Summary
The embryonic stem cell differentiation system was used to define the roles of the Activin/Nodal, BMP, and canonical Wnt signaling pathways at three distinct developmental stages during hematopoietic ontogeny: induction of a primitive streak-like population, formation of Flk1+ mesoderm, and induction of hematopoietic progenitors. Activin/Nodal and Wnt, but not BMP, signaling are required for the induction of the primitive streak. Although BMP is not required for primitive streak induction, it displays a strong posteriorizing effect on this population. All three signaling pathways regulate induction of Flk1+ mesoderm. The specification of Flk1+ mesoderm to the hematopoietic lineages requires VEGF and Wnt, but not BMP or Activin/Nodal signaling. Specifically, Wnt signaling is essential for commitment of the primitive erythroid, but not the definitive lineages. These findings highlight dynamic changes in signaling requirements during blood cell development and identify a role for Wnt signaling in the establishment of the primitive erythroid lineage.
Introduction
The establishment of the hematopoietic system involves multiple developmental steps ranging from induction and patterning of mesoderm to specification of the earliest blood cell progenitors. Mesoderm is formed during gastrulation from undifferentiated epiblast cells as they transit through the primitive streak (PS) (Tam and Behringer, 1997). The first mesodermal cells to be induced migrate proximally through extraembryonic tissue where they undergo specification to the hematopoietic and vascular lineages at approximately embryonic day 7.5, giving rise to the blood islands of the yolk sac (Moore and Metcalf, 1970; Palis et al., 1999). The predominant hematopoietic cells in the blood islands are known as primitive erythrocytes and are distinguished by their large size, by their production of an embryonic spectrum of globin chains, and by the fact that they retain their nuclei after entry into circulation. In addition to the primitive erythroid lineage, the mouse yolk sac also generates definitive erythroid and myeloid progenitors (Dieterlen-Lievre, 1975; Palis et al., 1999).
The developmental progression from PS formation through mesoderm induction to hematopoietic specification can be mapped, to some extent, by the expression of genes indicative of each individual stage. The formation of the PS is defined by the upregulation of brachyury, a conserved T box transcription factor that is expressed throughout this structure in diverse organisms, including Xenopus, zebrafish, and mammals (Kispert and Herrmann, 1994; Schute-Merker et al., 1997; Smith et al., 1991). Lineage tracing and reporter studies as well as in situ analyses have shown that expression of the receptor kinase Flk1 (VEGFR-2) is associated with the next developmental stage: the induction and patterning of distinct subsets of mesoderm, including those fated to the hematopoietic, vascular, and some cardiac and somitic precursors (Ema et al., 2006; Motoike et al., 2003). As mesoderm is specified to the hematopoietic lineages, Flk1 expression diminishes while CD41 expression increases. CD41 is expressed on all embryonic hematopoietic progenitors, although levels on the primitive erythroid progenitors are somewhat lower than those of the definitive lineages (Ferkowicz et al., 2003; Mikkola et al., 2003).
To understand the signaling pathways regulating the establishment of the hematopoietic system, it is necessary to focus on each specific stage independently, as critical inductive factors such as those from the TGFβ and Wnt families are known to display different functions on different cell populations. Studies using a variety of model systems have shown that Nodal and Wnt signaling are required for the proper formation of the PS and subsequent induction of mesoderm and endoderm (Conlon et al., 1994; Liu et al., 1999). The role of the BMP pathway on PS formation is less clear. Targeting studies have shown that all BMP receptor 2 (bmpr2) null mice and a subpopulation of those deficient in either BMP receptor 1a (bmpr1a) or bmp4 fail to undergo gastrulation, suggesting a role for BMP signaling at this early stage (Beppu et al., 2000; Mishina et al., 1995; Winnier et al., 1995). In contrast to these observations, conditional inactivation of bmpr1a in the epiblast did not impact gastrulation, suggesting that BMP signaling may not be required for this process (Miura et al., 2006). The differences in these studies may be related to stage-specific requirements for this pathway in early development or due to compensatory functions of the bmpr1b in the latter study. Although these different models have defined key regulators that control PS formation, little is known about the induction of Flk1+ mesoderm and its specification to the hematopoietic lineage. Insights into the regulation of this developmental step have been provided by studies using the ES cell model that showed that BMP4 and Activin A, a nodal surrogate (Tada et al., 2005), can induce the formation of a Flk1+ population in developing embryoid bodies (EBs) in serum-free cultures (Park et al., 2004). However, as no distinction was made between PS formation and Flk1+ mesoderm induction, it is difficult to determine the specific target populations for these factors. Progression of Flk1+ mesoderm to the hematopoietic lineage is also poorly understood. The studies of Park et al. did show that the interaction of VEGF with Flk1 was important for this specification step. Whether or not other factors are involved at this stage remains to be determined. To further elucidate the regulatory pathways that control hematopoietic specification from ES cells, we have evaluated the combined roles of Activin/Nodal, Wnt, and BMP signaling in this process at discrete developmental stages. The findings from these studies indicate that Activin/Nodal and Wnt, but not BMP4, are required for PS formation, whereas all three factors appeared to function in the induction of Flk1+ mesoderm. As observed previously, hematopoietic specification of the Flk1+ mesoderm was dependent on VEGF/Flk1 signaling. Although BMP and Activin/Nodal signaling did not appear to impact this developmental stage, our studies uncovered an essential role for Wnt signaling in the specification of the primitive erythroid lineage from Flk1+ mesoderm. These findings highlight the power of the ES cell model in mapping regulatory pathways that control embryonic lineage commitment and in identifying lineage regulators that would be difficult to identify in the early embryo.
Results
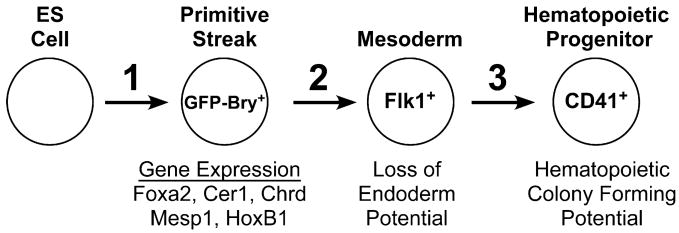
To define the key signaling pathways that regulate hematopoietic commitment in ES cell differentiation cultures, we examined three distinct developmental stages representing (1) the induction of a PS-like population, (2) the commitment of the PS-like population to Flk1+ mesoderm, and (3) the specification of Flk1+ mesoderm to the hematopoietic lineage (Figure 1). For these studies, we used an ES cell line with the green fluorescent protein (GFP) cDNA targeted to the brachyury locus (GFP-Bry) and the human CD4 cDNA targeted to the foxa2 locus (CD4-Foxa2) (Gadue et al., 2006) and focused our analysis on the BMP, Wnt, and Activin/Nodal/TGFβ pathways. ES cells were differentiated as EBs in the presence of the indicated inducers in the absence of fetal calf serum. Our previous studies have demonstrated that PS formation and germ layer induction in the EBs in the presence of appropriate inducers accurately recapitulates these developmental steps as they happen in the early embryo (Gadue et al., 2006).
