Abstract
The hyperthermophilic archaea Acidianus hospitalis, Aeropyrum pernix, Pyrobaculum aerophilum, Pyrobaculum calidifontis, and Sulfolobus tokodaii representing three different orders in the phylum Crenarchaeota were analyzed by flow cytometry and combined phase-contrast and epifluorescence microscopy. The overall organization of the cell cycle was found to be similar in all species, with a short prereplicative period and a dominant postreplicative period that accounted for 64 to 77% of the generation time. Thus, in all Crenarchaeota analyzed to date, cell division and initiation of chromosome replication occur in close succession, and a long time interval separates termination of replication from cell division. In Pyrobaculum, chromosome segregation overlapped with or closely followed DNA replication, and further genome separation appeared to occur concomitant with cellular growth. Cell division in P. aerophilum took place without visible constriction.
All extant cellular organisms can be divided into three main evolutionary lineages, the domains Archaea, Bacteria, and Eukarya (32). The domain Archaea is further subdivided into two main phyla, Crenarchaeota and Euryarchaeota. Additional phyla have also been proposed (2, 8, 14), although their phylogenetic validities are under discussion (7, 25).
The cell cycle of organisms belonging to the domain Bacteria is organized into three main stages: the prereplication (B), replication (C), and postreplication (D) periods. In eukaryotes a different nomenclature is used, and the cell cycle is divided into the G1 (gap 1), S (DNA synthesis), G2 (gap 2), and M (mitosis) stages. The organization and relative lengths of the cell cycle periods vary greatly between organisms in both domains.
The first cell cycle analyses of archaea were performed with organisms belonging to the crenarchaeal genus Sulfolobus. The cell cycle in these organisms is characterized by a short prereplication period and an extensive postreplication stage dominated by a long G2 period (3a, 5, 12, 24). The organization of the cell cycle in the Euryarchaeota Methanocaldococcus jannaschii and Methanothermobacter thermautotrophicus differs considerably from that in Sulfolobus species. In M. jannaschii, cells with high and uneven numbers of genome copies are present, and asymmetric genome segregation and cell division are observed (22). M. thermautotrophicus grows as filaments consisting of multiple cells, each containing a minimum of two genomes (21). After replication, the two newly formed chromosome pairs rapidly segregate into separate nucleoids without any discernible G2 stage, resulting in four spatially distinct chromosomes. In contrast to these methanogenic species, the sulfate-reducing euryarchaeon Archaeoglobus fulgidus has a cell cycle organization similar to that of Sulfolobus (20).
In stationary-phase Sulfolobus cultures all cells contain two genome copies (5), resulting in an increase in the average cellular DNA content relative to an exponentially growing culture (3a). In contrast, a dramatic reduction in DNA content is observed in stationary-phase M. jannaschii cells (22). The average filament length and thus the total number of genome copies per filament decrease with cell concentration in M. thermautotrophicus cultures (21). Stationary-phase A. fulgidus cells contain either one or two genome copies, and the ratio varies with the mode of cultivation (20).
Here, we studied the cell cycle characteristics of hyperthermophilic Crenarchaeota belonging to the orders Desulfurococcales (Aeropyrum pernix), Thermoproteales (Pyrobaculum aerophilum and Pyrobaculum calidifontis), and Sulfolobales (Acidianus hospitalis and Sulfolobus tokodaii). We show below that the overall organization of the cell cycle previously described for Sulfolobus species also occurs in other branches in the phylum Crenarchaeota. Also, microscopy analyses of P. aerophilum revealed a noncentral location for the nucleoids during most of the cell cycle, postreplicative nucleoid segregation concomitant with cellular growth, and an unusual cell division mechanism.
MATERIALS AND METHODS
Strains.
A. pernix DSM 11879, P. aerophilum DSM 7523, Sulfolobus solfataricus DSM 1617, Sulfolobus acidocaldarius DSM 639, and S. tokodaii DSM 16993 were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) (Braunschweig, Germany). P. calidifontis VA1 (1) and A. hospitalis (6) were kindly provided by Haruyuki Atomi and David Prangishvili, respectively.
Media and growth conditions.
