Summary
Information on the development and functions of rostral prefrontal cortex (PFC), or Brodmann area 10, has been gathered from different fields, from anatomical development to functional neuroimaging in adults, and put in relation to three particular cognitive and behavioural disorders. Rostral PFC is larger and has a lower cell density in humans than in other primates. It also has a large number of dendritic spines per cells and numerous connections to the supramodal cortex. These characteristics suggest that rostral PFC is likely to support processes of integration or coordination of inputs particularly developed in humans. The development of rostral PFC is prolonged, with decreases in grey matter and synaptic density continuing into adolescence. Functions attributed to rostral PFC, such as prospective memory, appear to similarly follow a prolonged development until adulthood. Neuroimaging studies have generally found reduced recruitment of rostral PFC, e.g. in tasks requiring response inhibition, in adults compared with children or adolescents, which is consistent with grey matter maturation. The examples of autism, attention deficit and hyperactivity disorder and schizophrenia show that rostral PFC could be affected in several disorders due to the susceptibility of its prolonged maturation to developmental abnormalities.
1. Introduction
Rostral prefrontal cortex (PFC), which corresponds approximately to Brodmann area 10 (BA10, (1, 2), is the largest single architectonic region of the frontal lobes of the human brain (3). In humans, this region continues developing throughout childhood and adolescence. Evidence about the cognitive function of rostral PFC has been scarce until the last decade, partly because its position within the skull prevents successful use of techniques such as electroencephalography, animal lesions or single cells recording. Moreover, the proximity to facial nerves makes electrical stimulations of the scalp used in techniques such as transcranial magnetic stimulation painful. Therefore, the recent data that have been obtained on the function of rostral PFC tend to come from human lesion studies and functional imaging. Several theories of rostral PFC function have been proposed on the basis of these data, mostly attributing to this region a role in certain executive functions.
The purpose of this article is to integrate these findings with what is known about the structural development of rostral PFC and research on cognitive and behavioural disorders. We hope that this review will be a useful tool to inform psychological studies of children and adolescents, show the relevance of this field of research to a range of psychological disorders, and indicate possible future areas of investigation. First, the anatomy and anatomical development of rostral PFC will be presented, before describing evidence obtained in adults on the function of this region, and some theories that have been proposed. Second, experimental psychology and neuroimaging studies that shed light on rostral PFC development will be reviewed. Finally, it is proposed that due to the prolonged development of rostral PFC, extending to adolescence, impairments in the functions associated to this region should be observed in a large range of developmental disorders. Autism spectrum disorders (ASD), attention deficit and hyperactivity disorder (ADHD), and schizophrenia, three cognitive and behavioural disorders with different ages of onsets, were studied to test this hypothesis. The results suggest indeed that rostral PFC function might be affected by developmental anatomical abnormalities in a large spectrum of psychological disorders.
2. Anatomy of rostral PFC
Recent anatomical investigations have provided detailed data on the localisation of BA10, its size and connections with other areas, revealing an apparent distinction of sub-regions of rostral PFC.
2.1. Rostral PFC across species
A century ago Brodmann (2) suggested that a homology between the human and monkey frontal cortical regions was very unclear. However, recently Semendeferi et al. (4) demonstrated the presence of a homologous area 10 in the frontal pole of humans and other primates (see also (5)). Area 10 in the right hemisphere of humans was estimated by Semendeferi et al. (4) to contain 254.4 million neurons, whereas the estimate for great apes is less than one third of that number, and is for the gibbon of 8 million neurons only. The reverse relationship held true for the density of neurons: the human area 10 was found to have the lowest density and the gibbon’s the highest. Additionally, neuropil, which consists of the tangle of dendrites, axons and glial processes that remain when cell bodies of neurons and glia are removed from grey matter, was found to represent a higher fraction of grey matter in BA10 in humans than in the others apes. These findings are consistent with the description of the frontal pole of the human brain as being cell-sparse and pale in its overall appearance in comparison to the surrounding areas (5). Jacobs et al. (6) similarly observed that the number of dendritic spines per cell and the spine density are higher in human rostral PFC than in other comparable areas of the cortex, but that the density of cell bodies is markedly low. In terms of volume, area 10 was found to be larger in the human brain than in the other hominoids: even in relative terms it may be up to twice the size in the human brain than in any of the great apes ((4), see (7) for discussion on this result).
2.2 Subdivisions of rostral PFC
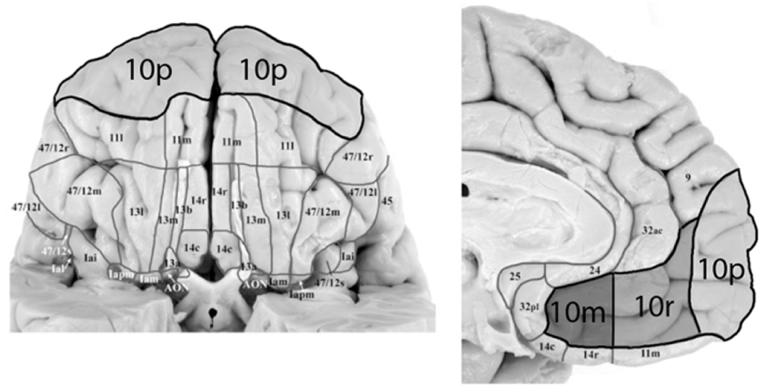
The large size of Brodmann area 10 suggests the possible differentiation of sub-regions. Semendeferi et al. (4) found no subdivisions of area 10 in the human brain. Öngür et al. (8), however, suggested that area 10 could be divided into different regions: caudally lie areas 10m and 10r, which form an area with relatively homogeneous granular structure, although axonal tracing experiments have suggested that the caudal and rostral portions have distinct connections (9-11); a large highly differentiated cortical area covers the frontal pole itself, and was named polar area 10 (10p) by the authors, to distinguish it from the monkeys area 10o, which occupies the rostral orbital region and the frontal pole (see (12)). Öngür et al. (8) found a marked difference between human and monkeys, with a striking expansion in the human brain of the granular cortex at the frontal pole, corresponding to area 10p (see Figure 1).
Figure 1.
Architectonic subdivisions of the human area 10. On the left is shown the orbital surface, on the right the medial surface. Adapted from (8), with kind permission from the authors and the publishers of Journal of Comparative Neurology, © 2003 Wiley-Liss, Inc.
2.3 Connections between rostral PFC and other brain regions
Some subdivisions of rostral PFC are also apparent from connectivity data. Öngür et al. (8) proposed that a “medial” network involves areas on the medial wall including area 10 and projects to visceral control centres in the hypothalamus and periaqueductal grey. These regions receive auditory or polymodal input from the superior temporal sulcal region (13, 14). This network has been suggested to be a “viscero-motor” or “emoto-motor” system that could modulate visceral activity in response to affective stimuli (15). Rostral PFC does not seem to be interconnected with “downstream” areas in the way that other prefrontal areas are. It is the only prefrontal region that is predominantly interconnected with supramodal cortex in the PFC (e.g. (16)), anterior temporal cortex (e.g. (17)) and cingulate cortex (e.g. (18)). In addition, its projections are broadly reciprocal (19) (see (20) for review).
Anatomical studies of rostral PFC thus suggest this area is more extended and differentiated in humans than in other primates. It has a low cell density, which may indicate that rostral PFC in humans has more space available for extrinsic and intrinsic connections (4). Rostral PFC connections are mostly limited to interconnections with the supramodal cortex. Finally, Brodmann area 10 also has a particularly high number of dendritic spines per cell, which indicates that the computational properties of rostral PFC are more likely than those of comparable areas to involve the integration or coordination of inputs (20).
3. Anatomical development of rostral PFC
Anatomical development studies have focused on different age groups and employed a wide range of methods, from post-mortem to neuroimaging studies. Studies of neuroanatomical changes have shown that different brain structures mature at different rates. In particular, recent evidence from studies in humans suggests that frontal brain regions, including rostral PFC, develop more slowly than other regions, maturing into late adolescence and beyond.
3.1. Dendritic systems
Post-mortem studies of the development of the dendritic systems in rostral PFC suggest they mature later than primary sensory and motor regions, and continue maturing until late adolescence. Travis et al. (21) observed that total dendritic length and dendritic spine number of certain pyramidal neurons of neonatal human cortex were greater in BA4 than in BA10. According to the authors, these results show a roughly inverse regional pattern of dendritic complexity in the neonate than in adults, and indicate that the developmental time courses of these dendritic systems are more protracted for supramodal BA10 than for primary and unimodal regions (BA1, BA2, BA3, BA4 and BA18). This suggestion is supported by findings of prolonged decrease of synaptic density (22, 23), and increase of dendritic arborisation of pyramidal neurons (24) in the middle frontal gyrus-the rostral part of which corresponds to BA10- during late childhood and adolescence.
3.2. White matter
Developmental changes in white matter, which reflect differences in myelination and/or the direction or density of fibre tracts, have been investigated in MRI studies of the human brain, but only a few studies provide information regarding prefrontal cortex. Increased white matter volume in adults (mean age 27 years (y.)) compared with children (mean age 10 y.) was reported in the frontal lobes (25). Nagy and colleagues (26) observed a positive correlation of white matter density and age in relatively deep white fibre tracks of the left inferior frontal cortex. Finally, Barnea-Goraly and colleagues (27) observed that a measure of the direction of fibre tracts (fractional anisotropy1) correlated positively with age (range: 6-19 y.) in a number of prefrontal regions, including right lateral BA10 and medial area 10 (see also (29)).
3.3 Grey matter
MRI studies have also provided information on the development of grey matter in rostral PFC, which seems to follow a non-linear time course. Sowell et al. (30) scanned 45 children twice (2 y. apart) between the ages of 5 and 11. They showed that grey matter thickness of the right frontal cortex, including right rostral PFC, decreased significantly in absolute terms over the 2 year period between the scanning sessions, with 77.8% of points on this surface showing a significant loss. Konrad et al. (31) observed a reduction in grey matter during adolescence, with a smaller grey matter volume in adults (20-34 y.) compared to children (8-12 y.) bilaterally in rostral PFC. Giedd et al. (32) found that frontal grey matter volumes peaked at around 11-13 y. and then decreased during adolescence and early adulthood. In line with these findings, Sowell et al. (33) observed a reduction in grey matter density between adolescence (12-16 y.) and adulthood (23-30 y.) in rostral PFC and other regions. O’Donnell et al. (34) studied cortical thickness both in rostral PFC and dorsolateral PFC (DLPFC) in children and adolescents. Cortical thickness was found to decrease with age from 8 to 20 y., but there was no difference in the rate of change between the two frontal regions. A study by Gogtay et al. (35), however, suggests that grey matter maturation over rostral PFC ends earlier than over the DLPFC (Figure 2). In this longitudinal study, MRI scans of children were obtained repeatedly (minimum 3 times) at 2 year intervals, in the range 4 to 21 y.
