Abstract
We have performed a detailed analysis of the recognition of melanoma Ags by the tumor-infiltrating lymphocytes (TIL) 1790, isolated from a patient who experienced a dramatic tumor regression following immunization with peptides from the gp100, MART-1, and tyrosinase Ags. This TIL was found to recognize HLA-A2-restricted CTL epitopes in tyrosinase-related protein (TRP)-2 (clone MR7) and NY-ESO-1 (clone M8). These epitopes were the same as the previously identified nonapeptide TRP-2: 180–188, and the overlapping NY-ESO-1 peptides, obtained by using lymphocytes from in vitro stimulation. We also cloned a previously unknown TRP-2 mRNA isoform (TRP-2-6b) that contained two novel exons alternatively spliced from the sixth intron between exons 6 and 7 of TRP-2 mRNA. The isoform encoded an HLA-A2-restricted antigenic epitope recognized by TIL clone MB4. An immunologic analysis of the patient’s PBMC obtained before treatment showed the presence of high reactivity against NY-ESO-1 and both TRP-2 Ags, but not the Ags used for immunization. Because immune response against these Ags was less pronounced, it is possible that NY-ESO-1, TRP-2, and TRP-2-6b may be of importance in the generation of CTL-mediated tumor destruction and may have played a role in the dramatic tumor regression seen in this patient.
Major histocompatibility complex class I-restricted CTL responses directed against a variety of tumor Ags have been demonstrated in several studies (1–3). These Ags can be grouped into four general categories based on their patterns of expression. The first group includes shared cancer/testis Ags such as MAGE and NY-ESO-1. These Ags are expressed on tumor cells of a variety of histologic types, including melanoma. However, they are not expressed in normal tissues except for the testis and placenta (3–5). In a recent study, HLA-A2-restricted CTL epitopes in NY-ESO-1 were identified using CTL from mixed lymphocyte tumor cultures (6). The second group of Ags result from mutations and are therefore unique to each patient. Some examples are β-catenin (7), CDK-4 (8), and MUM-1 (9). The third group of Ags are widely expressed in a variety of normal tissues but are selectively overexpressed on tumors. These include p15 (10) and PRAME (11).
The fourth group includes the melanoma differentiation Ags such as MART-1 (12, 13), gp100 (14), and tyrosinase (15). Other melanoma differentiation Ags include gp75/tyrosinase-related protein (TRP)2-1 (16) and TRP-2, which have recently been identified as melanoma Ags and contain antigenic epitopes recognized by specific CTL in the context of HLA-A31 and HLA-A33 alloantigens (17, 18). In other studies, HLA-A2-restricted melanoma-specific CTL epitopes in TRP-2 were identified using lymphocytes from repeated in vitro stimulation of PBMC from donors (19–22).
We conducted a melanoma vaccine trial that enrolled 16 patients with metastatic disease. Patients were vaccinated s.c. with peptides (gp100: 209–217 (210 M), gp100: 280–288 (288V), MART-1: 27–35, and tyrosinase 368–376) emulsified in IFA. Two patients experienced dramatic tumor regression. In this report, we studied and analyzed the immunologic reactivity of one of these patients, MM, and have isolated, for the first time to our knowledge, melanoma-specific tumor-infiltrating lymphocytes (TIL) that recognized TRP-2 and NY-ESO-1 epitopes restricted by HLA-A2. In addition, we also identified a novel TRP-2 mRNA isoform that contained an HLA-A2-restricted CTL epitope recognized by TIL. This mRNA transcript was derived from alternative splicing of the TRP-2 gene in which two novel exons were spliced from noncontiguous regions of the sixth intron between exons 6 and 7.
Surprisingly, the immunologic reactivity directed against NY-ESO-1 and the two TRP-2 Ags was present in patient MM even before treatment and thus may have played a role in the dramatic tumor regression seen in this patient.
Materials and Methods
Patient
MM is a 63-year-old female who underwent a wide local excision of a primary melanoma on her back in 1981. In May 1997, she developed a s.c. metastasis on her left chest wall, which was resected, and she was started on a chemoimmunotherapy regimen consisting of cisplatin, vinblastine, dacarbazine, IL-2, and IFN-γ. Because of disease progression, she was referred to the Surgery Branch of the National Cancer Institute (Bethesda, MD) in June 1998. At that time, she had developed metastatic disease in multiple sites including the lungs, liver, intrapelvic area, left abdominal wall, and left thigh. She was started on a four-peptide vaccination protocol using 1 mg each of gp100: 209–217 (210 M), gp100: 280–288 (288V), MART-1: 27–35, and tyrosinase: 368–376 in IFA s.c. every 3 wk. Most of her tumors completely regressed after 2 cycles of treatment (day 45), including complete resolution of a large tumor in her left thigh, an intrapelvic mass, a liver lesion, and most of the nodules in her lungs. She completed a total of six cycles of vaccinations in October 1998. A year later (October 1999), she developed a frontal lobe metastatic brain lesion and a s.c. nodule on her right chest wall, both of which were resected. Bulk TIL 1790 used in this study was grown from the chest wall lesion. In March 2000, she underwent a second right temporal craniotomy for resection of recurrent disease at the prior resection site, followed by whole brain irradiation. The patient is doing well at this time, with disease limited to three small lung lesions (~1 × 1 cm) that are slow growing.