P. aerophilum was grown under anaerobic conditions at 100°C in basal salts medium supplemented with 0.1% KNO3 and 0.05% yeast extract (30). P. calidifontis was grown aerobically at 90°C in TY medium (1% tryptone, 0.1% yeast extract, 0.3% sodium thiosulfate) (1). S. acidocaldarius and S. solfataricus were grown aerobically at 79°C in modified Allen mineral base medium supplemented with 0.2% tryptone (11). S. tokodaii was cultivated in Allen medium supplemented with 0.1% tryptone, 0.1% yeast extract, and 0.2% sucrose. A. pernix was grown aerobically at 90°C in Aeropyrum-JXT medium (DSMZ medium 820). A. hospitalis was grown at pH 3.5 in a medium containing 0.2% tryptone, 15 g/liter sulfur, 0.3 mM Ca(NO3)2, 1 mM MgCl2, 26 mM (NH4)2SO4, 2.2 mM K2HPO4, 1.4 mM KCl, 0.7 g/liter glycine, 4.5 μM MnCl2, 5.9 μM Na2B4O7, 0.08 μM ZnSO4, 0.15 μM CuCl2, 0.45 μM Na2MoO4, 0.06 μM VOSO4, 0.02 μM CoCO4, 0.02 μM NiSO4, 20 μg/liter biotin, 20 μg/liter folic acid, 100 μg/liter pyridoxamine hydrochloride, 50 μg/liter thiamine hydrochloride, 50 μg/liter riboflavin, 50 μg/ml nicotinic acid, 50 μg/liter dl-calcium pantothenate, 1 μg/liter cyanocobalamine, 50 μg/liter p-aminobenzoic acid, and 50 μg/liter lipoic acid. Growth was monitored by counting cells using a Thoma chamber (depth, 0.02 mm) or by determining the optical density at 600 nm for all species except P. calidifontis, for which the optical density at 660 nm was determined. The cultures were grown for up to 10 cell generations before sampling at mid-exponential phase, as well as in stationary phase.
Flow cytometry.
The cells were prepared for flow cytometry as described previously (5), and measurements were obtained with an A40 analyzer (Apogee Flow Systems). The relative lengths of the cell cycle periods were calculated from the flow cytometry DNA content distributions, using Winflow 2.0 software kindly provided by Flemming Hansen, with correction for the exponential age distribution of the populations (33). Flow cytometry reference samples from exponentially growing M. thermautotrophicus DSM 1053 cultures were obtained from experiments performed as described by Majernik et al. (21). Flow cytometry reference samples from rifampin-treated Escherichia coli MG1655 seqA::Tn10 cultures (23) were kindly provided by Jan Olsson.
Microscopy.
Samples for microscopy analysis were collected like the samples used for flow cytometry, and cell mounting was performed as described previously (24). Microscopy was performed with a Nikon Optiphot-2 epifluorescence microscope.
RESULTS
Cell cycle characteristics.
To generate an overview of cell cycle features in the phylum Crenarchaeota, the following species belonging to three different orders were analyzed: A. hospitalis and S. tokodaii belonging to the Sulfolobales, A. pernix belonging to the Desulfurococcales, and P. aerophilum and P. calidifontis belonging to the Thermoproteales. These species represent branches that are widely separated in the phylogenetic tree and have different cell morphologies, pH requirements, optimal growth temperatures, generation times, and genome sizes (Table 1).
TABLE 1.
Characteristics of the different archaea studied
| Species | Order | Doubling time (min) | Genome size (Mb) | Prereplicative period (%/min) | Replicative period (%/min) | Postreplicative period (%/min) | Optimum temp (°C) | Optimum pH | Oxygen tolerance | Morphology |
|---|---|---|---|---|---|---|---|---|---|---|
| A. hospitalis | Sulfolobales | NAa | 1.8b | 4b/NA | 27b/NA | 69b/NA | 80f | Acidic (pH 3.0)f | Aerobef | Irregular coccif |
| A. pernix | Desulfurococcales | 200b | 1.67c (1.75b) | 4/9b | 31/63b | 64/128b | 95g | Neutral (pH 7.0)g | Aerobeg | Irregular coccig |
| P. aerophilum | Thermoproteales | 190b | 2.20d | NA | NA | NA | 100h | Neutral (pH 7.0)h | Facultative anaerobeh | Rodsh |
| P. calidifontis | Thermoproteales | 330b | 1.8b | 1/5b | 13/43b | 75/157b | 90i | Neutral (pH 7.0)i | Facultative anaerobei | Rodsi |
| S. tokodaii | Sulfolobales | 480b | 2.7e (2.5b) | 1/5b | 22/105b | 77/370b | 80j | Acidic (pH 2.5)j | Aerobej | Irregular coccij |
Flow cytometry revealed that in exponential phase, cells with two fully replicated genome copies (postreplicative cell cycle phase) dominated the growing population for all species analyzed (Fig. 1). The peak representing the population of cells with a single genome copy (prereplicative) was small and overlapped extensively with the peak representing the population that contained between one and two genome equivalents (replicative phase), showing that in all cases the G1 (B) period was short. The DNA content distributions were used to calculate the relative lengths of the cell cycle phases (see Materials and Methods), and the values obtained are shown in Table 1.
