Abstract
Injury to the central nervous system triggers glial calcium waves in both vertebrates and invertebrates. In vertebrates the pannexin1 ATP-release channel appears to provide for calcium wave initiation and propagation. The innexins, which form invertebrate gap junctions and have sequence similarity with the pannexins, are candidates to form non-junctional membrane channels. Two leech innexins previously demonstrated in glia were expressed in frog oocytes. In addition to making gap junctions, innexins also formed non-junctional membrane channels with properties similar to those of pannexons. In addition, carbenoxolone reversibly blocked the loss of carboxyfluorescein dye into the bath from the giant glial cells in the connectives of the leech nerve cord, which are known to express the innexins we assayed.
Keywords: pannexin, innexin, calcium wave, ATP, glia, evolution
1. Introduction
Intercellular calcium waves are widespread and, in addition to signaling injury to the nervous system, serve important physiological functions, including synchronization of ciliary beats, modulation of synaptic transmission, ossification, and control of vascular perfusion [1–4]. Calcium waves involve gap junction channels for propagation from cell to cell [1]. However, they also involve the release of ATP from the cytoplasm to the extracellular space [5]. Binding of ATP to P2 receptors results in further ATP release and propagation of the wave. Several mechanisms for ATP release have been proposed, including vesicular release and channel-mediated release through a variety of membrane channels (cit.ref. [6]). Although a large body of literature suggests that a non-junctional membrane channel with properties of gap junction “hemichannels” represents the ATP release channel, the identity of the channel is questionable. Various vertebrate gap junction proteins, in particular connexins 43 and 32, have been implicated in ATP release [7,8]. But calcium waves can occur in a connexin free environment. The waves are not restricted to vertebrates but are found in a broad spectrum of phyla that include invertebrates [9–11]. The invertebrate organisms do not express connexins, but the unrelated innexin family of gap junction proteins [12,13].
Recent evidence indicates that pannexins [14], a second family of vertebrate gap junction proteins that exhibit sequence homology to innexins, are a plausible alternative to connexins as the ATP release channel [6]. We therefore tested whether innexins, like vertebrate pannexins, are capable of forming channels in the non-junctional membrane. Different leech (Hirudo sp.) innexins, expressed by glial cells or by neurons, were expressed in Xenopus oocytes and analyzed by whole cell voltage clamp and patch clamp.
2. Materials and Methods
Oocytes
Oocytes were prepared as described previously [15]. In brief, Xenopus laevis oocytes were isolated by incubating small pieces of ovary in 2 mg ml−1 collagenase in calcium free oocyte Ringer’s solution (OR2) and stirring at 1 turn/second for 3 hours at room temperature. After being thoroughly washed with regular OR2, oocytes devoid of follicle cells and having uniform pigmentation were selected and stored in OR2 at 18 °C.
In vitro transcription of mRNAs
Hirudo innexins 2 and 3 (Hminx2 and Hminx3) had been cloned into the expression vector pCR-BluntII-TOPO. mRNA was transcribed by SP6 (Hminx2) or T7 (Hminx3) RNA polymerase from 10 μg of XbaI- (Hminx2) or SpeI- (Hminx3) linearized plasmid using the mMessage mMachine kit (Ambion). mRNA was quantified by absorbance (260nm), and the proportion of full-length transcripts was checked by agarose gel electrophoresis. 20 nl of mRNA (50 ng/μl) was injected into oocytes.
Solutions
OR2 solution in mM: 82.5 NaCl, 2.5 KCl, 1.0 MgCl2, 1.0 CaCl2, 1.0 Na2HPO4, 5.0 HEPES, antibiotics (Penicillin, 10,000 units/ml; Streptomycin, 10 mg/ml), pH7.5). Patch pipette KGlu solution: 140 mM potassium gluconate, 10 mM KCl, 5.0 mM TES, pH 7.5)
Voltage clamp
Whole cell voltage clamp recording was performed with two intracellular microelectrodes as described [15].
