Abstract
Unlike other eukaryotes studied to date, yeast has two genes for pyruvate carboxylase coding for very similar, but not identical, isozymes (Pyc1 and Pyc2), both of which are located in the cytoplasm. We have found that there are marked differences in the kinetic properties of the isozymes potentially leading to differential regulation of Pyc1 and Pyc2 activity by both activators and substrates. For example, Pyc2 is only activated 3.7-fold by acetyl CoA, and 9.6 fold by NH4+, whilst the figures for Pyc1 are 16-fold and 14.6-fold respectively. Pyc1 and Pyc2 display different allosteric properties with respect to acetyl CoA activation and aspartate inhibition, with Pyc1 showing a higher degree of cooperativity than Pyc2, even in the absence of aspartate. We have investigated the locus of action in the amino acid sequence of the isozymes of this activator by measuring its regulation of various chimeric constructs of the two isozymes. In this way we conclude that the main locus of action of acetyl CoA lies in the N-terminal half of the enzyme, within the biotin carboxylation domain, between amino acids 99 and 478 of Pyc1.
Keywords: pyruvate carboxylase, isozyme, enzyme kinetics, regulation of enzyme activity, biotin, acetyl CoA activation, chimera
1. INTRODUCTION
Pyruvate carboxylase (EC 6.4.1.1) is a biotin-dependent enzyme that catalyses the carboxylation of pyruvate using bicarbonate as a substrate, with ATP cleavage used to drive the carboxylation of biotin by bicarbonate to form an enzyme-carboxybiotin intermediate which then carboxylates the pyruvate to form oxaloacetate (for reviews see Attwood, 1995; Jitrapakdee & Wallace 1999; Attwood & Wallace, 2002). The enzyme has an anaplerotic role in that it supplies oxaloacetate for gluconeogenesis and to replenish tricarboxylic acid cycle intermediates that have been removed for synthetic purposes. In vertebrates, the enzyme is primarily regulated by the allosteric activator, acetyl CoA, whereas in some microorganisms, acetyl CoA is not regulatory (e.g. Aspergillus niger) whilst in others there is a complete dependence on its presence (e.g. Bacillus thermodenitrificans) (Wallace, 1985). In many microorganisms, aspartate is a negative regulator of pyruvate carboxylase activity (Wallace, 1985). Monovalent cations such as K+ and NH4+ have also been shown to activate pyruvate carboxylase (Barden & Scrutton, 1975; McClure, Lardy & Kneifel, 1971), although the physiological relevance of this regulation is not clear.
In S. cerevisiae there are two isozymes of pyruvate carboxylase (Pyc1 and Pyc2), each encoded by a separate gene (Stucka, Dequin, Salmon & Gancedo, 1991; Walker, Val, Rohde, Devenish & Wallace, 1991). Both isozymes are expressed in the cytoplasm, unlike the pyruvate carboxylase of higher eukaryotes, where the enzyme is found in the mitochondrial matrix (Rhode, Lim & Wallace, 1991). The amino acid sequences of the two isozymes are 91% identical (Stucka, Dequin, Salmon & Gancedo, 1991; Val, Chapman-Smith, Walker, Cronan & Wallace, 1995). Fig. 1 shows the major domain structure of Pyc1 in relation to the amino acid sequence and shows that the most N-terminal of the domains is the ATP/HCO3-binding domain, where biotin carboxylation occurs. This is followed by the pyruvate-binding domain where oxaloacetate formation occurs and then the biotinyl domain that contains Lys1112 to which biotin is covalently bonded.
Fig. 1.
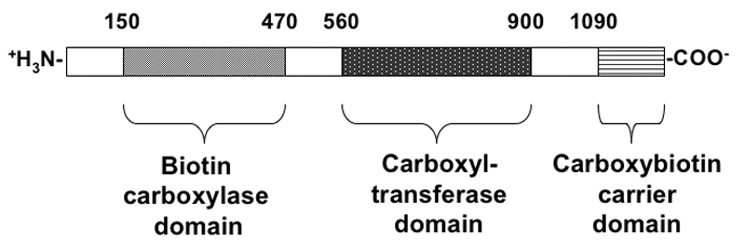
Schematic representation of the primary structure of yeast pyruvate carboxylase, showing regions of homology with other, related enzymes, based on the work of Lim, Morris, Occhiodoro & Wallace (1988).
The expression of the two isozymes is differentially regulated, depending on factors such as growth phase, carbon source and nitrogen source, especially ammonium ions (Brewster, Val, Walker & Wallace, 1994; Huet, Menendez, Gancedo & Francois, 2000). This differential regulation of gene expression and the co-localisation of Pyc1 and Pyc2 in the cytoplasm would suggest that Pyc1 and Pyc2 have different kinetic or regulatory properties. All of the early characterisation of pyruvate carboxylase from S. cerevisiae was performed on enzyme preparations from baker's yeast, which potentially contained a mixture of both Pyc1 and Pyc2. The kinetic parameters of Pyc1 in terms of substrate Kms have been recently determined (Branson, Nezic, Wallace & Attwood, 2002; Branson, Nezic, Jitrapakdee, Wallace & Attwood., 2004). However, the kinetics and regulation of purified Pyc2 have not been investigated.
In the current work we have measured the Kms for each of the substrates of Pyc2 and the dependence of its activity on acetyl CoA concentration, including the Ka. We compare the kinetic parameters of Pyc1 and Pyc2 and their regulation by acetyl CoA, NH4+ and aspartate. We discuss the differences in kinetic parameters and regulation of the two isozymes with respect to the differences in the regulation of their expression. In addition, we have investigated the locus of action of acetyl CoA in the amino acid sequences of the isozymes by examining the regulation of various chimeric constructs of Pyc1 and Pyc2.
2. MATERIALS AND METHODS
2.1 Chemicals and reagents
The sodium salt of acetyl CoA was purchased from Sigma and all other reagents were of analytical quality.
2.2 Yeast strains and constructs
S. cerevisiae DM18 (MATα, ura3, trp1, Δpyc1: LEU2, Δpyc2: HIS3) (Brewster, Val, Walker & Wallace, 1994) harboring pVT100-PYC1 (Branson, Nezic, Wallace & Attwood, 2002) was used as a source to prepare PYC1 isozyme.
