Abstract
Background and purpose:
Nitric oxide (NO) acts on receptors coupled to guanylyl cyclase (GC), leading to cGMP accumulation. The NO binding site is a haem group, oxidation or loss of which diminishes NO-stimulated activity. Agonists reportedly engaging both these NO-insensitive forms have emerged. Here we characterize the effect of a prototype compound (BAY 58-2667) and use it to assess the haem status of cellular GC.
Experimental approach:
GC activity measurements were made on the purified protein and on rat platelets.
Key results:
Experiments on purified GC showed that the target for BAY 58-2667 is the haem-free GC, not the haem-oxidized form. The efficacy of BAY 58-2667 was about half that shown normally by NO. In platelets, BAY 58-2667 was a potent GC activator (EC50 ∼15 nM) but the maximum effect was only about 1% of that achievable with NO. Nevertheless, it was enough to evoke cGMP-dependent protein phosphorylation. Profound (85 %) desensitization of NO-evoked GC activity did not alter the effectiveness of BAY 58-2667. Haem oxidation, however, increased the efficacy of BAY 58-2667 by 22-fold, implying that about half the cellular GC was then haem-free. Oxidation appeared to enhance the rate of haem dissociation from purified GC.
Conclusions and implications:
Compounds such as BAY 58-2667 are useful for probing the occupancy of the haem pocket of NO receptors in cells but not for distinguishing oxidized from reduced haem. In vivo, such compounds are likely to be particularly effective in conditions where there is deficient haem incorporation or enhanced haem loss.
Keywords: nitric oxide, guanylyl cyclase, cGMP, BAY 58-2667, HMR-1776, ODQ, desensitization, haem, platelet
Introduction
Nitric oxide (NO) affects the functioning of most organs in the body. For example, in the cardiovascular system, NO regulates blood flow and platelet function; in the peripheral nervous system, it performs a neurotransmitter-like role influencing the gastrointestinal and urogenital systems; and in the central nervous system, it participates in sensory and motor pathways and in synaptic plasticity (Moncada et al., 1991; Garthwaite and Boulton, 1995; Rand and Li, 1995). Most physiological NO signal transduction occurs through binding to specialized intracellular receptors having intrinsic GC activity, leading to cGMP generation and the engagement of one or more downstream pathways. The receptors are αβ-heterodimers containing a prosthetic ferrous (Fe2+) haem, which constitutes the NO-binding site, and a catalytic site where GTP is converted to cGMP (Ignarro, 1991; Denninger and Marletta, 1999; Koesling et al., 2004).
Because of its importance in health and disease, the NO–cGMP pathway is an attractive target for new medicines and, during the last several years, pharmacological agents interacting with various sites on NO receptors have emerged. The compounds 1-H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and NS 2028 inhibit NO receptor function by oxidizing the haem group, preventing NO binding (Garthwaite et al., 1995; Schrammel et al., 1996; Olesen et al., 1998). Allosteric agonists such as YC-1 and BAY 41-2272 have little activity on their own but greatly potentiate NO-evoked GC activity, apparently by binding to a site distinct from the haem (Ko et al., 1994; Stasch et al., 2001). Most recently, stimulatory compounds with a different mode of action have been discovered. BAY 58-2667, HMR-1766 and S-3448 are purported to target NO receptor proteins whose haem iron is in its oxidized state (Fe3+ instead of Fe2+), or whose haem group is missing (Stasch et al., 2002; Schmidt et al., 2004; Evgenov et al., 2006; Schindler et al., 2006). The pronounced effect of these compounds in tissue preparations and in vivo indicates that NO receptors in one or other of these states exist physiologically and may become more prevalent under pathological conditions (Dumitrascu et al., 2006; Stasch et al., 2006; Boerrigter et al., 2007).
Despite their potential therapeutic utility, the precise target of these compounds (the haem-oxidized or haem-free species, or both) remains unclear, as does the effect of such compounds on cellular GC activity, nor it is known if alterations in the haem association or oxidation state might represent a physiological regulatory mechanism serving to alter the sensitivity of cells to NO. Of note, here is the finding that GC-coupled NO receptors in cells desensitize rapidly on exposure to NO, a state from which they recover quite slowly (Bellamy et al., 2000; Bellamy and Garthwaite, 2002; Wykes et al., 2002). This phenomenon is not observed in lysed preparations or with the purified protein and so requires some cellular factor. As speculated previously (Brandes et al., 2000), haem oxidation or haem loss could account for the desensitization because both result in a loss of responsiveness to NO. In cells, moreover, inhibition by ODQ or NS 2028 is reversible (Garthwaite et al., 1995; Olesen et al., 1998; Bellamy and Garthwaite, 2002), presumably by re-reduction of the haem (Gupte et al., 1999; Iesaki et al., 1999), a phenomenon consistent with an oxidation–reduction cycle being physiological. In addition, recovery from ODQ occurs at a similar rate to resensitization measured in the same cells (Wykes et al., 2002).
Here, we have primarily used the most potent of the new compounds, BAY 58-2667, to try to define the mechanism of action of this class of agent more exactly and subsequently to investigate the haem status of native NO receptors in cells and the possible changes taking place during different functional states of the receptor. As a model cell system, we have used rat platelets, which have unsurpassed merit for such purposes (Mo et al., 2004). Apart from being highly enriched in NO receptors, they have a radius of only 0.5 μm, minimizing problems of diffusion and subcellular compartmentation inherent in the use of larger cells; they exist naturally as a suspension, which is ideal for kinetic studies; and, finally, the resulting cGMP is hydrolysed by a single phosphodiesterase isoform, phosphodiesterase-5, whose inhibition by sildenafil allows precise control over cGMP breakdown.