Figure 1. Schematic of Hematopoietic Development Depicting the Stages of Development Analyzed in This Study.
Stage 1: The Role of BMP Signaling in ES Cell-Derived PS Induction
We recently demonstrated that the induction of a PS-like population in ES cell differentiation cultures requires both Wnt and Activin/Nodal/TGFβ signaling, findings consistent with the requirement of these pathways in the establishment of the PS in the early embryo (Gadue et al., 2006). In addition to these pathways, targeting studies in mice suggest that BMP signaling may also play a role in the formation of the PS (Beppu et al., 2000; Mishina et al., 1995; Winnier et al., 1995). To evaluate the role of BMP signaling in PS formation in the ES cell model, ES cells were differentiated with BMP4 alone or with BMP4 and DKK1 or SB-431542 (SB), inhibitors of Wnt and Activin/Nodal/TGFβ signaling, respectively (Inman et al., 2002; Semenov et al., 2001). The concentration of inhibitors used was based on their ability to completely inhibit the induction of CD4-Foxa2 and GFP-Bry by exogenously added Activin A or Wnt3a (Figure S1 available online). When added to ES cell differentiation cultures in the absence of other factors, BMP4 induced a GFP-Bry population (GFP-Bry+/CD4-Foxa2lo/−) that appeared to represent the posterior PS (Figure 2A). The BMP-induced population expressed high levels of the posterior PS markers hoxB1 and mesp1 (Gadue et al., 2006; Saga et al., 1996) and low levels of the anterior markers chrd and cer1 (Bachiller et al., 2000; Gadue et al., 2006) (Figures 2B and 2C). To determine if the Wnt and Activin/Nodal/TGFβ signaling pathways are involved in the formation of the BMP-induced GFP-Bry+ population, DKK1 or SB was added together with BMP4 to the differentiation cultures. The addition of either inhibitor completely blocked the development of the BMP4-induced posterior streak population (Figure 2A). As expected, expression of other PS markers, including hoxb1 and mesp1, was also inhibited by addition of DKK1 and SB to the cultures (Figure 2B). These findings demonstrate that the induction of the GFP-Bry+/CD4-Foxa2lo/− population by BMP4 is dependent on both Wnt and Activin/Nodal/TGFβ signaling and suggest that BMP4 functions through the upregulation of factors from both pathways at this stage of development. To determine if this is the case, BMP4-induced populations were analyzed for expression of wnt3 and nodal. As shown in Figure 2D, genes encoding these cytokines were induced by BMP4.
Figure 2. PS Formation during ES Cell Differentiation.

ES cells were differentiated as EBs for 4 days in serum-free media in the presence of BMP4 (0.3 ng/ml), Activin A (2 ng/ml), DKK1 (300 ng/ml), SB-431542 (6 uM), Wnt3a (3 ng/ml), and BMPR1A-Fc plus BMPR1B-Fc (500 ng/ml each) as indicated.
(A) Flow cytometric analysis of day 4 EBs.
(B–D) Quantitative RT-PCR gene expression analysis of ES cells differentiated in (A). Average expression normalized to actb is shown. (B and C) Numbers on vertical axis represent expression levels relative to Activin A and Wnt3a-treated samples. (B) Expression of posterior PS markers. (C) Expression of anterior PS markers. (D) Expression of nodal and wnt3 after BMP induction.
(E) Flow cytometric analysis of intracellular-phosphorylated Smad1/5 in day 4 EBs induced with the indicated combinations of factors. Filled histograms represent anti-GST control; open histograms are cells stained with anti-phospho-Smad1/5. Errors bars indicate ± SEM (n = 3). (Act, Activin A; R, BMPR1A-Fc and BMPR1B-Fc; SB, SB-431542; and −Stim, minus stimulation).
To examine the interplay between BMP, Activin/Nodal, and Wnt signaling in PS induction, ES cells were differentiated in the presence of Activin A and low concentrations of Wnt3a together with either BMP4 or BMPR1A-Fc and BMPR 1B-Fc fusion proteins, which are inhibitors of BMP signaling (Natsume et al., 1997). As shown previously, the combination of Activin A and Wnt3a efficiently induced a PS-like population that expressed GFP-Bry together with a broad range of CD4-Foxa2 expression (Figure 2A) (Gadue et al., 2006). The addition of BMP4 with Activin A and Wnt3a enhanced GFP-Bry induction but completely inhibited the development of the anterior streak CD4-Foxa2hi subpopulation, suggesting that the BMP pathway exerts a dominant effect in promoting posterior streak formation (Figure 2A). In addition to Foxa2, chrd and cer1 were also down-regulated, whereas the posterior markers mesp1 and hoxB1 were enhanced by BMP4 treatment (Figures 2B and 2C). The dominant effect of BMP4 was blocked by the addition of the BMP inhibitors to the cultures (+BMP&R). Addition of the soluble BMPRs to the Activin A/Wnt3a-induced cultures (+R) had no discernable effect on the development of the GFP-Bry/CD4-Foxa2 population or on the expression of other PS markers (Figures 2A–2C). To confirm that BMP signaling was efficiently inhibited by the addition of the soluble receptors, we evaluated the level of phosphorylation of Smad1 and/or Smad5, mediators of BMP signaling (Derynck and Zhang, 2003), by intracellular flow cytometry. As shown in Figure 2E, the addition of BMP4 to the Activin A and Wnt3a-induced cultures resulted in a robust phospho-Smad1/5 signal, which was completely inhibited by the addition of the soluble BMPRs. Taken together, these findings clearly demonstrate that BMP signaling is not required for the development of the PS population in ES cell differentiation cultures. They also show that BMP exerts a dominant effect over Activin A and Wnt3a in promoting the formation of the posterior streak population.