FIG. 1.
Flow cytometry cell size and DNA content distributions for exponentially growing and stationary-phase cell populations of four crenarchaeal species.
Stationary phase.
In stationary-phase P. calidifontis and S. tokodaii cultures, most cells contained two fully replicated genome copies (Fig. 1L and 1P). Peaks corresponding to four genome copies were also observed at a low frequency. This probably resulted either from incomplete cell separation after division or from cell aggregation, since no single cells in which the fluorescence signal was significantly higher than the average signal could be detected by quantitative epifluorescence microscopy (not shown). For P. calidifontis, a small population with either one genome copy or between one and two genome copies was still observed long after the optical density had ceased to increase, indicating that chromosome replication did not run to completion in all cells under the conditions used. In contrast to the other organisms, the proportion of cells with a single chromosome increased in stationary-phase A. pernix cultures (Fig. 1H) compared to exponential phase (Fig. 1F), indicating that there was preferential arrest in the prereplicative phase, although cells with a higher DNA content also remained in the culture. For A. hospitalis, cells with a DNA content of less than one chromosome equivalent appeared in stationary phase (Fig. 1D), indicating either that DNA degradation took place or that chromatin restructuring in nongrowing cells resulted in a reduced dye binding capacity (5, 24).
The average light scatter decreased for S. tokodaii in stationary phase (compare Fig. 1O to Fig. 1M). No large changes were apparent for A. hospitalis, A. pernix, and P. calidifontis (compare Fig. 1C, 1G, and 1K to Fig. 1A, 1E, and 1I, respectively). However, light scatter reflects not only cell size, since this parameter also is affected by cell morphology and composition. Between species there was an obvious lack of correlation between light scatter and cell size; A. pernix (Fig. 1E and 1G) and P. calidifontis (Fig. 1I and 1K) displayed much lower light refraction than A. hospitalis (Fig. 1A and 1C) and S. tokodaii (Fig. 1M and 1O), although they were in the same size range. In the cases where DNA content distributions indicated incomplete replication and/or chromosome degradation (see above), changes in light scatter may have occurred as a result of cell shrinkage or swelling as a consequence of loss of cell envelope integrity in stationary phase under the extreme growth conditions used (3, 12).
Genome size estimation.
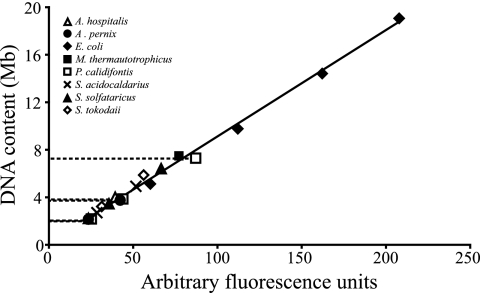
In flow cytometry, the fluorescence signal from stained chromosomes is linearly proportional to the amount of intracellular DNA, although the G+C content and chromatin structure may affect the absolute signal strength (5, 9). Thus, for the species with unknown genome sizes, estimates could be obtained by calibration with samples from organisms with known genome sizes and DNA contents (A. pernix, E. coli seqA::Tn10, M. thermautotrophicus, S. acidocaldarius, S. solfataricus, and S. tokodaii), as previously described (4, 21). The genome size was estimated to be 1.8 Mb for both P. calidifontis and A. hospitalis (Fig. 2 and Table 1).
FIG. 2.
Correlation between cellular DNA content and flow cytometry fluorescence signal strength for organisms whose genome sizes have been determined by complete genome sequencing, including A. pernix, E. coli, M. thermautotrophicus, S. acidocaldarius, S. solfataricus, and S. tokodaii. In addition, the DNA contents estimated for A. hospitalis and P. calidifontis are indicated by dashed lines.
Nucleoid structure and localization in P. aerophilum.