Cell lines
Melanoma-reactive CTL were derived from bulk TIL cultures grown in IMDM (Life Technologies, Gaithersburg, MD) containing 6000 IU/ml human rIL-2 (Chiron, Emeryville, CA) as previously described (23). CTL clones were derived from bulk TIL cultures by limiting dilution with the addition of anti-CD3 Ab (OKT-3; Ortho-McNeil Pharmaceuticals, Raritan, NJ). Briefly, 5 × 104 irradiated (3000–3400 cG) PBMC from three allogeneic donors were plated in round-bottom 96-well plates with 0.5–90 T cells per well. Cells were cultured in RPMI 1640 medium (Life Technologies) containing 20% heat-inactivated FBS (Life Technologies) and 30 ng/ml OKT-3 Ab with 300 IU/ml IL-2. The same dose of IL-2 was added on day 7, and clones were tested for recognition of HLA-A2+ vs HLA-A2− melanoma cell lines 14 days after stimulation. After testing, the remainder of T cells were expanded by plating them in a T25 flask with 2.5 × 107 irradiated PBMC from three allogeneic donors in 25 ml of RPMI 1640 medium containing 10% FBS and 30 ng/ml OKT-3 Ab. Subsequent expansion was similarly done, but with 1–2 × 106 CTL and 2.5 × 108 allogeneic PBMC plated in an upright T162 flask in 150 ml of medium.
The melanoma cell lines 624–28, 624–38, 938, 526, 888, 888A2, F002S, and F002R were established in the Surgery Branch (National Cancer Institute). 624–28 and 624–38 cell lines were derived from the same parental cell line, 624-mel, and share a similar pattern of Ag and HLA allele expression, except for HLA-A0201 Ag, which was expressed by 624–38 cells but not by 624–28 cells because of aberrant pre-mRNA splicing (24). 888A2-mel cell line was obtained by stable transfection of 888-mel with HLA-A2 cDNA (pCDNA3 plasmid; Invitrogen, San Diego, CA). F002S cell line is deficient in gp100 expression. F002R cell line was derived from F002S cell line by in vitro immunoselection for loss of MART-1 expression (22). T2 cells, deficient in transporter-associated protein, were used to test HLA-A2-restricted peptides for CTL activity. 293 cell line was derived from primary human embryonal kidney cells and was used for transfection purposes. 293A2 cell line was obtained by stable transfection of 293 cells with HLA-A2 cDNA. All cell lines were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 10 mM HEPES buffer, 100 U/ml penicillin-streptomycin (Biofluids, Rockville, MD), 2 mM L-glutamine (Biofluids). This medium is referred to as complete medium (CM) in this paper.
Cytokine release assays
CTL cells (5 × 104) were plated with 1 × 105 target cells in 96-well round-bottom plates in 200 μl of CM. After 18–24 h of incubation at 37°C, the supernatant was harvested for detection of IFN-γ release using ELISA kits (Endogen, Cambridge, MA). For the MHC blocking assays, target cells were incubated with the appropriate mAb at a final concentration of 50 μg/ml (except for BB7.2, which was used at 25% final volume) for 1 h at 37°C before the addition of CTLs. W6/32 (anti-HLA class I) was obtained from the American Type Culture Collection (Manassas, VA). TU39 (anti-HLA class II) and L243 (anti-HLA-DR) mAbs were purchased from BD PharMingen (San Diego, CA). Anti-HLA-A2 mAbs, SB00-60 and BB7.2, were a kind gift from Dr. F. Marincola (Department of Transfusion Medicine, National Cancer Institute) and Dr. E. Bergmann-Leitner (Department of Immunology, Walter Reed Army Institute of Research, Silver Spring, MD), respectively. For the 12-day in vitro sensitization assay, PBMC were plated at 3 × 106 cells in 24-well plates in 2 ml of IMDM, prepared as the CM but with 10% heat-inactivated human AB serum. On day 0, peptides were added at a final concentration of 1 μM and the plates were incubated at 37°C. On day 1 and every 3–4 days thereafter, 300 IU/ml human rIL-2 was added. On day 12, cells were harvested for testing. Targets were T2 cells that had been pulsed with peptides at 1 μM for 2 h at 37°C and washed once. Detection of IFN-γ release was done as described above, but with 1 × 105 CTL instead of 5 × 104.
