Abstract
Tuberoinfundibular peptide of 39 residues (TIP39) was identified as the endogenous ligand of parathyroid hormone 2 receptor. We have recently demonstrated that TIP39 expression in adult rat brain is confined to the subparafascicular area of the thalamus with a few cells extending laterally into the posterior intralaminar thalamic nucleus (PIL), and the medial paralemniscal nucleus (MPL) in the lateral pontomesencephalic tegmentum. During postnatal development, TIP39 expression increases until postnatal day 33 (PND-33), then decreases, and almost completely disappears by PND-125. Here, we report the expression of TIP39 during early brain development. TIP39-immunoreactive (TIP39-ir) neurons in the subparafascicular area first appeared at PND-1. In contrast, TIP39-ir neurons were detectable in the MPL at embryonic day 14.5 (ED-14.5), and the intensity of their labeling increased thereafter. We also identified TIP39-ir neurons between ED-16.5 and PND-5 in two additional brain areas, the PIL and the amygdalo-hippocampal transitional zone (AHi). We confirmed the specificity of TIP39 immunolabeling by demonstrating TIP39 mRNA using in situ hybridization histochemistry. In the PIL, TIP39 neurons are located medial to the CGRP group as demonstrated by double immunolabeling. All TIP39-ir neurons in the AHi and most TIP39-ir neurons in the PIL disappear during early postnatal development. The adult pattern of TIP39-ir fibers emerge during postnatal development. However, fibers emanating from PIL can be followed in the supraoptic decussations towards the hypothalamus at ED-18.5. These TIP39-ir fibers disappear by PND-1. The complex pattern of TIP39 expression during early brain development suggests the involvement of TIP39 in transient functions during ontogeny.
Keywords: neuropeptide, transient expression, ontogeny, medial paralemniscal nucleus, amygdalo-hippocampal transitional zone, posterior intralaminar thalamic nucleus
Introduction
Tuberoinfundibular peptide of 39 residues (TIP39) was purified from bovine hypothalamus (Usdin et al., 1999) as an agonist of the parathyroid hormone 2 receptor (PTH2R) (Usdin et al., 1995). Mouse, rat, and human TIP39 were subsequently cloned (Dobolyi et al., 2002; John et al., 2002). Mouse and rat TIP39 sequences are identical, and share only 4 and 6 amino acid residues with parathyroid hormone-related peptide (PTHrP) and parathyroid hormone (PTH), respectively (Usdin et al., 2000). TIP39 is a potent agonist of rat and human PTH2 receptors and binds to the rat and human receptors with high affinity (Usdin et al., 1999). In contrast, PTH is only a low potency partial agonist at the rat PTH2R while PTHrP does not activate the PTH2R at all (Hoare et al., 1999). In turn, PTH and PTHrP are established ligands of the PTH 1 receptor (PTH1R) (Gensure et al., 2005) while TIP39 has little or no effect on the PTH1R (Usdin et al., 1999). These pharmacological data together with similarities in distribution between TIP39 and the PTH2R (Dobolyi et al., 2006a; Faber et al., 2007) suggest that TIP39 is the endogenous ligand of the PTH2R (Usdin, 2000; Dobolyi et al., 2006a; Faber et al., 2007). Initial functional studies implicate TIP39 and the PTH2R in the modulation of some aspects of spinal nociceptive signaling (Dobolyi et al., 2002). Furthermore, c-fos activation associated with specific sexual or maternal functions in brain areas expressing TIP39 suggests that TIP39 neurons may be involved in regulation of reproduction related processes (Lin et al., 1998; Li et al., 1999; Holstege et al., 2003; Coolen et al., 2004; Wang et al., 2006a) and the audiogenic stress response (Palkovits et al., 2004). In addition, intracerebroventricular injection of TIP39 in rats produced effects that include the apparent modulation of an affective component of nociception (LaBuda and Usdin, 2004) and the regulation of the release of pituitary hormones (Ward et al., 2001; Sugimura et al., 2003; Usdin et al., 2003), as well as anxiolytic- and antidepressant-like effects (LaBuda et al., 2004).
The expression and distribution of TIP39 in adult rodents have been investigated in detail (Dobolyi et al., 2003b). Reverse-transcription PCR showed relatively strong expression of TIP39 in the testis and the brain (Dobolyi et al., 2002). Within the brain, cells expressing TIP39 mRNA were concentrated in only two regions, the subparafascicular area of the thalamus, and the medial paralemniscal nucleus in the lateral pons (Dobolyi et al., 2003b). The distribution of TIP39-immunoreactive cell bodies was the same as that of TIP39 mRNA expressing cells (Dobolyi et al., 2003b). While the TIP39 neurons in the medial paralemniscal nucleus form a compact cluster, the topography of the subparafascicular TIP39 neurons is more complex. The vast majority of subparafascicular TIP39 neurons were located medially between the midline and the fasciculus retroflexus in and around the magnocellular subparafascicular nucleus. However, a few TIP39 neurons were located caudolaterally above the medial lemniscus in the parvicellular (lateral) subparafascicular nucleus extending through the posterior intralaminar thalamic nucleus as far lateral as the area ventromedial to the medial geniculate body (Dobolyi et al., 2003b) in the posterior intralaminar complex of the thalamus as defined previously (Ledoux et al., 1987). The medial part of this area demonstrates Fos expression following male ejaculation (Coolen et al., 2003; Coolen et al., 2004) while the lateral part, which contains the CGRP neurons of the thalamus does not (Coolen et al., 2003; D’Hanis et al., 2007) supporting the compartmentalization of the area (Coolen et al., 2003). However, the small number of labeled TIP39 neurons in the posterior intralaminar complex of the thalamus of the adult rat did not allow the comparison of the topographical distribution of TIP39 and CGRP neurons in the area (Dobolyi et al., 2005). Rather, the small number of these laterally positioned TIP39 neurons of the adult rat have been described as a caudolateral extension of the medial subparafascicular cell group (Usdin et al., 2003; Wang et al., 2006b) even though the projections of these cells are somewhat different from those of the major TIP39 cell group located medially in and around the magnocellular subparafascicular nucleus (Dobolyi et al., 2003a). Based on lesion studies, TIP39 neurons project to limbic, endocrine, and auditory brain regions, with more pronounced hypothalamic projections by laterally positioned TIP39 neurons (Dobolyi et al., 2003a).