Materials and methods
Preparation of platelets
Platelets were prepared from rat blood as described previously (Mo et al., 2004) to give a final concentration of 0.5 mg protein per ml (protein being measured using the bicinchoninic acid method). The platelet suspension was incubated in 1–2 ml volumes in a solution containing (mM) NaCl (137), KCl (2.7), MgCl2 (0.5), NaH2PO4 (0.55), Tris (25) and D-glucose (5.6) for at least 1 h at 37 °C before use. L-Nitroarginine (100 μM) was included at the start of the incubation to eliminate possible complications arising from endogenous NO synthesis.
Delivery of NO
For most experiments with platelets, clamped NO concentrations were delivered using the method developed in our laboratory (Griffiths et al., 2003). Briefly, spermine NONOate was used as the source of NO and 2-4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (CPTIO; 200 μM) was included as an NO sink, serving to translate different rates of NO release from the donor into proportionate steady-state concentrations. With the CPTIO concentration chosen, steady-state NO concentrations are achieved within about a second. Urate (300 μM) was added to convert nitrogen dioxide radicals (the product of the reaction between NO and CPTIO) into nitrite ions. Superoxide dismutase (1000 U ml−1) was included as a precautionary measure to remove any superoxide ions that would otherwise react with NO. The NO concentration was measured using an electrochemical probe (ISO-NO MkII; World Precision Instruments, Sarasota, FL, USA). In some experiments, brief (20 s) pulses of NO were applied using the combination of the fast-acting NO donor, proline NONOate and CPTIO (50 μM), as described previously (Roy and Garthwaite, 2006), and in other experiments, the NO source was diethylamine NONOate (DEA/NO).
Preparation of haem-oxidized and haem-free GC
GC purified from bovine lung (soluble GC) was diluted to a final concentration of 5 μg ml−1 in buffer containing Tris (50 mM), dithiothreitol (1 mM) and BSA (5 mg ml−1) and incubated for 15 min at 37 °C with ODQ (10 μM) to oxidize the haem, the ODQ solvent (0.5% dimethyl sulphoxide) or Tween-20 (0.5%) to remove the haem (Foerster et al., 1996). An additional 180 μl of buffer was then added and the samples were centrifuged (12 500 g, 10 min, 4 °C) through 30 kDa size-exclusion filters (Microcon YM-30; Millipore UK Ltd, Watford, UK). The retained protein was eluted from the filter by a further 5 min centrifugation and resuspended in its original volume of buffer.
Measurement of cGMP and GC activity
As described before (Mo et al., 2004), aliquots of the platelet suspension were withdrawn before and at various times after addition of spermine NONOate or BAY 58-2667 and transferred immediately into inactivation buffer (50 mM Tris, 4 mM EDTA, pH 7.4) at 100 °C for at least 10 min. When used, 10 μM ODQ was added to the platelets 15 min before addition of spermine NONOate. Purified GC activity was measured at a final concentration of 50 ng ml−1 in assay buffer (50 mM Tris, 100 μM EDTA, 1 mM NaGTP, 1.3 mM MgCl2, 50 μg ml−1 BSA, pH 7.4), stimulated with DEA/NO (10 μM) or BAY 58-2667 (1 μM), enzyme inactivation being performed as with platelets. The levels of cGMP were measured by radioimmunoassay and expressed relative to the amount of protein. Three independent runs were carried out in each experiment and the resulting data presented as means±s.e.mean.
Analysis of rates of cGMP synthesis and degradation
The level of cGMP at any time is governed by the rates of synthesis and degradation. For platelets, these parameters were extracted by fitting the cGMP accumulation curve to a generalized hyperbola or to a pulse function (Origin 7.5; OriginLab Corporation, Northampton, MA, USA) as described (Bellamy et al., 2000; Mo et al., 2004). As found before (Mo et al., 2004), the maximum levels of cGMP varied by up to twofold on different days, but the kinetic profiles were indistinguishable. Accordingly, direct comparisons were made only on data from the same batch of platelets.
Vasodilator-stimulated phosphoprotein phosphorylation
Platelets were incubated for 2 min with or without NO (10 μM DEA/NO) in the presence or absence of BAY 58-2667 or ODQ as described above. Aliquots were boiled in Laemmli buffer and the proteins were separated for 2 h at 30 mA on a 7.5% Tris-HCl gel (Bio-Rad UK, Hemel Hempstead, UK). Proteins were electroblotted to a polyvinylidene difluoride membrane (GE Healthcare UK Ltd, Little Chalfont, UK) for 2 h at 50 V in the presence of 10 mM N-cyclohexyl-3-aminopropanesulphonic acid buffer (pH 10.5). The membrane was treated for 1 h at room temperature with blocking solution containing 3% BSA, 1% polyvinyl pyrrolidone and 1% polyethylene glycol in wash buffer (10 mM Tris-HCl, 150 mM NaCl, 0.05% Tween-20, 0.2% Triton X-100, pH 7.5). The monoclonal antibody, anti-vasodilator-stimulated phosphoprotein (VASP) phosphorylated on Ser239 (clone 16C2; Axxora (UK) Ltd, Nottingham, UK), was prepared at 0.25 μg ml−1 in blocking solution diluted 1:1 in wash buffer. The blot was incubated with antibody for 12 h at 4 °C and washed six times in wash buffer (5 min each wash). The blot was probed for 1 h at room temperature with goat anti-mouse secondary antibody conjugated to horseradish peroxidase (product no. 31430; Pierce Biotechnology Inc., Rockford, IL, USA) and diluted 1:40 000 in wash buffer. The bands were developed by enhanced chemiluminescence (Supersignal West Pico, product no. 34080; Pierce Biotechnology Inc.) and scanned with a Bio-Rad GS-800 imaging densitometer. For quantitation, the optical densities of the phospho-VASP bands (46 and 50 kDa) were added together and the mean density of an identical contour just above and below the bands was subtracted to correct for background. The density recorded from control (untreated) platelets served as the reference for normalization (set at 100).
For statistical testing, the data were subjected to the arcsine transformation (to correct for the skewed distribution of normalized data) and then analysed using one-way ANOVA and Tukey's test for significance at the P<0.05 level in Origin 7.5.