Patch clamp technique
Single innexon channels were studied by the patch-clamp technique [16] using a WPC-100 amplifier (E.S.F. Electronics, Goettingen, Germany). Currents were filtered at 5 kHz, digitized using a VR-10B digital data recorder, and stored on video tape. The recordings were transferred to a Power Macintosh (Apple) computer using an ITC-18 Computer Interface (Instrutech Corporation) and analyzed. Acquisition and analysis were done with the Acquire and TAC programs (both from Bruxton Corporation, Seattle, WA).
Details of patch clamp techniques used, including application of negative pressure, can be found elsewhere [17,18].
Extracellular ATP measurements from oocytes
ATP assay solutions (Luciferin/Luciferase, Sigma, St. Louis) were mixed with supernatants collected from hm-inx2 injected and uninjected cells treated with oocyte Ringer’s solution (OR2) or potassium gluconate (KGlu) in the presence of 0–100 μM carbenoxolone (CBX). Oocytes were 4 days post-injection. Innexin expression and cell viability were confirmed electrophysiologically. Cells were pretreated for 10 minutes in experimental solutions and then isolated for 10 minutes in 150 μL of the same experimental solutions. 100 μL supernatant was obtained for each condition. Each condition was done in quintuplicate. Luminescence readings were obtained with a Victor 1420 multilabel counter (PerkinElmer, Waltham, MA) on a 96 well culture plate. ANOVA with Post-Hoc Fisher test (Statistica, StatSoft Inc., Tulsa, OK) was used for comparison and statistical analysis of luminescence.
In situ dye release
6-Carboxyfluorescein was injected into a single glia cell of the leech connective and dye release was followed under a fluorescence microscope. Details of the technique are given in Supplement 1 and Supplemental Figure 1.
3. Results
Innexins form innexons
Both Hminx2 and Hminx3 formed patent “hemichannels” (innexons) in the non-junctional plasma membrane (Fig. 1a, b). Like pannexin 1, but in contrast to “hemichannels” formed by some connexins, no change of extracellular calcium was required to observe innexon channel activity. The channels were closed at the resting membrane potential (−40 mV) and opened when the membrane was depolarized to –20 mV or higher (Fig. 1a, b). Cytoplasmic acidification, induced by perfusing the oocytes with CO2-saturated Ringer’s solution, closed the channels (Fig. 1c, d). This effect slowly reversed upon washout (Supplemental Figure 2). Moreover, carbenoxolone closed the channels in a dose dependent way (Fig. 1e, Supplemental Figure 3). In Figure 1b, as shown previously [19], sham injected oocytes did not exhibit a current that was activated in this voltage range and that was sensitive to cytoplasmic acidification.