Investigation of the postnatal development of TIP39 provided important data on the ontogeny of TIP39 neurons (Dobolyi et al., 2006b), which provides a foundation for experiments on its function in reproduction. TIP39 was present in newborn rats in the subparafascicular area and medial paralemniscal nucleus. A significant increase was found in the mRNA and protein levels of TIP39 between postnatal days (PND)-1 and 14 both in the subparafascicular area and the medial paralemniscal nucleus (Dobolyi et al., 2006b). In addition, a surprising finding was the dramatic decrease in the level of TIP39 between postnatal days 33 and 125, in both TIP39-expressing brain regions. The remaining TIP39 mRNA and protein levels were somewhat greater in females although they were near the limits of detection in both genders (Dobolyi et al., 2006b). The transient expression of TIP39 in subparafascicular and medial paralemniscal neurons during postnatal development suggest temporal specific functions of TIP39 in these neurons. In addition, it suggests that there may be a different pattern of expression of TIP39 during embryonic development. In order to fully describe the ontogenic development of TIP39 in the rat brain, we investigated the expression and distribution of TIP39 during embryonic and early postnatal development. Specifically, we investigated 1, the appearance of TIP39-immunoreactivity in the subparafascicular area and the medial paralemniscal nucleus; 2, the early development of subparafascicular vs. posterior intralaminar TIP39 neurons; 3, the topographical relationship of posterior intralaminar TIP39 neurons to those expressing CGRP in the area; 4, the embryonic expression of TIP39 in additional brain regions, which do not contain TIP39 in the adult brain. TIP39-immunoreactivity was mapped by immunocytochemistry at embryonic days (ED)-14.5, 16.5, 18.5, 20.5, and postnatal days (PND)-1 and 5. The specificity of TIP39-immunoreactivity in brain areas not previously known to express TIP39 was confirmed by the detection of TIP39 mRNA with in situ hybridization histochemistry.
Materials and methods
Animals
All procedures involving rats were carried out according to experimental protocols approved by the Animal Examination Ethical Council of the Animal Protection Advisory Board at the Semmelweis University, Budapest and were conducted in accordance with international standards on animal welfare as defined by the European Communities Council Directive of 24 November 1986 (86/609/EE) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Four adult male (300–350 g in body weight) and 12 female Wistar rats (250–290 g) were purchased from Charles Rivers Laboratories. Rats were housed in plastic cages and kept on a 12:12 light-dark cycle at a temperature of 22 ± 1 °C throughout the study. Food and water were freely available at all times and adequate measures were taken to minimize the pain and discomfort of the animals.
At least one week after the arrival of the rats in the animal facility, a male rat was placed at 6 pm in a cage housing 3 female rats. Vaginal smears were taken from female rats at 8 am the following morning. Female rats with sperm-containing vaginal smears were housed individually and the date recorded as the beginning of pregnancy (1 am was considered as zero hour). This procedure was repeated until all females were separated.
Tissue collection
Pregnant rats were deeply anesthetized with an intramuscular injection (0.3 ml/300 g body weight) of an anesthetic mix containing ketamine (60 mg/ml) and xylazine (8 mg/ml) on day 14, 16, 18, and 20 days of pregnancy. Embryos (embryonic day (ED)-14.5, 16.5, 18.5 and 20.5) were taken by Cesarean section at 1–2 pm from 2 rats per age group. The sizes of the embryos were measured to double-check their age. Embryos were immersion fixed in 4% paraformaldehyde for 3 days, and then their brains were removed. Newborn (postnatal day (PND)-1) and PND-5 rats, 5 males and 5 females per age group, were deeply anesthetized at 1–2 pm and their brains were removed.
TIP39 immunocytochemistry
For immunocytochemistry, the brains were fixed in 4% paraformaldehyde for 3 days, stored in sodium-azide-containing phosphate buffer (PB) at 4 °C, and transferred PB to containing 30% sucrose for 2 days before sectioning. Serial coronal sections were cut at 20 μm on cryostat (Leica CM3050S) from the olfactory bulb to the spinal cord, collected on positively charged slides (Superfrost Plus, Fisher Scientific, Pittsburgh, PA), and stored at 4 °C until further processing.
Sections were immunolabeled for TIP39 as described previously using an affinity-purified rabbit polyclonal antibody to rat TIP39, which can be absorbed with synthetic TIP39 (Dobolyi et al., 2002; Dobolyi et al., 2003a). Briefly, brain sections were pretreated in PB containing 0.5% Triton X-100 and 3% bovine serum albumin for 1 hour. Then they were incubated with a primary antibody against TIP39 (1:3000) in PB containing 3% bovine serum albumin for 48 hours at room temperature. Sections were then incubated in biotin-conjugated donkey anti-rabbit secondary IgG at 1:600 (Jackson Immunoresearch, West Grove, PA) for 1 hour, followed by incubation in avidin-biotin-horseradish peroxidase complex (ABC) at 1:500 (Vectastain ABC Elite kit, Vector Laboratories, Burlingame, CA) for 2 hours. Then TIP39-immunoreactive cell bodies and fibers in most sections were visualized by incubation in 0.02% 3,3-diaminobenzidine (DAB; Sigma), 0.08% nickel (II) sulfate and 0.0012% hydrogen peroxide in Tris hydrochloride buffer (0.1 M; pH 8.0) for 10 minutes. Sections were mounted and some sections were counterstained with nuclear red (Vector Laboratories). Finally, the sections were dehydrated and coverslipped with Cytoseal 60 (Stephens Scientific, Riverdale, NJ). Alternatively, in some other sections, TIP39-immunoreactive cell bodies and fibers were visualized using fluorescein isothiocyanate (FITC)-tyramide amplification immunofluorescence as described previously (Hunyady et al., 1996). Following incubation in ABC, the sections were treated with FITC-tyramide (1:10,000) and 0.001% hydrogen peroxide in Tris hydrochloride buffer (0.1 M; pH 8.0) for 6 minutes. After washes, the sections were coverslipped with antifade medium (Prolong Antifade Kit; Molecular Probes, Eugene, OR).