Flk1 Induced by BMP Signaling Is a Marker of Hematopoietic Mesoderm Commitment
As Flk1 has been shown to represent one of the earliest markers of prehematopoietic mesoderm, we next evaluated its expression on ES cells induced with BMP4, Activin A, and Wnt3a as in Figure 2. Induction with BMP4 alone resulted in the development of a Flk1+ population, a finding consistent with previous studies of Park et al. (2004) (Figure 3A). As expected, the development of this Flk1+ population was completely inhibited by the addition of either DKK1 or SB given that both pathways are required for PS formation, the stage prior to the onset of Flk1 expression. Activin A and low levels of Wnt3a did not induce substantial levels of Flk1 expression, and the combination of the three factors did not induce a larger Flk1+ population than BMP4 alone. Flk1 expression at this stage was completely inhibited by the addition of the soluble BMPRs (Figure 3A). These observations indicate that BMP4 signaling is important for the induction of Flk1 at this stage of development. To be able to use Flk1 as a marker of committed mesoderm, it is essential to demonstrate that the fate of this population is fixed with respect to germ layer potential. We have previously shown that the Activin A/Wnt3a-generated GFP-Bry+/Foxa2lo posterior streak-like population can be induced to form endoderm by treatment with high levels of Activin A (Gadue et al., 2006), indicating that this population is not fully committed to the mesoderm lineage. To determine if the Flk1+ population can still undergo conversion to an endoderm fate, GFP-Bry+/Flk1+ or GFP-Bry+/Flk1− cells induced with the combination of Activin A, Wnt3a, and BMP4 were isolated by cell sorting and reaggregated in the presence or absence of Activin A for 2 or 3 days. After this time, the aggregates were harvested, dissociated, and analyzed for the expression of CD4-Foxa2, sox17, foxa3, pdx1, and hnf4a as a measure of endoderm potential and CD31 as a marker of mesoderm (Albelda et al., 1991; Friedman and Kaestner, 2006; Gadue et al., 2006; Kanai-Azuma et al., 2002). When cultured for 2 or 3 days with Activin A, the majority of the cells derived from the GFP-Bry+/Flk1− fraction expressed high levels of CD4-Foxa2 (Figure 3B). This population also expressed the above spectrum of endoderm genes, suggesting that it had undergone commitment to endoderm (Figure 3C). In contrast, the GFP-Bry+/Flk1+-derived population did not upregulate CD4-Foxa2 or any other genes indicative of endoderm development (Figures 3B and 3C). Rather, these cells generated a significant CD31+ population after culture with Activin A, suggesting that they underwent maturation to the endothelial lineage (Figure 3B). The outcome of these studies strongly suggests that upregulation of Flk1 is indicative of a mesoderm commitment step, in that the population is no longer able to generate endoderm in response to Activin A. As Flk1 is expressed on all posterior mesoderm that gives rise to the yolk sac blood islands (Ema et al., 2006), our findings demonstrate that Flk1 provides a valid marker for monitoring hematopoietic mesoderm induction in the ES cell model. Although Flk1 is expressed on all posterior mesoderm, it is found only on a subpopulation of other mesodermal cells, indicating that is it not a general mesodermal marker (Ema et al., 2006).
Figure 3. Expression of Flk1 Defines Mesoderm Commitment in the ES Cell Differentiation Cultures.

(A) ES cells differentiated as in Figure 2 were analyzed for Flk1 versus Brachyury expression by flow cytometry at day 4 of differentiation.
(B and C) ES cells were differentiated for 4 days with Activin A (2 ng/ml), Wnt3a (3 ng/ml), and BMP4 (0.3 ng/ml), and the resulting GFP-Bry+/Flk1− or GFP-Bry+/Flk1+ populations were isolated by cell sorting. These populations were reaggregated in serum-free media in the presence and absence of Activin A (10 ng/ml). (B) Flow cytometric analysis of CD4-Foxa2 and CD31 expression on these reaggregated populations after 2 or 3 days of culture. (C) Gene expression analysis from the samples in B by RT-PCR. (n = 3) (Act, Activin A; R, BMPR1A-Fc and BMPR1B-Fc; SB, SB-431542; −Stim, minus stimulation; and Rg, reaggregation).
Stage 2: From PS to Flk1+ Hematopoietic Mesoderm
The above studies suggest that BMP4, but not low levels of Wnt3a and Activin A, can induce the development of Flk1 mesoderm when added to the cultures at the onset of differentiation. These findings also indicate that BMP4 likely exerts its effect at a stage later than induction of the PS, as it is not required for the development of this population. To further dissect the role of Wnt, Activin/Nodal, and BMP signaling in Flk1 mesoderm induction from the brachyury-positive PS population, we isolated GFP-Bry+/Flk1− cells from Activin A/Wnt3a-induced cultures and reaggregated them with different combinations of the three factors with or without inhibitors of these pathways (Figure 4A). The optimal dose of each factor in inducing Flk1 and hematopoietic commitment were determined by titration (data not shown). The levels of Flk1 were measured after 1 additional day of culture, and hematopoietic potential was assayed as follows. After reaggregation for 1 day, the aggregates were harvested, washed, and cultured in the presence of VEGF for an additional 2 days (Figure 4A). Treatment with VEGF, the ligand for Flk1, has been shown to be important for hematopoietic development from Flk1+ mesoderm (Park et al., 2004). After culture with VEGF, hematopoietic commitment was assayed by expression of CD41, a marker of embryonic hematopoietic commitment (Ferkowicz et al., 2003; Mikkola et al., 2003). In this way, mesodermal commitment can be assayed by Flk1 upregulation, and the hematopoietic potential of the Flk1+ population can be analyzed by CD41 upregulation upon VEGF treatment.
Figure 4. Activin/Nodal/TGFβ, Wnt, and BMP Signaling Pathways Are Required for the Induction of Flk1+ Hematopoietic Mesoderm.
(A) Schematic representation of the stages of development analyzed and the assays used for the evaluation of Flk1+ mesoderm development. GFP-Bry+/Flk1− cells were isolated from day 4 EBs differentiated in the presence of Activin A (2 ng/ml) and Wnt3a (3–5 ng/ml). These PS cells were reaggregated for 24 hr with BMP4 (0.3 ng/ml), Activin A (2 ng/ml), DKK1 (300 ng/ml), Wnt3a (50 ng/ml), SB-431542 (6 μM), and BMPR1A-Fc plus BMPR1B-Fc (500 ng/ml each) or combinations of these factors as indicated.