Figure 1.
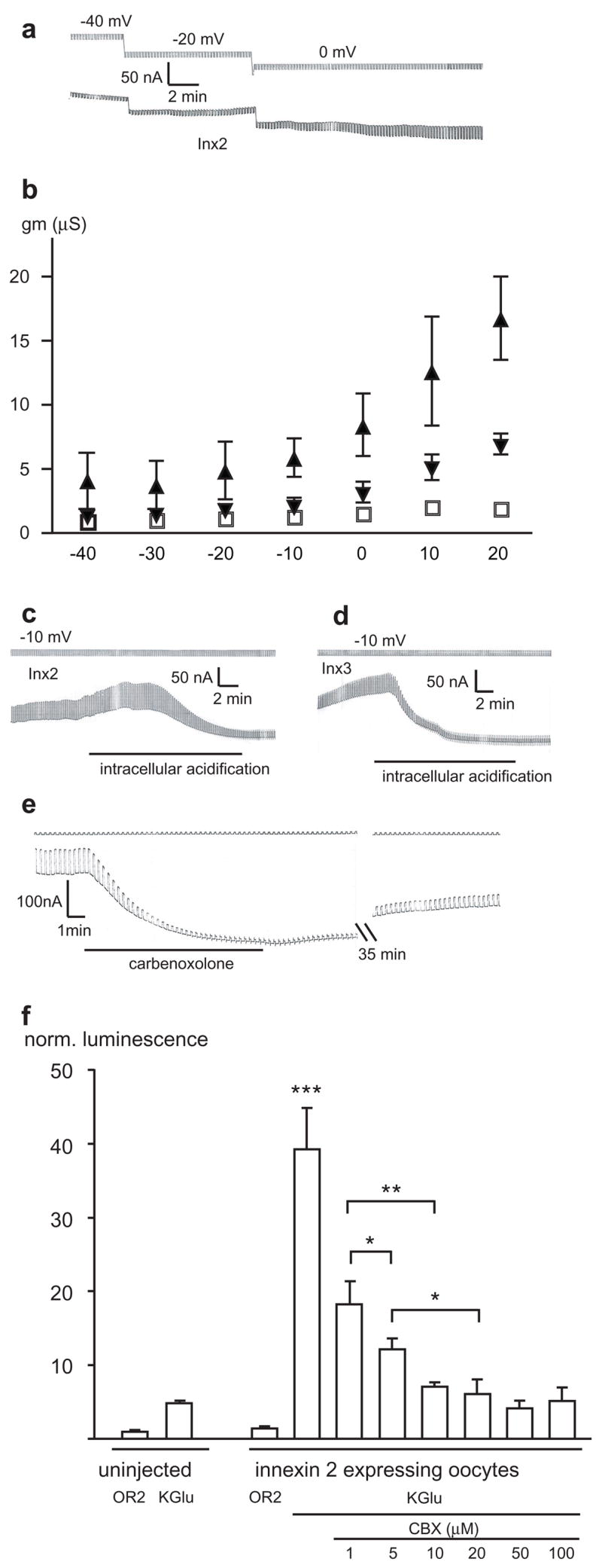
Macroscopic membrane currents. (a): The membrane potential of an Hminx2--expressing oocyte was held at the potentials indicated and 5 mV hyperpolarizing voltage pulses to test membrane resistance were applied (top trace) at a rate of 6/min. The corresponding currents are shown in the bottom trace. (b) Voltage dependence of membrane conductance (gm) of oocytes expressing Hminx2 ( ),Hminx3 ( ) or uninjected oocytes (◽). Means ±SD (n=4, 5, or 5, respectively) are plotted. The membrane potential was held at the respective voltages for 5 minutes and conductance was determined with 5 mV depolarizing pulses. (c) & (d): Application of CO2 to the bath solution reduced membrane currents of oocytes expressing Hminx2 (c) or Hminx3 (d). (e): Addition of 100 μM carbenoxolone increased membrane resistance and blocked the current in an oocyte expressing Hminx2. Note the slow time course of recovery. (f) ATP release mediated by Hminx2is inhibited by carbenoxolone. Release of ATP to the extracellular medium by oocytes was measured by luminometry using a luciferase assay. Depolarization of the cells with high potassium solution (KGlu) resulted in a small ATP release from uninjected oocytes and a significantly larger release from Hminx2injected oocytes. Carbenoxolone attenuated this larger release in a dose dependent fashion. Means ± SEM are given (n=5). ***= p< 0001, **=p<0.001, *=p<0.05.
To test whether the formation of innexons might be a general property of innexins, two other innexins, Hminx1 and Hminx6, which are found in leech neurons, were also expressed in oocytes. Both formed non-junctional membrane channels with properties similar to those of the channels formed by Hminx2 and Hminx3 (Supplemental Figure 4).
To test for ATP permeability of innexons, the efflux of ATP from oocytes expressing Hminx2 was measured by a luminometric luciferase assay. As reported previously, uninjected control oocytes released some ATP when depolarized by a high potassium solution (KGlu) (Figure 1f), [18,20] which probably represents the Brefeldin sensitive vesicular release [20]. Hminx2 expressing oocytes had the same basal release as control oocytes. However, when depolarized with high potassium solution a significant increase in ATP release was observed (Figure 1f). Carbenoxolone inhibited this ATP release in a dose dependent way (Figure 1f).