Double fluorescent labeling of TIP39 and CGRP
First, immunolabeling for TIP39 was performed as described above for single TIP39 immunostaining except that visualization was always achieved with FITC-tyramide amplification. Subsequently, the sections were incubated in goat anti-rat CGRP (1:500 dilution; Biogenesis, Kingston, NH) for 2 days at room temperature followed by Alexa Fluor 594 donkey anti-goat secondary antibody (1:400 dilution; Molecular Probes) for 2 hours. After washes, the sections were coverslipped with antifade medium (Prolong Antifade Kit; Molecular Probes).
In situ hybridization histochemistry
Brains of 2 male and 2 female rats per age group were processed for in situ hybridization histochemistry at PND-1 and -5. After dissection, brains were immediately frozen on dry ice and stored at −80 °C until further processing. Serial coronal sections were cut at 12 μm with a cryostat (Leica CM3050S) from the olfactory bulb to the spinal cord, collected on positively charged slides (Superfrost Plus, Fisher Scientific), dried, and stored at −80 °C until further processing. In situ hybridization protocols are described in detail at http://intramural.nimh.nih.gov/lcmr/snge/Protocols/ISHH/ISHH.html. 35S-UTP-labeled riboprobes were generated using a MAXIscript transcription kit (Ambion, Austin, TX) from PCR-amplified fragments of the TIP39 cDNA subcloned into the vector pBluescript (Stratagene, La Jolla, CA). Antisense or sense (control) riboprobes were prepared using T7 or T3 RNA polymerase, respectively. A region of the rat TIP39 cDNA sequence corresponding to amino acids −55 to 37, where amino acid 1 is the first residue of mature TIP39, was used to generate probes. After hybridization and washes, slides were dipped in NTB nuclear track emulsion (Eastman Kodak) and stored at 4°C for 3 weeks. Then, the slides were developed and fixed with Kodak Dektol developer and Kodak fixer, respectively, dried, counterstained with Giemsa, and coverslipped with Cytoseal 60 (Stephens Scientific).
Data analysis
Sections were examined using an Olympus BX60 light microscope also equipped with fluorescent epi-illumination and dark-field condensor. Images were captured at 2048 X 2048 pixel resolution with a SPOT Xplorer digital CCD camera (Diagnostic Instruments, Sterling Heights, MI) using 4–40 X objectives.
Contrast and sharpness of the images were adjusted using the “levels” and “sharpness” commands in Adobe Photoshop CS 8.0. Full resolution was maintained until the photomicrographs cropped and assembled for printing, at which point images were adjusted to a resolution of 300 dpi. Drawings were prepared by aligning the pictures with corresponding schematics adapted from the developing rat brain atlas of Paxinos et al. (Paxinos et al., 1991).
Results
TIP39 in the subparafascicular area
TIP39 immunoreactive neurons are not detected in the subparafascicular area during embryonic development except for a few faintly immunolabeled cells at ED-20.5 (Fig. 1A). However, a number of TIP39-ir neurons appear at PND-1 between the 3rd ventricle and the fasciculus retroflexus. The intensity of immunolabeling and also the number of TIP39-ir neurons increases by PND-5 (Fig. 1B) and no gender difference was observed. The position and distribution of TIP39 neurons in the subparafascicular area during early postnatal development is similar to that previously reported in adult rats.
Fig. 1.
TIP39 neurons in the subparafascicular area. A: Only a few TIP39-ir neurons are faintly labeled in the subparafascicular area (SPF) between the third ventricle (3V) and the fasciculus retroflexus (fr) at ED-20.5. B: TIP39-ir neurons are distinctly labeled in the subparafascicular area at PND-5. C: A drawing of a coronal brain section (Paxinos et al., 1991) indicates the position of the SPF at PND-1. The framed area corresponds to panels A and B. Additional abbreviations: DM – dorsomedial hypothalamic nucleus, f - fornix, ml – medial lemniscus, PH –posterior hypothalamic nucleus, VPM – ventral posteromedial thalamic nucleus. Scale bars = 250 μm for A and 300 μm for B.