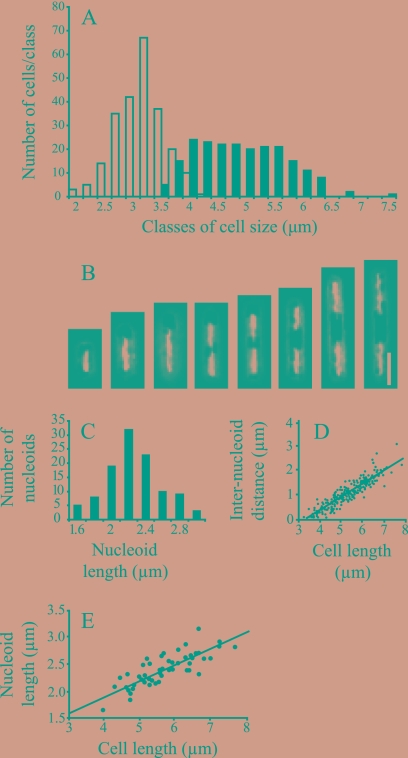
The nucleoids in P. aerophilum were usually clearly separated and preferentially distributed toward the cell poles (Fig. 3B, 3D, and 4B). Small P. aerophilum cells contained a single nucleoid, whose length gradually increased with cell length, until two separated nucleoids could be distinguished (Fig. 4A and 4B). Two nucleoids were present in 48% of the cells (Table 2), and the cells were between 3.5 and 7.5 μm long (Fig. 4A). The minimal nucleoid length in cells with two nucleoids (1.6 μm) (Fig. 4C) was slightly less than one-half the minimal length in cells in which two separated nucleoids could be detected (3.5 μm) (Fig. 4D). Nucleoid length appeared to increase further after the formation of two distinct fluorescence foci (Fig. 4B and 4E). P. calidifontis displayed similar characteristics, with 38% of the cells containing segregated nucleoids during exponential growth (not shown).
FIG. 3.
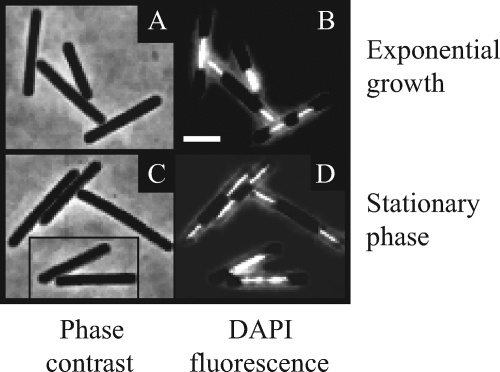
Microscopy of exponentially growing and stationary-phase P. aerophilum cultures. The cells were visualized by phase-contrast microscopy (left panels) and epifluorescence microscopy (right panels). For nucleoid visualization (right panels), the DNA was stained with 1 μg/μl DAPI (4′,6-diamidino-2-phenylindole). Bar = 2 μm.
FIG. 4.
Cell lengths, nucleoid lengths, and numbers of nucleoids in exponentially growing P. aerophilum cells. (A) Size class distribution for 234 cells containing one nucleoid (open bars) and 209 cells with two nucleoids (filled bars). (B) Examples of nucleoid number and length for cells of different sizes. Bar = 2 μm. (C) Nucleoid length class distribution for 59 cells having two separated nucleoids. (D) Correlation between cell length and distance between nucleoids for 250 cells. (E) Correlation between cell length and average length of the two nucleoids for 52 cells. In panels A and C, the values indicated on the x axis are the lower limits for the classes.
TABLE 2.
Proportions of cells with different numbers of nucleoids in exponentially growing P. aerophilum populations
| Sample | No. of cells counted | % of cells with one nucleoid | % of cells with two nucleoids |
|---|---|---|---|
| 1 | 1,100 | 52.7 | 47.3 |
| 2 | 430 | 50.5 | 49.5 |
| 3 | 270 | 52.4 | 47.6 |
| 4 | 330 | 53.5 | 46.5 |
| Avg | 52.3 | 47.7 |
P. aerophilum cell division.
In most prokaryotes, cell division occurs by gradual invagination of the cytoplasmic membrane and the surrounding cell envelope, resulting in a constriction. However, no constrictions were observed in P. aerophilum. Instead, microscopy analysis revealed the presence of bent cells (Fig. 5A), with a bend angle between 0° and 45°. The bending occurred in cells longer than 5 μm with clearly segregated nucleoids. Divided cells that had not separated completely were also detected (Fig. 5B), indicating that the bent cells split in a rapid process. The split was not always centered, so that the daughter cell sizes in unseparated pairs ranged from 2.3 to 3.4 μm. An unusual feature was the presence of short particles that had the same diameter as regular cells but were devoid of DNA (Fig. 5C). The lengths of these particles ranged from 0.7 to 2 μm (average length for 70 short cells measured, 1.1 μm).