Materials
CPTIO, DEA/NO, spermine NONOate, proline NONOate and soluble GC were obtained from Axxora (UK) Ltd. Sildenafil was supplied by the chemistry division of the Wolfson Institute for Biomedical Research, BAY 58-2667 by Bayer Healthcare (Wuppertal, Germany) and HMR-1766 by Sanofi-Aventis Deutschland GmbH (Frankfurt, Germany). ODQ was obtained from Tocris Bioscience (Bristol, UK) and the remaining chemicals were from Sigma-Aldrich (Poole, Dorset, UK). Stock solutions of ODQ, BAY 58-2667 and HMR-1766 were in 50% dimethyl sulphoxide. Stock solutions of NO donors were made up in 10 mM NaOH and kept on ice. The other chemicals were dissolved in deionized water.
Results
Mechanism of action of BAY 58-2667
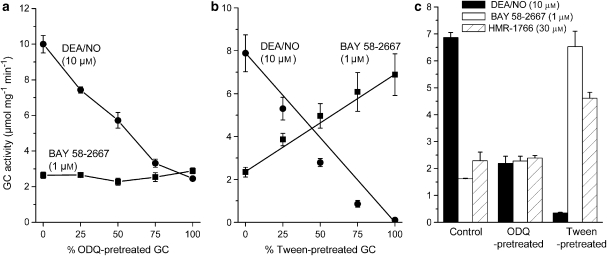
To test if BAY 58-2667 has a preferential activity towards haem-oxidized or haem-free GC, purified protein in both forms was prepared by pretreatment with ODQ or Tween-20, respectively (see Materials and methods). The samples were incubated in various mixtures with control (sham-pretreated) protein and were then exposed for 2 min to BAY 58-2667 or NO (10 μM DEA/NO). Predictably, as the proportion of ODQ-pretreated GC was increased, the NO-stimulated activity decreased (Figure 1a), maximally by about 80%. Incomplete inhibition by ODQ at high NO concentrations accords with previous findings (Garthwaite et al., 1995; Zhao et al., 2000). BAY 58-2667 at a maximally effective concentration of 1 μM (Stasch et al., 2002) stimulated control GC activity to about 25% of that observed with NO and, contrasting with the stimulation by NO, this level of activity remained constant as the proportion of ODQ-pretreated GC was increased (Figure 1a). Repeating these experiments using haem-free GC, the other purported target for BAY 58-2667, induced a progressive loss of response to NO and a progressive increase in the effect of BAY 58-2667 as the amount of haem-free protein increased (Figure 1b). With 100% haem-free protein, the NO-stimulated activity was very low (0.1 μmol mg−1 min−1), whereas the activity in the presence of BAY 58-2667 was almost as high as that observed with NO under control conditions (8 μmol mg−1 min−1).
Figure 1.
Mechanism of action of BAY 58-2667 and HMR-1766. Purified GC was treated with either (a) 10 μM ODQ to oxidize the GC haem or (b) 0.5% Tween-20 to render GC haem-free. The treated GC preparations were mixed in varying proportions with sham-treated GC and exposed to either 1 μM BAY 58-2667 or 10 μM DEA/NO for 2 min. (c) The GC activity evoked by DEA/NO (10 μM), BAY 58-2667 (1 μM) and HMR-1766 (30 μM) is compared under control conditions and after pretreatment with ODQ (10 μM) or Tween-20 (0.5%). DEA/NO, diethylamine NONOate; ODQ, 1-H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one.
These findings suggest that the sole target of BAY 58-2667 is the haem-free receptor. The activity of BAY 58-2667 in the native and ODQ-treated GC, therefore, is likely to reflect the presence of haem-free protein in these preparations. The data in Figure 1b could be reasonably well explained by assuming that BAY 58-2667 maximally stimulates haem-free GC to a rate of 6.9 μmol mg−1 min−1 (the value recorded using 100% Tween-20-pretreated samples), that the maximal NO-evoked activity of the haem-containing protein is 11.8 μmol mg−1 min−1 and that 33% of the protein under control conditions is haem-free (Figure 1b, lines; the deviations of both sets of data from the lines at intermediate dilutions, while moderate, suggest additional uncontrolled haem loss; see below).
Comparison of HMR-1766 with BAY 58-2667
The compound HMR-1766, which appears to act similarly to BAY 58-2667, has been suggested to activate predominantly the haem-oxidized receptor rather than the haem-free form (Schindler et al., 2006). In our experiments, HMR-1766 at a near-maximally effective concentration of 30 μM (Schindler et al., 2006) evoked an activity in control preparations similar to that of 1 μM BAY 58-2667 (20–30% of the response to DEA/NO) and was likewise without effect on haem-oxidized (ODQ-pretreated) GC (Figure 1c). Following removal of the haem, HMR-1766 became more effective but was less so than BAY 58-2667. These results indicate that HMR-1766 and BAY 58-2667 share the same mechanism of action, although they may differ in their efficacies.
Time-dependent haem loss from native and haem-oxidized GC
To check for stability of the haem under normal conditions, purified GC was incubated at 37 °C in the absence of GTP for 0 or 18 min and then stimulated with DEA/NO or BAY 58-2667 for 2 min in the presence of GTP (1 mM). The preincubation resulted in doubling of the GC activity stimulated by BAY 58-2667 and a halving of the DEA/NO-evoked activity (Figure 2a), suggesting that the proportion of haem-free enzyme had increased twofold. In a more detailed time course (with GTP present at the start), an observable increase in the rate of cGMP accumulation in the presence of BAY 58-2667 occurred after only 2–4 min preincubation (Figure 2b), indicating quite a rapid rate of haem dissociation. With ODQ present, the initial GC activity in response to BAY 58-2667 was similar to the control (about 2.5 μmol mg−1 min−1; see broken line in Figure 2b) but then became greater, suggesting that ODQ increased the rate of haem dissociation.
Figure 2.