TIP39 in the posterior intralaminar thalamic nucleus
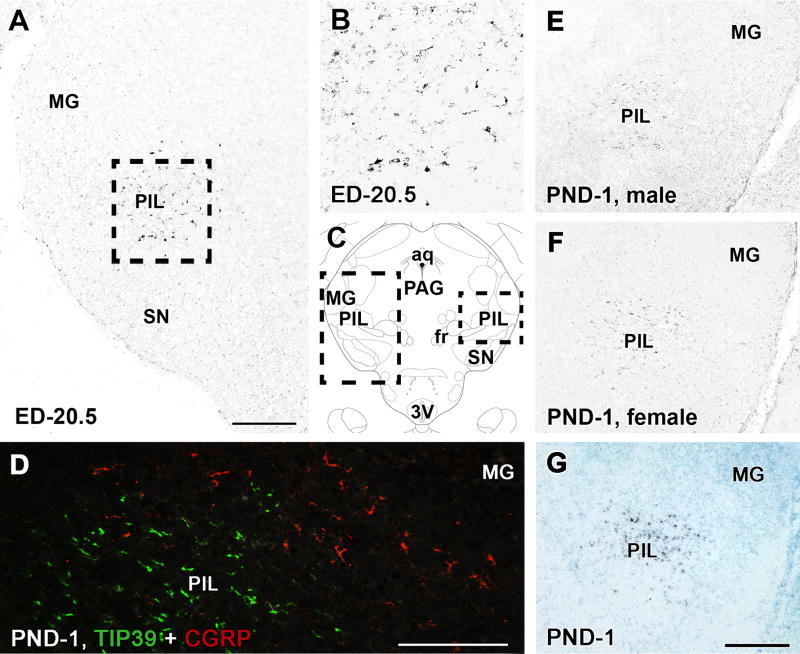
A few TIP39-ir neurons are present in the presumptive posterior intralaminar thalamic nucleus at ED-14.5 (Fig. 2A). The number of TIP39-ir neurons increases markedly by ED-16.5 (Fig. 2B). At this age, TIP39-ir neurons occupy a large area in and around the presumptive posterior intralaminar thalamic nucleus. A few cells are also seen more rostromedially, in the area of the presumptive parvicellular (lateral) subparafascicular nucleus and caudolaterally in the area ventromedial to the medial geniculate body thereby occupying large parts of the posterior intralaminar complex of the thalamus. However, TIP39-ir cell bodies are not distributed evenly in all parts of the posterior intralaminar complex of the thalamus because they are located medially to CGRP neurons (Fig. 3D), which are known to be localized throughout the lateral part of the posterior intralaminar complex of the thalamus (Coolen et al., 2003; Dobolyi et al., 2005). Between ED-16.5 and PND-5, a gradual decrease in the number of posterior intralaminar TIP39-ir neurons as well as in the intensity of immunolabeling in the area occurs. At PND-1, and even at PND-5, TIP39 immunoreactivity is still visible in the posterior intralaminar thalamic nucleus (Fig. 3) although the intensity of the labeling is significantly decreased as compared to that at ED-16.5 and ED-18.5. The distribution of TIP39 neurons and the intensity of their immunolabeling is the same in male (Fig. 3E) and female rats (Fig. 3F). The distribution of mRNA of TIP39 examined at PND-1 (Fig. 3G) is the same as that of TIP39-ir cell bodies (Fig. 3E,F) suggesting specific immunolabeling of TIP39 in posterior intralaminar neurons.
Fig. 2.
TIP39 neurons in the posterior intralaminar thalamic and the medial paralemniscal nuclei between ED-14.5 and ED-18.5. A: At ED-14.5, a few TIP39-ir neurons are visible in the brain areas where the posterior intralaminar thalamic (PIL) and the medial paralemniscal (MPL) nuclei will be formed. B: The number of TIP39-ir neurons increases markedly by ED-16.5 in both brain regions. The density of TIP39-ir neurons and the intensity of immunolabeling are similar in the two brain regions at this age. C: TIP39-ir cells are present in both brain regions at ED-18.5. D: A high magnification photomicrograph of the area framed in panel C around the posterior intralaminar thalamic nucleus. E: A high magnification photomicrograph of the area framed in panel C around the medial paralemniscal nucleus. F: A drawing of a coronal brain section at ED-16.5 (Paxinos et al., 1991). The framed area corresponds to panels A, B, and C. G: The position of the plane of the coronal sections in the other panels (Altman and Bayer, 1995). The curvature of the embryonic brain causes the posterior intralaminar thalamic nucleus and the medial paralemniscal nuclei appear on the same section until ED-18.5. Additional abbreviations: CG –periaqueductal central gray, MG – medial geniculate body, M5 – motor trigeminal nucleus, PnO – oral part of the pontine reticular formation, R – red nucleus, VLL – ventral nucleus of the lateral lemniscus, 3V - third ventricle, 4V - fourth ventricle, 7 – motor facial nucleus. Scale bars = 300 μm for A, B, and C, and 50 μm for D and E.
Fig. 3.
TIP39 neurons in the posterior intralaminar thalamic nucleus at ED-20.5 and PND-1. A: A coronal brain section immunolabeled with TIP39 at ED-20.5. Relatively faintly labeled TIP39-ir neurons are visible in and around the posterior intralaminar thalamic nucleus (PIL). B: A high magnification photomicrograph of the area framed in panel A shows TIP39-ir cell bodies in the PIL. C: A drawing of a coronal brain section at PND-1 (Paxinos et al., 1991). The framed area on the left corresponds to panels A while the framed area on the right corresponds to panels E, F and G. D: A photomicrograph of a double fluorescent labeled coronal section demonstrates that TIP39 neurons (green) are located medial to CGRP neurons (red) in the posterior intralaminar complex of the rat at PND-1. E: A coronal brain section of a male rat immunolabeled with TIP39 at PND-1. Faintly labeled TIP39-ir neurons are visible in the posterior intralaminar thalamic nucleus. F: A coronal brain section of a female rat immunolabeled with TIP39 at PND-1. The number of TIP39-ir neurons and the intensity of immunolabaling in the posterior intralaminar thalamic nucleus is similar to that in male. G: A coronal brain section of a female rat labeled with in situ hybridization histochemistry for TIP39 (black dots) demonstrates that the distribution of TIP39 mRNA in the posterior intralaminar thalamic nucleus is the same as that of TIP39-ir cell bodies at PND-1. Additional abbreviations: aq – cerebral aqueduct, fr – fasciculus retroflexus, MG – medial geniculate body, PAG – periaqueductal gray, SN – substantia nigra, 3V - third ventricle. Scale bars = 300 μm for A and G, and 200 μm for D.
TIP39 neurons in the posterior intralaminar thalamic nucleus elaborate TIP39-ir fibers at ED16.5. The intensity of TIP39 immunoreactivty in these fibers increases by ED-18.5. By ED-20.5, the intensity of TIP39 immunolabeling decreases in the fibers, and they are no longer detectable postnatally. At ED-18.5, the TIP39-ir fibers can be followed in serial coronal sections rostral to the posterior intralaminar thalamic nucleus (Fig. 4). They project rostrally in the zona incerta, then turn ventrally and join the supraoptic decussations. Here, TIP39-ir varicosities can also be observed on the fibers (Fig. 4C, D). Some TIP39-ir fibers turn dorsally and terminate in the lateral hypothalamic area while others continue to course rostrally and medially along the supraoptic decussations. A few TIP39-ir fibers can be observed above the optic chiasm as they cross the midline (Fig. 4D). During embryonic development, we observed no other TIP39-ir fibers in the rat brain.