(B) Flow cytometric analysis showing the average proportion of cells that express Flk1 after 24 hr of culture ± SEM (n = 6).
(C) Cells treated as in (B) were washed in serum-free media and cultured for 2 additional days in the presence of VEGF (5 ng/ml) to induce hematopoietic development. The average proportion of cells that express CD41 was determined by flow cytometry.
(D–H) Cells were harvested, and gene expression analysis was performed by quantitative RT-PCR. Average expression normalized to actb is shown. Number on vertical axis represents relative expression levels. (D) Expression of bmp4, bmp2, and chrd in the GFP-Bry+/Flk1− cells after 24 hr of culture in the presence (Rg1; Act) or absence (Rg1; −Stim) of Activin A (from [B] above) or in day 4 EBs induced with Wnt3a and Activin A as in Figure 3A. (E–H) Expression of gata1, scl, meox1, and nkx2.5 in the populations stimulated with different factors (from [C] above). Errors bars indicate ± SEM (n = 6, [B] and [C]) (n = 3, [D] and [E]). (Act, Activin A; R, BMPR1A-Fc and BMPR1B-Fc; Rg, reaggregation; SB, SB-431542; and −Stim, minus stimulation).
In the first set of analyses, Activin A, BMP4, or high levels of Wnt3a were tested alone for their ability to induce the development of Flk1+ cells from the GFP-Bry+/Flk1− population. Activin A induced a population in which 50% of the cells expressed Flk1 after 24 hr of culture and 25% expressed CD41 after 48 hr of VEGF treatment (Figures 4B and 4C). BMP4 or high levels of Wnt3a alone were considerably less efficient at inducing Flk1 and CD41 expression, with ∼20% of the cells expressing Flk1 (Figure 4B) and less than 10% expressing CD41 (Figure 4C). To determine if BMP or Wnt signaling is required for the Activin A-induced expression of Flk1, inhibitors of these pathways were included in the cultures. In combination with Activin A, the Wnt inhibitor DKK1 completely blocked Flk1 expression, whereas the BMP inhibitor decreased its expression by more than 3-fold (Figure 4B). Both inhibitors completely blocked the upregulation of CD41 (Figure 4C). These data suggest that multiple pathways are involved in Flk1 hematopoietic mesoderm induction. To further investigate this hypothesis, combinations of the three factors were used to induce Flk1 and CD41 expression (Figures 4B and 4C). The combination of Wnt3a and BMP4 was not more effective than either factor alone. In contrast, Activin A with either BMP4 or Wnt3a or all three factors together induced robust Flk1 (>60%) and CD41 expression (∼60%). The CD41 expression levels were more than 2-fold above that observed with Activin A alone (Figure 4C). Taken together, these results strongly support the interpretation that all three pathways are important for the development of Flk1 hematopoietic mesoderm from GFP-Bry+ PS-like cells.
Considering the inability of Activin A and low levels of Wnt3a to induce Flk1 when added to the cultures at the onset of differentiation (Figure 3A), it is somewhat surprising that Activin A alone displayed this potential on the GFP-Bry+/Flk1− population (Figure 4B). Our earlier studies demonstrated that BMP4 is an important regulator of Flk1 induction, when added prior to the induction of the PS. The differences in the ability of Activin A to generate Flk1+ cells could relate to the endogenous levels of BMPs and/or BMP inhibitors in the two populations after induction. A comparison of the expression levels of bmp2, bmp4, and the BMP inhibitor chrd in the GFP-Bry+/Flk1−-reaggregated cultures with or without Activin A and the day 4 Activin A-induced PS population described in Figure 3A revealed interesting differences consistent with this interpretation. As shown in Figure 4D, Activin A induced the expression of bmp2 and bmp4 and inhibited the expression of chrd in the reaggregation cultures. In contrast, the expression of chrd was higher in the day 4 PS population than in the GFP-Bry+/Flk1−-reaggregated cells, whereas the levels of bmp2 and bmp4 were lower. These findings suggest that the differential response to Activin A may be due to differential upregulation of the BMP factors and chrd in the two populations. They also highlight the importance of analyzing distinct developmental intermediates when defining the signaling pathway requirements for a complex developmental program.
To further characterize the mesodermal populations generated by the different inducers, the cultures were evaluated for the expression of gata1 (Pevny et al., 1991) and scl (Begley et al., 1989) as an additional demonstration of hematopoietic potential, the expression of meox1 as an indication of somitic specification (Candia et al., 1992), and nkx2.5 to track early cardiac development (Lints et al., 1993). As expected, gata1 and scl displayed expression patterns very similar to that of CD41, with the highest levels detected in the Activin A plus Wnt3a and/or BMP-stimulated populations (Figures 4E and 4F). The patterns of meox1 and nkx2.5 were distinct from those of the hematopoietic genes. meox1 expression was highest in the Wnt3a and the unstimulated populations, whereas nkx2.5 was induced at higher levels in the Activin A and BMP4-stimulated populations (Figures 4G and 4H). Together, these findings demonstrate that Activin A together with BMP and/or Wnt signaling efficiently induces hematopoietic mesoderm from the GFP-Bry+/Flk1− PS population. Individual factors such as Wnt3a or BMP4 appear to promote the specification of other cell fates.
Stage 3: Specification of Flk1+ Mesoderm to the Hematopoietic Lineage
The findings from the previous set of experiments demonstrate that Flk1+ mesodermal progenitors induced with the combination of Activin A and Wnt3a or BMP4 are highly enriched for cells with hematopoietic potential (Figure 4). Although these three pathways are important to induce Flk1+ cells that can respond to VEGF and generate CD41+ cells, their role in the hematopoietic specification from the Flk1+ mesodermal cells is not known.
To determine if individual factors can induce the progression of Flk1+ mesoderm to the hematopoietic lineage, Flk1+ cells were isolated by cell sorting and reaggregated with different factors from 1 to 3 days. To avoid multiple cell sorting steps, the ES cells were induced with the combination of Activin A, BMP4, and Wnt3a. By day 4, 90% of the cells within the EBs expressed GFP-Bry, and of these, 50% expressed Flk1 (data not shown). At this stage, GFP-Bry+/Flk1+ cells were sorted and assayed for hematopoietic colony-forming ability in methylcellulose. This GFP-Bry+/Flk1+ population did not give rise to any hematopoietic colonies when plated directly into methylcellulose (data not shown). When reaggregated in the presence of VEGF for 1 or more days, the GFP-Bry+/Flk1+ population acquired the potential to generate hematopoietic colonies. As shown in Figure S2, the highest number of primitive erythroid colonies was obtained from aggregates exposed to VEGF for 2 days. Given these observations, all subsequent analyses were carried out on cells aggregated for 2 days in the presence of different factors. In the absence of VEGF, the addition of either Activin A, BMP4, or Wnt3a did not lead to significant hematopoietic colony formation (Figure 5A). In addition, only treatment with VEGF prevented significant cell death (Figure S3). These data indicate that VEGF has an important role in hematopoietic specification and/or survival that cannot be substituted by Activin A, BMP4, or Wnt3a. Relative colony numbers per 10,000 cells plated are shown in Figure S4; these results are very similar to the total colonies per culture shown in Figure 5.