Haem loss under GC assay conditions. (a) DEA/NO (10 μM) or BAY 58-2667 (1 μM) was added simultaneously with substrate (GTP, 1 mM) to GC after 0 or 18 min incubation at 37 °C and cGMP measured after a further 2 min. (b) GC was incubated with substrate and with 1 μM BAY 58-2667 in the absence or presence of 10 μM ODQ; cGMP was measured at the indicated time points. DEA/NO, diethylamine NONOate; ODQ, 1-H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one.
Characterization of BAY 58-2667 activity in platelets
The first experiments were carried out with the relevant phosphodiesterase activity (that of phosphodiesterase-5) effectively eliminated by sildenafil (100 μM) so as to remove complications arising from variations in cGMP breakdown (Mo et al., 2004). Exposure to a near-maximal clamped NO concentration (50 nM) led to a rapid increase in cGMP that, starting from almost nothing, plateaued after about 20 s at around 1700 pmol per mg protein (Figure 3a). Under the same conditions, a saturating concentration (see below) of BAY 58-2667 (10 μM) gave a low-amplitude cGMP response that was linear over the whole time course (Figure 3a), reaching a level after 2 min that was 14% of that achieved by NO after only 20 s. With a combination of 50 nM NO and 10 μM BAY 58-2667, the response was very similar to that observed with NO alone. In the presence of 10 μM ODQ, the effect of BAY 58-2667 was greatly enhanced. Although still slower than with 50 nM NO, BAY 58-2667 plus ODQ ultimately gave the same plateau level of about 1700 pmol per mg protein (Figure 3a).
Figure 3.
Comparative effects of NO and BAY 58-2667 on platelet GC activity. (a) Time course of cGMP accumulation in response to a clamped NO concentration (50 nM) with or without BAY 58-2667 (BAY, 10 μM) and to BAY 58-2667 in the absence or presence of ODQ (10 μM, 15 min preincubation), all in the presence of 100 μM sildenafil. Data were fitted to generalized hyperbolae or a straight line. (b) Kinetic profiles of GC activity extracted from the data in panel a. NO, nitric oxide; ODQ, 1-H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one.
These different cGMP profiles can be converted into profiles of GC activity with time after correcting for the rate of cGMP degradation, which, in the presence of 100 μM sildenafil, is almost negligible (Mo et al., 2004). With NO (with or without BAY 58-2667), cGMP synthesis peaked after 2–3 s at about 250 pmol per mg protein per s and then desensitized rapidly down to almost zero after 30 s (Figure 3b), corresponding to the attainment of the cGMP plateau in Figure 3a. With BAY 58-2667 plus ODQ, the peak GC activity was lower by a factor of about 5 and was slower to peak and desensitize. The activity with BAY 58-2667 on its own was very low (2.1 pmol per mg protein per s), corresponding to only 0.77% of the maximum NO-evoked activity and 3.7% of the activity with ODQ and, as shown by the linear time course (Figure 3a), was non-desensitizing.
Concentration–response curves for BAY 58-2667 were constructed after 1 min exposure in the presence of sildenafil (Figure 4a). Without ODQ, the EC50 was 18 nM, and in the presence of ODQ the potency of BAY 58-2667 was not significantly different, the EC50 being 13 nM. In both cases, the Hill coefficient was 1.0, indicative of a single binding site.
Figure 4.
Concentration–response curves for BAY 58-2667 and NO in platelets. (a) Curve for BAY 58-2667 in rat platelets in the presence or absence of 10 μM ODQ (15 min preincubation). The exposure time was 1 min. (b) NO transients of peak concentration ranging from 0.1 to 150 nM were delivered to unstimulated platelets (control) or platelets 1 min after desensitization was induced by a single NO transient peaking at 100 nM (desensitized). The apparent GC activity (ordinate) corresponds to half that measured after 2 s exposure to NO in the presence of sildenafil (100 μM). The elevated baseline following desensitization largely reflects extracellular cGMP generated from the initial exposure to NO (Mo et al., 2004). All data are fitted by the Hill equation, giving the indicated EC50 values and Hill coefficients (nH). NO, nitric oxide; ODQ, 1-H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one.
Effect of desensitization on the NO concentration–response curve
Determining if desensitization is associated with a change in the potency of NO or a change in efficacy (or both) requires sequential administration of NO, the first to induce desensitization and the second to measure its effect on GC activity. Recently, we have developed a method for delivering NO pulses of known amplitude and duration (Roy and Garthwaite, 2006), allowing the experiment to be performed. Partial desensitization was imposed by giving a pulse peaking at 100 nM NO (peak at 2 s; duration ∼20 s), and GC activity was assessed 1 min later by adding a second pulse of varying amplitude in the presence of sildenafil (100 μM) and measuring cGMP accumulation after 2 s. The control concentration–response curve was very similar to that reported before with this technique (Roy and Garthwaite, 2006) or when using clamped NO concentrations (Mo et al., 2004), the EC50 being about 10 nM (Figure 4b). Previous exposure to the 100 nM NO pulse led to a 65% loss in maximal GC activity but no change in the EC50 for NO (Figure 4b). The slope of the concentration–response curve was, however, steepened (Hill coefficient of 2.4 versus 1.2 in the controls), as noted beforehand from experiments on brain cell suspensions (Bellamy and Garthwaite, 2001). The results show that desensitization is associated with a loss of active NO receptor, not simply a change in the potency of NO, consistent with the possibility that loss of the haem NO binding site is responsible.