The PYC2 construct was generated by PCR-amplification of PYC2 gene from S. cerevisiae W303 chromosomal DNA. The gene was cloned from two overlapping PCR fragments using two pairs of primer designed from the PYC2 gene sequence deposited in the Saccharomyces genome database (http://www.yeastgenome.org). [PYC2-F1 (5’cccaagcttaagtaaaatatgagcagtagcaag-3’, underline indicates HindIII restriction site, bold indicates an ATG initiation codon) and PYC2-R1 (5’-accaccggccttcttgacagcattc-3’) were synthesized to generate a 1.9 kb 5’-end of PYC2 gene while PYC2-F2 (5’-agtgctactggaaaagggaccatg -3’) and PYC2-R2 (5’-ccctctagattaacgagtaaaaattactttttttggggatgg -3’; underline indicates XbaI restriction site) were synthesized to generate a 1.8 kb 3’-end of PYC2 gene. These two fragments were sequenced and assembled by digestion with HindIII/BamHI and with BamHI/XbaI respectively, and cloned into HindIII/XbaI-digested pVT100u (Vernet, Dignard & Thomas, 1987), producing pVT100-PYC2 construct. Pyc2 construct with N-terminal 9His tag was constructed by PCR-amplifying a 0.8 kb 5’-end of Pyc1 gene using 9His F primer (5’-gcccctcgagatgcaccaccaccatcatcaccaccatcacagcagtagcaagaaattggccggtctt-3’, bold indicates codons for 9His) and Pyc2-R primer (5’-atagcgtcacgaacttcacgg-3’). This PCR product was cloned into pDrive cloning system (Qiagen) and sequenced. The insert was excised from pDrive vector by digesting with XhoI, located upstream of an initiation codon and an AccI, which located in the middle of PCR product, and replaced the corresponding fragment in pVT100-PYC2. Pyc1 construct with N-terminal 9His was generated by PCR-amplifying a 0.9 kb 5’-end of Pyc2 gene using 5’-His Pyc1 primer (5’ gccctcgagtaaaaataatgcatcatcatcaccaccatcatcatggctcgcaaagaaaattcgccggcttg-3’; bold indicates codons for 9His) and PYC1-R (5’-tctgtgatggtatgttccacttgg-3’) using pV100-PYC1 as template. This PCR product was cloned into pDrive cloning system and sequenced. Finally, the insert was excised from pDrive by digesting with XhoI, locating at the most 5’-end of initiation codon, and EcoRI, locating in the middle of the PCR product, and replaced the corresponding fragment in pVT100-PYC1.
Six Pyc1/Pyc2 chimeras were generated as follows: Chimera 1 containing the N-terminal 616 residues of Pyc1 and the C-terminal 562 residues of Pyc2 and chimera 2 containing the N-terminal 617 residues of Pyc2 and the C-terminal 562 residues of Pyc1 were generated by utilizing a unique BamHI site presence between codons 616 and 617 of Pyc1, and between codons 617 and 618 of Pyc2 genes, allowing these two fragments to be swapped in frame. These two chimeras were constructed by digesting Pyc1 and Pyc2 genes with XhoI, locating at the most 5’-end of the gene and BamHI. These XhoI-BamHI fragments were then replaced each other to form chimeras 1 and 2, respectively. Chimera3 containing the N-terminal 437 residues of Pyc1 and the C-terminal 741 residues of Pyc2 and chimera 4 containing the N-terminal 438 residues of Pyc2 and the C-terminal 741 residues of Pyc1 were generated by introducing a SacII site between codons 437 and 438 of Pyc1 and between codons 438, and 439 of Pyc2 genes, without altering their encoded amino acids and reading frames by site-directed mutagenesis. The mutagenic primers used to introduce SacII site in Pyc1 gene were PYC1/SacII-Forward (5’-aatcgagttcagaatccgcggtgtcaagaccaac-3’; underline indicates SacII restriction site) and PYC1/SacII-Reverse (5’-gttggtcttgacaccgcggattctgaactcgatt-3’) and in Pyc2 gene were PYC2/SacII-Forward (5’-atcgaattcagaatccgcggtgtgaagaccaac-3’) and PYC2-SacII-Reverse (5’-gttggtcttcacaccgcggattctgaattcgat-3’). These SacII-modified Pyc1 and Pyc2 constructs cloned in pBluescript SK II plasmid (Stratagene) were then excised with XhoI locating at the most 5’-end of both Pyc1 and Pyc2 genes, and SacII, and replaced each other to produce chimeras 3 and 4, respectively. Chimera 5 containing the N-terminal 95 residues of Pyc1 and the C-terminal 1,083 residues of Pyc2 and chimera 6 containing the N-terminal 96 residues of Pyc2 and the C-terminal 1,083 residues of Pyc1 were generated by introducing SalI site between codons 95 and 96 of Pyc1 and between codons 96 and 97 of Pyc2 genes, without altering their encoded amino acids and reading frames. The mutagenic primers used to introduce a SalI site in Pyc1 gene were Pyc1-MluI-Forward (5’-ccaaaaacaccaggtcgacttcatccatccaggt-3’) and Pyc1-MluI-Reverse (5’-acctggatggatgaagtcgacctggtgtttttgg-3’) and for Pyc2 gene are Pyc2-MluI-Forward (5’-aaagaagcataaggtcgacttcatccatccaggt-3’) and Pyc2-MluI-Reverse (5’-acctggatggatgaagtcgaccttatgcttcttt-3’). The SalI-modified Pyc1 and Pyc2 constructs cloned in pBluescript SK II plasmid were then excised with XhoI and SalI and replaced each other to produce chimeras 5 and 6, respectively. Finally, all of the above chimeric Pyc1-Pyc2 fragments were excised from pBluescript and cloned into the equivalent sites in pVT100u. The positions of various regions of Pyc1 and Pyc2 being swapped to form these six chimeras are shown in Table 2 and Table 3. All of these constructs were transformed into S. cerevisiae W303 where the pyc1 gene had been disrupted with a HIS3 cassette by homologous recombination (Brachmann et al., 1998).
Table 2.