Figure 2.

Right lateral view of the dynamic sequence of grey matter maturation over the cortical surface in the age range 9-16 obtained by Gogtay et al. (35). The legend shows a shade representation of 4 categories of grey matter volume units. Rostral PFC appears to undergo earlier maturation than the nearby dorsolateral PFC. Adapted from (35), with kind permission from the authors and the publishers of Proceedings of the National Academy of Science of the USA, © 2004 The National Academy of Sciences of the USA.
Decreases in grey matter volume during adolescence have been attributed to synaptic pruning (e.g. (22)). However, it is important to note that developmental changes in grey matter volume could in addition be a consequence of the increase in cortical myelination (see (35-37)).
3.4 Brain growth
Sowell et al. (30) evaluated another measure of brain development: brain growth, assessed using an adapted “distance from the centre” method2. This measure does not indicate increases in grey or white matter but simply reflects radial expansion of the exterior cortical surface presumably caused by changes in the underlying grey and white matter. The results indicated that rostral PFC is one of the regions with the highest rate of brain growth in this age range (5-11 y.), with rates of growths between 0.5 and 1 mm per year in right lateral and medial rostral PFC.
Rostral PFC has thus been found to continue developing during childhood and adolescence (and beyond), with a reduction in synaptic density, reduction in grey matter density, and particularly fast brain growth. The protracted development of rostral PFC, as well as its anatomical characteristics (see section 2), are two reasons to suppose that rostral PFC may support cognitive processing that is especially important to humans (see (38)).
4. Cognitive functions supported by rostral PFC: studies in adults
The results of neuropsychological and neuroimaging studies of rostral PFC function will be summarised below and followed by a brief discussion of some of the theories of PFC function (for more details, see (39)).
4.1. Evidence from human lesion studies
Despite having profound problems in everyday life, patients with a lesion in rostral PFC often pass a wide range of cognitive tasks in the laboratory. A typical example is the case AP from Shallice & Burgess (40) (called “NM” in (41)). AP sustained a head injury that led to almost complete removal of the rostral PFC. However, on standard neuropsychological measures of intellectual functioning, memory, perception and even traditional tests of executive function, AP performed within the superior range (see (42)). This lack of general cognitive impairment has been replicated in several different patients (40, 42-46).
Some specific impairments, however, have been described: lesions in rostral PFC seem to lead to deficits when patients need to coordinate the performance of a number of tasks (multitasking), or in ill-structured situations. Multitasking typically involves maintaining super- or sub-ordinate goals whilst performing another task (see (44) for more details). Accordingly, one of AP’s most noticeable impairments in everyday life was a marked multitasking problem, which manifested itself as tardiness and disorganisation and which, despite his excellent intellect and social skills, ensured he never managed to return to work at the level he had enjoyed premorbidly (40-42). Shallice & Burgess (40) designed two new tests of multitasking to assess these problems, a real-life multitasking test based around a shopping exercise, the “Multiple Errands Test”, and a multitasking test for use in the laboratory: the “Six Element Test” (44). Despite excellent general cognitive skills, AP and the other cases reported by Shallice and Burgess (40) all performed these tasks below the 5% level compared with age- and IQ-matched controls (see (44) for a review of further cases reported in the literature). Additionally, rostral PFC lesions have also been found to impair disproportionately performance in “ill-structured” situations (e.g. (46, 47)), i.e. when the optimal way of behaving is not precisely signalled by the situation, so one has to impose one’s own structure.
To summarise, patients with rostral PFC lesions may have a generally preserved IQ, episodic memory and normal performance on standard tests of intelligence and executive functions. However, they are often impaired in multitasking, and in “ill-structured” situations (i.e. those where there are many possible ways of behaving, and the most advantageous is not immediately apparent).
4.2. Evidence from neuroimaging
Another approach which has been used to study rostral PFC function is neuroimaging. The studies mentioned here have used either positron emission topography (PET) or functional magnetic resonance imaging (fMRI). fMRI measures blood-oxygen dependent (BOLD) signal change, while positron emission topography usually measures cerebral blood flow differences (rCBF). These techniques have shown that BOLD signal or rCBF changes in rostral PFC occur during a wide range of cognitive tasks (48), from the simplest paradigms (e.g. conditioning paradigms (49)) to highly complex tests involving memory and judgment (e.g. (50-53) or problem-solving (e.g. (3)), but also during rest (e.g. (54)). Indeed, one can find recruitment of the rostral PFC in just about any kind of task (see (39)). However, there does seem to be some evidence for functional specialisation within rostral PFC. A recent meta-analysis of the changes in BOLD signal or rCBF observed in BA10 (55) showed that some paradigms show lateral or medial BA10 BOLD signal or rCBF differences depending on condition manipulations (e.g. working memory, episodic memory), while others tend to lead to the increase of BOLD signal or rCBF in a unique sub-region of rostral PFC.
In particular, two types of task seem to elicit BOLD signal or rCBF changes that occur both in the lateral and medial portions of BA10; further these changes appear to reveal a dissociation of these two regions depending on the conditions that are contrasted. (1) Gilbert et al. (55) defined a “Multitask” category which included studies involving the performance of more than one task within any given block of trials, e.g. tasks where one has to “bear something in mind” whilst doing something else (voluntary task switching after a delay (e.g. (53, 56)), prospective memory (PM; e.g. (51, 57, 58)). There is an increased recruitment of the lateral parts of BA10 in PM or multitasking conditions compared to a control task, while in medial BA10 the BOLD signal or rCBF are higher in the control task. (2) A similar dissociation of medial/lateral recruitment was observed in the different conditions of attention shifting (e.g. (59, 60)).
Some tasks appear to lead to changes in the recruitment of specific sub-regions of BA10, suggesting a functional specialisation of the underlying brain regions. Gilbert and colleagues (see (55) for full references) found that lateral changes in rCBF or BOLD signal were more likely in “working memory” tasks (86% of reported BOLD or rCBF signal changes in these tasks were lateral), defined in the manner of Cabeza & Nyberg (61) and including studies involving the phonological loop (verbal/numerical maintenance), the visuospatial sketchpad (maintenance of object or spatial information), and the central executive (problem solving) (e.g. (3, 62, 63)). The recruitment of lateral rostral PFC was also more likely in “episodic retrieval” tasks (86% of reported BOLD or rCBF signal changes in these tasks were lateral), i.e. those involving the search, access, and monitoring of stored information about personally experienced past events, as well as to the sustained mental set underlying these processes (e.g. (64, 65)). A reverse pattern was observed by Gilbert et al. (55) in “mentalising” tasks, i.e. tasks requiring reflection on one’s mental states and those of other agents, which tended to recruit medial rostral PFC rather than lateral PFC (88% of reported BOLD or rCBF signal changes in these tasks were medial) (e.g. (66-68) (see Figure 3).
Figure 3.

Partitioning of rostral PFC according to a classification algorithm that predicted the task category from the absolute x coordinate and y coordinate of each peak of BOLD signal or rCBF change. The algorithm predicted Episodic Retrieval, Mentalising, or Multitask on 92% occasions, so only these three categories are presented (leaving out the categories Perception, Attention, Language, Working memory and Other memory). The algorithm used the absolute x coordinate to make predictions, thus left- and right-hemisphere shadings overlays are mirror images of one another. Results are plotted on an axial slice at z=0. Adapted from (55), with kind permission from the authors and the publishers of Journal of Cognitive Neuroscience, © 2006 Massachusetts Institute of Technology.
Gilbert et al. (55) also observed a further subdivision of rostral PFC: conditions requiring mentalising tended to be associated with increases in BOLD signal or rCBF in a medial region with a y coordinate lower (i.e. more posterior) than the mean of all studies, while multitasking tended to involve a rostral PFC region with a y coordinate higher (i.e. more anterior) than the mean of all studies. In other words, mentalising tasks recruit a medial rostral PFC region located posteriorly within area 10, whereas multitasking tests tend to involve a medial rostral PFC region that is relatively polar (i.e. anterior) (see Figure 3).
To summarise, neuroimaging studies have found rostral PFC BOLD signal or rCBF changes in a large variety of tasks. These activities tend to be independent of the precise characteristics of the response mode or stimuli. PM and attention paradigms reveal a dissociation of BOLD signal or rCBF changes in lateral and medial BA10. In other tasks only one sub-region tends to show increased BOLD signal or rCBF: episodic memory and working memory paradigms mostly lead to the recruitment of lateral rostral PFC, while mentalising tasks mostly recruit medial rostral PFC. Additionally, mentalising BOLD signal or rCBF changes have been found to be more caudal, and multitasking changes more rostral (see (69) for more details).
4.3. Theories of rostral PFC function
A number of theories of rostral PFC function have been put forward in recent years on the basis of the neuropsychological and neuroimaging results. These theories will be presented succinctly below. The aim of this section is to provide an overview of the possible functions of rostral PFC that will be used to approach the developmental and clinical data.
As presented in the section above, rostral PFC changes in rCBF or BOLD signal have been observed in a wide range of tasks. The recruitment of rostral PFC observed during episodic memory studies have led to the suggestion that rostral PFC is involved in aspects of memory retrieval (70), including retrieval mode (71-75), success monitoring (or retrieval verification) and source memory, i.e. remembering details of events that were not central to the event at the time, such the temporal order of them, or thoughts and feelings that were provoked by them ((65), see (20, 39) for reviews), or contextual recollection (50, 65, 76, 77). Independently, direct empirical studies of mentalising also suggest this region may be engaged when we attend to our own or others’ mental states ((52), see (78, 79) for reviews).