Effect of desensitization in the absence of sildenafil on BAY 58-2667 activity
In the initial characterization of the activity of BAY 58-2667 on platelets (above), desensitization to NO appeared unaltered by the presence of BAY 58-2667, but this result was obtained in the presence of sildenafil, which led to prolonged very high cGMP levels, so that increases in the proportion of activity that is sensitive to BAY 58-2667 could have been lost in the noise. To address this problem, desensitization was monitored in the absence of sildenafil. In this condition, exposure of platelets to a constant NO concentration generates only a transient increase of cGMP (Figure 5a), as a result of the combined influence of receptor desensitization and a time-dependent enhancement of phosphodiesterase-5 activity (Mo et al., 2004). The degree of desensitization can be gauged by adding sildenafil after the blip and charting the rate of cGMP accumulation. When sildenafil was added 60 s after initiating the NO exposure (50 nM), the initial rate was greatly slowed compared with the control (Figures 5a and b), showing persistent desensitization despite the return of cGMP to near basal levels well before this time. As reported previously (Mo et al., 2004), the capacity for cGMP accumulation after late addition of sildenafil was also almost half that seen when sildenafil was added at the start. Extracting the kinetics showed that GC was about 85% desensitized (Figure 5b), agreeing with previous findings (Mo et al., 2004). Since it gave a greater degree of desensitization at a lower NO concentration than was obtained using the NO pulse method (see above), constant exposure to NO was used in the following experiments.
Figure 5.
Effect of GC desensitization on activity of BAY 58-2667. (a) Platelets were exposed to 50 nM NO, and cGMP accumulation was followed over time. Sildenafil (100 μM) was added at either t=0 or 60 s. Data in the presence of sildenafil were fitted to generalized hyperbolae. (b) GC activity profiles derived from the data in panel a. (c) cGMP accumulation after addition of 50 nM NO in the presence or absence of 100 μM sildenafil, and after addition of 10 μM BAY 58-2667 (10 μM) in the presence or absence of ODQ (10 μM) at either t=0 or t=60 s, just after NO had been scavenged using haemoglobin (Hb, 30 μM). As a control, haemoglobin (30 μM) was also added immediately before the initial exposure to BAY 58-2667 alone. All exposures to BAY 58-2667 were in the presence of sildenafil. Preincubation with ODQ was for 15 min. The data were fitted using pulse, hyperbolic or linear functions. (d) GC activity profiles derived from the data in panel c. NO, nitric oxide; ODQ, 1-H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one.
Examining the response to BAY 58-2667 on different states of the receptor was rather complex (Figure 5c), but the strategy was to supply NO for sufficient time to generate the transient cGMP increase and for the receptor to adopt a steady-state desensitized level (60 s exposure; Mo et al., 2004). Then, free NO was scavenged using haemoglobin, and BAY 58-2667 and sildenafil were added, and the rate of increase of cGMP was measured against a control without previous NO exposure. First, control recordings were made of the cGMP response to NO (50 nM) with and without sildenafil, giving the usual large plateau and transient blip, respectively. Control responses to BAY 58-2667 with and without ODQ, both in the presence of sildenafil, showed, as before (Figure 3), a very low rate with BAY 58-2667 on its own (less than 1% of the maximum NO-stimulated activity) and a large enhancement in the presence of ODQ (to near 20% of maximum), as seen in the extracted kinetics (Figure 5d). Under desensitized conditions, the rate of GMP accumulation in response to BAY 58-2667 (1.1 pmol per mg protein per s) was identical to that occurring in the absence of a previous NO exposure, as evidenced by the parallel increases in cGMP (Figure 5c). As a further control, the effect of BAY 58-2667 in the presence of ODQ after an equivalent NO exposure was examined and this response was also unchanged (Figures 5c and d). Comparable with the previous experiment (Figure 3), the GC activity in the presence of BAY 58-2667 alone was 0.86% of maximal NO-evoked activity and 5.6% of the mean activity of the compound in the presence of ODQ.
Effect of BAY 58-2667 on VASP phosphorylation
That the GC activity evoked by BAY 58-2667 in platelets under normal conditions is maximally so low (about 1% of the maximal NO-stimulated activity) raises questions about how the compound could exert biological activity. One way that the NO–cGMP pathway is transduced in platelets is through cGMP-dependent protein kinase, leading to increased phosphorylation of VASP (Smolenski et al., 1998). Exposure of the platelets to BAY 58-2667 (10 μM, 2 min) resulted in a significant increase in phospho-VASP, similar to that produced by exposure to 10 μM DEA/NO for the same duration (Figure 6). Inclusion of ODQ did not significantly increase the response to BAY 58-2667, whereas sildenafil (100 μM) augmented the levels of phospho-VASP in the presence of BAY 58-2667, DEA/NO and BAY 58-2667 plus ODQ (Figure 6).
Figure 6.
VASP phosphorylation in platelets. Bars signify total amounts of phospho-VASP following 2 min incubation without anything (Control), 10 μM BAY 58-2667 (BAY), 10 μM BAY 58-2667+100 μM sildenafil (BAY+Sild), 10 μM DEANO (DEA/NO), 10 μM DEANO+100 μM sildenafil (DEA/NO+Sild), 10 μM BAY 58-2667+10 μM ODQ (BAY+ODQ) or 10 μM BAY 58-2667+10 μM ODQ+100 μM sildenafil (BAY+ODQ+Sild). Band density per unit area was measured in digital light units (DLUs) and the readings were normalized to the control densities (100%). All test conditions were significantly higher than the control (P<0.05) and sildenafil significantly increased the band densities in all cases (P<0.05). A representative immunoblot of phosphorylated VASP (two bands migrating at ∼46 and ∼50 kDa representing the singly and doubly phosphorylated species) is displayed at the top; the experimental condition for each lane corresponds to that of the underlying bar. DEA/NO, diethylamine NONOate; ODQ, 1-H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; VASP, vasodilator-stimulated phosphoprotein.