Analysis of effects of NH4Cl on Pyc1, Pyc1 and Chimeras 1 & 2
| Isozyme/Chimera | Ka (mM) | KI (mM²x 10−4) | Vmax (u mg−1) | Vo (% Vmax) |
|---|---|---|---|---|
| 11.1 ± 0.6 | 7.8 ± 0.3 | 5.8 | 6.8 | |
| 7.5 ± 0.8 | 19.8 ± 1.7 | 7.9 | 10.4 | |
| 13.7 ± 1.7 | 4.5 ± 0.5 | 8.0 | 9.7 | |
| 9.2 ± 1.0 | 17.8 ± 1.5 | 3.8 | 11.6 | |
Values of Ka, KI and Vmax are derived from the fits of equation (ii) to the data as shown in Fig. 3.
Values of vo were obtained by expressing the velocity in the absence of NH4Cl as percentage of Vmax.
Table 3.
Analysis of acetyl CoA activation of Pyc1, Pyc2 and Chimeras 1–6 and the effects of aspartate on this activation
| Isozyme/Chimerad | aKa (µM) | Vmax (u mg−1) | bvo (% max vel) | cn (0 mM Asp) | cn (2.6 mM Asp) | cn (5.0 mM Asp) |
|---|---|---|---|---|---|---|
| 16.5±0.6 | 8.3 | 6.1 | 1.20±0.04 | 1.86±0.04 | 2.13±0.04 | |
| 8.0±1.7 | 4.0 | 26.7 | 0.99±0.02 | 1.32±0.01 | 1.51±0.03 | |
| 6.9±0.3 | 5.1 | 11.3 | 1.18±0.04 | ND | ND | |
| 4.0±0.2 | 2.1 | 20.0 | 0.98±0.15 | ND | ND | |
| 10.9±0.7 | 4.5 | 10.5 | 1.12±0.06 | ND | ND | |
| 1.9±0.1 | 2.3 | 22.2 | 0.94±0.07 | ND | ND | |
| 7.6±0.4 | 1.4 | 15.2 | 1.18±0.07 | ND | ND | |
| 1.8±0.1 | 0.8 | 22.3 | 1.07±0.04 | ND | ND | |
| 5.7±0.4 | 0.06 | 17.1 | 1.03±0.06 | ND | ND | |
| 3.4±0.1 | 1.3 | 11.9 | 1.37±0.03 | ND | ND | |
Ka is the activation constant for acetyl CoA in the absence of aspartate ± standard error derived from the fit of equation (iv) to the data.
vo is the velocity of the reaction in the absence of acetyl CoA (and aspartate) given as a percentage of the maximum velocity of the reaction at saturating [acetyl CoA].
n is the Hill coefficient of cooperativity ± standard error estimated by linear regression analysis of the Hill plot.
Diagrams representing the polypeptide chains of the various constructs of Pyc1, Pyc2 and the chimeras where the numbers indicate the amino acid position in Pyc1 at the boundary between the Pyc1 and Pyc2 sequences.
ND is not determined
2.3 Preparation and purification of isozymes and chimeras
Preparation and purification of the untagged Pyc1 and Pyc2 isozymes and the untagged chimeras was essentially as described by Branson, Nezic, Jitrapakdee, Wallace & Attwood. (2004) using anion exchange chromatography on DEAE Sepharose CL-6B and affinity chromatography on avidin-Sepharose as a last purification step. The 9xHis-tagged chimeras were purified by affinity chromatography on Ni-NTA resin, using a linear imidazole gradient (0 – 250 mM). Purified enzymes were stored at −80°C in storage buffer comprising 20% (v/v) glycerol in 0.1M Tris-Cl, pH 7.8. Prior to assay, the enzyme was transferred to 0.1M Tris-Cl, pH 7.8 by centrifuging through Sephadex G-25 as described by Helmerhorst & Stokes (1980).
2.3 Assays of pyruvate carboxylase activity
The conditions for this spectrophotometric assay where oxaloacetate formation is measured in a coupled reaction with malate dehydrogenase, were essentially as described by Attwood & Cleland (1986). One unit of enzyme activity is defined as the amount of enzyme required to catalyze the formation of 1 µmol of oxaloacetate min−1 at 30°C. All assays were performed at 30°C. Assays were performed in 0.1 mM Tris-Cl, pH7.8 with the following concentrations of substrates unless stated otherwise: 2.5 mM ATP; 20 mM NaHCO3; 7 mM MgCl2; 10 mM pyruvate; 0.21 mM NADH. Note that in the experiment where [ATP] was varied, the concentration of free Mg2+ was maintained at 4.5 mM and in all experiments where substrate concentrations were varied the reactions were performed in the presence of 0.25 mM acetyl CoA. Assays were initiated by the addition of either isozymes or chimeras and activities were calculated as units ml−1 of enzyme solution.
2.4 Data analysis
The kinetic parameters given in Table 1 were obtained from non-linear regression fits of the Michaelis-Menten equation to the data. The ammonium chloride activation/inhibition data were analysed using non-linear least-squares regression analysis to fit of the following equations (i and ii) to the data:
| (i) |
| (ii) |
where: v is the measured velocity of the reaction; vo is the measured velocity of the reaction in the absence of NH4Cl; V is the velocity of the reaction if all of the enzyme subunits had a single NH4+ bound (E.NH4, see Fig. 3); Ka is the activation constant for the binding of NH4+ to the enzyme (see Fig. 3); KI is the inhibition constant for the subsequent binding of NH4+ (see Fig. 3) and has units of mM in (i) and mM² in (ii).
Table 1.
Comparison of kinetic parameters of Pyc1 and Pyc2.
| Kinetic parameter | Value of parameter for Pyc1 (± S.E. †) (mM)* for Km or (s−1)* for kcat | Value of parameter for Pyc2 (S.E. †) (mM) for Km or (s−1) for kkcat |
|---|---|---|
| Km for HCO3− | 1.36 ± 0.12 | 21.5 ± 1.8 |
| Km for pyruvate | 0.50 ± 0.06 | 0.79 ± 0.04 |
| Km for MgATP | 0.07 ± 0.01 | 0.17 ± 0.02 |
| kcat (with saturating substrates and acetyl CoA) | 60 ± 2 | 47 ± 4 |
Standard errors were obtained from the non-linear least squares regression analysis of the kinetic data.