cDNA library screening
624-mel cDNA expression library was kindly prepared by Dr. R.-F. Wang (Baylor College of Medicine, Houston, TX). Briefly, total RNA was extracted from 624-mel cells using TRIzol reagent (Life Technologies). Poly(A) RNA was purified from total RNA by the poly(A) tract isolation system (Promega, Madison, WI) and converted to cDNA using an oligo(dT) primer. The cDNA was ligated to BstXI adapters and digested with NotI, then ligated to the expression vector pEAK 8. The cDNA was electroporated into DH10B cells (Life Technologies). Pools containing ~100 cDNA clones were prepared from bacteria. The plasmid DNA (200 ng) were transfected into 293A2 cells with Effectene reagent (Qiagen, Valencia, CA). Briefly, 4 × 104 cells per well were plated in flat-bottom 96-well plates in complete medium the day before transfection. On the day of transfection, plasmid DNA (200 ng) from each pool were diluted in 29 μl of buffer EC. Next, 0.8 μl of Enhancer was added and mixed. The mixture was incubated at room temperature for 5 min. After the incubation, 5 μl of Effectene reagent was diluted in 17.5 μl of buffer EC and then added to and mixed with the DNA-Enhancer mixture. The mixture was incubated for 10 min at room temperature. During this time, old medium from 293A2 plates was removed and replaced with 100 μl of fresh CM. After the incubation, the DNA-Enhancer-Effectene mixture was added drop-wise onto the cells. The plates were then incubated at 37° C/5% CO2 for 24 h. The following day, the old medium was removed and replaced with 100 μl of fresh CM. A total of 5 × 104 CTL in 100 μl of CM were added to each well, and the plates were incubated at 37°C for 24 h. ELISA for IFN-γ was performed as described.
DNA sequencing
Sequencing of the isolated cDNA clone was performed with an ABI Prism 310 automated capillary electrophoresis instrument (PerkinElmer, Foster City, CA) using the Dye Terminator Cycle Sequencing Ready Reaction kit (PerkinElmer). Searches for sequence homology were done with the Gen-Bank database using the basic local alignment search tool algorithm.
Peptide synthesis
Peptides were synthesized using a solid-phase method based on standard F-moc chemistry on a multiple peptide synthesizer (Gilson, Worthington, OH). Peptide identity was verified by laser desorption mass spectrometry (Biosynthesis, Lewisville, TX). Lyophilized peptides were solubilized in DMSO at a 10 mg/ml concentration.
Results
Characterization of TIL 1790
The 1790 TIL line was isolated from a metastatic s.c. lesion in the right chest wall of patient MM, who was HLA-typed as A0201, A0301, B15, B35, C3, and C4. The 1790 TIL line, in four separate experiments, recognized a panel of melanoma cell lines matched for HLA-A2 but did not recognize non-HLA-A2 cell lines (Table I). In addition, TIL 1790 was tested against peptide-pulsed T2 cells and was found to recognize MART-1 27–35 but not the tyrosinase or two gp100 peptides against which patient MM was immunized. Of interest, TIL 1790 recognized both MART-1 peptide and F002R cells, a melanoma cell line that does not express MART-1.
Table I.
Specific activity of TIL 1790a
| Target | HLA-A2 | IFN-γ(pg/ml) |
|---|---|---|
| 526 | + | >1000 |
| 624–38 | + | >1000 |
| 888A2 | + | 884 |
| 697 | + | 69 |
| 1102 | + | 361 |
| F002R | + | >1000 |
| F002S | + | >1000 |
| 293A2 | + | 22 |
| 938 | − | 4 |
| 624–28 | − | 18 |
| 888 | − | 3 |
| T2 alone | + | 22 |
| T2-gp154 | + | 47 |
| T2-gp209 | + | 56 |
| T2-gp280 | + | 40 |
| T2-MART-1 | + | >1000 |
| T2-tyrosinase | + | 26 |
T cells (2 × 104) were incubated with 1 × 105 targets for 24 h, and the release of IFN-γ (measured in picograms per milliliter) was measured in an ELISA gp154, gp100: 154–162; pg209, pg100: 209–217; gp280, gp100: 280–288.
The lack of MART-1 expression on F002R cells was confirmed in the same experiment by the lack of recognition of F002R cells by a control anti-MART-1 CTL clone (clone V2C8) (data not shown). This demonstrated that besides MART-1, 1790 TIL line recognized at least one other melanoma Ag.
Ag specificity of TIL clones MR7, MB4, and M8
In an attempt to identify the Ag(s) recognized by TIL 1790, a number of CTL clones were generated from this TIL line by limiting dilution. CTL clones MR7, MB4, and M8 were selected for further study based on their ability to specifically release cytokine (IFN-γ) in response to HLA-A2 melanoma cell lines but not to cell lines that did not express HLA-A2 (Table II), and based on the lack of recognition of T2 cells pulsed with peptides from MART-1, gp-100, and tyrosinase (data not shown). The fact that non-HLA-A2 melanoma cell lines such as 888 stimulated cytokine release from clones MR7 and MB4 after being stably transfected with the cDNA encoding HLA-A2 Ag indicated that they expressed the Ag(s) of interest and that MR7 and MB4 recognized a melanoma Ag restricted by HLA-A2. Clone M8 recognized neither 888 nor 888A2; however, it reacted with 624–38, but not the HLA-A2 negative variant, 624–28. This suggested that M8 also recognized a melanoma Ag restricted by HLA-A2. This HLA-A2 restriction was confirmed with Ab blocking studies performed on clone MR7. Anti-class I Ab (W6/32) caused a 100% reduction in the activity of clone MR7, whereas anti-class II and anti-DR Abs had a minimal effect (6 and 13%, respectively). In addition, anti-HLA-A2 Abs, SB00-60 and BB7.2, blocked the activity of clone MR7 at 99 and 77% efficiency, respectively. These same Abs exerted a minimal effect (20 and 0% reduction, respectively) on a control, HLA-A24-restricted CTL clone (clone G5G10), which was specific for a mutated β-catenin epitope. Clones MR7, M8, and MB4 were tested for recognition of previously described Ags in a coculture assay using 293A2 cells transfected with cDNA encoding these Ags (Table III). Clone MR7 recognized TRP-2, and clone M8 recognized NY-ESO-1, whereas clone MB4 did not recognize any of the known Ags tested. In addition, a cDNA clone coding for TRP-2 was isolated and sequenced when CTL clone MR7 was used to screen the 624-mel library (data not shown). The nonapeptide TRP-2: 180–188, and the overlapping NY-ESO-1 peptides NY-ESO-1: 157–167, NY-ESO-1: 157–165, and NY-ESO-1: 155–163 were found to be the epitopes recognized by CTL MR7 and CTL M8, respectively (Table IV).