Discussion and conclusion
The initial issue addressed was the mechanism of action of BAY 58-2667 and the similarly acting compound, HMR-1766. In an earlier report (Schmidt et al., 2004), binding, functional and molecular modelling studies gave coherent evidence that BAY 58-2667 is a haem mimetic able to bind with high affinity to GC when the native haem (the NO binding site) is removed, haem oxidation (with ODQ) serving to increase the rate of haem dissociation and thereby increase the efficacy of the compound. Subsequent reports, however, have emphasized the ability of the compound to act selectively on the haem-oxidized form of the receptor (Dumitrascu et al., 2006; Stasch et al., 2006; Boerrigter et al., 2007), and this was also the primary mechanism attributed to HMR-1766 and the chemically related compound S-3448, despite evidence being presented for an ability to stimulate the haem-free protein (Schindler et al., 2006). Our results show that BAY 58-2667 and HMR-1766 cannot distinguish between the haem-reduced (Fe2+) and haem-oxidized (Fe3+) species but are effective agonists for the haem-free entity. The moderate efficacy of the compounds in untreated purified GC preparations is likely to result from loss of haem during the purification process (Craven and DeRubertis, 1978). In addition, our results point to a time-dependent loss of receptor haem when the protein is in the dilute solutions used in GC assays and an acceleration of this process when the haem is oxidized by ODQ. This factor would help explain why HMR-1766 and S-3448 appeared to have a higher efficacy than NO in a partially purified GC preparation subjected to very long (1 h) incubation (Schindler et al., 2006). Moreover, the notion that the compounds act on the haem-oxidized form may have arisen because the speed of dissociation of the oxidized haem in cell-free preparations was not fully appreciated. In accordance with the original description (Schmidt et al., 2004), we would conclude that, whatever the cause, the lack of a haem group is the condition that allows the compounds to bind to GC and thereby promote its activation.
The second aim was to analyse quantitatively the action of BAY 58-2667 on cellular GC. The potency of BAY 58-2667 in platelets (EC50 ∼15 nM) was very similar to estimates made on purified recombinant GC (Stasch et al., 2002; Schmidt et al., 2004, 2005). This, in itself, is noteworthy because the potency of NO as an agonist for the receptors is about 10-fold lower in intact platelets compared to lysates or purified enzyme preparations (10 versus 1 nM; Mo et al., 2004). The alteration in the potency of NO could reflect kinetic differences in the NO binding step and/or in the subsequent conformational change (Colquhoun, 1998), but the lack of this disparity with BAY 58-2667, which substitutes for the NO-bound haem, suggests that the kinetics of the conformational change is similar in platelets and in the purified protein. Thus, it is likely to be at the NO binding step where the difference lies, as was suggested from theoretical considerations (Garthwaite, 2005).
This interpretation assumes that the concentration of BAY 58-2667 to which the platelets were exposed rapidly equilibrates with the cell interior to give equal concentrations on either side of the membrane. Although there is no direct evidence to bear on this issue, the GC activity on addition of BAY 58-2667 showed no obvious lag and remained linear for at least 2 min, implying rapid equilibration with the cytosol. The membrane permeability of BAY 58-2667 has not been reported to our knowledge but can be estimated from its molecular weight and polar surface area (Clark, 1999) to be about 5 × 10−6 cm s−1 (a polar surface area of 95 Å2 was calculated using ChemDraw Ultra 9.0; CambridgeSoft, Cambridge, MA, USA). Representing platelets as spheres of radius 0.5 μm, the rate constant for equilibration of BAY 58-2667 across the platelet membrane (permeability multiplied by surface area-to-volume ratio) comes to 0.3 s−1. Thus, when 10 M BAY 58-2667 is applied externally, a maximally effective intracellular concentration (for example, 1 M; Figure 4a) would be attained in less than 0.4 s. These calculations suggest that equilibration across the platelet membrane would not be rate limiting, in accordance with experimental observations.
As expected (Stasch et al., 2002; Schmidt et al., 2004), oxidation of the haem with ODQ increased the efficacy of BAY 58-2667 and did so to a greater degree than observed in cell-free conditions, indicating that the haem occupancy in a normal intracellular environment is high and stable, and that ODQ promotes haem loss. In ODQ-treated platelets, BAY 58-2667 generated about a fivefold lower maximum activity compared with that normally elicited by NO, whereas the difference in efficacy with purified GC was estimated to be nearer twofold. The operation of mechanisms in a cellular environment that re-reduce the haem (Gupte et al., 1999; Iesaki et al., 1999; Bellamy and Garthwaite, 2002), preventing its dissociation (or promoting its re-association), is likely to contribute to this disparity.
Our data allow an estimate to be made of the amount of GC protein that exists in cells in a haem-deficient form. Assuming the true efficacy of BAY 58-2667 on haem-free GC to be half that normally achieved by NO (from data on purified protein; Figure 1), the observation that BAY 58-2667 maximally evoked about 1% of the peak NO-stimulated activity suggests that about 2% of the protein is normally haem-free. The 22-fold increase in maximum activity of BAY 58-2667 observed in the presence of ODQ (the average from all three experiments) indicates that about half the protein is then haem-free. The figure we obtain for normal platelets is an order of magnitude lower than the 20% deduced for rat aortic smooth muscle cells (Schindler et al., 2006). The value of 20% originated in the finding that cGMP in smooth muscle cells was four- to fivefold higher when HMR-1766 was given in the presence of ODQ compared with its absence. The interpretation is probably erroneous, however, because it was based on comparison of cGMP levels at a single time point (10 min). Such measurements cannot be translated into relative GC activities unless the cGMP is shown to accumulate linearly with time under both conditions, which is highly unlikely. To illustrate this point, the cGMP level stimulated by BAY 58-2667 alone as a proportion of the level achieved by BAY 58-2667 plus ODQ varies constantly with time (Figures 3a and 5c). Only by measuring GC activity itself can proper calculations be performed.
If only 2% of the available GC protein is normally available to compounds such as BAY 58-2667, it is perhaps surprising that they are so effective at producing functional responses, such as smooth muscle relaxation, in isolated tissues and in vivo (Stasch et al., 2002; Dumitrascu et al., 2006; Schindler et al., 2006). Transduction through cGMP-dependent protein kinase, however, requires only relatively low rates of cGMP generation, as shown by the 20-fold higher potency for NO, to induce VASP phosphorylation compared with cGMP accumulation in rat platelets (Mo et al., 2004) and the fact that NO can still evoke vascular relaxation through cGMP when 94% of NO-activated GC has been genetically deleted (Mergia et al., 2006). Thus, physiological GC activity probably corresponds to that found near the base of the NO concentration–response curve. Viewed quantitatively, 1% of the maximal NO-stimulated GC activity corresponds to a rate of cGMP formation of around 1 μM s−1 in rat platelets (Mo et al., 2004), which, bearing in mind the submicromolar affinity of cGMP for cGMP-dependent protein kinase (Francis and Corbin, 1999), should be biologically significant, particularly as binding to the kinase protects cGMP from degradation by phosphodiesterase-5 (Kotera et al., 2003). In accordance, BAY 58-2667 evoked significant VASP phosphorylation in rat platelets, a result that agrees with findings using S-3448 in rat aorta (Schindler et al., 2006).