Fig. 4.

TIP39-ir fibers project from the posterior intralaminar thalamic nucleus to the hypothalamus at ED-18.5. Coronal brain sections from the same animal are approximately 300–400 μm from each other. A: TIP39-ir cell bodies are shown in the posterior intralaminar thalamic nucleus (PIL). B: Cross-sectioned fibers are visible in the zona incerta (ZI) approximately 300–400 μm rostral to the plane of panel A. C: A further rostral section shows varicose TIP39-ir fibers and fiber terminals in the supraoptic decussations and dorsal to it in the hypothalamus. D: Fibers can be followed even more medially and rostrally as they cross the midline over the optic chiasm. Additional abbreviations: cp – cerebral peduncle, LH – lateral hypothalamic area, MG –medial geniculate body, ml – medial lemniscus, ot – optic tract, ox – optic chiasm, 3V - third ventricle. Scale bars = 500 μm for A, 400 μm for B, and 200 μm for C and D.
TIP39 in the medial paralemniscal nucleus
TIP39-ir cell bodies in the medial paralemniscal nucleus appear at ED-14.5 immediately medial to the presumptive ventral nucleus of the lateral lemniscus (Fig. 2A). The number of TIP39-ir neurons as well as the intensity of their labeling increases thereafter. By PND-1, the distribution of medial paralemniscal TIP39-ir neurons is the same as that in adult and shows no gender difference. The TIP39-ir neurons occupy an area immediately medial to the ventral nucleus of the lemniscus lateralis and dorsal to the rubrospinal tract. At PND-5, TIP39-ir fibers can be observed in the medial paralemniscal nucleus as they leave TIP39-ir cell bodies and project dorsally and ventrally (Fig. 5).
Fig. 5.
TIP39 neurons in the medial paralemniscal nucleus at ED-20.5 and PND-5. A: A coronal brain section shows TIP39-ir neurons in the medial paralemniscal nucleus (MPL) at ED-20.5. B: A high magnification photomicrograph of the area framed in panel A around the medial paralemniscal nucleus shows TIP39-ir cell bodies. C: A coronal brain section immunolabeled with TIP39 at PND-5. The medial paralemniscal nucleus situated between the oral part of the pontine reticular formation (PnO) and the ventral nucleus of the lateral lemniscus (VLL) contains intensely labeled TIP39 neurons. D: A drawing of a coronal brain section at PND-1 (Paxinos et al., 1991) that corresponds to panels A and C. Additional abbreviations: PAG – periaqueductal gray, py – pyramidal tract, rs – rubrospinal tract, SC – superior colliculus, 5n – root of the trigeminal nerve. Scale bars = 1 mm for A, 100 μm for B, and 500 μm for C.
TIP39 in the amygdalo-hippocampal transitional zone
A group of TIP39-ir neurons appeared in the amygdala at ED-16.5 (Fig. 6). These neurons were located in the anterolateral subdivision of the amygdalo-hippocampal transitional zone dorsal to the posterior part of the cortical amygdaloid nucleus and lateral to the posterior part of the medial amygdaloid nucleus (Fig. 6) with some cells located in the adjacent posterior subdivision of the basomedial amygdaloid nucleus. The intensity of TIP39 immunolabeling decreased from ED-16.5. By PND-1, only faintly labeled TIP39-ir neurons were visible in the amygdalo-hippocampal transitional zone in both genders. These TIP39-ir neurons have not been described previously. Therefore, non-specific labeling was a possibility. The specificity of immunolabeling by the antiserum we used has been evaluated by comparing the location of TIP39-ir neurons to that of TIP39-mRNA expressing neurons in the subparafascicular area, the posterior intralaminar thalamic nucleus and the medial paralemniscal nucleus. We performed the comparison in the amygdalo-hippocampal transitional zone at PND-1. Immunolabeling was performed using amplification immunofluorescence to reliably label TIP39-ir cell bodies at PND-1 (Fig. 7A, B). Radioactive in situ hybridization histochemistry detected TIP39 mRNA at the same position as TIP39-ir cell bodies in the anterolateral subdivision of the amygdalo-hippocampal transitional zone (Fig. 7C, D).
Fig. 6.
TIP39 neurons in the amygdalo-hippocampal transitional zone at ED-16.5 and ED-18.5. A: A coronal brain section shows TIP39-ir neurons in the amygdalo-hippocampal transitional zone (AHi) at ED-16.5. B: A high magnification photomicrograph of the area framed in panel A shows TIP39-ir cell bodies in the AHi. C: A coronal brain section shows TIP39-ir neurons in the amygdalo-hippocampal transitional zone (AHi) at ED-18.5. D: A drawing of a coronal brain section at PND-1 (Paxinos et al., 1991). The framed area corresponds to panels A and C. Additional abbreviations: BL – basolateral amygdaloid nucleus, CoA – cortical amygdaloid nucleus, LV – lateral ventricle, MD – mediodorsal thalamic nucleus, MeA – medial amygdaloid nucleus, ot – optic tract, VMH – ventromedial hypothalamic nucleus, VPL – ventral posterolateral thalamic nucleus. Scale bars = 200 μm for A, 50 μm for B, and 200 μm for C.
Fig. 7.
TIP39-ir cell bodies and TIP39 mRNA-expressing neurons in the amygdalo-hippocampal transitional zone at PND-1. A: A coronal brain section immunolabaled for TIP39 with fluorescent amplification shows the position of TIP39-ir neurons in the amygdalo-hippocampal transitional zone (AHi). B: A high magnification photomicrograph of the area framed in panel A shows TIP39-ir cell bodies in the AHi. C: A dark-field photomicrograph of a coronal brain section demonstrates TIP39 mRNA expression in the amygdalo-hippocampal transitional zone (AHi). The field corresponds to that in panel A. TIP39 mRNA-expressing neurons have the same location as TIP39-ir neurons in panel A. D: A high magnification bright-field photomicrograph of the area framed in panel C shows autoradiographic grains above cell bodies demonstrating TIP39 mRNA expressing neurons in the AHi. Additional abbreviations: CoA – cortical amygdaloid nucleus, MeA – medial amygdaloid nucleus. Scale bars = 300 μm for A and C, 30 μm for B, and 50 μm for D.