FIG. 5.
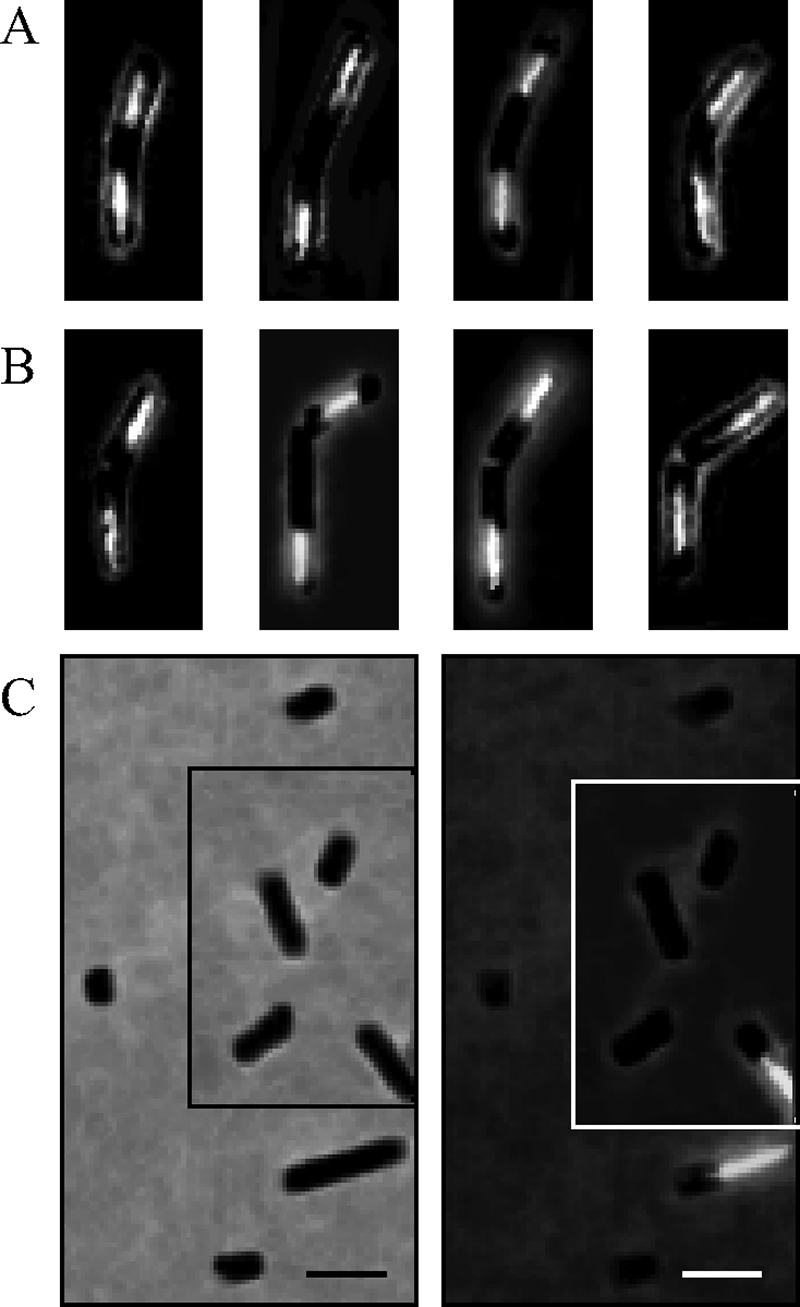
Microscopy images of DAPI-stained P. aerophilum cells. (A) Examples of bent cells prior to division. (B) Examples of split cells still attached to each other. (C) Assembled micrographs of DNA-less particles with diameters similar to the diameters of DNA-containing cells. The left and right panels show phase-contrast and epifluorescence images, respectively, of the same microscopy fields. Bar, 2 μm.
DISCUSSION
Prereplication period.
The prereplication period is short (<5% of the cell cycle) in S. acidocaldarius and S. solfataricus (5). Here, we found that this period is also short in A. hospitalis, A. pernix, P. calidifontis, and S. tokodaii. Thus, replication is initiated shortly after cell division in all Crenarchaeota studied to date. It is possible that completion of cell division could provide a positive signal for replication initiation or could release a replication block that allows subsequent initiation.