Table II.
Recognition of melanoma cell lines by TIL clones MR7, MB4, and M8a
| IFN-γ (pg/ml)
|
||||
|---|---|---|---|---|
| Expt. 1
|
Expt. 2
|
|||
| Stimulator Cell Line | HLA-A2 | MR7 | MB4 | M8 |
| 624–38 | + | >1000 | >1000 | >1000 |
| 526 | + | >1000 | >1000 | 81 |
| 888A2 | + | 206 | 196 | 101 |
| F002S | + | >1000 | >1000 | >1000 |
| F002R | + | >1000 | 260 | 834 |
| 1760 | + | 516 | 456 | >1000 |
| 624–28 | − | 57 | 26 | 83 |
| 938 | − | 50 | 62 | 95 |
| 888 | − | 43 | 47 | 101 |
| None | 40 | 59 | 152 | |
T cells (5 × 104) were incubated with 1 × 105 target cells for 24 h, and the release of IFN-γ (measured in picograms per milliliter) was measured in an ELISA.
Table III.
Recognition of gene transfectants by TIL clones MR7, MB4, and M8a
| IFN-γ (pg/ml)
|
|||
|---|---|---|---|
| Expt. 1
|
Expt. 2
|
||
| Transfected Gene | MR7 | MB4 | M8 |
| gp100 | 8 | 94 | 0 |
| MART-1 | 4 | 92 | 0 |
| TYR | 9 | 87 | 0 |
| TRP-1 | 5 | 44 | 0 |
| TRP-2 | >1000 | 114 | 0 |
| MAGE-1 | 5 | 67 | 0 |
| MAGE-3 | 0 | 109 | 0 |
| AIM | 7 | 53 | 0 |
| NY-ESO-1 | 5 | 9 | >1000 |
Cytokine release (measured in picograms per milliliter) following coincubation of T cells with 293A2 cells transfected with cDNA encoding known Ags for 24 h was measured using an IFN-γ ELISA (background activity from 293 transfectants has been subtracted).
Table IV.
Peptide recognition of TIL clones MR7 and M8
| Clone | Target | IFN-γ (pg/ml) |
|---|---|---|
| MR7 | T2-MART-1 | 41 |
| T2-TRP-2: 180–188 | >1000 | |
| T2-TRP-2: 455–463 | 10 | |
| None | 0 | |
| M8 | T2-NY-ESO-1: 155–163 | 407 |
| T2-NY-ESO-1: 157–165 | >1000 | |
| T2-NY-ESO-1: 157–167 | >1000 | |
| T2-gp154a | 32 |
gp154, gp100: 154–162.
Isolation of a cDNA clone recognized by TIL clone MB4
A cDNA library prepared from 624-mel cells was next used to screen for a gene encoding reactivity with CTL MB4. Plasmid DNA from pools containing ~100 cDNA clones was transiently transfected into 293A2 cells, a human embryonal kidney cell line transfected with the cDNA encoding HLA-A2 Ag. From 400 pools, the cDNA in one pool stimulated strong IFN-γ release from CTL MB4. By subcloning the positive pool, one cDNA clone (cDNA 198) was identified which induced a high level of IFN-γ secretion from TIL clone MB4 (Table V).
Table V.
Stimulation of TIL clone MB4 by 293A2 cells transfected with cDNA 198a
| Stimulator | IFN-γ (pg/ml) |
|---|---|
| 293A2 + cDNA 198 | >1000 |
| 293 + cDNA 198 | 2 |
| 293A2 + GFP | 8 |
| 293 + GFP | 9 |
| 293A2 | 0 |
| 293 | 2 |
293 cells transfected with cDNA 198 or green fluorescent protein (GFP) and 293A2 cells transfected with GFP were used as control.
