scholar]
- [23].Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- [24].Triglia T, Peterson MG, Kemp DJ. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988;16:8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dalby B, Pereira AJ, Goldstein LS. An inverse PCR screen for the detection of P element insertions in cloned genomic intervals in Drosophila melanogaster. Genetics. 1995;139:757–766. doi: 10.1093/genetics/139.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ballinger DG, Benzer S. Targeted gene mutations in Drosophila. Proc. Natl. Acad. Sci. USA. 1989;86:9402–9406. doi: 10.1073/pnas.86.23.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Horst M, Jeno P, Kronidou NG. Isolation of protein import components from Saccharomyces cerevisiae mitochondria. Methods Enzymol. 1995;260:232–241. doi: 10.1016/0076-6879(95)60141-4. [DOI] [PubMed] [Google Scholar]
- [28].Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal. Biochem. 2000;285:194–204. doi: 10.1006/abio.2000.4753. [DOI] [PubMed] [Google Scholar]
- [29].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [30].Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tirouvanziam R, Davidson CJ, Lipsick JS, Herzenberg LA. Fluorescence-activated cell sorting (FACS) of Drosophila hemocytes reveals important functional similarities to mammalian leukocytes. Proc. Natl. Acad. Sci. USA. 2004;101:2912–2917. doi: 10.1073/pnas.0308734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sinenko SA, Kim EK, Wynn R, Manfruelli P, Ando I, Wharton KA, Perrimon B, Mathey-Prevot N. Yantar, a conserved arginine-rich protein is involved in Drosophila hemocyte development. Dev. Biol. 2004;273:48–62. doi: 10.1016/j.ydbio.2004.05.022. [DOI] [PubMed] [Google Scholar]
- [33].Hammond SM. Dicing and slicing: the core machinery of the RNA interference pathway. FEBS Lett. 2005;579:5822–5829. doi: 10.1016/j.febslet.2005.08.079. [DOI] [PubMed] [Google Scholar]
- [34].Kavi HH, Fernandez HR, Xie W, Birchler JA. RNA silencing in Drosophila. FEBS Lett. 2005;579:5940–5949. doi: 10.1016/j.febslet.2005.08.069. [DOI] [PubMed] [Google Scholar]
- [35].Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- [36].Tsuneizumi K, Nakayama T, Kamoshida Y, Kornberg TB, Christian JL, Tabata T. Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature. 1997;389:627–631. doi: 10.1038/39362. [DOI] [PubMed] [Google Scholar]
- [37].Royet J, Reichhart JM, Hoffmann JA. Sensing and signaling during infection in Drosophila. Curr. Opin. Immunol. 2005;17:11–17. doi: 10.1016/j.coi.2004.12.002. [DOI] [PubMed] [Google Scholar]
- [38].Evans CJ, Hartenstein V, Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev. Cell. 2003;5:673–690. doi: 10.1016/s1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- [39].Tzou P, De GE, Lemaitre B. How Drosophila combats microbial infection: a model to study innate immunity and host-pathogen interactions. Curr. Opin. Microbiol. 2002;5:102–110. doi: 10.1016/s1369-5274(02)00294-1. [DOI] [PubMed] [Google Scholar]
- [40].Elrod-Erickson M, Mishra S, Schneider D. Interactions between the cellular and humoral immune responses in Drosophila. Curr. Biol. 2000;10:781–784. doi: 10.1016/s0960-9822(00)00569-8. [DOI] [PubMed] [Google Scholar]
- [41].Irving P, Troxler L, Heuer TS, Belvin M, Kopczynski C, Reichhart JM, Hoffmann JA, Hetru C. A genome-wide analysis of immune responses in Drosophila. Proc. Natl. Acad. Sci. USA. 2001;98:15119–15124. doi: 10.1073/pnas.261573998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].De Gregorio E, Spellman PT, Rubin GM, Lemaitre B. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc. Natl. Acad. Sci. USA. 2001;98:12590–12595. doi: 10.1073/pnas.221458698. [DOI] [PMC free article] [PubMed] [Google Scholar]