Discussion
This study follows a previous study that described a dramatic change in the expression of TIP39 during postnatal development (Dobolyi et al., 2006b). Therefore, we relate the new data to previously published data on the postnatal development of TIP39. Next, we discuss the expression of TIP39 in neurons, which do not express TIP39 postnatally. Finally, we speculate on the possible functions of TIP39 during brain development.
Correspondence with postnatal development of TIP39
The two major sites of TIP39 expression in adult rat are the subparafascicular area and the medial paralamniscal nucleus (Dobolyi et al., 2003b). The amount of TIP39 mRNA and the intensity of TIP39 immunoreactivity increased similarly in both regions from PND-1 to PND-14 (Dobolyi et al., 2006b). Since the degree and the time course of decline was also similar in these two regions, the same regulation of TIP39 expression was previously suggested (Dobolyi et al., 2006b). In contrast, we found marked differences in the expression of TIP39 between the subparafascicular area and the medial paralemniscal nucleus during embryonic development. While TIP39 is not expressed in the subparafascicular area until ED-20.5, TIP39 appears in the presumptive medial paralemniscal nucleus as early as ED-14.5 and TIP39 maintains a significant level of expression here continuously thereafter. The dramatically different developmental stages of the appearance of TIP39 suggest different regulation of its expression within the subparafascicular area and the medial paralemniscal nucleus, which could imply its involvement in different types of brain function.
The relatively few TIP39-expressing neurons in the parvicellular (lateral) subparafascicular nucleus and the posterior intralaminar thalamic nucleus in the adult and young rats was previously considered a lateral extension of the subparafascicular area (Usdin et al., 2003; Dobolyi et al., 2006b; Wang et al., 2006b) because the density of TIP39-expressing neurons was higher medially around the magnocellular subparafascicular nucleus and because some TIP39 neurons were continuously distributed laterally in the direction of the posterior intralaminar thalamic nucleus. The significant expression of TIP39 in and around the posterior intralaminar thalamic nucleus during embryonic development changes this view. Since the time course of expression of TIP39 is dramatically different in the subparafascicular area and the posterior intralaminar thalamic nucleus, it is likely that they represent two independent groups of TIP39 neurons. This separation is consistent with a study of TIP39 neuronal projections that shows predominantly hypothalamic projections of posterior intralaminar TIP39 neurons as opposed to projections to hypothalamic but also to septal and limbic cortical regions from the subparafascicular area (Dobolyi et al., 2003a).
Transient expression of TIP39 during embryonic brain development
There are two groups of TIP39-ir neurons that are visible as early as ED-16.5 in the embryonic rat brain and then disappear during early postnatal development. The first group of neurons showing such transient TIP39 expression is located in the amygdala. Based on the distribution of the 20–30 TIP39-ir neurons per section immediately dorsal to the cortical amygdaloid nucleus and lateral to the medial amygdaloid nucleus, we identified their location as the anterolateral subdivision of the amygdalo-hippocampal transitional zone with some cells located in the posterior subdivision of the basomedial amygdaloid nucleus. The present study is the first identification of these TIP39-expressing neurons, as they were not apparent in previous postnatal studies. The posterior part of the amygdala is a relatively little studied brain region. Based on the expression of steroid receptors within it and its neuronal connections (Canteras et al., 1992), the amygdalo-hippocampal transitional zone has been suggested to play a role in conveying hormonal information toward reproductive brain centers (Simerly, 2002).
The second group of neurons expressing TIP39 transiently during embryonic development is located in and around the posterior intralaminar thalamic nucleus. Although TIP39-ir neurons are present in the postnatal and even in the adult posterior intralaminar thalamic nucleus, the number of labeled neurons is considerably higher during embryonic development. These TIP39-ir neurons are located medial to CGRP neurons, which define the lateral subdivision of the posterior intralaminar complex of the thalamus. Therefore, TIP39-ir neurons can be localized in the medial subdivision of the posterior intralaminar complex of the thalamus. In addition, TIP39-ir fibers can be traced from the posterior intralaminar thalamic nucleus towards the hypothalamus. The presence of these projections supports the functional significance of TIP39 expression in the posterior intralaminar thalamic nucleus during development. However, the TIP39-ir fibers also disappear by PND-1, well before the adult patterns of TIP39-ir fibers emerges by PND-14. Because we visualized these fibers only by TIP39 immunolabeling, it is not possible to tell from our data if the fibers themselves degenerate or only their TIP39 content disappears. Similarly, the disappearance of TIP-ir cell bodies only means that TIP39 is not detectable in them, and these data provide no information regarding whether TIP39 neurons degenerate during development or whether their TIP39 expression decreases. We have not yet identified any independent markers for these neurons. An additional possibility is that the position of the cell body changes with migration. For example, on the basis of our data, we cannot exclude the possibility that some TIP39-ir neurons that differentiate in and around the posterior intralaminar thalamic nucleus during embryonic development subsequently migrate medially to the subparafascicular area during early postnatal development.