Figure 5. Canonical Wnt Signaling Is Required for Primitive Erythroid Development.
(A–C) GFP-Bry+/Flk1+ cells were isolated by cell sorting from day 4 EBs differentiated with Activin A (2 ng/ml), Wnt3a (3–5 ng/ml), and BMP4 (0.3 ng/ml). These mesoderm cells were reaggregated and cultured for 2 days with VEGF (5 ng/ml), BMP4 (0.3 ng/ml), Activin A (2 ng/ml), DKK1 (300 ng/ml), Wnt3a (50 ng/ml), SB-431542 (6 μM), and BMPR1A-Fc plus BMPR1B-Fc (500 ng/ml each) or combinations of these factors as indicated. Methylcellulose hematopoietic colony assays were performed on each population after 2 days of culture. Primitive erythroid (∼550 per 10,000 cells in VEGF treated) and definitive hematopoietic (both myeloid and erythroid combined; ∼550 per 10,000 cells in VEGF treated) total colony numbers per treatment were determined. All data are normalized to the VEGF-treated cells. (A) Culture with individual factors or inhibitors. (B) Culture with VEGF plus inhibitors as indicated. (C) Culture with VEGF plus inducers as indicated.
(D) Flk1+ cells isolated from Activin A, Wnt3a, and BMP4-induced EBs generated from the A2lox.sbcat ES cell line were cultured for 2 days with VEGF in the presence and absence of doxycycline and/or DKK1. Methylcellulose hematopoietic colony assays were performed as above. Numbers shown are normalized to the uninduced control.
Errors bars indicate ± SEM (n = 4, [A]−[C]) (n = 3, [D]) (**p value < 0.0004, *p value < 0.05). (Act, Activin A; R, BMPR1A-Fc and BMPR1B-Fc; Def, definitive hematopoietic colonies; Dox, doxycycline; Prim, primitive erythrocyte colonies; SB, SB-431542; −Stim, minus stimulation; and V, VEGF).
Although the findings above indicate that treatment with VEGF alone can lead to hematopoietic progenitor formation, endogenous Activin/Nodal, BMP, or Wnt secreted by the cells in culture may also play a role. To define the requirement of these pathways in the context of VEGF stimulation, inhibitors of Activin/Nodal, BMP, and Wnt were used in combination with VEGF during the reaggregation of Flk1+ mesodermal cells. The addition of DKK1, SB, and BMPRs to the VEGF-induced population did not significantly impact the frequency (Figure S4B) or total number of definitive hematopoietic progenitors that developed (Figure 5B). In contrast, primitive erythroid colony formation was completely blocked by the addition of these inhibitors. To determine which pathway was responsible for the block in primitive erythroid development, Flk1+ cells were reaggregated with VEGF plus each individual inhibitor of Activin/Nodal, BMP, or Wnt signaling. Addition of DKK1 completely blocked primitive erythroid development. The frequency and total number of definitive colony progenitors were also reduced compared to the VEGF-treated group; however, the observed reduction was not statistically significant (Figure S4B and Figure 5B). The addition of SB to block Activin/Nodal signaling or soluble BMPRs to block BMP signaling had no effect on either primitive or definitive colony formation. These results suggest that the maturation of Flk1+ mesoderm into cells capable of forming definitive hematopoietic colonies is independent of Activin/Nodal, BMP, and Wnt signaling while primitive erythropoiesis has an absolute requirement for canonical Wnt signaling.
The addition of Activin A or Wnt3a together with VEGF consistently resulted in an increase in both the frequency (Figure S4C) and total number (Figure 5C) of primitive erythroid progenitors. Although the increase in total numbers did not reach statistically significant levels, the change in the frequency of progenitors induced with Wnt3a and VEGF was significant (Figure S4C). The addition of BMP4 did not significantly impact the number of primitive erythroid progenitors. None of the factors significantly altered the frequency or total number of definitive progenitors. Taken together, these findings indicate that Wnt signaling in the Flk1+ population is absolutely required in primitive erythroid development, whereas Activin/Nodal and BMP signaling are dispensable.
To confirm the importance of Wnt signaling on specification of the primitive erythroid lineage from Flk1+ mesoderm, the canonical Wnt pathway was induced in an ES cell line that overexpresses a stabilized form of β-catenin in a doxycycline-inducible manner (Lindsley et al., 2006). Induction of β-catenin (+Dox) in the Flk1+ cells reaggregated in the presence of VEGF resulted in more than a 3-fold increase in the frequency and total number of primitive erythroid progenitors compared to uninduced Flk1+ cells reaggregated in the presence of VEGF alone (Figure S4D and Figure 5D). The induction of β-catenin had no effect on the specification of the definitive lineages. Expression of the stabilized β-catenin was also able to reverse the block in primitive erythroid development mediated by DKK1, indicating that these inhibitory effects on specification of the primitive erythroid lineage were due to suppression of Wnt signaling. These findings are consistent with those in the Wnt3a or DKK1-treated cultures and further demonstrate a role for canonical Wnt signaling in the specification of the primitive erythroid lineage from Flk1+ mesoderm.
Discussion
During embryogenesis, development is controlled by the concerted actions of many signaling pathways that function together to specify a given cell fate. To understand the molecular mechanisms driving differentiation, it is essential to examine the complexities of these interactions on isolated populations that represent distinct stages of development. The ES cell differentiation system is well suited to this approach, as it provides access to such populations and it is amenable to the activation and suppression of multiple pathways at once using appropriate agonists and antagonists. Taking advantage of the power of this model, we have investigated the roles of Activin/Nodal, BMP, and Wnt signaling at distinct stages along the differentiation program of ES cells to blood. The outcome of these studies has provided a basic understanding of the factors regulating different developmental steps and in doing so has uncovered a role for Wnt signaling in the specification of the primitive erythroid lineage (Figure 6).