Values of kinetic parameters for Pyc1 are taken from Branson, Nezic, Jitrapakdee, Wallace & Attwood (2004)
Fig. 3.

The effects of NH4+ concentration on the activity of (a) Pyc1, (b) Pyc2, (c) chimera 1, (d) chimera 2 in the absence of acetyl CoA. Reactions were performed in 0.125M Tris-Cl at pH7.8 and 30°C, using the normal components of the spectrophotometric assay (see Materials and Methods: 2.3), in the presence of different concentrations of NH4Cl. The dashed lines represent fits of equation (i) to the activity data, the solid lines represent fits of equation (ii).
Equation (i) and Equation (ii) were derived based on the respective reaction schemes shown in Fig. 3 with the assumptions that E.NH4 represents the maximally active form of the enzyme and that E.(NH4)2 and E.(NH4)3 have no enzymic activity.
The data for activation of the isozymes and chimeras by acetyl CoA, in the presence or absence of aspartate were initially analysed by linear regression according to the Hill equation (iii):
| (iii) |
where: v is the velocity of the reaction at any acetyl CoA concentration; Vmax is the velocity of the reaction at saturating acetyl CoA; n is the Hill coefficient of cooperativity; Ka is the activation constant.
In order to obtain an estimate of Ka with a standard error, the data were re-analysed according to equation (iv):
| (iv) |
where the value of n used was taken from the Hill plot analysis.
3. RESULTS AND DISCUSSION
3.1 Kinetic parameters for the reaction catalysed by Pyc1 and Pyc2
The values of the Kms for the substrates and kcat values are shown in Table 1. The value of kcat in the presence of saturating concentrations of acetyl CoA and the substrates of the reaction for Pyc2 is 78% of that for Pyc1 indicating that Pyc2 is somewhat less catalytically active than Pyc1. In addition, the Km for pyruvate of Pyc2 is somewhat higher than that of Pyc1. The largest differences between Pyc1 and Pyc2 however, occur in their Km values for MgATP and HCO3−.
The Km for MgATP is 2.4 fold higher for Pyc2 compared to that of Pyc1 and is closer to the value of 0.2 mM reported by Myers, Tolbert & Utter. (1983) for a preparation of pyruvate carboxylase from S. cerevisiae, which may have contained a mixture of Pyc1 and Pyc2. This may indicate that there is a greater degree of control of the activity of Pyc2 than that of Pyc1 activity by the concentration of ATP.
The Km for HCO3−, of Pyc2 is nearly 16 times that of Pyc1. The value of the Km for HCO3− of Pyc2 lies closer to the value reported by Myers Tolbert & Utter, (1983) (19.1 mM) in the presence of acetyl CoA than that measured by Ruiz-Amil, de Torrontegui, Palacián, Catalina & Losada, (1965) (2.4 mM) for enzyme isolated from S. cerevisiae, which in both cases may have contained a mixture of Pyc1 and Pyc2. However, in the presence of 8.4 mM K+ the enzyme preparation studied by Myers, Tolbert & Utter, (1983) exhibited a Km for HCO3− of 3.1 mM and in the presence of a combination of acetyl CoA and K+, this Km was reduced further. Thus in the yeast cell, where the concentration of K+ is estimated to be about 17 mM (Myers, Tolbert & Utter, 1983) and in the presence of acetyl CoA, the Km for HCO3− may be much closer to that measured for Pyc1. When we repeated the kinetic experiments varying [NaHCO3] in the presence of 17 mM KCl, we found that at concentrations of NaHCO3 above about 50 mM, inhibition was evident (data not shown). Estimates of the Kms obtained from a fit of an equation of the form similar to that of eq (i) to the data were 4.1 ± 1.8 mM for Pyc2 and 34 ± 10 mM for Pyc1. The inhibition was somewhat similar to that seen with increasing concentrations of NH4+ (see below) suggests that there may be both activatory and inhibitory effects of the monovalent cations (K+ and Na+) (McClure, Lardy& Kneifel, 1971). that differ between Pyc11 and Pyc2 and that make determination of the true Km for HCO3− difficult.
3.2 Effect of NH4+ on the activity of Pyc1, Pyc2 and Chimeras 1 and 2
In view of the amino sequence similarity between Pyc1 and Pyc2 (92.8% sequence identity), it seemed feasible to prepare chimeras of Pyc1 and Pyc2 without incurring any great disruption of the structure or function of the enzyme whilst enabling the investigation as to which domain(s) was associated with the regulatory properties with respect to acetyl CoA and NH4+. To perform an initial investigation, two chimeras were constructed, each containing roughly half the amino acid sequence of each isozyme (see Table 2). Both chimeras were expressed as 9His-tagged recombinant proteins.
Fig. 3 shows the effects of increasing concentration of NH4+ on the activity of Pyc1, Pyc2 and chimeras 1 and 2. Enzyme activity increases with [NH4+] up to about 75 mM. However, above this concentration, the NH4Cl becomes inhibitory. The estimated Kas and KIs for NH4+ and velocities in the absence of NH4+ are given in Table 2. Both Pyc1 and chimera 1 are somewhat less sensitive to activation by NH4+, with their Kas being about 49% higher than those for Pyc2 and chimera 2 respectively. On the other hand, both Pyc2 and chimera 2 are less sensitive to inhibition by NH4+, with their KIs being 2.5 and 4 fold higher than those of Pyc1 and chimera 1 respectively. Whilst the vo values for Pyc1 and chimera 1 are lower than those for Pyc2 and chimera 2 respectively, the maximum velocity for Pyc1 is lower than that for Pyc2 whilst the reverse is the case for the chimeras.
Huet, Menendez, Gancedo & Francois (2000) found a 4–5 fold up-regulation of the expression of Pyc1, but little change in Pyc2 expression when the yeast was grown with ammonium as the nitrogen source compared to aspartate. Huet, Menendez, Gancedo & Francois (2000) offered the explanation that with NH4+ as the nitrogen source there would be a high demand for α-ketoglutarate as a substrate for glutamate dehydrogenase which catalyses the formation of glutamine, using NH4+ as the other substrate. Pyruvate carboxylase activity would therefore need to be increased to replenish α-ketoglutarate in the tricarboxylic acid cycle removed for this purpose. One hypothesis that might explain the difference in regulation of expression Pyc1 compared to Pyc2 is that Pyc1 activity is more highly regulated by the intracellular concentration of NH4+. However, our data do not really support this hypothesis, since the differences in activation of the two isozymes by NH4+ are relatively small and indeed the Ka of activation for Pyc1 is higher than that for Pyc2.