Other theories have focused on rostral PFC recruitment in multitasking situations, which are consistent with the impairments in multitasking observed in patients with lesions in this area (44). These theories concentrate on the different demands made by multitasking. (1) Burgess et al. (57) have proposed that rostral PFC is crucial for PM, which allows an intended act to be executed after a delay (51, 58, 80-82). (2) Pollman and colleagues proposed that rostral PFC is involved in controlling the reallocation of attention, which can be necessary when the most salient stimulus, or the most salient feature of a stimulus, is not the most relevant for a task, i.e. where intentional selection of perceptual information is required (83-85). (3) Another set of hypotheses of rostral PFC function focuses on the ability to manage more than one behavioural goal. Koechlin and colleagues (53, 86) proposed that the observation of selective bilateral activity in rostral PFC when volunteers were required to keep in mind a main goal (a working memory task) while performing concurrent sub-goals (dual-task performance) suggests this region mediates “cognitive branching”, or the ability to “hold in mind goals while exploring and processing secondary goals”. Braver & Bongiolatti (56) obtained similar data, but proposed that rostral PFC is involved in the integration of the results of the sub-goal processing with the main goal, rather than in the storage of information (see also (20)). (4) Finally, in a review of reasoning and episodic memory functional neuroimaging studies, Christoff and Gabrieli (87) proposed that rostral PFC is specifically involved in “self-referential evaluation”, a process that would be critical when non-routine cognitive strategies have to be generated and selected in the context of novel tasks or activities. This process would be particularly involved in multitasking, where strategies are used to perform goals and sub-goals, and could be related to impairments in ill-structured situations observed in patients with rostral PFC lesions.
Burgess and colleagues recently proposed a further theory of rostral PFC function (see (39, 69, 88, 89) for more details) which attempts to integrate the findings from human lesion studies with those from functional neuroimaging. The authors suggest that the impairments of patients with rostral PFC lesions in multitasking and ill-structured situations and the recruitment of rostral PFC during PM indicate that rostral PFC is involved in coordinating the allocation of attention either towards perceptually-derived information (the current stimuli) or towards stimulus-independent information that has been internally generated (e.g. the characteristics of the PM condition), or the overall goal. To perform PM tasks or to multitask subjects need regularly to switch between these two types of representation. This theory could account for the recruitment of rostral PFC in a wide range of studies, as it is proposed that any type of task that requires either regular reallocation of attention between perceptually-derived information and self-generated information (e.g. between cues and episodic memories) or forceful attending to a particular type of thoughts (e.g. attending to the less salient feature of a stimulus) would lead to the recruitment of rostral PFC.
To summarise this section, while lesion studies show only limited impairments associated with rostral PFC lesions, principally in multitasking and dealing with novel situations, neuroimaging data demonstrate the recruitment of this region in a wide range of tasks. On the basis of these data, a number of theories of rostral PFC function have been proposed, attributing to this region a role in: episodic memory, mentalising, PM, reallocation of attention, cognitive branching, self-referential evaluation or allocation of attention towards perceptually-derived or self-generated information. In the following two sections, psychological and neuroimaging data relating to the development of some of these functions will be presented.
5. Development of cognitive abilities supported by rostral PFC
This section will first present behavioural studies of development in children and adolescents, and then neuroimaging studies which provide some information regarding the recruitment of rostral PFC during development in different cognitive tasks.
5.1 Behavioural studies
The study of the development of executive functions, which is relatively recent (14, 90, 91), has provided evidence that the development of attentional and executive functions is a multistage process: different components have been found to develop at different times, beginning in infancy and continuing at least until adolescence (e.g. (92)). Because of the breadth of evidence obtained in this field, this section will focus on two particular cognitive functions which have been associated with rostral PFC: PM and mentalising. PM is an ability recruited during multitasking, or branching, and has been suggested to be supported by rostral PFC on the basis of neuroimaging and lesion studies (see above). Mentalising has been associated in imaging studies with changes in BOLD signal or rCBF in medial rostral PFC ((52), see (55) for a review).
5.1.2 Prospective memory (PM)
11- to 12-months-old infants are able to carry out an intention on the basis of stored information (93, 94). Performance on PM tasks continues to improve during childhood (95) and adolescence, with significant differences between late childhood and adolescence (96-98) and between adolescence and adulthood (99). Children become increasingly skilled at using external cues (95, 100) and time-checking strategies (97). Ward et al. (101) studied the performance of children (7-10 y.), adolescents (13-16) and young adults (18-21) on PM tasks. Children were worse at PM tasks than were adolescents and adults. However, despite their similar performances, adolescents and adults differed in the strategies they reported using. Adolescents reported keeping the intention in mind and looking out for the PM cues more often than adults, while most adults reported remembering an intention only when they saw the PM cues (see Table 1). This suggests that the use of cognitive resources vary between adolescence and adulthood in PM tasks.
Table 1.
Group frequencies of remembering strategies in a prospective memory task. Table from (101), with kind permission from the authors and the publishers of Child Neuropsychology, © 2005 Taylor & Francis Inc
| Group Frequencies (%) | |||
|---|---|---|---|
| Strategy used | Children (age 7-10) | Adolescents (age 13-16) | Adults (age 18-21) |
| Don’t know/can’t remember | 3.3 | 0.0 | 0.0 |
| Remembered only when saw the cues | 73.3 | 37.9 | 73.3 |
| Thought about all the time/looked out for the cues | 23.3 | 48.9 | 20.0 |
| Thought about at the start, but with all the other things to think about, switched to remembering only with the cues | 0.0 | 10.3 | 3.3 |
| Remembered only when saw the cues at the start but, with the reminders, began to think about all the time and looked out for the cues | 0.0 | 3.4 | 3.3 |
5.1.2 Theory of Mind and mentalising
Frith & Frith (52) reviewed behavioural studies of mentalising development, and proposed the existence of two developmental spurts in mentalising. First, children develop an understanding of desires, goals and intentions at around 18 months, which suggest that the understanding of many mental states such as wanting, knowing, pretending or believing is available in implicit form to 2-year-olds. The second developmental spurt is proposed to take place between the ages of 4 and 6 years. Typical tests of mentalising at these ages involve false belief paradigms such as those used by Wimmer & Perner (102), where children’s understanding of two sketches was tested: “in each sketch subjects observed how a protagonist put an object into a location x and then witnessed that in the absence of the protagonist the object was transferred from x to location y. Since this transfer came as a surprise they had to assume that the protagonist still believed that the object was in x. Subjects had to indicate where the protagonist will look for the object at his return.” (p. 103) Children of around 4 years old start to understand this scenario (102). At age 6 years all typically developing children understand the task (e.g. (103)), and also understand tasks involving more complex scenarios (104, 105).
Some studies have investigated the development of PM and mentalising, which have both been associated with rostral PFC. PM, although present in a rudimentary form very early on, continues to develop throughout childhood and adolescence, in particular in relation to the use of strategies (101). There have been very few studies investigating mentalising ability after age 6. However, it is logical to predict that, due to the development of the neural substrates of mentalising during late childhood and adolescence, subtle changes in mentalising ability might occur during this period of life.
5.2 Functional neuroimaging studies
In this section we review neuroimaging studies that provide information on developmental changes in the recruitment of rostral PFC in a range of tasks.
5.2.1. Response inhibition
Studies of inhibition-related BOLD or rCBF changes during development do not always observe variations in rostral PFC recruitment (e.g. (106, 107)), however when they do, they consistently report decreases of BOLD or rCBF signal in adulthood compared to younger ages. Indeed, in go-no go paradigms, Casey et al. (108) observed that the middle frontal gyrus (BA9/10/46) was less strongly recruited and over a smaller region in adults (21-24 y.) than in children (7-12 y.), and Booth et al. (109) observed reduced medial BA10 involvement in adults (20-30 y.) compared to children (9-12 y.). In a paradigm of emotional self-regulation, Levesque and colleagues (110, 111) found that voluntary suppression of emotion when shown sad film excerpts was associated with the recruitment of more prefrontal regions, including bilateral and medial BA10, in a children study (8-10 y.) than in an adult (20-30 y) study. Interestingly Casey et al. (112) focused on a young age group and observed that over this age range (9-11 year olds) BOLD signal was found to increase with age in the middle and inferior fontal gyri.
These results suggest a change in the recruitment of rostral PFC in situations of response inhibition during late childhood and adolescence. An initial increase of BOLD signal in this region (112) followed by a decrease in BOLD signal (108-111) appears consistent with the anatomical findings suggesting that grey matter volumes in the frontal cortex peak during early adolescence (32).
5.2.2. Response competition
Similarly to what has been observed for response inhibition, a study has shown evidence of decrease recruitment of rostral PFC during response competition during development. Konrad et al. (31) observed reduced BOLD signal in left lateral BA10 in adults (20-34 y.) compared to children (8-12 y.) when comparing incongruent targets to congruent targets in an Eriksen flanker task’s type of paradigm (113), where distractors can be congruent or incongruent with the target placed between them. Inferior frontal gyrus showed the reverse pattern. Two other contrasts: “reorienting”, comparing invalidly cued trials to validly cued trials, and “alerting”, comparing double cue trials (no direction) to no cue trials, did not show differences of BA10 BOLD signal between adults and children. However other studies did not find an effect of age on rostral PFC involvement when testing response competition with the Stroop task (114, 115) or in another study that employed the flanker task (116).
5.2.3. Working memory
Studies of working memory tasks do not appear to follow the same pattern of decrease recruitment in adults compared to children and adolescents, although only a few studies have investigated the involvement of PFC during development in these tasks. Kwon et al. (117) tested subjects from 7 to 22 years old in a 2-back spatial working memory task and found that the BOLD signal in bilateral rostral PFC increased with age during the working memory task compared with a control task. More specifically, both the size of these clusters, and the t-value of the contrast, increased with age, while accuracy and reaction times were not significantly different. Other studies did not find age effects on rostral PFC recruitment in tasks with a working memory component. Thomas et al. (118) found that when comparing an n-back task to a motor condition, a similar right lateral BA10/46 region showed increased BOLD signal both in adults (19-26 y.) and children (8-10 y.) (see also (119)). Crone et al. (120) observed a greater recruitment of right DLPFC during the delay period, when the working memory items had to be manipulated rather than only maintained, in adolescents (13-17 y.) and adults (18-25 y.) but not in children (8-12 y.), however no effect was found in rostral PFC.
5.2.4. Intelligent quotient (IQ)
Shaw et al. (121) obtained MRI scans of a large number of children in a longitudinal study and correlated cortical thickness and IQ, on the basis of Wechsler intelligence scales (122). The results showed that the relationship between cortical thickness and IQ changes during the development, and also differs across brain regions (see also (123, 124)). Of particular interest here was a comparison between the “superior” and “average intelligence” groups that showed that from around 10 years old there is a rapid increase in cortical thickness in the superior intelligence group compared to the average intelligence group, which peaks at age 13 and wanes in late adolescence. This effect is observed particularly strongly and over an extended period, over the rostral PFC.