Consistent with the macroscopic membrane currents, expression of Hminxs1,2,3 and 6 in single Xenopus oocytes resulted in the appearance of a novel type of membrane channel, with a large unitary conductance of about 500 pS (Hminxs2,3 and 6) and about 250 pS (Hminx1) in symmetrical 150 mM KCl (Fig. 2a, Supplemental Figure 4). The channels were closed at negative potentials and open at positive potentials. The voltage gate was slow, and consequently the channel remained open for tens of seconds after switching from positive to negative potential. The channel activity was attributable to innexins because such large conductance channels were not observed in control oocytes and because of the distinct features of the channel, which were quite similar to pannexin 1 channels expressed in oocytes [23]. Like pannexin 1 channels, all leech innexin channels tested here, when active, exhibited multiple subconductance states at which they mainly dwelled, while sojourns to the full open and closed states were infrequent (Fig. 2a, Supplemental Figure 4). For a more detailed characterization of the channels we concentrate hereafter on those formed by Hminx2.
Figure 2.
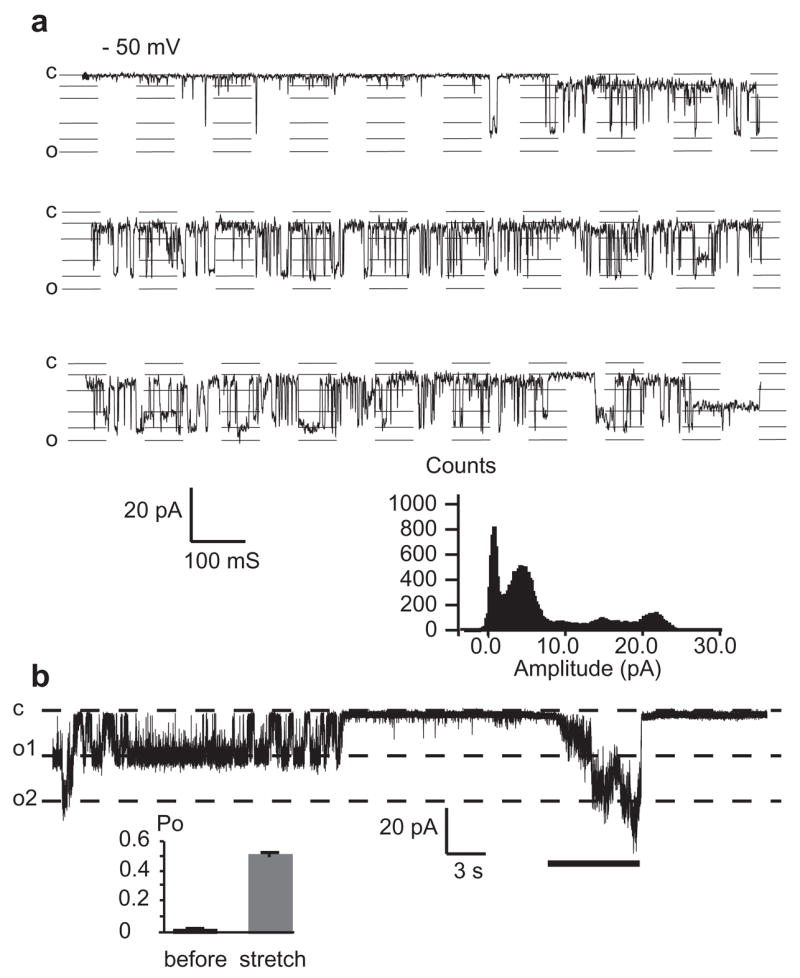
Single channel currents of leech Hminx2. (a) The membrane potential was held at positive potential to activate the channel before shifting to –50 mV. Like human pannexin 1 channels, the channel has a large unit conductance (~500 pS in 150 mM KCl) and exhibits many subconductance levels (compare to Figure 1 in Bao et al., 2004). Histogram shows distribution of current amplitudes, with intermediate peaks at subconductance levels. (b) Patch clamp recording from a membrane patch of an oocyte exogenously expressing Hminx2. After activation of the channels at +20 mV, the membrane potential was held at −50 mV. At this potential, channel activity continued for a period of time and then subsided, as is typical for this holding potential. Negative pressure (~40 mbar) was applied by suction to the patch pipette during the time indicated by the line. That the patch contained at least three channels can be seen by the steps during negative pressure application.