We did find a larger difference between the isozymes in terms of their inhibition at high NH4Cl concentrations, with Pyc2 being less sensitive to inhibition. However, the concentrations of NH4Cl at which this inhibition has a pronounced effect on enzymic activity are unlikely to be of physiological relevance and may be a salt concentration effect rather than a specific effect of NH4+ binding.
In general, chimera 2 is more like Pyc2 in being somewhat more sensitive to activation and less sensitive to inhibition by NH4+ than chimera 1, which is more like Pyc1. In terms of the velocities in the absence of NH4+ (vo) chimera 1 is more similar to Pyc1 in having a somewhat lower vo than chimera 2, however the maximum velocity of chimera 1 is lower than that of chimera 2 whilst the reverse is true for Pyc1 and Pyc2. Thus, in the majority of ways, the effects of NH4+ on yeast pyruvate carboxylase appear to be determined by the N-terminal half of the amino acid sequence, although the maximum velocity may be influenced by the carboxy-terminal sequence.
3.3 Activation of Pyc1 and Pyc2 by acetyl CoA and the effects of aspartate
Fig. 4a and b show Hill plots of the activation of Pyc1 and Pyc2 respectively, by acetyl CoA in the presence and absence of aspartate. Table 3 shows the Hill coefficients of cooperativity calculated from these Hill plots, the Kas of activation by acetyl CoA in the absence of aspartate and the velocities of the reaction catalysed by Pyc1 and Pyc2 in the absence of acetyl CoA as a percentage of those in the presence of saturating acetyl CoA. In the absence of aspartate, the Hill coefficient for Pyc2 is not significantly different from 1 indicating that there is that there is no cooperativity in the binding of acetyl CoA. However, that for Pyc1 in the absence of aspartate is significantly higher than 1, indicating that there is some positive cooperativity in this case. With both Pyc1 and Pyc2, the Hill coefficients increase in the presence of increasing aspartate concentration however, those for Pyc1 are higher than for Pyc2 and indicate a higher degree of cooperativity in the binding acetyl CoA to Pyc1 in the presence of aspartate and thus a greater inhibitory effect of aspartate on Pyc1. The values of the Hill coefficient for Pyc1 are similar to those observed in pyruvate carboxylase prepared from baker’s yeast (Cazzulo & Stoppani, 1968).
Fig. 4.

Hill plots showing the effects of [aspartate] on the activation of Pyc1 (a) and Pyc2 (b) by acetyl CoA. Reactions were performed in the absence of aspartate (■) in the presence of 2.6 mM mM (▲) or 5 mM (●) aspartate. Plots were as described by Cazzulo and Stoppani (1968), where v is the velocity of the reaction at any [acetyl CoA] minus that at zero [acetyl CoA]. V is the maximum velocity of the reaction minus that at zero [acetyl CoA](for the reactions in the presence of aspartate, it was assumed that the maximum velocity was the same as that in the absence of aspartate). The solid lines are linear least squares fits of the Hill equation (iii).
The original acetyl CoA binding studies on the chicken liver enzyme indicated that there were four acetyl CoA binding sites per tetramer (Frey & Utter, 1977). However, a more recent study on the yeast enzyme in which more accurate determinations of protein concentration were performed, revealed only 2 binding sites per tetramer (Chapman-Smith, Booker, Clements, Wallace & Keech, 1991). If this latter figure is correct, this suggests that at 5 mM aspartate, acetyl CoA binding is completely cooperative in Pyc1.
If we consider the classical model of allostery, in the absence of any ligand there is an equilibrium between relaxed (R) and taut (T) conformers of the enzyme, with the affinity of the R form for the ligand being higher than that of the T form. In the case of Pyc1 and Pyc2, the ligand is acetyl CoA and in the absence of aspartate the equilibrium between R and T forms of the enzymes would appear to be very much in favour of the R form. Thus, in Pyc2, in the absence of aspartate, all the binding sites for acetyl CoA are already in the high affinity state and thus there is no cooperativity of acetyl CoA binding. Aspartate, however shifts the equilibrium between R and T conformers in favour of the T form and hence, as the concentration of acetyl CoA increases, this equilibrium is shifted back towards the R form, giving rise to the sigmoidal activation kinetics. In Pyc1 there is a significant presence of the T form in the equilibrium between R and T forms in the absence of aspartate and acetyl CoA, with increasing concentrations of aspartate shifting this equilibrium in favour of the T form. This difference between Pyc1 and Pyc2 may also, in part at least explain, the much higher activity (vo) of Pyc2 in the absence of activators compared to Pyc1, with the R form having a higher intrinsic activity than the T form.
The activation constant for acetyl CoA (Ka) in the absence of aspartate for Pyc1 is about twice that for Pyc2 and vo for Pyc2 is about 4 times greater than that for Pyc1 suggesting that Pyc2 is more active at zero and low concentrations of acetyl CoA than Pyc1. Huet, Menendez, Gancedo & Francois (2000) suggested that Pyc2 is important for glycolytic growth under anaerobic conditions, where acetyl CoA concentrations would be low, and a major role of Pyc2 would be to produce oxaloacetate that could then be reduced by malate dehydrogenase, with concomitant oxidation of NADH to NAD+. This supply of NAD+ would allow the continuation of glycolysis in the early stages of fermentative growth.
3.5 Activation of chimeras 1 and 2 by acetyl CoA
Fig. 5a shows Hill plots of the activation of chimeras 1 and 2 by acetyl CoA. Table 3 shows the Hill coefficients of cooperativity calculated from these Hill plots, the Kas of activation by acetyl CoA in the absence of aspartate and the velocities of the reaction catalysed by chimeras 1 and 2 in the absence of acetyl CoA as a percentage of those in the presence of saturating acetyl CoA. The Hill coefficient for chimera 2 is not significantly different from one, indicating that there is no cooperativity in the binding of acetyl CoA. However, the Hill coefficient for chimera 1 is significantly higher than one, indicating that there is some positive cooperativity in this case. In this respect, chimera 1 is similar to Pyc1 while chimera 2 is similar to Pyc2.