Sequence determination
The sequence of cDNA 198 was identical to that of the TRP-2 gene with the exception of a 99-nt insert between exon 6 and exon 7 of the TRP-2 gene (Fig. 1). This extra insert was composed of two separate sequences, named 6b and 6c, and each showed complete homology with two noncontiguous sequences in intron 6 of the TRP-2 gene. The deduced amino acid sequence was translated in-frame with the TRP-2 product. The two polypeptides encoded by cDNA 198 and the cDNA encoding the previously described TRP-2 were identical except for an in-frame insert of a 33-aa sequence following amino acid 393. 6b and 6c were coding sequences and their flanking regions conformed to the intron consensus motif GT-AG (data not shown); therefore, 6b and 6c were identified as novel exons, alternatively spliced from intron 6 of TRP-2. In addition to the insertion in the coding region, the sequence of cDNA 198 differed from TRP-2 in the noncoding regions. The initial 122 bp (of 414 bp) of the 5′ untranslating region (5′ UTR) of TRP-2 was deleted in cDNA 198. However, the 3′ UTR of TRP-2 was further extended with a 10-bp sequence in cDNA 198. The protein encoded by cDNA 198 was named TRP-2-6b.
FIGURE 1.

Sequence of TRP-2-6b. The ATG start codon of the TRP-2 cDNA and the stop codon TAG are boxed. The extra 99-bp sequence is in parentheses. Exons 6b and 6c are separated by a slash. The nucleotide sequence coding for exons 6b and 6c is translated in the corresponding amino acid sequence using the single letter amino acid code. The antigenic epitope recognized by TIL clone MB4 is underlined.
Identification of the T cell epitope in TRP-2-6b that was recognized by TIL clone MB4
The fact that CTL MB4 did not recognize 293A2 cells transfected with TRP-2 cDNA suggested that the antigenic epitope may be encoded by the 99-bp sequence of the new exons 6b and 6c. Using a peptide-HLA motif search program, 22 peptides were generated with predicted HLA-A2 binding, from the new 33-aa sequence (encoded by exons 6b and 6c) and 10 amino acids upstream and downstream of it. These peptides were pulsed on T2 cells and tested for recognition by CTL MB4. Two peptides, ATTNILEHV and NATTNILEHV, designated TRP-2-6b: 403–411 and TRP-2-6b: 402–411, respectively, were recognized by CTL MB4 when pulsed on T2 cells (Table 6). The decapeptide TRP-2-6b: 402–411 was able to sensitize target cells for recognition by CTL MB4 at 5 ng/ml, whereas the nonapeptide TRP-2-6b: 403–411 was recognized at 50 pg/ml (Fig. 2).
Table VI.
Candidate HLA-A2-restricted peptides from TRP-2-6b and their recognition by CTL MB4 as measured by IFN-γ releasea
| Peptide | Sequence | IFN-γ (pg/ml) |
|---|---|---|
| 9 mer | ||
| 399–407 | LLYNATTNI | 6 |
| 427–435 | VLHSFTDAI | 4 |
| 386–394 | AANDPIFVV | 7 |
| 385–393 | SAANDPIFV | 8 |
| 419–427 | ELPSLHVLV | 6 |
| 426–434 | LVLHSFTDA | 5 |
| 418–426 | KELPSLHVL | 6 |
| 415–423 | KATKELPSL | 3 |
| 392–400 | FVVISNRLL | 6 |
| 403–411 | ATTNILEHY | >1000 |
| 424–432 | HVLVLHSFT | 6 |
| 10 mer | ||
| 399–408 | LLYNATTNIL | 10 |
| 398–407 | RLLYNATTNI | 5 |
| 418–427 | KELPSLHVLV | 5 |
| 425–434 | VLVLHSFTDA | 4 |
| 426–435 | LVLHSFTDAI | 6 |
| 385–394 | SAANDPIFVV | 4 |
| 394–403 | VISNRLLYNA | 41 |
| 419–428 | ELPSLHVLVL | 2 |
| 402–411 | NATTNILEHV | >1000 |
| 309–399 | PIFVVISNRL | 2 |
| 416–425 | ATKELPSLHV | 4 |
Using a HLA peptide motif search program (http://www.bimas.dcrt.nih.gov/cgi-bin/molbio/ken_parker_comboform), 22 peptides with predicted HLA-A2 binding were made from the new 33-aa sequence as well as 10 amino acids upstream and downstream of the sequence.
FIGURE 2.
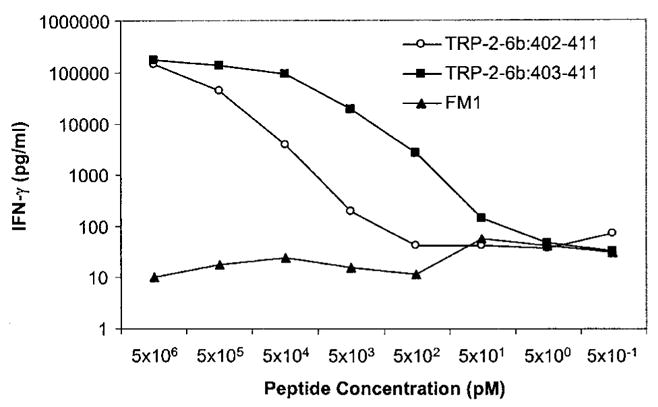
IFN-γ release by TIL clone MB4 cocultured with T2 cells pulsed with decreasing concentration of peptides TRP-2-6b: 402–411 and 403–411. The flu A matrix protein M1 peptide (FM1) was used as a negative control.