Innexon channels are mechanosensitive
It has been reported that pannexon channels are sensitive to mechanical stress [18]. To test whether innexons are also mechanosensitive, we used cell-attached and excised membrane patches from oocytes expressing Hminx2. After identification of the innexons, based on single channel conductance, voltage dependence and gating properties (archetypal subconductance levels), the channels were subjected to mechanical stress by suction applied to the patch pipette [21]. Fig. 2b shows a typical response of Hminx2 channels to stretch. When the patch, containing at least three channels, was held at −50 mV, mechanical stress caused activity to increase. The channel activity initially was in the form of subconductance states before full openings were discerned. For quantitative analysis, open probability was determined before and during stretch (Fig. 2b). During prolonged stretch, channel activity often subsided spontaneously (not shown).
Oocytes are known to express an endogenous stretch activated channel [22–24]. However, the channel properties including unit conductance (~50pS), kinetics, and calcium block of this endogenous channel [17,24] are distinct from those of the large conductance channel seen only in innexin mRNA injected oocytes (Figure 2).
Innexon channels are activated by cytoplasmic calcium
Pannexin1 channels can be activated by cytoplasmic calcium. We tested whether the functional similarity of pannexin and innexin channels also extend to this property. Oocytes endogenously express a calcium activated chloride channel that could potentially interfere with the analysis. However, the innexon channels can be discriminated easily from the endogenous calcium activated chloride channel based on their unitary conductances of ~500 pS and 3 pS [25], respectively.
After identification of innexon channels at potentials permissive for channel opening, the channels were held at −50 mV. At this potential, channels were closed or stayed in low subconductance states. Application of calcium to the intracellular side of the channels contained in excised inside-out membrane patches resulted in channel activity that ceased upon washout of the calcium containing solution (Supplemental Fig. 5). Free calcium concentrations in the micromolar range were sufficient to elicit this effect. Current recordings from inside-out patches containing multiple Hminx2 channels were used to determine the Ca2+ dose-response relationship (Supplemental Fig. 5b). When the free [Ca2+] was 10−6 M or less, the channels opened briefly and resided in low subconductance states. When the free [Ca2+] was 10−5 M or higher, multiple channels opened, resulting in rapid increases in patch currents.
Repeated applications of 10−5 M [Ca2+] to the intracellular side resulted in decreased current amplitudes and slower activation kinetics (Supplemental. Fig. 6). It is unclear whether this adaptation phenomenon was due to complete inactivation of individual channels or whether the majority of channels were driven into lower subconductance states.
Innexons function in vivo
Although measurements using frog oocytes show that Hminx1, 2, 3 and 6 form innexons, direct study of the leech nervous system is required to determine whether innexins normally form innexons in vivo. One measure of the presence of pannexons and other “hemichannels” has been the uptake or loss across the plasmalemma of small molecules of dye that can be specifically blocked by drugs such as carbenoxolone [26], which we showed above blocks Hminx2 innexon activity in frog oocytes (Fig. 1e,f).