5.2.5. Mentalising
A recent fMRI study investigated the development of communicative intent using an irony comprehension task and found that children (aged between 9 and 14) engaged frontal regions (medial PFC and left inferior frontal gyrus) more than did adults in this task (125). A similar result was revealed in a study in which we investigated the development during adolescence of the neural network underlying thinking about intentions (126). In this study, 19 adolescent participants (aged 12.1 - 18.1 years), and 11 adults (aged 22.4 - 37.8 years), were scanned using fMRI. In both adults and adolescents, answering questions about intentional causality versus physical causality activated the mentalising network, including medial PFC, superior temporal sulcus (STS) and temporal poles. In addition, there was a significant interaction between group and task in the medial PFC. During intentional relative to physical causality, adolescents activated part of the medial PFC more than did adults and adults activated part of the right STS more than did adolescents. These results suggest that the neural strategy for thinking about intentions changes between adolescence and adulthood. Although the same neural network is active, the relative roles of the different areas change, with activity moving from anterior (medial prefrontal) regions to posterior (temporal) regions with age.
To summarise, because of the novelty of the field of neuroimaging of brain development, there is only limited evidence regarding changes in rostral PFC recruitment. However when changes have been observed, they have quite consistently shown reduced recruitment of this region between late childhood-adolescence and adulthood. These results should be considered in relation to the anatomical findings of decrease grey matter volume in rostral PFC during adolescence presented above (31, 33, 34). Indeed, the progressive growth of the nervous system during development results in an overabundance of connections. Several mechanisms have been found to play a role in removing or modifying the exuberant connections to ensure that a functional organization of circuitry is established. These include cell death (127), synapses elimination (128) and the fine tuning of axon terminals and elimination of long axon collaterals, or “axon pruning” (129, 130). Maturation of the neural networks through such mechanisms during late childhood and adolescence could lead to an increase of the efficiency of the cerebral regions involved in particular tasks, and thus lead to a reduction of the observed increases in BOLD signal or rCBF during the performance of these tasks.
6. Role of rostral PFC in adult and childhood psychological disorders
As we have seen, rostral PFC is a region that undergoes protracted development that continues through adolescence in humans. Thus developmental abnormalities occurring at any point from conception through adolescence could affect rostral PFC function. It has been suggested that rostral PFC can be subdivided into regions based on both anatomical and functional differences (see also (69)). It is possible that these regions mature at different rates. Thus, abnormal development of early-developing and late-developing subregions might result in different disorders. In this section, we briefly present three developmental disorders that have different onsets and have been associated with abnormal functioning of BA10.
6.1. Autism spectrum disorders
Autism spectrum disorders (ASD) are characterised by impairments in reciprocal social interaction and communication, together with the presence of stereotyped or repetitive behaviours (DSM-IV (131)). ASDs are usually diagnosed in early childhood, but rarely before age 2, and are expressed throughout life (132). Neuro-anatomical differences between children with ASD and controls have been observed as early as 2-3 years of age (133). One recent finding is that people with autism have a greater total brain volume (e.g. (134)), which appears during the first few years of life and disappears from adolescence onwards (133, 135). One speculative theory is that brain enlargement might be due to a lack of early synaptic pruning (136). Some neuroanatomical and regional cerebral blood flow studies have pointed to abnormalities in rostral PFC, although the direction of the effects are inconsistent (e.g. (137-140)).
A number of cognitive abilities that have been associated with rostral PFC are impaired in ASD. These include multitasking (141, 142), episodic memory (143, 144, 145, 146-148), performance in novel situations (149, 150, 151) and mentalising (e.g. (103, 152, 153, 154, 155, 156, 157)). Hill & Bird (142) found that impairment of individuals with Asperger’s syndrome (a disorder at the high functioning end of the autism spectrum), on a multitasking paradigm correlated with the severity of their symptoms. PM does not appear to have been studied, but Farrant et al. (157) found that children with autism were not particularly impaired in reflecting on memory strategies, including PM ones.
6.2. Attention deficit and hyperactivity disorder
ADHD is a neuropsychiatric disorder with onset at preschool age, characterised by hyperactivity, inattentiveness and impulsivity, which can persist into adulthood ((158), DSM-IV (131)). Structural studies show that children with ADHD have reduced total brain volume compared with control children (159). Reduced volumes of grey and/or white matter in the PFC have been observed, although no study has found a specific abnormality in rostral PFC (see (158, 160, 161) for reviews). In relation to possible rostral PFC dysfunction, individuals with ADHD appear to be impaired in multitasking and PM (162-166). Further, one of these studies found a significant correlation between PM performance and a clinical measure of ADHD (166). Mentalising abilities have not been shown to be impaired in one study found (167), while evidence is mixed regarding episodic memory impairments (148, 168-170).
6.3 Schizophrenia
Schizophrenia is a disorder in which individuals experience positive symptoms such as auditory hallucinations and delusions as well as negative symptoms including loss of motivation, poverty of thought and emotional blunting (DSM-IV (131)). People with schizophrenia often have executive and memory deficits (171). Schizophrenia appears to have a significant developmental component, with abnormalities possibly taking place at different stages of development (172), including before birth (173, 174). Although the onset of psychosis tends to be during early adulthood, many children who go on to develop schizophrenia tend to display early neurological and cognitive problems (e.g. (175-178)). It has recently been suggested that schizophrenia may be associated with synaptic abnormalities that occur during cortex maturation in adolescence (179, 180). Structural imaging studies suggest some abnormalities in rostral medial PFC/orbito-frontal cortex, including a reduction in grey matter (181-183) and increased mean diffusivity (184). Harrison and colleagues (185) have suggested that the decreased cortical volume observed in schizophrenia is due to reduced neuropil and neuronal size, in particular in the prefrontal cortex (see also (186)). Reduced neuropil fraction has been observed in Brodmann area 10 (187), as well as reduced grey-level index (GLI), possibly associated to a reduced neuropil fraction (188).
A number of cognitive abilities associated with rostral PFC function have been observed to be impaired in schizophrenia, and one might speculate that there would be a relation with particular symptoms. For instance, mentalising impairments (see (189, 190) for reviews) may relate to delusions of persecution (e.g. (191-196)). Episodic memory impairment also seems to be associated with current symptoms (e.g. (197), see (198) for a review), and some have argued that source memory impairment could be associated with hallucination symptoms (199-202). PM impairment (203-207) is however mainly present in patients with other executive functions deficits (208). Finally, multitasking impairments have been observed in schizophrenic patients (209, 210), particularly in patients with a high level of negative symptoms ((211), see also (212, 213)).
The overview above suggests that ASDs, many of which are diagnosed in early development, are associated with widespread impairment of cognitive abilities associated with rostral PFC function. Thus it is possible that ASDs are associated with abnormal early development of rostral PFC. ADHD, which has a later onset, appears to be associated with less widespread impairment: for example, mentalising appears to be preserved and episodic memory has less consistently been found to be impaired. This would support the idea that mentalising abilities are well in place, and episodic memory possibly partly so, by the time ADHD-related developmental abnormalities occur. The case of schizophrenia appears to be more complicated, as developmental abnormalities at different stages overlap. Similarly to ASDs, a wide range of cognitive abilities are impaired in schizophrenia, although generally these impairments are associated with specific symptomatology. The different symptoms observed might be the consequences of different childhood and adolescent developmental abnormalities.
8. Conclusion
We have presented the current knowledge of the development of rostral PFC in relation to theories of the cognitive functions supported by this region. Anatomical studies show that rostral PFC is larger and more differentiated in humans than in other primates. This region has a low cell density and numerous connections to the supramodal cortex. Additionally, there is evidence for a sub-division of rostral PFC along a posterior-anterior axis. Anatomical and MRI studies reveal prolonged development of BA10, with rapid brain expansion during late childhood, decreases in grey matter and synaptic density and increases of dendritic arborisation during childhood and adolescence. In adults, lesion studies suggest a role of rostral PFC in multitasking and novel situations. Neuroimaging studies, on the other hand, show widespread involvement of this region in multiple tasks. A meta-analysis of these studies (55) suggests a subdivision of this region along the posterior-anterior axis, between the areas recruited preferentially in mentalising and multitasking paradigms respectively. An additional subdivision along a lateral-medial axis is suggested, which has not been observed anatomically, and which would differentiate between episodic and working memory tasks and mentalising tasks. On the basis of the neuropsychological and neuroimaging data, a number of theories have been proposed, attributing to rostral PFC a role in episodic and prospective memory, mentalising, allocation of attention, cognitive branching and self-referential evaluation.
In this review, we have attempted to link experimental psychology and neuroimaging findings of developmental studies to these proposed functions of rostral PFC. Psychological studies indicate that certain executive function capacities undergo extended development, with the presence early on of a certain level of ability and prolonged improvements in performance in the more complex tasks until late adolescence. This pattern thus follows the prolonged development, in particular reductions of grey matter volumes and densities, observed in rostral PFC during childhood and adolescence. Neuroimaging data of response inhibition, response competition and working memory, although limited, suggest that when a change during development is observed in rostral PFC, it tends to be in the direction of a reduction of the recruitment of this region between late childhood-adolescence and adulthood. Again these results are consistent with the anatomical findings of decrease grey matter volume in rostral PFC. Moreover, the study by Shaw and colleagues (121) suggests that processes of cortical extension and pruning might play an important role in the variations of intellectual abilities between individuals. Studying the development of functions attributed to rostral PFC, and their impairments, could help tease apart different components of this brain region. This would be particularly relevant for rostral PFC, as neuroimaging and anatomical studies already suggest subdivisions of this region.
Footnotes
Fractional anisotropy is a measure of the diffusion along fiber tracks (from 0 when diffusion is isotropic, i.e. equal in all directions, to 1 when there is maximum anisotropic diffusion, i.e. diffusion along one axis (see 28)).
In this “distance from the centre” method, a measure of radial expansion is calculated in millimetres from the centre of the brain to each point on the lateral surfaces, and from the centre of the hemispheres to each point on the medial surfaces.
Contributor Information
Iroise Dumontheil, Medical Research Council-Cognition and Brain Sciences Unit, Cambridge, UK.
Paul W. Burgess, Institute of Cognitive Neuroscience and Department of Psychology, University College London, UK.
Sarah-Jayne Blakemore, Institute of Cognitive Neuroscience and Department of Psychology, University College London, UK.