Fig. 5.

Hill plots showing the activation of: (a) chimeras 1 (■) and 2 (▲); (b) chimeras 3 (●) and 4 (△); (c) chimeras 5 (○) and 6 (□) by acetyl CoA. Plots were as described by Cazzulo and Stoppani (1968), where v is the velocity of the reaction at any [acetyl CoA] minus that at zero [acetyl CoA]. V is the maximum velocity of the reaction minus that at zero [acetyl CoA](for the reactions in the presence of aspartate, it was assumed that the maximum velocity was the same as that in the absence of aspartate). The solid lines are linear least squares fits of the Hill equation (iii).
In terms of the values of vo, chimera 1 is similar to Pyc1 and chimera 2 is more like Pyc2. Similarly, chimera 2 has a lower activation constant (Ka) than chimera 1, again making it more like Pyc2 than Pyc1. However, the absolute values of Ka for both chimeras 1 and 2 are 1.5 and 4 fold lower than those for Pyc1 and Pyc2 respectively. To investigate whether the His tag on the chimeras was affecting the activation constant for acetyl CoA, N-terminally 9His-tagged constructs of Pyc1 and Pyc2 were expressed and purified and their activation by acetyl CoA measured as for Pyc1 and Pyc2. The parameters for the activation of these constructs are also shown in Table 3. Thus the Ka for 9HisPyc1 was found to be less than that of Pyc1, suggesting that the 9His tag is affecting the activation of the enzyme by acetyl CoA, primarily by reducing the Ka. Similarly, the Ka for 9HisPyc2 was reduced compared to Pyc2. In addition, the values of Vmax of the 9His-tagged constructs were lower than those of the untagged isozymes and similar to those of the corresponding chimeras whilst the vo and n values appear not to be markedly affected.
From the studies of chimeras 1 and 2 and the comparisons with the Pyc1 and Pyc2 and their 9His-tagged forms, we can conclude that the major determinants of the response of the enzyme to acetyl CoA activation and the effects of NH4Cl lie in the N-terminal half of the amino acid sequence. This is in agreement with the study by Islam, Sueda & Kondo, (2005) who made a chimera of the biotin carboxylation subunit (472 amino acids) of the Aquifex aeolicus enzyme and the C-terminal sequence of the Bacillus thermodenitrificans enzyme. The A. aeolicus enzyme activity is independent of acetyl CoA and Islam, Sueda & Kondo (2005) found that the activity of the chimera was also acetyl CoA-independent. From our study, however, it is important to note the marked effect of the 9His tag on the Ka for acetyl CoA in both Pyc1 and Pyc2 and illustrates the potential dangers of using such tags which, although extremely useful for the purposes of enzyme purification, can have unforseen effects on enzyme action.
3.6 Further investigation of the locus of activation of the Pyc isozymes by acetyl CoA
In order to further investigate the locus of action of acetyl CoA, two more sets of chimeras were created (see Table 3). As the 9His tag on both Pyc1 and Pyc2 appeared to affect Ka for acetyl-CoA, the next sets of chimeras were constructed without this N-terminal tag. In chimeras 3 and 4, the N-terminal section comprises the first 437 amino acids from Pyc1 and Pyc2 respectively. This N-terminal sequence encompasses the whole biotin carboxylation domain (see Fig. 1) and a sequence of about 100 amino acids, N-terminal to this. In chimeras 5 and 6, the N-terminal section comprises most of this N-terminal sequence i.e. the first 95 amino acids from Pyc1 and Pyc2 respectively.
Figs. 5b and c show the Hill plots for activation of these chimeras by acetyl CoA and Table 3 shows parameters of activation of these chimeras by acetyl CoA. The parameters for chimeras 3 and 4 most resemble those of Pyc1 and Pyc2, respectively with chimera 4 showing little cooperativity, having a higher vo than chimera 3 and a lower Ka than chimera 3. The absolute values of Ka for both chimeras are however, lower than those for Pyc1 (about 50%) and Pyc2 (about 23%) and the vo value for chimera 3 is 2.5 times that for Pyc1, whilst that for chimera 4 is similar to that of Pyc2.
The parameter values for chimeras 5 and 6 in Table 3, show that chimera 5 exhibits little cooperativity, while chimera 6 shows significant cooperativity. In addition, the vo value for chimera 5 is larger than that for chimera 6. In these respects chimera 6 behaves like Pyc1 and chimera 5 more like Pyc2. However, the Ka value for chimera 5 is still larger than that for chimera 6, although there is less than a two-fold difference between them. The Ka value for chimera 6 is almost double that of chimera 4 and 43% of that of Pyc2, while that of chimera 5 is 75% of that of chimera 3 and 35% of that of Pyc1. The low Vmax values observed with chimeras 3– 6 suggest that fusion of parts of the isozyme sequences has introduced some structural instability. This is especially severe in chimera 5, suggesting that there may be some structurally important interactions in Pyc1 that have been severely disrupted in this chimera.
Analysis of the activation of chimeras 3 and 4 by acetyl CoA indicate that, while overall they behave in a similar manner to Pyc1 and Pyc2 respectively, there are some marked differences. The Hill coefficients for chimeras 3 and 4 are essentially the same as for Pyc1 and Pyc2 respectively and the vo value for chimera 4 is similar to that of Pyc2, the vo value for chimera 3 is considerably higher than for Pyc1. In addition, the values of Ka for chimeras 3 and 4 are lower than for Pyc1 and Pyc2 respectively. This suggests that there is part of the amino acid sequence between amino acids 437 and 616 that contributes to the action of acetyl CoA on the enzyme. However, analysis of Pyc1 and Pyc2 sequences between these regions reveal only three amino acid differences i.e. V449 in Pyc1 (I450 in Pyc2), A604 (S605) and R608(K608) (see Fig. 6).
Fig. 6.