A final issue addressed in our experiments was whether the haem-deficient component of GC is fixed or can vary under physiological conditions. Our emphasis was on desensitization because this is a clear example of a sudden loss of NO-stimulated GC activity that reverses at a similar rate to the recovery from inhibition by ODQ. However, the results were very clear in showing no change in the proportion of haem-free GC (as indicated using BAY 58-2667) under conditions where 85% desensitization had occurred. Incidentally, the desensitization kinetics found in the presence of BAY 58-2667 plus ODQ (in the presence of sildenafil) was similar to when NO is applied at a concentration (3 nM) giving a comparable GC activity (Mo et al., 2004). This result indicates that desensitization is independent of the nature of the agonist and so is likely to be connected with the associated GC activity, as suggested previously (Wykes et al., 2002).
In conclusion, the novel class of activators typified by BAY 58-2667 does not have a dual action on both haem-oxidized and haem-depleted GC but acts only on the protein with an unoccupied haem pocket. The use of BAY 58-2667 as a tool for probing the haem occupancy of GC in cells indicated that in rat platelets only a small proportion (2%) of enzyme normally exists in this state and that this proportion does not change during prolonged NO exposure associated with a loss of 85% of NO-evoked GC activity, excluding haem loss as a mechanism. However, the proportion of haem-free enzyme in platelets increased 22-fold under conditions of haem oxidation. This event, or a deficiency in the incorporation of haem into the protein, could account for the enhanced potency of BAY 58-2667 observed in spontaneous and experimental pathologies (Dumitrascu et al., 2006; Stasch et al., 2006; Boerrigter et al., 2007).
Acknowledgments
The work was funded by The Wellcome Trust; BR holds a Medical Research Council (UK) PhD studentship. We thank Dr Johannes-Peter Stasch (Bayer Healthcare) and Dr Ursula Schindler (Sanofi-Aventis Deutschland GmbH) for generously supplying BAY 58-2667 and HMR-1766, respectively, and Dr David Selwood (Wolfson Institute for Biomedical Research, University College London) for assistance with molecular descriptors.
Abbreviations
- CPTIO
2-4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- DEA/NO
diethylamine NONOate
- ODQ
1-H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
Conflict of interest
The authors state no conflict of interest.
References
- Bellamy TC, Garthwaite J. Sub-second kinetics of the nitric oxide receptor, soluble guanylyl cyclase, in intact cerebellar cells. J Biol Chem. 2001;276:4287–4292. doi: 10.1074/jbc.M006677200. [DOI] [PubMed] [Google Scholar]
- Bellamy TC, Garthwaite J. Pharmacology of the nitric oxide receptor, soluble guanylyl cyclase, in cerebellar cells. Br J Pharmacol. 2002;136:95–103. doi: 10.1038/sj.bjp.0704687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy TC, Wood J, Goodwin DA, Garthwaite J. Rapid desensitization of the nitric oxide receptor, soluble guanylyl cyclase, underlies diversity of cellular cGMP responses. Proc Natl Acad Sci USA. 2000;97:2928–2933. doi: 10.1073/pnas.97.6.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerrigter G, Costello-Boerrigter LC, Cataliotti A, Lapp H, Stasch JP, Burnett JC., Jr Targeting heme-oxidized soluble guanylate cyclase in experimental heart failure. Hypertension. 2007;49:1128–1133. doi: 10.1161/HYPERTENSIONAHA.106.083832. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Kim D, Schmitz-Winnenthal FH, Amidi M, Godecke A, Mulsch A, et al. Increased nitrovasodilator sensitivity in endothelial nitric oxide synthase knockout mice: role of soluble guanylyl cyclase. Hypertension. 2000;35:231–236. doi: 10.1161/01.hyp.35.1.231. [DOI] [PubMed] [Google Scholar]
- Clark DE. Rapid calculation of polar molecular surface area and its application to the prediction of transport phenomena. 1. Prediction of intestinal absorption. J Pharm Sci. 1999;88:807–814. doi: 10.1021/js9804011. [DOI] [PubMed] [Google Scholar]
- Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure–activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol. 1998;125:924–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven PA, DeRubertis FR. Restoration of the responsiveness of purified guanylate cyclase to nitrosoguanidine, nitric oxide, and related activators by heme and hemeproteins. Evidence for involvement of the paramagnetic nitrosyl-heme complex in enzyme activation. J Biol Chem. 1978;253:8433–8443. [PubMed] [Google Scholar]
- Denninger JW, Marletta MA. Guanylate cyclase and the NO/cGMP signalling pathway. Biochim Biophys Acta. 1999;1411:334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- Dumitrascu R, Weissmann N, Ghofrani HA, Dony E, Beuerlein K, Schmidt H, et al. Activation of soluble guanylate cyclase reverses experimental pulmonary hypertension and vascular remodeling. Circulation. 2006;113:286–295. doi: 10.1161/CIRCULATIONAHA.105.581405. [DOI] [PubMed] [Google Scholar]
- Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster J, Harteneck C, Malkewitz J, Schultz G, Koesling D. A functional heme-binding site of soluble guanylyl cyclase requires intact N-termini of alpha 1 and beta 1 subunits. Eur J Biochem. 1996;240:380–386. doi: 10.1111/j.1432-1033.1996.0380h.x. [DOI] [PubMed] [Google Scholar]