References
- 1.Brodmann K. Beitraege zur histologischen Lokalisation der Grosshirnrinde. VI. Mitteilung: Die Cortex-gliederung des Menschen. Journal PsycholNeurol(Lzp) 1908;10:231–46. [Google Scholar]
- 2.Brodmann K. Vergleichende localisationslehre der grosshirnrinde in ihren prinzipien dargestellt auf grund des zellenbaues. Leipzig Barth. 1909 [Google Scholar]
- 3.Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, et al. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14(5):1136–49. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- 4.Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: a comparative study of area 10. AmJPhysAnthropol. 2001;114(3):224–41. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 5.Petrides M, Pandya DN, Boller F, Grafman J. Handbook of neuropsychology. Amsterdam: Elsevier Science; 1994. Comparative architectonic analysis of the human and the macaque frontal cortex; pp. 17–58. [Google Scholar]
- 6.Jacobs B, Schall M, Prather M, Kapler E, Driscoll L, Baca S, et al. Regional dendritic and spine variation in human cerebral cortex: a quantitative golgi study. CerebCortex. 2001;11(6):558–71. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- 7.Holloway RL. Brief communication: how much larger is the relative volume of area 10 of the prefrontal cortex in humans? AmJPhysAnthropol. 2002;118(4):399–401. doi: 10.1002/ajpa.10090. [DOI] [PubMed] [Google Scholar]
- 8.Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. JComp Neurol. 2003;460(3):425–49. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 9.Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. JComp Neurol. 1996;371(2):179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 10.An X, Bandler R, Ongur D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. JComp Neurol. 1998;401(4):455–79. [PubMed] [Google Scholar]
- 11.Ongur D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. JComp Neurol. 1998;401(4):480–505. [PubMed] [Google Scholar]
- 12.Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. JComp Neurol. 1994;346(3):366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- 13.Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. JComp Neurol. 1995;363(4):642–64. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 14.Romanski LM, Bates JF, Goldman-Rakic PS. Auditory belt and parabelt projections to the prefrontal cortex in the rhesus monkey. JComp Neurol. 1999;403(2):141–57. doi: 10.1002/(sici)1096-9861(19990111)403:2<141::aid-cne1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 15.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. CerebCortex. 2000;10(3):206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 16.Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. EurJNeurosci. 1999;11(3):1011–36. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 17.Moran MA, Mufson EJ, Mesulam MM. Neural inputs into the temporopolar cortex of the rhesus monkey. JComp Neurol. 1987;256(1):88–103. doi: 10.1002/cne.902560108. [DOI] [PubMed] [Google Scholar]
- 18.Arikuni T, Sako H, Murata A. Ipsilateral connections of the anterior cingulate cortex with the frontal and medial temporal cortices in the macaque monkey. NeurosciRes. 1994;21(1):19–39. doi: 10.1016/0168-0102(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 19.Passingham RE, Stephan KE, Kotter R. The anatomical basis of functional localization in the cortex. NatRevNeurosci. 2002;3(8):606–16. doi: 10.1038/nrn893. [DOI] [PubMed] [Google Scholar]
- 20.Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. NatRevNeurosci. 2004;5(3):184–94. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 21.Travis K, Ford K, Jacobs B. Regional dendritic variation in neonatal human cortex: a quantitative Golgi study. DevNeurosci. 2005;27(5):277–87. doi: 10.1159/000086707. [DOI] [PubMed] [Google Scholar]
- 22.Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 23.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. JComp Neurol. 1997;387(2):167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 24.Schade JP, van GW. Structural organization of the human cerebral cortex. 1. Maturation of the middle frontal gyrus. Acta Anat(Basel) 1961;47:74–111. doi: 10.1159/000141802. [DOI] [PubMed] [Google Scholar]
- 25.Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport. 1999;10(13):2817–21. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- 26.Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. JCogn Neurosci. 2004;16(7):1227–33. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- 27.Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. CerebCortex. 2005;15(12):1848–54. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- 28.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. JMagn ResonB. 1996;111(3):209–19. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 29.Woo J, Asato M, Terwilliger R, Teslovich T, Olagunju-Jones Y, Velanova K, et al. White matter development from childhood to adulthood corresponding to cognitive development; CNS Conference; New York. 2007. [Google Scholar]
- 30.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. JNeurosci. 2004;24(38):8223–31. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konrad K, Neufang S, Thiel CM, Specht K, Hanisch C, Fan J, et al. Development of attentional networks: an fMRI study with children and adults. Neuroimage. 2005;28(2):429–39. doi: 10.1016/j.neuroimage.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 32.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. NatNeurosci. 1999;2(10):861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 33.Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. NatNeurosci. 1999;2(10):859–61. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- 34.O’Donnell S, Noseworthy MD, Levine B, Dennis M. Cortical thickness of the frontopolar area in typically developing children and adolescents. Neuroimage. 2005 Feb 15;24(4):948–54. doi: 10.1016/j.neuroimage.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. ProcNatlAcadSciUSA. 2004;101(21):8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9(2):60–8. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. JNeurosci. 2001;21(22):8819–29. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amati D, Shallice T. On the emergence of modern humans. Cognition. 2006 doi: 10.1016/j.cognition.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Burgess PW, Simons JS, Dumontheil I, Gilbert SJ, Duncan J, Philips L, et al. Measuring the mind: Speed, control, and age. Oxford: Oxford University Press; 2005. The Gateway Hypothesis of Rostral Prefrontal Cortex (Area 10) Function; pp. 217–48. [Google Scholar]
- 40.Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114(Pt 2):727–41. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- 41.Metzler C, Parkin AJ. Reversed negative priming following frontal lobe lesions. Neuropsychologia. 2000;38(4):363–79. doi: 10.1016/s0028-3932(99)00097-4. [DOI] [PubMed] [Google Scholar]
- 42.Wood RL, Rutterford NA. Relationships between measured cognitive ability and reported psychosocial activity after bilateral frontal lobe injury: An 18-year follow-up. Neuropsychological Rehabilitation. 2004;14(3):329–50. [Google Scholar]
- 43.Bird CM, Castelli F, Malik O, Frith U, Husain M. The impact of extensive medial frontal lobe damage on ‘Theory of Mind’ and cognition. Brain. 2004;127(Pt 4):914–28. doi: 10.1093/brain/awh108. [DOI] [PubMed] [Google Scholar]
- 44.Burgess PW. Strategy application disorder: the role of the frontal lobes in human multitasking. PsycholRes. 2000;63(3-4):279–88. doi: 10.1007/s004269900006. [DOI] [PubMed] [Google Scholar]
- 45.Goldstein LH, Bernard S, Fenwick PB, Burgess PW, McNeil J. Unilateral frontal lobectomy can produce strategy application disorder. JNeurolNeurosurgPsychiatry. 1993;56(3):274–6. doi: 10.1136/jnnp.56.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goel V, Grafman J. Role of the right prefrontal cortex in ill-structured planning. Cognitive Neuropsychology. 2000;17(5):415–36. doi: 10.1080/026432900410775. [DOI] [PubMed] [Google Scholar]
- 47.Grafman J, Stuss DT, Knight RT. Principles of frontal lobe function. New York: Oxford University Press; 2002. The structured event complex and the human prefrontal cortex; pp. 292–310. [Google Scholar]
- 48.Grady CL, Miller BL, Cummings JL. The Human Frontal Lobes: Function and Disorders. New York: Guilford Press; 1999. Neuroimaging and activation of the frontal lobes; pp. 196–230. [Google Scholar]
- 49.Blaxton TA, Zeffiro TA, Gabrieli JD, Bookheimer SY, Carrillo MC, Theodore WH, et al. Functional mapping of human learning: a positron emission tomography activation study of eyeblink conditioning. JNeurosci. 1996;16(12):4032–40. doi: 10.1523/JNEUROSCI.16-12-04032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burgess N, Maguire EA, Spiers HJ, O’Keefe J. A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage. 2001;14(2):439–53. doi: 10.1006/nimg.2001.0806. [DOI] [PubMed] [Google Scholar]
- 51.Burgess PW, Scott SK, Frith CD. The role of the rostral frontal cortex (area 10) in prospective memory: a lateral versus medial dissociation. Neuropsychologia. 2003;41(8):906–18. doi: 10.1016/s0028-3932(02)00327-5. [DOI] [PubMed] [Google Scholar]
- 52.Frith U, Frith CD. Development and neurophysiology of mentalizing. PhilosTransRSocLond B BiolSci. 2003;358(1431):459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399(6732):148–51. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- 54.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. ProcNatlAcadSciUSA. 2001;98(7):4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. JCogn Neurosci. 2006;18(6):932–48. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- 56.Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. Neuroimage. 2002;15(3):523–36. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- 57.Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39(6):545–55. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- 58.Okuda J, Fujii T, Yamadori A, Kawashima R, Tsukiura T, Fukatsu R, et al. Participation of the prefrontal cortices in prospective memory: evidence from a PET study in humans. NeurosciLett. 1998;253(2):127–30. doi: 10.1016/s0304-3940(98)00628-4. [DOI] [PubMed] [Google Scholar]
- 59.Pollmann S, Weidner R, Muller HJ, von Cramon DY. A fronto-posterior network involved in visual dimension changes. JCogn Neurosci. 2000;12(3):480–94. doi: 10.1162/089892900562156. [DOI] [PubMed] [Google Scholar]