Figure 6. Schematic Representation of Hematopoietic Development during ES Cell Differentiation Summarizing the Role of the Different Signaling Pathways Identified in This Study.
Studies using mouse mutants have demonstrated an essential role for nodal and Wnt signaling at the earliest stages of primary germ layer formation, as nodal−/− and wnt3−/− embryos all fail to gastrulate (Conlon et al., 1994; Liu et al., 1999). A similar requirement for these pathways in gastrulation and germ layer specification was demonstrated in both zebrafish and Xenopus (Feldman et al., 1998; Jones et al., 1995; Vonica and Gumbiner, 2002). In contrast to these pathways, the role of BMP signaling in PS formation is less clear. Although mutations in bmp4, bmpr1a, or bmpr2 all compromise gastrulation to some extent (Beppu et al., 2000; Mishina et al., 1995; Winnier et al., 1995), inactivation of bmpr1a specifically in the epiblast does not disrupt PS formation (Miura et al., 2006). Our previous studies with the ES cell system demonstrated that Wnt and Activin/Nodal are essential for the establishment of the PS in culture, indicating that regulation of key developmental events is recapitulated in this model (Gadue et al., 2006). The findings presented in this report show that BMP signaling is not essential for the induction of the in vitro PS-like population. BMP4 was found to induce a brachyury expressing PS population, but it does so indirectly, through the endogenous activation of the Nodal and Wnt pathways. A similar scenario may take place in vivo where BMP signaling from the extraembryonic ectoderm may be required to initiate the Wnt and Nodal pathways. Disruption of signaling at this stage would significantly impact gastrulation. Once Nodal and Wnt3 are induced, however, gastrulation could proceed in the absence of BMP, as observed in the epiblast-specific bmpr1a-deficient animals. Although BMP is not required for the development of the PS-like population in vitro, it does exert a “dominant posteriorizing” effect as demonstrated by the inhibition of the development of the Activin A-induced anterior PS population. These findings are consistent with studies in the mouse embryo that revealed that inhibitors of BMP signaling are expressed in the region of the anterior PS (Beddington and Robertson, 1999).
To track the establishment of hematopoietic mesoderm from the PS, we monitored the development of Flk1+ and CD41+ populations and the upregulation of scl and gata1. Flk1 was initially identified as a marker of the vascular lineage (Yamaguchi et al., 1993). Subsequent studies demonstrated that this receptor is expressed on posterior mesoderm, on the hemangioblast, and on the earliest hematopoietic and vascular progenitors (Choi et al., 1998; Huber et al., 2004; Kabrun et al., 1997). Lineage tracing experiments and marker analyses indicated that the expression of Flk1 extends beyond the hematopoietic and vascular system and demonstrated that it is expressed at early stages during the patterning of cardiac and somitic mesoderm (Ema et al., 2006; Motoike et al., 2003). In this report, we showed that Flk1 expression is associated with establishment of the mesoderm fate, as Flk1+ cells could no longer generate endoderm after Activin A treatment. Using the GFP-Bry+/Flk1− PS cells as a target population, we show that the optimal induction of Flk1+ hematopoietic mesoderm required the combination of Activin/Nodal, Wnt, and BMP signaling. In contrast, stimulation with either Activin A or BMP4 alone resulted in the upregulation of nkx2.5, indicative of cardiac development, whereas unstimulated or Wnt3a-induced cultures led to the expression of meox1, a gene associated with somitic mesoderm development. These findings are consistent with the above lineage tracing studies, which show that Flk1+ cells can contribute to many mesodermal lineages, including skeletal muscle and heart (Ema et al., 2006; Motoike et al., 2003).
A comparison of the regulation of induction of Flk1 from the PS cells to that of the prestreak epiblast-like population revealed interesting and important differences. BMP4 appeared to be more efficient at upregulating Flk1 when added to the day 2 epiblast-like population compared to the induction from GFP-Bry+/Flk1− PS-like cells. Park et al. also observed that BMP4 alone could induce Flk1 expression when included in the cultures early during the differentiation protocol (Park et al., 2004). Although BMP4 was effective in Flk1 induction from early stage cells, our gene expression analyses and inhibitor studies indicate that it mediates this induction through the upregulation of wnt3 and nodal, suggesting that all three pathways are involved. The low levels of Flk1 expression induced by BMP4 from the PS (GFP-Bry+) cells may reflect a reduced ability to induce the other pathways in this population. The reverse pattern was observed with Activin A, which was considerably more effective at inducing Flk1 from the PS-like population than from the early epiblast-like cells. In this case, the differential effects appear to be associated with the relative levels of endogenous bmp4, bmp2, and the BMP inhibitor chrd. These observations demonstrate that the environment within the developing EBs changes with time and highlight the importance of examining the role of signaling pathways on isolated populations rather than on intact EBs. The cytokine environments that are established within the EBs during these early developmental stages may reflect a certain level of spatial organization of the developing populations within these structures. Support for this interpretation is provided by in situ hybridization analysis demonstrating that brachyury expressing cells are distributed throughout the developing EBs, whereas those expressing Flk1 are located at the outer edge of the EB (Robertson et al., 2000). Although the significance of this spatial segregation is presently unclear, it may play a role in determining the factors that are produced and the populations that are induced within intact EBs.
The analysis of the final step, the specification of Flk1+ mesoderm to a hematopoietic fate, uncovered a role for Wnt signaling in the establishment of the primitive erythroid lineage. Although considerable effort has been directed at understanding the regulation and maturation of the definitive hematopoietic program (Baron and Fraser, 2005), little is known about the signaling pathways that regulate primitive erythropoiesis. Our studies using the Wnt inhibitor, Wnt3a, and the inducible activated β-catenin expressing ES cell line clearly demonstrate that activation of a β-catenin signaling pathway is essential for the establishment of the primitive, but not the definitive, hematopoietic lineage. Although comparable stage-specific evaluation of these signaling pathways is difficult, if not impossible, in the early embryo, the expression of Wnt3, Wnt3a, and Wnt8a in the posterior PS and the early yolk sac suggests that Wnt signaling may play a similar role in the establishment of the primitive erythroid lineage in vivo (Yamaguchi, 2001). The role of Wnt in hematopoietic commitment has not been investigated in any detail. In contrast to Wnt, BMP signaling does not appear to be required for hematopoietic specification of Flk1 mesoderm. This finding is consistent with recent in vivo studies demonstrating that deletion of bmpr1a in Flk1 expressing cells had no effect on hematopoiesis (Park et al., 2006). Although a compensatory role of other type 1 receptors could not be excluded in these in vivo studies, our ability to inhibit all BMP signaling with the soluble receptors BMPR1A and 1B strongly suggests that this pathway is not required for hematopoietic specification.