Immunologic reactivity against NY-ESO-1, TRP-2, TRP-2-6b, and the four peptides (MART-1, two gp100 peptides, and tyrosinase) used for immunization of patient MM, before and during the course of treatment
Patient MM’s PBMC collected before and during the course of treatment were tested for reactivity against NY-ESO-1, TRP-2, TRP-2-6b, and the four peptides that patient MM was immunized with, by stimulating the PBMC in vitro for 12 days with the un-modified epitopes from these Ags (Fig. 3). The nonapeptide from TRP-2-6b was used in this experiment. HLA-A2-restricted telomerase: 540–548 was used as an irrelevant peptide control. The patient’s pretreatment PBMC (day 0) exhibited strong reactivity against NY-ESO-1 and the two TRP-2 isoforms, none of which she had been vaccinated against (Fig. 3). The reactivity against the TRP-2 Ags increased and persisted over time. In contrast, the immune responses directed against MART-1, gp100: 209–217, gp100: 280–288, and tyrosinase, the peptides she had been vaccinated with, were not present in the prevaccination PBMC (Fig. 3).
FIGURE 3.

Immunologic reactivity to TRP-2, TRP-2-6b, NY-ESO-1, MART-1, gp100: 209–217, gp100: 280–288, and tyrosinase detected in patient MM’s PBMC obtained before and during her treatment as measured by a 12-day in vitro sensitization assay. Day 0 PBMC were obtained a few hours before her first vaccination. Regression of most of patient MM’s tumors was observed at day 45. Last cycle of treatment was day 129.
Discussion
We have identified the Ags recognized by a TIL line, TIL-1790, obtained from a patient who had experienced a dramatic tumor regression following immunization with a combination of MART-1, gp100, and tyrosinase peptides. One of the Ags recognized by TIL-1790 was an HLA-A2-restricted epitope from TRP-2, TRP-2: 180–188, identified by Parkhurst et al. (19) using CTL generated by in vitro stimulation. We have also isolated, for the first time, TIL that specifically recognized an HLA-A2-restricted epitope from NY-ESO-1, a cancer/testis Ag originally identified by the SEREX (serological expression cloning of recombinant cDNA libraries of human tumors) method (25). Abs and CTL reactivity directed against NY-ESO-1 were detected in ~50% of patients whose tumors expressed NY-ESO-1 (6, 26). TIL clone M8 recognized the overlapping epitopes from NY-ESO-1 previously identified by Jager et al. (6).
In addition, TIL-1790 recognized TRP-2-6b, a novel TRP-2 mRNA isoform. The original TRP-2 protein is a membrane-bound melanosomal enzyme (DOPAchrome tautomerase) involved in melanin biosynthesis. Of the TRP-2 isoforms reported so far, TRP-2-6b is unique in that it contains an in-frame insertion of two extra exons, with no deletion in the coding regions. Because exon 8 encodes a putative transmembrane domain (27–29), which seems to be critical for the DOPAchrome tautomerase activity of TRP-2 (30), the novel isoform TRP-2-6b, which contains an intact exon 8, is most likely localized to the melanosomal membrane and to have functional DOPAchrome tautomerase activity. However, the full functional status of TRP-2-6b is still not known. In contrast, the isoform TRP-2-INT2, which contains an HLA-A68-restricted CTL epitope, encodes a shortened protein of 237 amino acids because of an early stop site located in the retained intron 2 (31). Two other isoforms, TRP-2-8b and TRP-2-LT, have been described but have not been shown to contain any novel CTL epitopes (30). TRP-2-8b contains a novel exon 8b but lacks exon 8 and therefore, like TRP-2-INT2, is devoid of transmembrane domain as well as the DOPAchrome tautomerase activity. The isoform TRP-2-LT is identical to TRP-2 except for a long stretch of nucleotides (2315 bp) in the 3′ UTR.
The novel exon 6b of TRP-2-6b encoded an antigenic epitope recognized by melanoma-specific HLA-A2-restricted TIL. Both the nonapeptide (TRP-2-6b: 403–411) and decapeptide (TRP-2-6b: 402–411) of this epitope sensitized target cells for recognition by TIL clone MB4 at low concentrations, 50 pM and 5 nM, respectively. Even though the predicted HLA-A2 binding affinity of TRP-2-6b: 403–411 was low, the extremely low concentration it required to stimulate TIL clone MB4 indicated that clone MB4 might be high-affinity effectors.