- Francis SH, Corbin JD. Cyclic nucleotide-dependent protein kinases: intracellular receptors for cAMP and cGMP action. Crit Rev Clin Lab Sci. 1999;36:275–328. doi: 10.1080/10408369991239213. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Boulton CL. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- Garthwaite J. Dynamics of cellular NO-cGMP signaling. Front Biosci. 2005;10:1868–1880. doi: 10.2741/1666. [DOI] [PubMed] [Google Scholar]
- Griffiths C, Wykes V, Bellamy TC, Garthwaite J. A new and simple method for delivering clamped nitric oxide concentrations in the physiological range: application to activation of guanylyl cyclase-coupled nitric oxide receptors. Mol Pharmacol. 2003;64:1349–1356. doi: 10.1124/mol.64.6.1349. [DOI] [PubMed] [Google Scholar]
- Gupte SA, Rupawalla T, Phillibert D, Jr, Wolin MS. NADPH and heme redox modulate pulmonary artery relaxation and guanylate cyclase activation by NO. Am J Physiol. 1999;277:L1124–L1132. doi: 10.1152/ajplung.1999.277.6.L1124. [DOI] [PubMed] [Google Scholar]
- Iesaki T, Gupte SA, Wolin MS. A flavoprotein mechanism appears to prevent an oxygen-dependent inhibition of cGMP-associated nitric oxide-elicited relaxation of bovine coronary arteries. Circ Res. 1999;85:1027–1031. doi: 10.1161/01.res.85.11.1027. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ. Signal transduction mechanisms involving nitric oxide. Biochem Pharmacol. 1991;41:485–490. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- Ko FN, Wu CC, Kuo SC, Lee FY, Teng CM. YC-1, a novel activator of platelet guanylate cyclase. Blood. 1994;84:4226–4233. [PubMed] [Google Scholar]
- Koesling D, Russwurm M, Mergia E, Mullershausen F, Friebe A. Nitric oxide-sensitive guanylyl cyclase: structure and regulation. Neurochem Int. 2004;45:813–819. doi: 10.1016/j.neuint.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Kotera J, Grimes KA, Corbin JD, Francis SH. cGMP-dependent protein kinase protects cGMP from hydrolysis by phosphodiesterase-5. Biochem J. 2003;372:419–426. doi: 10.1042/BJ20030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergia E, Friebe A, Dangel O, Russwurm M, Koesling D. Spare guanylyl cyclase NO receptors ensure high NO sensitivity in the vascular system. J Clin Invest. 2006;116:1731–1737. doi: 10.1172/JCI27657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo E, Amin H, Bianco IH, Garthwaite J. Kinetics of a cellular nitric oxide/cGMP/phosphodiesterase-5 pathway. J Biol Chem. 2004;279:26149–26158. doi: 10.1074/jbc.M400916200. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Olesen S-P, Drejer J, Axelsson O, Moldt P, Bang L, Nielsen-Kudsk JE, et al. Characterization of NS 2028 as a specific inhibitor of soluble guanylyl cyclase. Br J Pharmacol. 1998;123:299–309. doi: 10.1038/sj.bjp.0701603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand MJ, Li CG. Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanism of transmission. Annu Rev Physiol. 1995;57:659–682. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- Roy B, Garthwaite J. Nitric oxide activation of guanylyl cyclase in cells revisited. Proc Natl Acad Sci USA. 2006;103:12185–12190. doi: 10.1073/pnas.0602544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler U, Strobel H, Schonafinger K, Linz W, Lohn M, Martorana PA, et al. Biochemistry and pharmacology of novel anthranilic acid derivatives activating heme-oxidized soluble guanylyl cyclase. Mol Pharmacol. 2006;69:1260–1268. doi: 10.1124/mol.105.018747. [DOI] [PubMed] [Google Scholar]
- Schmidt PM, Rothkegel C, Wunder F, Schroder H, Stasch JP. Residues stabilizing the heme moiety of the nitric oxide sensor soluble guanylate cyclase. Eur J Pharmacol. 2005;513:67–74. doi: 10.1016/j.ejphar.2005.02.046. [DOI] [PubMed] [Google Scholar]
- Schmidt PM, Schramm M, Schroder H, Wunder F, Stasch JP. Identification of residues crucially involved in the binding of the heme moiety of soluble guanylate cyclase. J Biol Chem. 2004;279:3025–3032. doi: 10.1074/jbc.M310141200. [DOI] [PubMed] [Google Scholar]
- Schrammel A, Behrends S, Schmidt K, Koesling D, Mayer B. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol Pharmacol. 1996;50:1–5. [PubMed] [Google Scholar]
- Smolenski A, Bachmann C, Reinhard K, Honig-Liedl P, Jarchau T, Hoschuetzky H, et al. Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J Biol Chem. 1998;273:20029–20035. doi: 10.1074/jbc.273.32.20029. [DOI] [PubMed] [Google Scholar]
- Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, et al. NO-independent regulatory site on soluble guanylate cyclase. Nature. 2001;410:212–215. doi: 10.1038/35065611. [DOI] [PubMed] [Google Scholar]
- Stasch JP, Schmidt P, Alonso-Alija C, Apeler H, Dembowsky K, Haerter M, et al. NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br J Pharmacol. 2002;136:773–783. doi: 10.1038/sj.bjp.0704778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, AK HS, Meurer S, et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest. 2006;116:2552–2561. doi: 10.1172/JCI28371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes V, Bellamy TC, Garthwaite J. Kinetics of nitric oxide-cyclic GMP signalling in CNS cells and its possible regulation by cyclic GMP. J Neurochem. 2002;83:37–47. doi: 10.1046/j.1471-4159.2002.01106.x. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Brandish PE, DiValentin M, Schelvis JP, Babcock GT, Marletta MA. Inhibition of soluble guanylate cyclase by ODQ. Biochemistry. 2000;39:10848–10854. doi: 10.1021/bi9929296. [DOI] [PubMed] [Google Scholar]