A single ~5 mm-long, glial cell expressing both Hminx2 and Hminx3 ensheathes all the axons of each connective between ganglia along the nerve cord of the leech (Supplementary Figure 1a) [27]. To determine whether Hminx2 and Hminx3 form non-junctional innexon channels in the living nervous system as they do in oocytes, dye release studies were done on live connective glial cells. The dye 6-carboxyfluorescein (6-CF), which is gap junction permeable for leech glia, was directly injected into the connective glial cell using small negative currents (−2 nA). Injection was restricted to one of the two glial cells of the connective; the second served as control. Both cells were imaged at 5 minute intervals to determine the rate of dye loss (see Methods). In leech saline solution, approximately 15% of the dye was lost from the connective every 5 minutes. This dye loss was almost completely blocked in saline with 10 μM carbenoxolone. This effect was reversed by rinsing the carbenoxolone from the tissue, as shown for a representative example in Figure 3a. Figure 3b shows the average of 6 experiments. Carbenoxolone’s attenuation of dye release demonstrated that non-junctional channels were involved in the passage of dye across the membrane.
Figure 3.

Carbenoxolone reversibly inhibits 6- carboxyfluorescein dye loss from the connective glial cell. (a) Representative time course of dye loss from a 1 mm piece of 6-carboxyfluorescein (6-CF) injected connective glial cell (see Supplemental Figure 1b). Carbenoxolone (CBX, 10μM) was added and washed out as indicated. (b) Quantification of average dye loss before, during, and after CBX treatment. (*= p<.01, **= p<0.001, n=6) Error bars represent SEM. (c): A schematic diagram showing that innexins share the roles both of pannexins in forming channels to the extracellular space and of connexins in forming gap junctions.
4. Discussion
These results indicate that Hminxs1,2, 3 and 6 when expressed in Xenopus oocytes, form functional innexons that permit the exchange between cytoplasm and extracellular space of molecules in the size range of second messengers. This is in addition to the established role of innexins to form gap junction channels, which has been demonstrated for Hm-inx2 [28]. With only hints towards its existence [6,29], innexon channel activity has not yet been described for innexins; previous studies were mostly limited to addressing the fundamental question of which protein participates in gap junctional function in invertebrates [12,30]. Whether the capacity to form innexon channels is a general property of all innexins remains to be determined, but it is of interest to note that all four leech innexins thus far tested do.
The channels formed by the Hirudo sp.innexins share several properties with human and mouse pannexin 1 channels. The unitary conductances of the channels are similar, yet distinct. In the full open state pannexin 1 channels have a single channel conductance of 550 pS in 150 mM potassium chloride solution [18]. Under identical conditions the single channel conductance of Hminxs2, 3 and 6 channels is ~500 pS and that of Hminx1 is ~250 pS.
Like pannexin 1 channels, the innexin channels exhibit multiple subconductance levels and at voltages permissive for channel opening, excursions to the full closed as well to the full open state are scarce. The single prominent subconductance state of connexin 46 channels alters the permeability limits of the channel in a voltage dependent way [31]. Whether the multiple subconductance states of innexin and pannexin channels have functional implications is untested. Because the subconductance levels appear over a broad voltage range, including positive and negative voltages, a voltage dependent function similar to that of connexin 46 is unlikely. Instead, the subconductance levels could simply be a sign of a slowly gating large channel that cannot rapidly switch its conformation from a fully closed to a fully open state.
Both innexin and pannexin 1 channels open with depolarization. However, while pannexin channels need to be held at positive potentials to observe voltage dependent channel activation, innexin channels open upon depolarization to –20 mV or more positive levels.
Cytoplasmic acidification by exposing cells to CO2 inhibited the innexin induced currents just as it does for pannexin 1 currents [32]. Channel closure in response to reduced cytoplasmic pH is characteristic of all gap junction channels and “hemichannels” irrespective of their protein makeup. However, the sensitivity of the channels formed by the various gap junction proteins varies considerably, with a range covering almost a whole pH unit. At least for Cx46 connexons it has been demonstrated that protons most likely interact directly with the channel protein [33,34].