Potential functions of TIP39 during development
Previous studies suggest that TIP39 neurons might be involved in nociceptive information processing (Dobolyi et al., 2002; LaBuda and Usdin, 2004), auditory functions (Palkovits et al., 2004), emotional changes (LaBuda et al., 2004), and endocrine regulation (Ward et al., 2001; Sugimura et al., 2003; Usdin et al., 2003). Areas that contain TIP39 neurons were suggested to be involved in arousal (Schiff et al., 2007) as well as sexual and maternal functions. In the subparafascicular nucleus, Fos activation has been reported in lactating female rats (Lin et al., 1998). More laterally, in the posterior intralaminar complex of the thalamus, Fos activation has been reported following male ejaculation in rat (Coolen et al., 1997; Coolen et al., 2004; Wang et al., 2006a) and the mating-activated cells projected to other regions that show Fos expression with ejaculation (Heeb and Yahr, 2001). In addition, an increase in regional cerebral blood flow during ejaculation has been reported in the subparafascicular area in human (Holstege et al., 2003). Furthermore, the neuronal connections of the amygdalo-hippocampal transitional zone (Canteras et al., 1992) and its sexual steroid receptor content lead to the suggestion that the amygdalo-hippocampal transitional zone regulates sexual behaviors (Simerly, 2002). In an additional region of TIP39 expression, the medial paralemniscal nucleus, induction of Fos expression has been reported in postpartum female rats in response to suckling stimulus (Li et al., 1999). Combined, these data suggest that TIP39 might have a role in central regulation of reproduction-associated functions. Bearing in mind that a TIP39 homologue, parathyroid hormone-related peptide, has well-established effects on the development of many tissues (Kronenberg et al., 1998) and that TIP39 can affect the proliferation of some cell types in vitro (Misiano et al., 2003), the transient expression of TIP39 during early development suggests that this peptide may be involved in developmental aspects of the above mentioned brain functions. Furthermore, it will be interesting to examine the re-appearance of TIP39 in adult related to the above mentioned functions, including sexual activity, pregnancy and lactation, in neurons of the posterior intralaminar thalamic nucleus and the amygdalo-hippocampal transitional zone.
In conclusion, we described a new TIP39-expressing cell group in the amygdalo-hippocampal transitional zone, we provided evidence that subparafascicular and posterior intralaminar thalamic TIP39 neurons form separate cell groups, and we identified 3 different temporal sequences of TIP39 expression during ontogeny in the rat brain: 1, early postnatal appearance and late postnatal disappearance of TIP39 characteristic of subparafascicular TIP39 neurons; 2, early embryonic appearance and late postnatal disappearance of TIP39 characteristic of medial paralemniscal and some posterior intralaminar thalamic TIP39 neurons; 3, early embryonic appearance and early postnatal disappearance of TIP39 characteristic of all TIP39 neurons in the amygdalo-hippocampal transitional zone and most TIP39 neurons in the posterior intralaminar complex of the thalamus. By revealing the temporal patterns of TIP39 expressions in different brain areas, our data provide a basis for investigating the specific functions of TIP39 during ontogeny. In particular, transient expressions of TIP39 during development suggest age-specific functions for TIP39.
Acknowledgments
We appreciate the technical assistance of Erzsébet Tárnokné Vörös and Frigyesné Helfferich. Support was provided by the Hungarian Science Foundation (OTKA K67646). Arpád Dobolyi is a grantee of the Bolyai János Scholarship.
Support was provided by the Hungarian Science Foundation (OTKA K67646). Arpád Dobolyi is a grantee of the Bolyai János Scholarship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman J, Bayer SA. Atlas of prenatal rat brain development. CRC Press; London: 1995. [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Connections of the posterior nucleus of the amygdala. J Comp Neurol. 1992;324:143–179. doi: 10.1002/cne.903240203. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Peters HJ, Veening JG. Distribution of Fos immunoreactivity following mating versus anogenital investigation in the male rat brain. Neuroscience. 1997;77:1151–1161. doi: 10.1016/s0306-4522(96)00542-8. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Veening JG, Petersen DW, Shipley MT. Parvocellular subparafascicular thalamic nucleus in the rat: anatomical and functional compartmentalization. J Comp Neurol. 2003;463:117–131. doi: 10.1002/cne.10740. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Allard J, Truitt WA, McKenna KE. Central regulation of ejaculation. Physiol Behav. 2004;83:203–215. doi: 10.1016/j.physbeh.2004.08.023. [DOI] [PubMed] [Google Scholar]
- D’Hanis W, Linke R, Yilmazer-Hanke DM. Topography of thalamic and parabrachial calcitonin gene-related peptide (CGRP) immunoreactive neurons projecting to subnuclei of the amygdala and extended amygdala. J Comp Neurol. 2007;505:268–291. doi: 10.1002/cne.21495. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Ueda H, Uchida H, Palkovits M, Usdin TB. Anatomical and physiological evidence for involvement of tuberoinfundibular peptide of 39 residues in nociception. Proc Natl Acad Sci USA. 2002;99:1651–1656. doi: 10.1073/pnas.042416199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobolyi A, Palkovits M, Bodnar I, Usdin TB. Neurons containing tuberoinfundibular peptide of 39 residues project to limbic, endocrine, auditory and spinal areas in rat. Neuroscience. 2003a;122:1093–1105. doi: 10.1016/j.neuroscience.2003.08.034. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Palkovits M, Usdin TB. Expression and distribution of tuberoinfundibular peptide of 39 residues in the rat central nervous system. J Comp Neurol. 2003b;455:547–566. doi: 10.1002/cne.10515. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Irwin S, Makara G, Usdin TB, Palkovits M. Calcitonin gene-related peptide-containing pathways in the rat forebrain. J Comp Neurol. 2005;489:92–119. doi: 10.1002/cne.20618. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Irwin S, Wang J, Usdin TB. The distribution and neurochemistry of the parathyroid hormone 2 receptor in the rat hypothalamus. Neurochem Res. 2006a;31:227–236. doi: 10.1007/s11064-005-9011-9. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Wang J, Irwin S, Usdin TB. Postnatal development and gender-dependent expression of TIP39 in the rat brain. J Comp Neurol. 2006b;498:375–389. doi: 10.1002/cne.21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber CA, Dobolyi A, Sleeman M, Usdin TB. Distribution of tuberoinfundibular peptide of 39 residues and its receptor, parathyroid hormone 2 receptor, in the mouse brain. J Comp Neurol. 2007;502:563–583. doi: 10.1002/cne.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensure RC, Gardella TJ, Juppner H. Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem Biophys Res Commun. 2005;328:666–678. doi: 10.1016/j.bbrc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- Heeb MM, Yahr P. Anatomical and functional connections among cell groups in the gerbil brain that are activated with ejaculation. J Comp Neurol. 2001;439:248–258. doi: 10.1002/cne.1346. [DOI] [PubMed] [Google Scholar]
- Hoare SR, Bonner TI, Usdin TB. Comparison of rat and human parathyroid hormone 2 (PTH2) receptor activation: PTH is a low potency partial agonist at the rat PTH2 receptor. Endocrinology. 1999;140:4419–4425. doi: 10.1210/endo.140.10.7040. [DOI] [PubMed] [Google Scholar]
- Holstege G, Georgiadis JR, Paans AM, Meiners LC, van der Graaf FH, Reinders AA. Brain activation during human male ejaculation. J Neurosci. 2003;23:9185–9193. doi: 10.1523/JNEUROSCI.23-27-09185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John MR, Arai M, Rubin DA, Jonsson KB, Juppner H. Identification and characterization of the murine and human gene encoding the tuberoinfundibular peptide of 39 residues. Endocrinology. 2002;143:1047–1057. doi: 10.1210/endo.143.3.8698. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM, Lanske B, Kovacs CS, Chung UI, Lee K, Segre GV, Schipani E, Juppner H. Functional analysis of the PTH/PTHrP network of ligands and receptors. Recent Prog Horm Res. 1998;53:283–301. [PubMed] [Google Scholar]
- LaBuda CJ, Dobolyi A, Usdin TB. Tuberoinfundibular peptide of 39 residues produces anxiolytic and antidepressant actions. Neuroreport. 2004;15:881–885. doi: 10.1097/00001756-200404090-00030. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Usdin TB. Tuberoinfundibular peptide of 39 residues decreases pain-related affective behavior. Neuroreport. 2004;15:1779–1782. doi: 10.1097/01.wnr.0000134849.63755.15. [DOI] [PubMed] [Google Scholar]
- Ledoux JE, Ruggiero DA, Forest R, Stornetta R, Reis DJ. Topographic organization of convergent projections to the thalamus from the inferior colliculus and spinal cord in the rat. J Comp Neurol. 1987;264:123–146. doi: 10.1002/cne.902640110. [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS. Neural populations in the rat forebrain and brainstem activated by the suckling stimulus as demonstrated by cFos expression. Neuroscience. 1999;94:117–129. doi: 10.1016/s0306-4522(99)00236-5. [DOI] [PubMed] [Google Scholar]
- Lin SH, Miyata S, Matsunaga W, Kawarabayashi T, Nakashima T, Kiyohara T. Metabolic mapping of the brain in pregnant, parturient and lactating rats using fos immunohistochemistry. Brain Res. 1998;787:226–236. doi: 10.1016/s0006-8993(97)01484-4. [DOI] [PubMed] [Google Scholar]
- Misiano P, Scott BB, Scheideler MA, Garnier M. PTH2 receptor-mediated inhibitory effect of parathyroid hormone and TIP39 on cell proliferation. Eur J Pharmacol. 2003;468:159–166. doi: 10.1016/s0014-2999(03)01673-x. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Dobolyi A, Helfferich F, Usdin TB. Localization and chemical characterization of the audiogenic stress pathway. Ann N Y Acad Sci. 2004;1018:16–24. doi: 10.1196/annals.1296.002. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Törk I, Tecott LH, Valentino KL. Atlas of the developing rat brain. Academic Press; San Diego: 1991. [Google Scholar]
- Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, Fritz B, Eisenberg B, Biondi T, O’Connor J, Kobylarz EJ, Farris S, Machado A, McCagg C, Plum F, Fins JJ, Rezai AR. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Murase T, Ishizaki S, Tachikawa K, Arima H, Miura Y, Usdin TB, Oiso Y. Centrally administered tuberoinfundibular peptide of 39 residues inhibits arginine vasopressin release in conscious rats. Endocrinology. 2003;144:2791–2796. doi: 10.1210/en.2002-0017. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Gruber C, Bonner TI. Identification and functional expression of a receptor selectively recognizing parathyroid hormone, the PTH2 receptor. J Biol Chem. 1995;270:15455–15458. doi: 10.1074/jbc.270.26.15455. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Hoare SR, Wang T, Mezey E, Kowalak JA. TIP39: a new neuropeptide and PTH2-receptor agonist from hypothalamus. Nat Neurosci. 1999;2:941–943. doi: 10.1038/14724. [DOI] [PubMed] [Google Scholar]
- Usdin TB. The PTH2 receptor and TIP39: a new peptide-receptor system. Trends Pharmacol Sci. 2000;21:128–130. doi: 10.1016/s0165-6147(00)01455-3. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Wang T, Hoare SR, Mezey E, Palkovits M. New members of the parathyroid hormone/parathyroid hormone receptor family: the parathyroid hormone 2 receptor and tuberoinfundibular peptide of 39 residues. Front Neuroendocrinol. 2000;21:349–383. doi: 10.1006/frne.2000.0203. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Dobolyi A, Ueda H, Palkovits M. Emerging functions for tuberoinfundibular peptide of 39 residues. Trends Endocrinol Metab. 2003;14:14–19. doi: 10.1016/s1043-2760(02)00002-4. [DOI] [PubMed] [Google Scholar]
- Wang J, Coolen LM, Brown JL, Usdin TB. Neurons containing tuberoinfundibular peptide of 39 residues are activated following male sexual behavior. Neuropeptides. 2006a;40:403–408. doi: 10.1016/j.npep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Wang J, Palkovits M, Usdin TB, Dobolyi A. Forebrain projections of tuberoinfundibular peptide of 39 residues (TIP39)-containing subparafascicular neurons. Neuroscience. 2006b;138:1245–1263. doi: 10.1016/j.neuroscience.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Ward HL, Small CJ, Murphy KG, Kennedy AR, Ghatei MA, Bloom SR. The actions of tuberoinfundibular peptide on the hypothalamo-pituitary axes. Endocrinology. 2001;142:3451–3456. doi: 10.1210/endo.142.8.8308. [DOI] [PubMed] [Google Scholar]