Replication period and replication rate.
The replication period accounted for between 13 and 31% of the doubling time, depending on the species, allowing estimation of replication fork progress rates. The in vivo replication rates of S. solfataricus and S. acidocaldarius, both of which contain three replication origins in a single chromosome, are on the order of 80 to 110 bp/s (18). The origin recognition boxes associated with the origins in these species (26, 27) are also conserved in the S. tokodaii genome sequence (15), and it is therefore likely that this species also harbors three origins, resulting in a replication rate of around 70 bp/s. A. pernix has been shown to contain two origins (26), yielding an in vivo replication rate of 110 bp/s. We have shown that flow cytometry is a convenient tool for genome size determination (4, 21), even for the eukaryote Giardia lamblia. Estimation of the genome size for A. hospitalis and P. calidifontis (Table 1) suggested that these organisms synthesize DNA at rates of 210 and 390 bp/s, respectively, assuming that there is bidirectional replication from a single origin. It remains to be determined whether these species also may contain additional origins, which would reduce these estimated rates to values similar to those for the other species.
Postreplication period.
The G2 period, the time interval between termination of replication and initiation of genome segregation, is long in Sulfolobus species (12, 24). The crenarchaeal species characterized here also have a long postreplication period, and we consider it likely that these organisms, with the exception of Pyrobaculum (see below), also go through an extensive G2 period, in agreement with the low frequency of cells displaying clearly segregated nucleoids. Furthermore, since very few dividing cells were observed in exponentially growing S. tokodaii cultures, the cell division period is also short (≤5% of the doubling time) in this species, similar to the situation in S. acidocaldarius and S. solfataricus (5).
Chromosome segregation and cell division in Pyrobaculum.
The Pyrobaculum species displayed a long postreplicative phase, similar to the organisms representing the Desulfurococcales and Sulfolobales. However, a G2-like period appeared to be absent since approximately 48% of the cells in actively growing populations contained two clearly separated nucleoids (Table 2), indicating that genome segregation occurred during or shortly after replication. Furthermore, a correlation between cell length and distance between nucleoids was evident, and nucleoid length appeared to increase with cell length, indicating that genome segregation and nucleoid extension continued during the postreplicative period. It is, therefore, possible that cell growth contributes to nucleoid segregation in this organism, e.g., through nucleoid association with the inner cell surface. Addition of cell wall material only at the midsection could contribute to nucleoid separation but would not cause elongation of the nucleoids. Importantly, cell growth-coupled partitioning does not rule out the possibility that there is an active genome segregation mechanism that operates during or shortly after replication.
No constrictions were visible in actively growing P. aerophilum cells. Rapid splitting in the cell center after bending has been described for Thermoproteus tenax (13) and may be a common feature of Thermoproteales, including Pyrobaculum species. In T. tenax, invagination of the cytoplasmic membrane in the cell center, accompanied by new S layer synthesis, eventually results in two daughter cells apparently connected by a small compartment (31). It is possible that the DNA-free minicells detected in P. aerophilum cultures could correspond to this central region, released as a by-product of cell division.
Concluding remarks.
The overall cell cycle features were found to be similar in hyperthermophilic organisms representing three different orders in the Crenarchaeota, despite considerable evolutionary distance and vast differences in cellular properties and growth preferences. In contrast, organisms belonging to the phylum Euryarchaeota display more variable cell cycle architectures (19). It will be of significant interest to investigate to what extent the conservation of crenarchaeal cell cycle features extends to low-temperature species (17).
Acknowledgments
We thank Gabi Gmeinwieser and Sabine Diller for excellent technical assistance.
This work was supported by grants from the Swedish Research Council, the Swedish Institute, the European Molecular Biology Organization, the Deutscher Akademischer Austauschdienst, and the Fonds der Chemischen Industrie.
Footnotes
Published ahead of print on 23 May 2008.