Another similarity between the non-junctional membrane channels formed by pannexin 1 and innexin is the channel activation by cytoplasmic calcium in the micromolar range [32]. This is in contrast to channels formed by connexins, which either are non-responsive to increased cytoplasmic calcium or are closed, depending on their protein composition [35–38].
It would appear that at least some innexins have a dual function. In addition to their conventional role as gap junction forming proteins, they can form physiologically non-junctional membrane channels. Our in vivo study supports the presence of innexons in the intact leech nervous system. Like pannexin 1 channels, the Hminx2 innexin channels described here have qualities expected for the ATP release channel involved in initiation and propagation of calcium waves. These include mechanosensitivity, activation by cytoplasmic calcium, and ATP permeability. We propose that with the acquisition of connexins by deuterostomes, the gap junction role was assumed by these proteins while the innexin orthologs in deuterostomes, the pannexins, were retained for the non-junctional channel function (Fig. 5c).
Supplementary Material
Acknowledgments
We thank Dr. I. Dykes for preparing the Hminx2 and Hminx3 constructs.
Supported in part by NIH training grants and a Lois Pope Fellowship (SS), an AHA graduate fellowship (SL), NSF grant 0446346 (ERM) and NIH grants NS34927 (KJM), NS37025 (KJM) and GM48610 (GD) and an AHA grant (GD)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanderson MJ, Charles AC, Dirksen ER. Mechanical stimulation and intercellular communication increases intracellular Ca2+ in epithelial cells. Cell Regul. 1990;1:585–596. doi: 10.1091/mbc.1.8.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J Neurosci. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jorgensen NR, Geist ST, Civitelli R, Steinberg TH. ATP- and gap junction-dependent intercellular calcium signaling in osteoblastic cells. J Cell Biol. 1997;139:497–506. doi: 10.1083/jcb.139.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigurdson WJ, Sachs F, Diamond SL. Mechanical perturbation of cultured human endothelial cells causes rapid increases of intracellular calcium. Am J Physiol. 1993;264:H1745–752. doi: 10.1152/ajpheart.1993.264.6.H1745. [DOI] [PubMed] [Google Scholar]
- 5.Osipchuk Y, Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992;359:241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- 6.Dahl G, Locovei S. Pannexin: To Gap or not to Gap, is that a question? IUBMB Life. 2006;58:409–419. doi: 10.1080/15216540600794526. [DOI] [PubMed] [Google Scholar]
- 7.Cotrina ML, et al. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. Embo J. 2006;25:34–44. doi: 10.1038/sj.emboj.7600908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brehm P, Lechleiter J, Smith S, Dunlap K. Intercellular signalling as visualized by endogenous calcium-dependent bioluminescence. Neuron. 1989;3:191–198. doi: 10.1016/0896-6273(89)90032-9. [DOI] [PubMed] [Google Scholar]
- 10.Cooper MS. Intercellular signaling in neuronal-glial networks. Biosystems. 1995;34:65–85. doi: 10.1016/0303-2647(94)01450-l. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann B, Walz B. The mechanism mediating regenerative intercellular Ca2+ waves in the blowfly salivary gland. Embo J. 1999;18:3222–3231. doi: 10.1093/emboj/18.12.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phelan P, Starich TA. Innexins get into the gap. Bioessays. 2001;23:388–396. doi: 10.1002/bies.1057. [DOI] [PubMed] [Google Scholar]
- 13.Stout C, Goodenough DA, Paul DL. Connexins: functions without junctions. Curr Opin Cell Biol. 2004;16:507–512. doi: 10.1016/j.ceb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10:R473–474. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- 15.Dahl G. In: The Xenopus oocyte cell-cell channel assay for functional analysis of gap junction proteins. in: Cell-cell interactions. A practical approach. Stevenson B, Gallin W, Paul D, editors. IRL; Oxford: 1992. pp. 143–165. [Google Scholar]