- 60.Weidner R, Pollmann S, Muller HJ, von Cramon DY. Top-down controlled visual dimension weighting: an event-related fMRI study. CerebCortex. 2002;12(3):318–28. doi: 10.1093/cercor/12.3.318. [DOI] [PubMed] [Google Scholar]
- 61.Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. JCogn Neurosci. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 62.Braver TS, Barch DM, Kelley WM, Buckner RL, Cohen NJ, Miezin FM, et al. Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. Neuroimage. 2001;14(1 Pt 1):48–59. doi: 10.1006/nimg.2001.0791. [DOI] [PubMed] [Google Scholar]
- 63.Christoff K, Ream JM, Geddes LP, Gabrieli JD. Evaluating self-generated information: anterior prefrontal contributions to human cognition. BehavNeurosci. 2003;117(6):1161–8. doi: 10.1037/0735-7044.117.6.1161. [DOI] [PubMed] [Google Scholar]
- 64.Simons JS, Gilbert SJ, Owen AM, Fletcher PC, Burgess PW. Distinct roles for lateral and medial anterior prefrontal cortex in contextual recollection. JNeurophysiol. 2005;94(1):813–20. doi: 10.1152/jn.01200.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rugg MD, Fletcher PC, Chua PM, Dolan RJ. The role of the prefrontal cortex in recognition memory and memory for source: an fMRI study. Neuroimage. 1999;10(5):520–9. doi: 10.1006/nimg.1999.0488. [DOI] [PubMed] [Google Scholar]
- 66.Castelli F, Happe F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12(3):314–25. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- 67.Zysset S, Huber O, Samson A, Ferstl EC, von Cramon DY. Functional specialization within the anterior medial prefrontal cortex: a functional magnetic resonance imaging study with human subjects. NeurosciLett. 2003;335(3):183–6. doi: 10.1016/s0304-3940(02)01196-5. [DOI] [PubMed] [Google Scholar]
- 68.Zysset S, Muller K, Lohmann G, von Cramon DY. Color-word matching stroop task: separating interference and response conflict. Neuroimage. 2001;13(1):29–36. doi: 10.1006/nimg.2000.0665. [DOI] [PubMed] [Google Scholar]
- 69.Burgess PW, Gilbert SJ, Dumontheil I. Function and localization within rostral prefrontal cortex (area 10) Philos Trans R Soc Lond B Biol Sci. 2007 May 29;362(1481):887–99. doi: 10.1098/rstb.2007.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475–83. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 71.Tulving E. Elements of Episodic Memory. Oxford Clarendon Press; 1983. [Google Scholar]
- 72.Duzel E, Cabeza R, Picton TW, Yonelinas AP, Scheich H, Heinze HJ, et al. Task-related and item-related brain processes of memory retrieval. ProcNatlAcadSciUSA. 1999;96(4):1794–9. doi: 10.1073/pnas.96.4.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kapur S, Craik FI, Jones C, Brown GM, Houle S, Tulving E. Functional role of the prefrontal cortex in retrieval of memories: a PET study. Neuroreport. 1995;6(14):1880–4. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- 74.Nyberg L, McIntosh AR, Cabeza R, Habib R, Houle S, Tulving E. General and specific brain regions involved in encoding and retrieval of events: what, where, and when. ProcNatlAcadSciUSA. 1996;93(20):11280–5. doi: 10.1073/pnas.93.20.11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Velanova K, Jacoby LL, Wheeler ME, McAvoy MP, Petersen SE, Buckner RL. Functional-anatomic correlates of sustained and transient processing components engaged during controlled retrieval. JNeurosci. 2003;23(24):8460–70. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35(5):989–96. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- 77.Ranganath C, Johnson MK, D’Esposito M. Left anterior prefrontal activation increases with demands to recall specific perceptual information. JNeurosci. 2000;20(22):RC108. doi: 10.1523/JNEUROSCI.20-22-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. JCogn Neurosci. 2004;16(10):1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- 79.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. NatRevNeurosci. 2006;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 80.den Ouden HE, Frith U, Frith C, Blakemore SJ. Thinking about intentions. Neuroimage. 2005;28(4):787–96. doi: 10.1016/j.neuroimage.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 81.Simons JS, Scholvinck ML, Gilbert SJ, Frith CD, Burgess PW. Differential components of prospective memory? Evidence from fMRI. Neuropsychologia. 2006;44(8):1388–97. doi: 10.1016/j.neuropsychologia.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 82.Coull JT, Frith CD, Frackowiak RS, Grasby PM. A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia. 1996;34(11):1085–95. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- 83.Pollmann S. Switching between dimensions, locations, and responses: the role of the left frontopolar cortex. Neuroimage. 2001;14(1 Pt 2):S118–S24. doi: 10.1006/nimg.2001.0837. [DOI] [PubMed] [Google Scholar]
- 84.Pollmann S. Anterior prefrontal cortex contributions to attention control. ExpPsychol. 2004;51(4):270–8. doi: 10.1027/1618-3169.51.4.270. [DOI] [PubMed] [Google Scholar]
- 85.Lepsien J, Pollmann S. Covert reorienting and inhibition of return: an event-related fMRI study. JCogn Neurosci. 2002;14(2):127–44. doi: 10.1162/089892902317236795. [DOI] [PubMed] [Google Scholar]
- 86.Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181–5. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 87.Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28(2):168–86. [Google Scholar]
- 88.Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci. 2007 Jul;11(7):290–8. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 89.Burgess PW, Gilbert SJ, Okuda J, Simons JS, Prinz W, Sebanz N. Disorders of Volition. Cambridge, MA: MIT Press; 2006. Rostral prefrontal brain regions (Area 10): A gateway between inner thought and the external world? [Google Scholar]
- 90.Hughes C. Executive functions and development: emerging themes. Infant and Child Development. 2002;11(2):201–9. [Google Scholar]
- 91.Hughes C. Executive functions and development: why the interest? Infant and Child Development. 2002;11(2):69–71. [Google Scholar]
- 92.Brocki KC, Bohlin G. Executive functions in children aged 6 to 13: a dimensional and developmental study. Dev Neuropsychol. 2004;26(2):571–93. doi: 10.1207/s15326942dn2602_3. [DOI] [PubMed] [Google Scholar]
- 93.Diamond A. Development of the ability to use recall to guide action, as indicated by infants’ performance on AB. Child Dev. 1985 Aug;56(4):868–83. [PubMed] [Google Scholar]
- 94.Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Dev. 1987;58(3):601–22. [PubMed] [Google Scholar]
- 95.Guajardo NR, Best DL. Do preschoolers remember what to do? Incentive and external cues in prospective memory. Cognitive Development. 2000;15:75–97. [Google Scholar]
- 96.Ceci SJ, Bronfenbrenner U. “Don’t forget to take the cupcakes out of the oven”: prospective memory, strategic time-monitoring, and context. Child Dev. 1985 Feb;56(1):152–64. [PubMed] [Google Scholar]
- 97.Kerns KA. The CyberCruiser: an investigation of development of prospective memory in children. J Int Neuropsychol Soc. 2000 Jan;6(1):62–70. doi: 10.1017/s1355617700611074. [DOI] [PubMed] [Google Scholar]
- 98.Kvavilashvili L, Messer DJ, Ebdon P. Prospective memory in children: the effects of age and task interruption. DevPsychol. 2001;37(3):418–30. doi: 10.1037//0012-1649.37.3.418. [DOI] [PubMed] [Google Scholar]
- 99.Wang L, Kliegel M, Yang Z, Liu W. Prospective memory performance across adolescence. J Genet Psychol. 2006 Jun;167(2):179–88. doi: 10.3200/GNTP.167.2.179-188. [DOI] [PubMed] [Google Scholar]
- 100.Meacham JA, Colom JA. External retrieval cues facilitate prospective remembering in children. Journal of Educational Research. 1980;73(5):299–301. [Google Scholar]
- 101.Ward H, Shum D, McKinlay L, Baker-Tweney S, Wallace G. Development of prospective memory: tasks based on the prefrontal-lobe model. Child Neuropsychol. 2005;11(6):527–49. doi: 10.1080/09297040490920186. [DOI] [PubMed] [Google Scholar]
- 102.Wimmer H, Perner J. Beliefs about beliefs: representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition. 1983 Jan;13(1):103–28. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]
- 103.Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985 Oct;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- 104.Perner J, Wimmer H. ‘John thinks that Mary thinks that...’: attribution of second-order beliefs by 5- to 10-year-old children. Journal of Experimental Child Psychology. 1985;39(3):437–71. [Google Scholar]
- 105.Sullivan K, Zaitchik D, Tager-Flusberg H. Preschoolers can attribute second-order beliefs. Developmental Psychology. 1994;30(3):395–402. [Google Scholar]
- 106.Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. JAmAcadChild AdolescPsychiatry. 2002;41(10):1231–8. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 107.Durston S, Thomas KM, Yang YH, Ulug AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5(4):F9–F16. [Google Scholar]
- 108.Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A developmental functional MRI study of prefrontal activation during performance of a Go/No-go task. Journal of Cognitive Neuroscience. 1997;9:835–47. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- 109.Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, et al. Neural development of selective attention and response inhibition. Neuroimage. 2003;20(2):737–51. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- 110.Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, et al. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003 Mar 15;53(6):502–10. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- 111.Levesque J, Joanette Y, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, et al. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129(2):361–9. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 112.Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, et al. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2(3):221–9. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- 113.Eriksen BA, Eriksen CW. Effects of noise letters on the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–9. [Google Scholar]