Multiple sequence alignment of the N-terminal halves of amino acid sequences of Pyc1 (PYC1_YEAST) (Lim, Morris, Occhiodoro & Wallace., 1988), Pyc2 (PYC2_YEAST) (Stucka, Dequin, Salmon & Gancedo, 1991) and pyruvate carboxylase from Aspergillus niger (PYC_ASPNG) (Panneman, Ruijter, Van den Broeck & Visser, 1998) The alignment was performed using the ClustalW program. The consensus line represents the consensus amino acid sequence where, at those positions, the amino acids in two or more of the enzymes are identical. Amino acids are given in each individual enzyme sequence when the amino acid at that position is different from the consensus or where there is no consensus i.e. the amino acids at such a position are different in all three sequences. The position numbers given above the consensus sequence refer to the Pyc1 sequence and indicate points in the alignment where there is either no consensus or there is a non-conservative difference in amino acids between Pyc1 and Pyc2.
Chimera 5 shows essentially no cooperativity whilst chimera 6 exhibits positive cooperativity and the acetyl CoA-independent activity of chimera 5 is greater than that of chimera 6. In these respects chimera 5 is behaving more like Pyc2 and chimera 6 is more like Pyc1. On the other hand, the Ka for chimeras 5 is larger than that for chimera 6, although that for chimera 6 is almost twice that for chimera 4. Thus the main determinants for cooperativity and acetyl-CoA-independent activity appear to lie somewhere between amino acids 95 and 437. Analysis of Pyc1 and Pyc2 sequences shows 13 amino acid differences across this region including N141 (H142 in Pyc2), K145 (R146), E162 (Q163), E285 (V286), A330 (S331), S340 (T341), P342 (T343), F347 (L348), A357 (S357), A368 (S368), I378 (L379), T400 (A401) and I401 (T402). These sequence differences between Pyc1 and Pyc2 may form distinct structural motifs that mediate differential activation by acetyl-CoA.
Sueda, Islam & Kondo (2004) found that there is an interaction between the biotin carboxylase domains in the enzyme from B. thermodenitrificans and postulated that this was important for the formation of the tetrameric quaternary structure. Since cooperativity is dependent on inter-subunit interactions, this is in agreement with our data that the basis for the cooperativity lies in the biotin carboxylation domain.
The situation is more complex with respect to the Ka for acetyl CoA. The differences in the Ka values between all of the chimeras and the isozymes suggests that determinants of Ka are more dispersed and there is an interdependence between these determinants that affects Ka. The fact that the Ka values of chimeras 5 and 6 are still quite different from those of Pyc1 and Pyc2 indicates that one or more of these determinants lies in the N-terminal 95 amino acid sequence.
Fig. 6 shows a multiple sequence alignment between the N-terminal halves of the amino acid sequences of Pyc1, Pyc2 and pyruvate carboxylase from Aspergillus niger, whose activity is completely independent of acetyl CoA (Feir & Suzuki, 1969). As can be seen, there are relatively few differences between Pyc1 and Pyc2 alone and with the exception of the arginine in position 595 of Pyc1 where the corresponding residue in Pyc2 is alanine, the differences are fairly conservative in terms of amino acid properties. Where there are differences between the sequences of Pyc1 and Pyc2 that are not so conservative, these also coincide with different residues in the A. niger sequence. For example at the position corresponding to 71 in Pyc1 there is a valine in Pyc1, a glutamate in Pyc2 and lysine in the A. niger enzyme. Thus at this position, the range of amino acids varies from non-polar, to acidic to basic. There is a similar arrangement at position 88. At other positions there is a combination of polar, non-polar and basic or acidic residues (positions 1, 91, 94, 285,342 and 545) or aromatic, aliphatic and polar (positions 5 and 347). Thus in N-terminal region of the sequence these amino acid differences are scattered. The differences in the alignment at positions 285 and 342 of Pyc1 do lie within the span between positions 95-437 and will be of interest for future site-directed mutagenesis studies aimed at investigating the basis of acetyl CoA-independent activity and the cooperativity of acetyl CoA activation.
3.7 Conclusions
In conclusion, we have shown a number of differences between the kinetic properties of the isozymes Pyc1 and Pyc2 from S. cerevisiae, which may have physiological relevance in the differential control of their activities. However, in the current work all activation experiments were performed in the presence of high substrate concentrations and in the presence of a single activator and thus we are essentially studying activator effects on Vmax. In the yeast cell there is likely to be a combination of the effects of different activator concentrations and changing substrate and inhibitor concentrations that contribute to the fine differential regulation of Pyc1 and Pyc2 activities.
The main determinants of acetyl CoA activation of the enzyme lie in the N-terminal half of the amino acid sequence, with those that affect the degree of acetyl-CoA-independent activity and the cooperativity of acetyl CoA action being more localised to the biotin-carboxylation domain.
Fig. 2.

Reaction schemes for the binding of NH4+ to the isozymes or chimeras. In both schemes initial binding of a single NH4+ to each subunit results in enzyme activation, however the subsequent binding of (i) one or (ii) two NH4+ results in inhibition.