In summary, the findings presented in this study establish a roadmap of the minimal signaling pathways that regulate the developmental progression from ES cells to the hematopoietic lineage. By evaluating multiple pathways on isolated populations representing distinct stages of development, we were able to define stage-specific roles for these different pathways and uncover a role for Wnt signaling in the specification of the primitive erythroid lineage. The approach used and the outcome of this study highlight the strengths of the ES cell model for investigating primary germ layer induction and specification and establish a platform for future studies aimed at gaining a more detailed understanding of these and other developmental programs.
Experimental Procedures
ES Cell Maintenance and Differentiation
The GFP-Bry/CD4-Foxa2 ES cells were maintained in a modified serum-free/feeder-free culture system (Gadue et al., 2006; Ying et al., 2003) and differentiated in a serum-free media as described previously (Gadue et al., 2006). In brief, ES cells were trypsinized and cultured in serum-free differentiation media without any additional growth factors to form EBs for 48 hr. At this stage, the EBs were dissociated with trypsin and reaggregated in serum-free differentiation media with the addition of various growth factors or inhibitors as indicated. In most experiments, the EBs were harvested 2 days later (total of 4 days of differentiation), the cells dissociated, and then appropriate populations were isolated by cell sorting. For reaggregation, sorted cells were cultured at 250,000 cells/ml in low-cluster dishes (Costar). The ES line A2lox.sbcat, containing a stabilized β-catenin gene under control of a doxycycline-regulated promoter (Lindsley et al., 2006), was adapted to serum-free/feeder-free culture. These cells were differentiated as described above, with the exception that the initial stage of EB formation in the absence of growth factors was shorted from 48 to 24 hr and DKK1 was added after 6 hr of reaggregation to allow time for the doxycycline induction of β-catenin. Human Activin A, human BMP4, mouse BMPR1A-Fc, mouse BMPR1B-Fc, human DKK1, human VEGF, and mouse Wnt3a were purchased from R&D systems, and SB-431542 was obtained from Tocris.
RT-PCR and Quantitative Real-Time PCR
Total RNA was prepared with the RNeasy mini or micro kits (QIAGEN) and treated with RNase-free DNase (QIAGEN). One-hundred nanograms to 1 μg RNA was reverse transcribed into cDNA by using random hexamers with Superscript II Reverse Transcriptase (Invitrogen). PCR was performed with Taq polymerase (Promega) or Platinum Taq (Invitrogen). Real-time quantitative PCR was performed on a MasterCycler EP RealPlex (Eppendorf) or the ABI 7900HT (Applied Biosystems). All experiments were done in triplicate with Platinum SYBR Green qPCR SuperMix or SYBR GreenER qPCR SuperMix (Invitrogen). The oligonucleotide sequences and PCR cycle conditions are listed in the supporting text (Table S1). A 10-fold dilution series of mouse genomic DNA standards ranging from 50 ng/μl to 5 pg/μl was used to evaluate the efficiency of the PCR and calculate the copy number of each gene relative to the house keeping gene Actb.
Flow Cytometry and Cell Sorting
EBs generated from the ES cell differentiations were dissociated by incubation with trypsin for 1–2 min and stained for the following cell surface antigens: anti-mouse CD41-Alexa 647 (1B5), anti-mouse Flk1-biotin, anti-mouse CD31-phycoerythrin (PharMingen), anti-human CD4-phycoerythrin or -allophycocyanin (Caltag), and streptavidin-allophycocyanin or -phycoerythrin (PharMingen). To assess Smad1/5 phosphorylation, intracellular staining was performed as described previously (Krutzik and Nolan, 2003). In short, day 4 EBs treated as indicated in the text were dissociated with trypsin/EDTA, and the resulting single cells were fixed with 1.6% paraformaldehyde for 10 min at 37°C and permeabilized with 90% ice-cold methanol for 20 min. Staining was performed with rabbit monoclonal anti-phospho-smad1/5 or anti-GST as a control (Cell Signaling Technologies) and revealed with F(ab′)2 Donkey anti-rabbit IgG-phycoerythrin (Jackson ImmunoResearch). The cells were acquired with a LSR II or FACSCalibur flow cytometer (Becton Dickenson) or sorted on a MoFlo (Cytomation Systems) or FACSVantage (Becton Dickenson) cell sorter. Analysis was performed with FlowJo software (Tree Star Inc.).
Hematopoietic Progenitor Assay
For the hematopoietic progenitor assay, EBs induced under various conditions were dissociated by trypsin to a single-cell suspension and plated in methylcellulose containing hematopoietic growth factors as described previously (Kennedy and Keller, 2003). Colonies were scored based on their morphology: primitive erythrocyte, definitive erythrocyte, macrophage, erythrocyte-macrophage, erythrocyte-megakaryocyte, granulocyte-macrophage, and mixed colonies containing at least three cell types. Colony numbers were normalized to the VEGF-treated group, and the mean and standard errors of the mean of four independent experiments were calculated. ANOVA statistical analysis was used to evaluate the significance among the different groups. The Bonferroni/Dunn correction factor was applied to the statistical analysis, which restricted our level of significance to p values below 0.0004. Experiments using the A2lox.sbcat ES cell line were analyzed with a Student's t test.
Supplementary Material
Acknowledgments
We would like to thank Kenneth Murphy at Washington University School of Medicine for the β-catenin overexpressing ES cell line. We would like to thank members of the Keller laboratory for critical discussions of the manuscript.
Footnotes
Supplemental Data include four figures and one table and can be found with this article online at http://www.cellstemcell.com/cgi/content/full/2/1/60/DC1/.