REFERENCES
- 1.Amo, T., M. L. F. Paje, A. Inagaki, S. Ezaki, H. Atomi, and T. Imanaka. 2002. Pyrobaculum calidifontis sp. nov., a novel hyperthermophilic archaeon which grows under atmospheric air. Archaea 1113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barns, S. M., C. F. Delwiche, J. D. Palmer, and N. R. Pace. 1996. Perspectives on archaeal diversity, thermophily and monophyly from environmental rRNA sequences. Proc. Natl. Acad. Sci. USA 939188-9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernander, R. 2000. Chromosome replication, nucleoid segregation and cell division in Archaea. Trends Microbiol. 8278-283. [DOI] [PubMed] [Google Scholar]
- 3a.Bernander, R. 2007. The cell cycle of Sulfolobus. Mol. Microbiol. 66557-562. [DOI] [PubMed] [Google Scholar]
- 4.Bernander, R., J. E. D. Palm, and S. G. Svärd. 2001. Genome ploidy in different stages of the Giardia lamblia life cycle. Cell. Microbiol. 355-62. [DOI] [PubMed] [Google Scholar]
- 5.Bernander, R., and A. Poplawski. 1997. Cell cycle characteristics of thermophilic archaea. J. Bacteriol. 1794963-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bettstetter, M., X. Peng, R. A. Garrett, and D. Prangishvili. 2003. AFV1, a novel virus infecting hyperthermophilic archaea of the genus Acidianus. Virology 31568-79. [DOI] [PubMed] [Google Scholar]
- 7.Brochier, C., S. Gribaldo, Y. Zivanovic, F. Confalonieri, and P. Forterre. 2005. Nanoarchaea: representatives of a novel archaeal phylum or a fast-evolving euryarchaeal lineage related to Thermococcales? Genome Biol. 6R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brochier-Armanet, C., B. Boussau, S. Gribaldo, and P. Forterre. 2008. Mesophilic Crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nat. Rev. Microbiol. 6245-252. [DOI] [PubMed] [Google Scholar]
- 9.Crissman, H. A., and G. T. Hirons. 1994. Staining of DNA in live and fixed cells. Methods Cell Biol. 41195-209. [DOI] [PubMed] [Google Scholar]
- 10.Fitz-Gibbon, S. T., H. Ladner, U.-J. Kim, K. O. Stetter, M. I. Simon, and J. H. Miller. 2002. Genome sequence of the hyperthermophilic crenarchaeon Pyrobaculum aerophilum. Proc. Natl. Acad. Sci. USA 99984-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grogan, D. W. 1989. Phenotypic characterization of the archaebacterial genus Sulfolobus: comparison of five wild-type strains. J. Bacteriol. 1716710-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hjort, K., and R. Bernander. 1999. Changes in cell size and DNA content in Sulfolobus cultures during dilution and temperature shift experiments. J. Bacteriol. 1815669-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horn, C., B. Paulmann, G. Kerlen, N. Junker, and H. Huber. 1999. In vivo observation of cell division of anaerobic hyperthermophiles by using a high-intensity dark-field microscope. J. Bacteriol. 1815114-5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huber, H., M. J. Hohn, R. Rachel, T. Fuchs, V. C. Wimmer, and K. O. Stetter. 2002. A new phylum of Archaea represented by a nanosized hyperthermophilic symbiont. Nature 41763-67. [DOI] [PubMed] [Google Scholar]
- 15.Kawarabayasi, Y., Y. Hino, H. Horikawa, K. Jin-No, M. Takahashi, M. Sekine, S. Baba, A. Ankai, H. Kosugi, A. Hosoyama, S. Fukui, Y. Nagai, K. Nishijima, R. Otsuka, H. Nakazawa, M. Takamiya, Y. Kato, T. Yoshizawa, T. Tanaka, Y. Kudoh, J. Yamazaki, N. Kushida, A. Oguchi, K. Aoki, S. Masuda, M. Yanagii, M. Nishimura, A. Yamagishi, T. Oshima, and H. Kikuchi. 2001. Complete genome sequence of an aerobic thermoacidophilic crenarchaeon, Sulfolobus tokodaii strain 7. DNA Res. 8123-140. [DOI] [PubMed] [Google Scholar]
- 16.Kawarabayasi, Y., Y. Hino, H. Horikawa, S. Yamazaki, Y. Haikawa, K. Jin-No, M. Takahashi, M. Sekine, S. Baba, A. Ankai, H. Kosugi, A. Hosoyama, S. Fukui, Y. Nagai, K. Nishijima, H. Nakazawa, M. Takamiya, S. Masuda, T. Funahashi, T. Tanaka, Y. Kudoh, J. Yamazaki, N. Kushida, A. Oguchi, K. Aoki, K. Kubota, Y. Nakamura, N. Nomura, Y. Sako, and H. Kikuchi. 1999. Complete genome sequence of an aerobic hyper-thermophilic crenarchaeon, Aeropyrum pernix K1. DNA Res. 683-101, 145-152. [DOI] [PubMed] [Google Scholar]