It is interesting that patient MM was never vaccinated against NY-ESO-1 or TRP-2, and that she presented to us with a high level of immune reactivity against NY-ESO-1 and two different TRP-2 peptides before receiving the four-peptide treatment with peptides from gp100, MART-1, and tyrosinase (Fig. 3). The overall response directed against all Ags may have been responsible for the eradication of her tumors; however, anti-NY-ESO-1, anti-TRP-2, and anti-TRP-2-6b reactivity may have played a more dominant role in mediating the regression of her tumors. Vaccination with gp100, MART-1, and tyrosinase appeared to stimulate immunologic responses against the two TRP-2 Ags. Before treatment, patient MM had circulating precursors directed against NY-ESO-1, TRP-2, and TRP-2-6b, as evidenced from the immunologic reactivity seen in her prevaccination PBMC, even though she continued to have progression of her metastatic disease at multiple sites. We hypothesize that repeated vaccination with gp-100, MART-1, and tyrosinase peptides emulsified in IFA induced cytokine cascades both locally and systemically, resulting in activation and proliferation of anti-melanoma Ag precursors such as those against NY-ESO-1 and the two TRP-2 Ags, and infiltration of these effectors into tumors. This may explain the initial decrease in anti-NY-ESO-1 reactivity observed in PBMC collected at the second time point (day 45), when her tumors were decreasing. In our prior studies, we have shown an increase in IFN-γ message in tumors in patients immunized with the gp100: 209–217 (210 M) peptide (32), which was one of the peptides used to immunize this patient. Of interest, the immune reactivity against the two TRP-2 Ags persisted over time, whereas that against NY-ESO-1 waned over the course of follow-up. One possible explanation for the continued decrease in reactivity against NY-ESO-1 over time is that residual tumors were immunoselected in vivo for loss of NY-ESO-1 expression, but sufficient tissues were unavailable for this analysis.
These findings indicate that alternative splicing of TRP-2 or other Ags such as gp-100 may generate novel melanoma Ags, and that the immunologic responses directed against NY-ESO-1, TRP-2, and TRP-2-6b may have played a role in the dramatic tumor regression seen in this patient. This suggests that the HLA-A2-restricted CTL peptide epitopes from NY-ESO-1 and the TRP-2 Ags may be useful in the active immunotherapy of patients with melanoma.
Acknowledgments
We thank Dr. Paul F. Robbins and Dr. Zhiya Yu for helpful discussions and advice, Dr. Maria Parkhurst and John Riley for their help with peptide synthesis, Dr. Rong-Fu Wang for preparing the 624-mel cDNA library, and Yong Li for help with the automated DNA sequencer.
Footnotes
Abbreviations used in this paper: TRP, tyrosinase-related protein; TIL, tumor-infiltrating lymphocyte; UTR, untranslating region; CM, complete medium.
References
- 1.Hom SS, Schwartzentruber DJ, Rosenberg SA, Topalian SL. Specific release of cytokines by lymphocytes infiltrating human melanomas in response to shared melanoma antigens. J Immunother. 1993;13:18. doi: 10.1097/00002371-199301000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Van den Eynde BJ, van der Bruggen P. T cell defined tumor antigens. Curr Opin Immunol. 1997;9:684. doi: 10.1016/s0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281. doi: 10.1016/s1074-7613(00)80028-x. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Shichijo S, Noguchi M, Hirohata M, Itoh K. Iden-tification of MAGE-1 and MAGE-4 proteins in spermatogonia and primary spermatocytes of testis. Cancer Res. 1995;55:3478. [PubMed] [Google Scholar]
- 5.Chen YT, Scanlan MJ, Sahin U, Tureci O, Gure AO, Tsang S, Williamson B, Stockert E, Pfreundschuh M, Old LJ. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA. 1997;94:1914. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jager E, Chen YT, Drijfhout JW, Karbach J, Ringhoffer M, Jager D, Arand M, Wada H, Noguchi Y, Stockert E, et al. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med. 1998;187:265. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins PF, El-Gamil M, Li YF, Kawakami Y, Loftus D, Appella E, Rosenberg SA. A mutated β-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J Exp Med. 1996;183:1185. doi: 10.1084/jem.183.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfel T, Hauer M, Schneider J, Serrano M, Wolfel C, Klehmann-Hieb E, De Plaen E, Hankeln T, Meyer zum Buschenfelde KH, Beach D. p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 1995;269:1281. doi: 10.1126/science.7652577. [DOI] [PubMed] [Google Scholar]
- 9.Coulie PG, Lehmann F, Lethe B, Herman J, Lurquin C, Andrawiss M, Boon T. A mutated intron sequence codes for an antigenic peptide recognized by cytolytic T lymphocytes on a human melanoma. Proc Natl Acad Sci USA. 1995;92:7976. doi: 10.1073/pnas.92.17.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins PF, el-Gamil M, Li YF, Topalian SL, Rivoltini L, Sakaguchi K, Appella E, Kawakami Y, Rosenberg SA. Cloning of a new gene encoding an antigen recognized by melanoma-specific HLA-A24-restricted tumor-infiltrating lymphocytes. J Immunol. 1995;154:5944. [PubMed] [Google Scholar]
- 11.Ikeda H, Lethe B, Lehmann F, van Baren N, Baurain JF, de Smet C, Chambost H, Vitale M, Moretta A, Boon T, Coulie PG. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199. doi: 10.1016/s1074-7613(00)80426-4. [DOI] [PubMed] [Google Scholar]