- 114.Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, et al. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16(1):61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- 115.Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, et al. A developmental fMRI study of self-regulatory control. HumBrain Mapp. 2006 doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33(2):301–11. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuospatial working memory. ProcNatlAcadSciUSA. 2002;99(20):13336–41. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, et al. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10(3 Pt 1):327–38. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- 119.Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. JCogn Neurosci. 2002;14(1):1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- 120.Crone EA, Bunge SA, van der Molen MW, Ridderinkhof KR. Switching between tasks and responses: a developmental study. Dev Sci. 2006 May;9(3):278–87. doi: 10.1111/j.1467-7687.2006.00490.x. [DOI] [PubMed] [Google Scholar]
- 121.Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006 Mar 30;440(7084):676–9. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 122.Wechsler D. Manual for the Wechsler Adult Intelligence Scale. New York: The Psychological Corporation; 1955. [Google Scholar]
- 123.Passingham R. Cognitive science: brain development and IQ. Nature. 2006 Mar 30;440(7084):619–20. doi: 10.1038/440619b. [DOI] [PubMed] [Google Scholar]
- 124.Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119(Pt 5):1763–74. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- 125.Wang AT, Lee SS, Sigman M, Dapretto M. Developmental changes in the neural basis of interpreting communicative intent. Soc Cogn Affect Neurosci. 2006 Sep 1;1(2):107–21. doi: 10.1093/scan/nsl018. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Blakemore S-J, den Ouden H, Choudhury S, Frith C. Adolescent development of the neural circuitry for thinking about intentions. Soc Cogn Affect Neurosci. 2007 Jun 1;2(2):130–9. doi: 10.1093/scan/nsm009. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yuan J, Lipinski M. Diversity in the mechanisms of neuronal cell death. Neuron. 2003;40:401–13. doi: 10.1016/s0896-6273(03)00601-9. [DOI] [PubMed] [Google Scholar]
- 128.Goda Y, Davis GW. Mechanisms of synapse assembly and disassembly. Neuron. 2003 Oct 9;40(2):243–64. doi: 10.1016/s0896-6273(03)00608-1. [DOI] [PubMed] [Google Scholar]
- 129.Low LK, Cheng HJ. Axon pruning: an essential step underlying the developmental plasticity of neuronal connections. Philos Trans R Soc Lond B Biol Sci. 2006 Sep 29;361(1473):1531–44. doi: 10.1098/rstb.2006.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kantor DB, Kolodkin AL. Curbing the excesses of youth: molecular insights into axonal pruning. Neuron. 2003 Jun 19;38(6):849–52. doi: 10.1016/s0896-6273(03)00364-7. [DOI] [PubMed] [Google Scholar]
- 131.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 132.Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage. 2004;22(2):619–25. doi: 10.1016/j.neuroimage.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 133.Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001 Jul 24;57(2):245–54. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 134.Bolton PF, Roobol M, Allsopp L, Pickles A. Association between idiopathic infantile macrocephaly and autism spectrum disorders. Lancet. 2001 Sep 1;358(9283):726–7. doi: 10.1016/S0140-6736(01)05903-7. [DOI] [PubMed] [Google Scholar]
- 135.Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002 Jul 23;59(2):175–83. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- 136.Frith C. What do imaging studies tell us about the neural basis of autism? Novartis Found Symp. 2003;251:149–66. discussion 66-76, 281-97. [PubMed] [Google Scholar]
- 137.Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, et al. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport. 1999 Jun 3;10(8):1647–51. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- 138.Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, Uema T, et al. Abnormal regional cerebral blood flow in childhood autism. Brain. 2000 Sep;123(Pt 9):1838–44. doi: 10.1093/brain/123.9.1838. [DOI] [PubMed] [Google Scholar]
- 139.Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. Structural white matter deficits in high-functioning individuals with autistic spectrum disorder: a voxel-based investigation. Neuroimage. 2005 Jan 15;24(2):455–61. doi: 10.1016/j.neuroimage.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 140.Schmitz N, Rubia K, Daly E, Smith A, Williams S, Murphy DG. Neural correlates of executive function in autistic spectrum disorders. Biol Psychiatry. 2006 Jan 1;59(1):7–16. doi: 10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 141.Mackinlay R, Charman T, Karmiloff-Smith A. High functioning children with autism spectrum disorder: A novel test of multitasking. Brain Cogn. 2006 Jun;61(1):14–24. doi: 10.1016/j.bandc.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 142.Hill EL, Bird CM. Executive processes in Asperger syndrome: patterns of performance in a multiple case series. Neuropsychologia. 2006;44(14):2822–35. doi: 10.1016/j.neuropsychologia.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 143.Bowler DM, Gardiner JM, Grice SJ. Episodic memory and remembering in adults with Asperger syndrome. J Autism Dev Disord. 2000 Aug;30(4):295–304. doi: 10.1023/a:1005575216176. [DOI] [PubMed] [Google Scholar]
- 144.Salmond CH, Ashburner J, Connelly A, Friston KJ, Gadian DG, Vargha-Khadem F. The role of the medial temporal lobe in autistic spectrum disorders. Eur J Neurosci. 2005 Aug;22(3):764–72. doi: 10.1111/j.1460-9568.2005.04217.x. [DOI] [PubMed] [Google Scholar]
- 145.Millward C, Powell S, Messer D, Jordan R. Recall for self and other in autism: children’s memory for events experienced by themselves and their peers. J Autism Dev Disord. 2000 Feb;30(1):15–28. doi: 10.1023/a:1005455926727. [DOI] [PubMed] [Google Scholar]
- 146.Gardiner JM, Bowler DM, Grice SJ. Further evidence of preserved priming and impaired recall in adults with Asperger’s syndrome. J Autism Dev Disord. 2003 Jun;33(3):259–69. doi: 10.1023/a:1024450416355. [DOI] [PubMed] [Google Scholar]
- 147.Toichi M, Kamio Y. Long-term memory in high-functioning autism: controversy on episodic memory in autism reconsidered. J Autism Dev Disord. 2003 Apr;33(2):151–61. doi: 10.1023/a:1022935325843. [DOI] [PubMed] [Google Scholar]
- 148.Oie M, Sunde K, Rund BR. Contrasts in memory functions between adolescents with schizophrenia or ADHD. Neuropsychologia. 1999 Nov;37(12):1351–8. doi: 10.1016/s0028-3932(99)00043-3. [DOI] [PubMed] [Google Scholar]
- 149.Turner MA. Generating novel ideas: fluency performance in high-functioning and learning disabled individuals with autism. J Child Psychol Psychiatry. 1999 Feb;40(2):189–201. [PubMed] [Google Scholar]
- 150.Channon S, Charman T, Heap J, Crawford S, Rios P. Real-life-type problem-solving in Asperger’s syndrome. J Autism Dev Disord. 2001 Oct;31(5):461–9. doi: 10.1023/a:1012212824307. [DOI] [PubMed] [Google Scholar]
- 151.Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of Autistic Disorder. J Autism Dev Disord. 2005 Aug;35(4):445–60. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- 152.Ozonoff S, Pennington BF, Rogers SJ. Executive function deficits in high-functioning autistic individuals: relationship to theory of mind. J Child Psychol Psychiatry. 1991 Nov;32(7):1081–105. doi: 10.1111/j.1469-7610.1991.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 153.Kleinman J, Marciano PL, Ault RL. Advanced theory of mind in high-functioning adults with autism. J Autism Dev Disord. 2001 Feb;31(1):29–36. doi: 10.1023/a:1005657512379. [DOI] [PubMed] [Google Scholar]
- 154.Colle L, Baron-Cohen S, Hill J. Do Children with Autism have a Theory of Mind? A Non-verbal Test of Autism vs. Specific Language Impairment. J Autism Dev Disord. 2007 Apr;37(4):716–23. doi: 10.1007/s10803-006-0198-7. [DOI] [PubMed] [Google Scholar]
- 155.Baron-Cohen S, Ring H, Moriarty J, Schmitz B, Costa D, Ell P. Recognition of mental state terms. Clinical findings in children with autism and a functional neuroimaging study of normal adults. Br J Psychiatry. 1994 Nov;165(5):640–9. doi: 10.1192/bjp.165.5.640. [DOI] [PubMed] [Google Scholar]
- 156.Brent E, Rios P, Happe F, Charman T. Performance of children with autism spectrum disorder on advanced theory of mind tasks. Autism. 2004 Sep;8(3):283–99. doi: 10.1177/1362361304045217. [DOI] [PubMed] [Google Scholar]
- 157.Farrant A, Boucher J, Blades M. Metamemory in children with autism. Child Dev. 1999 Jan-Feb;70(1):107–31. doi: 10.1111/1467-8624.00009. [DOI] [PubMed] [Google Scholar]
- 158.Durston S. A review of the biological bases of ADHD: what have we learned from imaging studies? Ment Retard Dev Disabil Res Rev. 2003;9(3):184–95. doi: 10.1002/mrdd.10079. [DOI] [PubMed] [Google Scholar]
- 159.Eliez S, Reiss AL. MRI neuroimaging of childhood psychiatric disorders: a selective review. J Child Psychol Psychiatry. 2000 Sep;41(6):679–94. [PubMed] [Google Scholar]
- 160.Krain AL, Castellanos FX. Brain development and ADHD. Clin Psychol Rev. 2006 Aug;26(4):433–44. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 161.Schneider M, Retz W, Coogan A, Thome J, Rosler M. Anatomical and functional brain imaging in adult attention-deficit/hyperactivity disorder (ADHD)-A neurological view. Eur Arch Psychiatry Clin Neurosci. 2006;256(Suppl 1):i32–i41. doi: 10.1007/s00406-006-1005-3. [DOI] [PubMed] [Google Scholar]
- 162.Clark C, Prior M, Kinsella GJ. Do executive function deficits differentiate between adolescents with ADHD and oppositional defiant/conduct disorder? A neuropsychological study using the Six Elements Test and Hayling Sentence Completion Test. J Abnorm Child Psychol. 2000 Oct;28(5):403–14. doi: 10.1023/a:1005176320912. [DOI] [PubMed] [Google Scholar]
- 163.Chan RC, Guo M, Zou X, Li D, Hu Z, Yang B. Multitasking performance of Chinese children with ADHD. J Int Neuropsychol Soc. 2006 Jul;12(4):575–9. doi: 10.1017/s1355617706060693. [DOI] [PubMed] [Google Scholar]
- 164.Siklos S, Kerns KA. Assessing multitasking in children with ADHD using a modified Six Elements Test. Arch Clin Neuropsychol. 2004 Apr;19(3):347–61. doi: 10.1016/S0887-6177(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 165.Kliegel M, Ropeter A, Mackinlay R. Complex prospective memory in children with ADHD. Child Neuropsychol. 2006 Dec;12(6):407–19. doi: 10.1080/09297040600696040. [DOI] [PubMed] [Google Scholar]
- 166.Kerns KA, Price KJ. An investigation of prospective memory in children with ADHD. Child Neuropsychol. 2001 Sep;7(3):162–71. doi: 10.1076/chin.7.3.162.8744. [DOI] [PubMed] [Google Scholar]
- 167.Perner J, Kain W, Barchfeld P. Executive control and higher-order Theory of Mind in children at risk of ADHD. Infant and Child Development. 2002;11(2):141–58. [Google Scholar]