- 17.Könneke, M., A. E. Bernhard, J. R. de la Torre, C. B. Walker, J. B. Waterbury, and D. A. Stahl. 2005. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437543-546. [DOI] [PubMed] [Google Scholar]
- 18.Lundgren, M., A. Andersson, L. Chen, P. Nilsson, and R. Bernander. 2004. Three replication origins in Sulfolobus species: synchronous initiation of chromosome replication and asynchronous termination. Proc. Natl. Acad. Sci. USA 1017046-7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundgren, M., and R. Bernander. 2005. Archaeal cell cycle progress. Curr. Opin. Microbiol. 8662-668. [DOI] [PubMed] [Google Scholar]
- 20.Maisnier-Patin, S., L. Malandrin, N. K. Birkeland, and R. Bernander. 2002. Chromosome replication patterns in the hyperthermophilic euryarchaea Archaeoglobus fulgidus and Methanocaldococcus (Methanococcus) jannaschii. Mol. Microbiol. 451443-1450. [DOI] [PubMed] [Google Scholar]
- 21.Majernik, A. I., M. Lundgren, P. McDermott, R. Bernander, and J. P. Chong. 2005. DNA content and nucleoid distribution in Methanothermobacter thermautotrophicus. J. Bacteriol. 1871856-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malandrin, L., H. Huber, and R. Bernander. 1999. Nucleoid structure and partition in Methanococcus jannaschii: an archaeon with multiple copies of the chromosome. Genetics 1521315-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsson, J. A., K. Nordström, K. Hjort, and S. Dasgupta. 2003. Eclipse-synchrony relationship in Escherichia coli strains with mutations affecting sequestration, initiation of replication and superhelicity of the bacterial chromosome. J. Mol. Biol. 334919-931. [DOI] [PubMed] [Google Scholar]
- 24.Poplawski, A., and R. Bernander. 1997. Nucleoid structure and distribution in thermophilic archaea. J. Bacteriol. 1797625-7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson, C. E., J. K. Harris, J. R. Spear, and N. R. Pace. 2005. Phylogenetic diversity and ecology of environmental Archaea. Curr. Opin. Microbiol. 8638-642. [DOI] [PubMed] [Google Scholar]
- 26.Robinson, N. P., and S. D. Bell. 2007. Extrachromosomal element capture and the evolution of multiple replication origins in archaeal chromosomes. Proc. Natl. Acad. Sci. USA 1045806-5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson, N. P., I. Dionne, M. Lundgren, V. L. Marsh, R. Bernander, and S. D. Bell. 2004. Identification of two origins of replication in the single chromosome of the archaeon Sulfolobus solfataricus. Cell 11625-38. [DOI] [PubMed] [Google Scholar]
- 28.Sako, Y., N. Nomura, A. Uchida, Y. Ishida, H. Morii, Y. Koga, T. Hoaki, and T. Maruyama. 1996. Aeropyrum pernix gen. nov., sp. nov., a novel aerobic hyperthermophilic archaeon growing at temperatures up to 100°C. Int. J. Syst. Bacteriol. 461070-1077. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki, T., T. Iwasaki, T. Uzawa, K. Hara, N. Nemoto, T. Kon, T. Ueki, A. Yamagishi, and T. Oshima. 2002. Sulfolobus tokodaii sp. nov. (f. Sulfolobus sp. strain7), a new member of the genus Sulfolobus isolated from Beppu Hot Springs, Japan. Extremophiles 639-44. [DOI] [PubMed] [Google Scholar]
- 30.Völkl, P., R. Huber, E. Drobner, R. Rachel, S. Burggraf, A. Trincone, and K. O. Stetter. 1993. Pyrobaculum aerophilum sp. nov., a novel nitrate-reducing hyperthermophilic archaeum. Appl. Environ. Microbiol. 592918-292627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wildhaber, I., and W. Baumeister. 1987. The cell envelope of Thermoproteus tenax: three-dimensional structure of the surface layer and its role in shape maintenance. EMBO J. 61475-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woese, C. R., O. Kandler, and M. L. Wheelis. 1990. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA 874576-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wold, S., K. Skarstad, H. B. Steen, T. Stokke, and E. Boye. 1994. The initiation mass for DNA replication in Escherichia coli K-12 is dependent on growth rate. EMBO J. 132097-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]