- 12.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL, Miki T, Rosenberg SA. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA. 1994;91:3515. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coulie PG, Brichard V, Van Pel A, Wolfel T, Schneider J, Traversari C, Mattei S, DePlaen E, Lurquin C, Szikora JP, et al. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1994;180:35. doi: 10.1084/jem.180.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Appella E, Yannelli JR, Adema GJ, Miki T, Rosenberg SA. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci USA. 1994;91:6458. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brichard V, Van Pel A, Wolfel T, Wolfel C, De Plaen E, Lethe B, Coulie P, Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993;178:489. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang RF, Robbins PF, Kawakami Y, Kang XQ, Rosenberg SA. Identification of a gene encoding a melanoma tumor antigen recognized by HLA-A31-restricted tumor-infiltrating lymphocytes. J Exp Med. 1995;181:799. doi: 10.1084/jem.181.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang RF, Appella E, Kawakami Y, Kang X, Rosenberg SA. Identification of TRP-2 as a human tumor antigen recognized by cytotoxic T lymphocytes. J Exp Med. 1996;184:2207. doi: 10.1084/jem.184.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang RF, Johnston SL, Southwood S, Sette A, Rosenberg SA. Recognition of an antigenic peptide derived from tyrosinase-related protein-2 by CTL in the context of HLA-A31 and -A33. J Immunol. 1998;160:890. [PubMed] [Google Scholar]
- 19.Parkhurst MR, Fitzgerald EB, Southwood S, Sette A, Rosenberg SA, Kawakami Y. Identification of a shared HLA-A*0201-restricted T-cell epitope from the melanoma antigen tyrosinase-related protein 2 (TRP2) Cancer Res. 1998;58:4895. [PubMed] [Google Scholar]
- 20.Noppen C, Levy F, Burri L, Zajac P, Remmel E, Schaefer C, Luscher U, Heberer M, Spagnoli GC. Naturally processed and concealed HLA-A2.1-restricted epitopes from tumor-associated antigen tyrosinase-related protein-2. Int J Cancer. 2000;87:241. [PubMed] [Google Scholar]
- 21.Sun Y, Song M, Stevanovic S, Jankowiak C, Paschen A, Rammensee HG, Schadendorf D. Identification of a new HLA-A(*)0201-restricted T-cell epitope from the tyrosinase-related protein 2 (TRP2) melanoma antigen. Int J Cancer. 2000;87:399. doi: 10.1002/1097-0215(20000801)87:3<399::aid-ijc14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Harada M, Li YF, El-Gamil M, Rosenberg SA, Robbins PF. Use of an in vitro immunoselected tumor line to identify shared melanoma antigens recognized by HLA-A*0201-restricted T cells. Cancer Res. 2001;61:1089. [PubMed] [Google Scholar]
- 23.Topalian SL, Muul LM, Solomon D, Rosenberg SA. Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J Immunol Methods. 1987;102:127. doi: 10.1016/s0022-1759(87)80018-2. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Marincola FM, Rivoltini L, Parmiani G, Ferrone S. Selective histocompatibility leukocyte antigen (HLA)-A2 loss caused by aberrant pre-mRNA splicing in 624MEL28 melanoma cells. J Exp Med. 1999;190:205. doi: 10.1084/jem.190.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stockert E, Jager E, Chen YT, Scanlan MJ, Gout I, Karbach J, Arand M, Knuth A, Old LJ. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187:1349. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jager E, Nagata Y, Gnjatic S, Wada H, Stockert E, Karbach J, Dunbar PR, Lee SY, Jungbluth A, Jager D, et al. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proc Natl Acad Sci USA. 2000;97:4760. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouchard B, Del Marmol V, Jackson IJ, Cherif D, Dubertret L. Molecular characterization of a human tyrosinase-related-protein-2 cDNA: patterns of expression in melanocytic cells. Eur J Biochem. 1994;219:127. doi: 10.1111/j.1432-1033.1994.tb19922.x. [DOI] [PubMed] [Google Scholar]
- 28.Cassady JL, Sturm RA. Sequence of the human dopachrome tautomerase encoding TRP-2 cDNA. Gene. 1994;143:295. doi: 10.1016/0378-1119(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 29.Yokoyama K, Suzuki H, Yasumoto K, Tomita Y, Shibahara S. Molecular cloning and functional analysis of a cDNA coding for human DOPAchrome tautomerase/tyrosinase-related protein-2. Biochim Biophys Acta. 1994;1217:317. doi: 10.1016/0167-4781(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 30.Pisarra P, Lupetti R, Palumbo A, Napolitano A, Prota G, Parmiani G, Anichini A, Sensi M. Human melanocytes and melanomas express novel mRNA isoforms of the tyrosinase-related protein-2/DOPAchrome tautomerase gene: molecular and functional characterization. J Invest Dermatol. 2000;115:48. doi: 10.1046/j.1523-1747.2000.00023.x. [DOI] [PubMed] [Google Scholar]
- 31.Lupetti R, Pisarra P, Verrecchia A, Farina C, Nicolini G, Anichini A, Bordignon C, Sensi M, Parmiani G, Traversari C. Translation of a retained intron in tyrosinase-related protein (TRP) 2 mRNA generates a new cytotoxic T lymphocyte (CTL)-defined and shared human melanoma antigen not expressed in normal cells of the melanocytic lineage. J Exp Med. 1998;188:1005. doi: 10.1084/jem.188.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kammula US, Lee KH, Riker AI, Wang E, Ohnmacht GA, Rosenberg SA, Marincola FM. Functional analysis of antigen-specific T lymphocytes by serial measurement of gene expression in peripheral blood mononuclear cells and tumor specimens. J Immunol. 1999;163:6867. [PubMed] [Google Scholar]