- 168.Krauel K, Duezel E, Hinrichs H, Santel S, Rellum T, Baving L. Impact of emotional salience on episodic memory in Attention-Deficit/Hyperactivity Disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2007 Jun 15;61(12):1370–9. doi: 10.1016/j.biopsych.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 169.Felton RH, Wood FB, Brown IS, Campbell SK, Harter MR. Separate verbal memory and naming deficits in attention deficit disorder and reading disability. Brain Lang. 1987 May;31(1):171–84. doi: 10.1016/0093-934x(87)90067-8. [DOI] [PubMed] [Google Scholar]
- 170.Oie M, Rund BR. Neuropsychological deficits in adolescent-onset schizophrenia compared with attention deficit hyperactivity disorder. Am J Psychiatry. 1999 Aug;156(8):1216–22. doi: 10.1176/ajp.156.8.1216. [DOI] [PubMed] [Google Scholar]
- 171.Morrison PD, Murray RM. Schizophrenia. CurrBiol. 2005;15(24):R980–R4. doi: 10.1016/j.cub.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 172.Murray RM, McDonald C, Bramon E. Neurodevelopmental impairment, dopamine sensitisation, and social adversity in schizophrenia. World Psychiatry. 2002 Oct;1(3):137–45. [PMC free article] [PubMed] [Google Scholar]
- 173.Geddes JR, Verdoux H, Takei N, Lawrie SM, Bovet P, Eagles JM, et al. Schizophrenia and complications of pregnancy and labor: an individual patient data meta-analysis. Schizophr Bull. 1999;25(3):413–23. doi: 10.1093/oxfordjournals.schbul.a033389. [DOI] [PubMed] [Google Scholar]
- 174.Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002 Jul;159(7):1080–92. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- 175.Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994 Nov 19;344(8934):1398–402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 176.Erlenmeyer-Kimling L, Rock D, Roberts SA, Janal M, Kestenbaum C, Cornblatt B, et al. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: the New York High-Risk Project. Am J Psychiatry. 2000 Sep;157(9):1416–22. doi: 10.1176/appi.ajp.157.9.1416. [DOI] [PubMed] [Google Scholar]
- 177.Murray GK, Jones PB, Moilanen K, Veijola J, Miettunen J, Cannon TD, et al. Infant motor development and adult cognitive functions in schizophrenia. Schizophr Res. 2006 Jan 1;81(1):65–74. doi: 10.1016/j.schres.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 178.Cannon M, Jones P, Huttunen MO, Tanskanen A, Huttunen T, Rabe-Hesketh S, et al. School performance in Finnish children and later development of schizophrenia: a population-based longitudinal study. Arch Gen Psychiatry. 1999 May;56(5):457–63. doi: 10.1001/archpsyc.56.5.457. [DOI] [PubMed] [Google Scholar]
- 179.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17(4):319–34. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 180.Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994 May-Jun;28(3):239–65. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 181.Yamada M, Hirao K, Namiki C, Hanakawa T, Fukuyama H, Hayashi T, et al. Social cognition and frontal lobe pathology in schizophrenia: A voxel-based morphometric study. Neuroimage. 2007 Mar;35(1):292–8. doi: 10.1016/j.neuroimage.2006.10.046. [DOI] [PubMed] [Google Scholar]
- 182.Riffkin J, Yucel M, Maruff P, Wood SJ, Soulsby B, Olver J, et al. A manual and automated MRI study of anterior cingulate and orbito-frontal cortices, and caudate nucleus in obsessive-compulsive disorder: comparison with healthy controls and patients with schizophrenia. Psychiatry Res. 2005 Feb 28;138(2):99–113. doi: 10.1016/j.pscychresns.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 183.Narr KL, Toga AW, Szeszko P, Thompson PM, Woods RP, Robinson D, et al. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 2005 Jul 1;58(1):32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 184.Rose SE, Chalk JB, Janke AL, Strudwick MW, Windus LC, Hannah DE, et al. Evidence of altered prefrontal-thalamic circuitry in schizophrenia: an optimized diffusion MRI study. Neuroimage. 2006 Aug 1;32(1):16–22. doi: 10.1016/j.neuroimage.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 185.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999 Apr;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 186.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999 Jan 1;45(1):17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 187.Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, et al. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161(4):742–4. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- 188.Vogeley K, Tepest R, Schneider-Axmann T, Hutte H, Zilles K, Honer WG, et al. Automated image analysis of disturbed cytoarchitecture in Brodmann area 10 in schizophrenia. Schizophr Res. 2003 Jul 1;62(1-2):133–40. doi: 10.1016/s0920-9964(02)00325-0. [DOI] [PubMed] [Google Scholar]
- 189.Harrington L, Siegert RJ, McClure J. Theory of mind in schizophrenia: a critical review. Cognit Neuropsychiatry. 2005 Aug;10(4):249–86. doi: 10.1080/13546800444000056. [DOI] [PubMed] [Google Scholar]
- 190.Brune M. “Theory of mind” in schizophrenia: a review of the literature. Schizophr Bull. 2005 Jan;31(1):21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- 191.Frith CD, Corcoran R. Exploring ‘theory of mind’ in people with schizophrenia. Psychol Med. 1996 May;26(3):521–30. doi: 10.1017/s0033291700035601. [DOI] [PubMed] [Google Scholar]
- 192.Sarfati Y, Hardy-Bayle MC, Besche C, Widlocher D. Attribution of intentions to others in people with schizophrenia: a non-verbal exploration with comic strips. Schizophr Res. 1997 Jun 20;25(3):199–209. doi: 10.1016/s0920-9964(97)00025-x. [DOI] [PubMed] [Google Scholar]
- 193.Corcoran R, Cahill C, Frith CD. The appreciation of visual jokes in people with schizophrenia: a study of ‘mentalizing’ ability. Schizophr Res. 1997 Apr 11;24(3):319–27. doi: 10.1016/s0920-9964(96)00117-x. [DOI] [PubMed] [Google Scholar]
- 194.Harrington L, Langdon R, Siegert RJ, McClure J. Schizophrenia, theory of mind, and persecutory delusions. Cognit Neuropsychiatry. 2005 Mar;10(2):87–104. doi: 10.1080/13546800344000327. [DOI] [PubMed] [Google Scholar]
- 195.Greig TC, Bryson GJ, Bell MD. Theory of mind performance in schizophrenia: diagnostic, symptom, and neuropsychological correlates. J Nerv Ment Dis. 2004 Jan;192(1):12–8. doi: 10.1097/01.nmd.0000105995.67947.fc. [DOI] [PubMed] [Google Scholar]
- 196.Pickup GJ, Frith CD. Theory of mind impairments in schizophrenia: symptomatology, severity and specificity. Psychol Med. 2001 Feb;31(2):207–20. doi: 10.1017/s0033291701003385. [DOI] [PubMed] [Google Scholar]
- 197.Turetsky BI, Moberg PJ, Mozley LH, Moelter ST, Agrin RN, Gur RC, et al. Memory-delineated subtypes of schizophrenia: relationship to clinical, neuroanatomical, and neurophysiological measures. Neuropsychology. 2002 Oct;16(4):481–90. [PubMed] [Google Scholar]
- 198.Keri S, Janka Z. Critical evaluation of cognitive dysfunctions as endophenotypes of schizophrenia. Acta Psychiatr Scand. 2004 Aug;110(2):83–91. doi: 10.1111/j.1600-0047.2004.00359.x. [DOI] [PubMed] [Google Scholar]
- 199.Franck N, Rouby P, Daprati E, Dalery J, Michel M-C, Georgieff N. Confusion between silent and overt reading in schizophrenia. Schizophrenia Research. 2000;41(2):357–64. doi: 10.1016/s0920-9964(99)00067-5. [DOI] [PubMed] [Google Scholar]
- 200.Garrett M, Silva R. Auditory hallucinations, source monitoring, and the belief that “voices” are real. Schizophr Bull. 2003;29(3):445–57. doi: 10.1093/oxfordjournals.schbul.a007018. [DOI] [PubMed] [Google Scholar]
- 201.Keefe RS, Arnold MC, Bayen UJ, McEvoy JP, Wilson WH. Source-monitoring deficits for self-generated stimuli in schizophrenia: multinomial modeling of data from three sources. Schizophr Res. 2002 Sep 1;57(1):51–67. doi: 10.1016/s0920-9964(01)00306-1. [DOI] [PubMed] [Google Scholar]
- 202.Moritz S, Woodward TS, Ruff CC. Source monitoring and memory confidence in schizophrenia. Psychol Med. 2003 Jan;33(1):131–9. doi: 10.1017/s0033291702006852. [DOI] [PubMed] [Google Scholar]
- 203.Woods SP, Twamley EW, Dawson MS, Narvaez JM, Jeste DV. Deficits in cue detection and intention retrieval underlie prospective memory impairment in schizophrenia. Schizophr Res. 2007 Feb;90(1-3):344–50. doi: 10.1016/j.schres.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kumar D, Nizamie HS, Jahan M. Event-based prospective memory in schizophrenia. J Clin Exp Neuropsychol. 2005 Oct;27(7):867–72. doi: 10.1080/13803390490919100. [DOI] [PubMed] [Google Scholar]
- 205.Shum D, Ungvari GS, Tang WK, Leung JP. Performance of schizophrenia patients on time-, event-, and activity-based prospective memory tasks. Schizophr Bull. 2004;30(4):693–701. doi: 10.1093/oxfordjournals.schbul.a007123. [DOI] [PubMed] [Google Scholar]
- 206.Elvevag B, Maylor EA, Gilbert AL. Habitual prospective memory in schizophrenia. BMC Psychiatry. 2003 Jul 30;3:9. doi: 10.1186/1471-244X-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Meissner F, Hacker W, Heilemann H. Memory performance and instruction effects in schizophrenia. A comparative study of chronic schizophrenic patients and healthy probands. Psychiatr Prax. 2001 May;28(4):180–8. doi: 10.1055/s-2001-13266. [DOI] [PubMed] [Google Scholar]
- 208.Kondel TK. Prospective memory and executive function in schizophrenia. Brain Cogn. 2002 Mar-Apr;48(2-3):405–10. [PubMed] [Google Scholar]
- 209.Evans JJ, Chua SE, McKenna PJ, Wilson BA. Assessment of the dysexecutive syndrome in schizophrenia. Psychol Med. 1997 May;27(3):635–46. doi: 10.1017/s0033291797004790. [DOI] [PubMed] [Google Scholar]
- 210.Chan RC, Chen EY, Cheung EF, Chen RY, Cheung HK. The components of executive functioning in a cohort of patients with chronic schizophrenia: a multiple single-case study design. Schizophr Res. 2006 Jan 31;81(2-3):173–89. doi: 10.1016/j.schres.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 211.Thoma P, Zoppelt D, Wiebel B, Daum I. Context processing and negative symptoms in schizophrenia. J Clin Exp Neuropsychol. 2007 May;29(4):428–35. doi: 10.1080/13803390600744814. [DOI] [PubMed] [Google Scholar]
- 212.Chan RC, Chen EY, Cheung EF, Cheung HK. Executive dysfunctions in schizophrenia. Relationships to clinical manifestation. Eur Arch Psychiatry Clin Neurosci. 2004 Aug;254(4):256–62. doi: 10.1007/s00406-004-0492-3. [DOI] [PubMed] [Google Scholar]
- 213.Katz N, Tadmor I, Felzen B, Hartman-Maeir A. The Behavioural Assessment of the Dysexecutive Syndrome (BADS) in schizophrenia and its relation to functional outcomes. Neuropsychol Rehabil. 2007 Apr;17(2):192–205. doi: 10.1080/09602010600685053. [DOI] [PubMed] [Google Scholar]