ACKNOWLEDGEMENTS
This work was supported in part by the Australian Research Council grant DP0346807 to J.C.W., the National Institutes of Health grant 1 R01 GM070455 to W.W. Cleland., J.C.W. and P.V.A. and the Career Development Grant RSA460002 from the Thailand Research Fund to S.J
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Attwood PV. The structure and the mechanism of action of pyruvate carboxylase. International Journal of Biochemistry and Cell Biology. 1995;27:231–249. doi: 10.1016/1357-2725(94)00087-r. [DOI] [PubMed] [Google Scholar]
- Attwood PV, Cleland WW. Decarboxylation of oxalacetate by pyruvate carboxylase. Biochemistry. 1986;25:8191–8196. doi: 10.1021/bi00373a011. [DOI] [PubMed] [Google Scholar]
- Attwood PV, Wallace JC. Chemical and catalytic mechanisms of carboxyl transfer reactions in biotin-dependent enzymes. Accounts of Chemical Research. 2002;35:113–120. doi: 10.1021/ar000049+. [DOI] [PubMed] [Google Scholar]
- Barden RE, Scrutton MC. Pyruvate carboxylase from chicken liver. Effects of univalent and divalent cations on catalytic activity. Journal of Biological Chemistry. 1974;249:4829–4838. [PubMed] [Google Scholar]
- Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, Boeke JD. Designer Deletion Strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–132. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Branson JP, Nezic M, Wallace JC, Attwood PV. Kinetic characterization of yeast pyruvate carboxylase isozyme pyc1. Biochemistry. 2002;41:4459–4466. doi: 10.1021/bi011888m. [DOI] [PubMed] [Google Scholar]
- Branson JP, Nezic M, Jitrapakdee S, Wallace JC, Attwood PV. Kinetic Characterization of Yeast Pyruvate Carboxylase Isozyme Pyc1 and the Pyc1 Mutant, C249A. Biochemistry. 2004;43:1075–1081. doi: 10.1021/bi035575y. [DOI] [PubMed] [Google Scholar]
- Brewster NK, Val DL, Walker ME, Wallace JC. Regulation of pyruvate carboxylase isozyme (PYC1, PYC2) gene expression in Saccharomyces cerevisiae during fermentative and nonfermentative growth. Archives of Biochemistry and Biophysics. 1994;311:62–71. doi: 10.1006/abbi.1994.1209. [DOI] [PubMed] [Google Scholar]
- Cazzulo JJ, Stoppani AOM. The regulation of yeast pyruvate carboxylase by acetyl-coenzyme A and L-aspartate. Biochimica et Biophysica Acta. 1968;127:563–567. doi: 10.1016/0003-9861(68)90263-4. [DOI] [PubMed] [Google Scholar]
- Chapman-Smith A, Booker GW, Clements PR, Wallace JC. Further studies on the localization of the reactive lysyl residue of pyruvate carboxylase. Biochemical Journal. 1991;276:759–764. doi: 10.1042/bj2760759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feir HA, Suzuki I. Pyruvate carboxylase of Aspergillus niger: kinetic study of a biotin-containing carboxylase. Canadian Journal of Biochemistry. 1969;47:697–710. doi: 10.1139/o69-107. [DOI] [PubMed] [Google Scholar]
- Frey WH, Utter MF. Binding of acetyl CoA to chicken liver pyruvate carboxylase. Journal of Biological Chemistry. 1977;252:51–56. [PubMed] [Google Scholar]
- Helmerhorst E, Stokes GB. Microcentrifuge desalting: a rapid, quantitative method for desalting small amounts of protein. Analytical Biochemistry. 1980;104:130–135. doi: 10.1016/0003-2697(80)90287-0. [DOI] [PubMed] [Google Scholar]
- Huet C, Menendez J, Gancedo C, Francois JM. Regulation of pyc1 encoding pyruvate carboxylase isozyme I by nitrogen sources in Saccharomyces cerevisiae. European Journal of Biochemistry. 2000;267:6817–6823. doi: 10.1046/j.1432-1033.2000.01779.x. [DOI] [PubMed] [Google Scholar]
- Islam MN, Sueda S, Kondo H. Construction of new forms of pyruvate carboxylase to assess the allosteric regulation by acetyl CoA. Protein Engineering Design and Selection. 2005;18:71–78. doi: 10.1093/protein/gzi011. [DOI] [PubMed] [Google Scholar]
- Jitrapakdee S, Wallace JC. Structure, function and regulation of pyruvate carboxylase. Biochemical Journal . 1999;340:1–16. doi: 10.1042/bj3400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim F, Morris CP, Occhiodoro F, Wallace JC. Sequence and domain structure of yeast pyruvate carboxylase. Journal of Biological Chemistry. 1988;263:11493–11497. [PubMed] [Google Scholar]
- McClure WR, Lardy HA, Kneifel HP. Rat liver pyruvate carboxylase. I. Preparation, properties, and cation specificity. Journal of Biological Chemistry. 1971;246:3569–3578. [PubMed] [Google Scholar]
- Myers DE, Tolbert B, Utter MF. Activation of yeast pyruvate carboxylase: interactions between acyl coenzyme A compounds, aspartate, and substrates of the reaction. Biochemistry. 1983;22:5090–5096. doi: 10.1021/bi00291a007. [DOI] [PubMed] [Google Scholar]
- Panneman H, Ruijter GJG, Van den Broeck HC, Visser J. Aspergillus niger pyruvate carboxylase. Submitted to the EMBL/GenBank/DDBJ databases. 1998 [Google Scholar]
- Rohde M, Lim F, Wallace JC. Electron microscopic localization of pyruvate carboxylase in rat liver and Saccharomyces cerevisiae by immunogold procedures. Archives of Biochemistry and Biophysics. 1991;290:197–201. doi: 10.1016/0003-9861(91)90608-l. [DOI] [PubMed] [Google Scholar]
- Ruiz-Amil M, de Torrontegui G, Palacián E, Catalina L, Losada M. Properties and function of yeast pyruvate carboxylase. Journal of Biological Chemistry. 1965;240:3845–3492. [PubMed] [Google Scholar]
- Stucka R, Dequin S, Salmon JM, Gancedo C. DNA sequences in chromosomes II and VII code for pyruvate carboxylase isoenzymes in Saccharomyces cerevisiae: analysis of pyruvate carboxylase-deficient strains. Molecular Genes and Genetics. 1991;229:307–315. doi: 10.1007/BF00272171. [DOI] [PubMed] [Google Scholar]
- Sueda S, Islam MN, Kondo H. Protein engineering of pyruvate carboxylase. European Journal of Biochemistry. 2004;271:1391–1400. doi: 10.1111/j.1432-1033.2004.04051.x. [DOI] [PubMed] [Google Scholar]
- Val DL, Chapman-Smith A, Walker ME, Cronan JE, Jr, Wallace JC. Polymorphism of the yeast pyruvate carboxylase 2 gene and protein - effects on protein biotinylation. Biochemical Journal. 1995;312:817–825. doi: 10.1042/bj3120817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet T, Dignard D, Thomas DY. A family of yeast expression vectors containing the phage f1 intergenic region. Gene. 1987;52:225–233. doi: 10.1016/0378-1119(87)90049-7. [DOI] [PubMed] [Google Scholar]
- Walker ME, Val DL, Rohde M, Devenish RJ, Wallace JC. Biochemical and Biophysical Research Communications. 1991;176:1210–1217. doi: 10.1016/0006-291x(91)90414-3. [DOI] [PubMed] [Google Scholar]
- Wallace JC. Distribution and biological functions of pyruvate carboxylase in nature. In: Keech DB, Wallace JC, editors. Pyruvate carboxylase. Boca Raton: CRC Press; 1985. pp. 5–64. [Google Scholar]