- 16.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 17.Bao L, Sachs F, Dahl G. Connexins are mechanosensitive. Am J Physiol Cell Physiol. 2004;287:C1389–1395. doi: 10.1152/ajpcell.00220.2004. [DOI] [PubMed] [Google Scholar]
- 18.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Pfahnl A, Zhou XW, Werner R, Dahl G. A chimeric connexin forming gap junction hemichannels. Pflugers Arch. 1997;433:773–779. doi: 10.1007/s004240050344. [DOI] [PubMed] [Google Scholar]
- 20.Maroto R, Hamill OP. Brefeldin A block of integrin-dependent mechanosensitive ATP release from Xenopus oocytes reveals a novel mechanism of mechanotransduction. J Biol Chem. 2001;276:23867–23872. doi: 10.1074/jbc.M101500200. [DOI] [PubMed] [Google Scholar]
- 21.Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamill OP, McBride DW., Jr Rapid adaptation of single mechanosensitive channels in Xenopus oocytes. Proc Natl Acad Sci U S A. 1992;89:7462–7466. doi: 10.1073/pnas.89.16.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Methfessel C, Witzemann V, Takahashi T, Mishina M, Numa S, Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986;407:577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- 24.Yang XC, Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989;243:1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi T, Neher E, Sakmann B. Rat brain serotonin receptors in Xenopus oocytes are coupled by intracellular calcium to endogenous channels. Proc Natl Acad Sci U S A. 1987;84:5063–5067. doi: 10.1073/pnas.84.14.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 27.Coggeshall RE, Fawcett DW. The fine structure of the central nervous system of the leech, Hirudo medicinalis. J Neurophysiol. 1964;27:229–289. doi: 10.1152/jn.1964.27.2.229. [DOI] [PubMed] [Google Scholar]
- 28.Dykes IM, Freeman FM, Bacon JP, Davies JA. Molecular basis of gap junctional communication in the CNS of the leech Hirudo medicinalis. J Neurosci. 2004;24:886–894. doi: 10.1523/JNEUROSCI.3676-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chuang CF, Vanhoven MK, Fetter RD, Verselis VK, Bargmann CI. An innexin-dependent cell network establishes left-right neuronal asymmetry in C. elegans. Cell. 2007;129:787–799. doi: 10.1016/j.cell.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 30.Phelan P, Stebbings LA, Baines RA, Bacon JP, Davies JA, Ford C. Drosophila Shaking-B protein forms gap junctions in paired Xenopus oocytes. Nature. 1998;391:181–184. doi: 10.1038/34426. [DOI] [PubMed] [Google Scholar]
- 31.Qu Y, Dahl G. Function of the voltage gate of gap junction channels: selective exclusion of molecules. Proc Natl Acad Sci U S A. 2002;99:697–702. doi: 10.1073/pnas.022324499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Pfahnl A, Dahl G. Localization of a voltage gate in connexin46 gap junction hemichannels. Biophys J. 1998;75:2323–2331. doi: 10.1016/S0006-3495(98)77676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trexler EB, Bukauskas FF, Bennett MV, Bargiello TA, Verselis VK. Rapid and direct effects of pH on connexins revealed by the connexin46 hemichannel preparation. J Gen Physiol. 1999;113:721–742. doi: 10.1085/jgp.113.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loewenstein WR. Junctional intercellular communication: the cell-to-cell membrane channel. Physiological Reviews. 1981;61:829–912. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- 36.Peracchia C, Wang X, Li L, Peracchia LL. Inhibition of calmodulin expression prevents low-pH-induced gap junction uncoupling in Xenopus oocytes. European Journal of Physiology. 1996;431:379–387. doi: 10.1007/BF02207275. [DOI] [PubMed] [Google Scholar]
- 37.Unwin PN, Ennis PD. Calcium-mediated changes in gap junction structure: evidence from the low angle X-ray pattern. J Cell Biol. 1983;97:1459–1466. doi: 10.1083/jcb.97.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spray DC, Harris AL, Bennett MV. Comparison of pH and calcium dependence of gap junctional conductance. Kroc Foundation Series. 1981;15:445–461. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