References
- Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991;114:1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, et al. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- Baron MH, Fraser ST. The specification of early hematopoiesis in the mammal. Curr Opin Hematol. 2005;12:217–221. doi: 10.1097/01.moh.0000163217.14462.58. [DOI] [PubMed] [Google Scholar]
- Beddington RS, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. doi: 10.1016/s0092-8674(00)80560-7. [DOI] [PubMed] [Google Scholar]
- Begley CG, Aplan PD, Denning S, Haynes BF, Waldman TA, Kirsch SS. The gene SCL is expressed during early hematopoiesis and encodes a differentiation-related DNA-binding motif. Proc Natl Acad Sci USA. 1989;86:10128–10132. doi: 10.1073/pnas.86.24.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol. 2000;221:249–258. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- Candia AF, Hu J, Crosby J, Lalley PA, Noden D, Nadeau JH, Wright CV. Mox-1 and Mox-2 define a novel homeobox gene subfamily and are differentially expressed during early mesodermal patterning in mouse embryos. Development. 1992;116:1123–1136. doi: 10.1242/dev.116.4.1123. [DOI] [PubMed] [Google Scholar]
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Dieterlen-Lievre F. On the origin of haemopoietic stem cells in the avian embryo: an experimental approach. J Embryol Exp Morphol. 1975;33:607–619. [PubMed] [Google Scholar]
- Ema M, Takahashi S, Rossant J. Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood. 2006;107:111–117. doi: 10.1182/blood-2005-05-1970. [DOI] [PubMed] [Google Scholar]
- Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, Schier AF, Talbot WS. Zebrafish organizer development and germ-layer formation require nodal- related signals. Nature. 1998;395:181–185. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- Ferkowicz MJ, Starr M, Xie X, Li W, Johnson SA, Shelley WC, Morrison PR, Yoder MC. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development. 2003;130:4393–4403. doi: 10.1242/dev.00632. [DOI] [PubMed] [Google Scholar]
- Friedman JR, Kaestner KH. The Foxa family of transcription factors in development and metabolism. Cell Mol Life Sci. 2006;63:2317–2328. doi: 10.1007/s00018-006-6095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Jones CM, Kuehn MR, Hogan BL, Smith JC, Wright CV. Nodal-related signals induce axial mesoderm and dorsalize mesoderm during gastrulation. Development. 1995;121:3651–3662. doi: 10.1242/dev.121.11.3651. [DOI] [PubMed] [Google Scholar]
- Kabrun N, Buhring HJ, Choi K, Ullrich A, Risau W, Keller G. Flk-1 expression defines a population of early embryonic hematopoietic precursors. Development. 1997;124:2039–2048. doi: 10.1242/dev.124.10.2039. [DOI] [PubMed] [Google Scholar]
- Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PP, et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- Kennedy M, Keller GM. Hematopoietic commitment of ES cells in culture. Methods Enzymol. 2003;365:39–59. doi: 10.1016/s0076-6879(03)65003-2. [DOI] [PubMed] [Google Scholar]
- Kispert A, Herrmann BG. Immunohistochemical analysis of the Brachyury protein in wild-type and mutant mouse embryos. Dev Biol. 1994;161:179–193. doi: 10.1006/dbio.1994.1019. [DOI] [PubMed] [Google Scholar]
- Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry. 2003;A55:61–70. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
- Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993;119:969. doi: 10.1242/dev.119.3.969. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Mikkola HK, Fujiwara Y, Schlaeger TM, Traver D, Orkin SH. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood. 2003;101:508–516. doi: 10.1182/blood-2002-06-1699. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- Miura S, Davis S, Klingensmith J, Mishina Y. BMP signaling in the epiblast is required for proper recruitment of the prospective paraxial mesoderm and development of the somites. Development. 2006;133:3767–3775. doi: 10.1242/dev.02552. [DOI] [PubMed] [Google Scholar]
- Moore M, Metcalf D. Ontogeny of the hematopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970;18:279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Motoike T, Markham DW, Rossant J, Sato TN. Evidence for novel fate of Flk1+ progenitor: contribution to muscle lineage. Genesis. 2003;35:153–159. doi: 10.1002/gene.10175. [DOI] [PubMed] [Google Scholar]
- Natsume T, Tomita S, Iemura S, Kinto N, Yamaguchi A, Ueno N. Interaction between soluble type I receptor for bone morphogenetic protein and bone morphogenetic protein-4. J Biol Chem. 1997;272:11535–11540. doi: 10.1074/jbc.272.17.11535. [DOI] [PubMed] [Google Scholar]
- Palis J, Roberston S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- Park C, Afrikanova I, Chung YS, Zhang WJ, Arentson E, Fong Gh G, Rosendahl A, Choi K. A hierarchical order of factors in the generation of FLK1- and SCL-expressing hematopoietic and endothelial progenitors from embryonic stem cells. Development. 2004;131:2749–2762. doi: 10.1242/dev.01130. [DOI] [PubMed] [Google Scholar]
- Park C, Lavine K, Mishina Y, Deng CX, Ornitz DM, Choi K. Bone morphogenetic protein receptor 1A signaling is dispensable for hematopoietic development but essential for vessel and atrioventricular endocardial cushion formation. Development. 2006;133:3473–3484. doi: 10.1242/dev.02499. [DOI] [PubMed] [Google Scholar]
- Pevny L, Simon MC, Robertson E, Klein WH, Tsai SF, D'Agati V, Orkin SH, Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Robertson SM, Kennedy M, Shannon JM, Keller G. A transitional stage in the commitment of mesoderm to hematopoiesis requiring the transcription factor SCL/tal-1. Development. 2000;127:2447–2459. doi: 10.1242/dev.127.11.2447. [DOI] [PubMed] [Google Scholar]
- Saga Y, Hata N, Kobayashi S, Magnuson T, Seldin MF, Taketo MM. MesP1: a novel basic helix-loop-helix protein expressed in the nascent mesodermal cells during mouse gastrulation. Development. 1996;122:2769–2778. doi: 10.1242/dev.122.9.2769. [DOI] [PubMed] [Google Scholar]
- Schute-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Smith JC, Price BM, Green JB, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Tada S, Era T, Furusawa C, Sakurai H, Nishikawa S, Kinoshita M, Nakao K, Chiba T, Nishikawa S. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development. 2005;132:4363–4374. doi: 10.1242/dev.02005. [DOI] [PubMed] [Google Scholar]
- Tam PP, Behringer RR. Mouse gastrulation: the formation of a mammalian body plan. Mech Dev. 1997;68:3–25. doi: 10.1016/s0925-4773(97)00123-8. [DOI] [PubMed] [Google Scholar]
- Vonica A, Gumbiner BM. Zygotic Wnt activity is required for Brachyury expression in the early Xenopus laevis embryo. Dev Biol. 2002;250:112–127. doi: 10.1006/dbio.2002.0786. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP. Heads or tails: Wnts and anterior-posterior patterning. Curr Biol. 2001;11:R713–R724. doi: 10.1016/s0960-9822(01)00417-1. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.