Abstract
Inflammation produces marked changes in lipid metabolism, including increased serum fatty acids (FAs) and triglycerides (TGs), increased hepatic TG production and VLDL secretion, increased adipose tissue lipolysis, and decreased FA oxidation in liver and heart. Lipopolysaccharide (LPS) also increases TG and cholesteryl ester levels in kidneys. Here we confirm these findings and define potential mechanisms. LPS decreases renal FA oxidation by 40% and the expression of key proteins required for oxidation of FAs, including FA transport protein-2, fatty acyl-CoA synthase, carnitine palmitoyltransferase-1, medium-chain acyl-CoA dehydrogenase, and acyl-CoA oxidase. Similar decreases were observed in peroxisome proliferator-activated receptor α (PPARα)-deficient mice. LPS also caused a reduction in renal mRNA levels of PPARα (75% decrease), thyroid hormone receptor α (TRα) (92% decrease), and TRβ (84% decrease), whereas PPARβ/δ and γ were not altered. Expression of PGC1 α and β, coactivators required for PPARs and TR, was also decreased in kidneys of LPS-treated mice, as were mitochondrial genes regulated by PGC1 (Atp5g1, COX5a, Idh3a, and Ndufs8). Decreased renal FA oxidation could be a by-product of the systemic coordinated host response to increase FAs and TGs available for host defense and/or tissue repair. However, the kidney requires energy to support its transport functions, and the inability to generate energy via FA oxidation might contribute to the renal failure seen in severe sepsis.
Keywords: acute phase, infection, inflammation, peroxisome proliferator-activated receptor, PGC1, thyroid hormone receptor, carnitine palmitoyltransferase 1
Infection and inflammation induce an acute-phase response, which results in increases in specific plasma proteins (positive acute-phase proteins) and decreases in other plasma proteins (negative acute-phase proteins) (1). In addition to changes in plasma protein levels, there are also marked changes in circulating lipid and lipoprotein levels, with characteristic findings of increases in serum-free FAs and triglyceride (TG) levels and decreases in serum HDL levels (2). Underlying these changes in serum lipid levels are alterations in lipid metabolism, particularly FA metabolism. In adipose tissue, there is enhanced lipolysis and TG mobilization (3, 4), whereas in the liver and heart, there is a decrease in FA oxidation (5–8). In the liver, the decrease in FA oxidation is accompanied by an increase in FA synthesis and the enhanced reesterification of FAs into TG, leading to an increase in VLDL production and secretion (2, 3).
The molecular mechanisms underlying these acute-phase changes in FA and TG metabolism are not fully understood, but recent studies have suggested that decreases in the expression of nuclear hormone receptors, particularly retinoid X receptors (RXRs), peroxisome proliferator-activated receptors (PPARs), and thyroid hormone receptors (TRs) play a key role (2, 9, 10). There are three peroxisome proliferator-activated receptor (PPAR) isoforms, PPARα, PPARβ/δ, and PPARγ; all three receptors are activated by FAs and then stimulate the transcription of specific genes that govern various aspects of FA metabolism (11). PPARs heterodimerize with RXRs, and bind to specific response elements present in the promoters of their target genes. Among these PPARs, PPARα activates a program of genes involved in FA oxidation, including carnitine palmitoyl transferase 1 (CPT1), medium-chain acyl CoA dehydrogenase (MCAD), and acyl-CoA oxidase (ACO) (11, 12). Moreover, PPARα also activates the genes that mediate the uptake of FAs, such as FA transport proteins (FATPs), and their initial derivatization for entry into the β-oxidation pathway, fatty acyl-CoA synthase (FACS) (11, 12). Consistent with its role in regulating β-oxidation, PPARα is primarily expressed in the tissues with high mitochondrial and β-oxidation activity, such as liver, heart, muscle, and kidney (11, 12). In addition, activation of TR, another nuclear hormone receptor, which forms heterodimers with retinoid X receptor (RXR), also stimulates FA oxidation by increasing the expression of CPT1 (13–15).
In previous studies, we have shown that the expression of RXR, PPARα, and TRα and -β and the key coactivator PGC1 is decreased in both liver and heart following the administration of lipopolysaccharide (LPS) (9, 10, 16). In addition, PPARα and TR target genes that are involved in FA metabolism in these tissues are also markedly reduced, suggesting that the decrease in these nuclear hormone receptors and coactivators plays an important role in regulating lipid metabolism during the acute-phase response (2, 9, 10, 16). Similarly, in adipose tissue, RXR and PPARγ expression is decreased during the acute-phase response, which could contribute to the increase in lipolysis and circulating FFAs that characterize the acute-phase response (17).
Recent studies have shown that LPS administration results in a marked increase in TG concentrations in the kidney (18). In addition, studies have shown that in the kidney, the mRNA levels of diacylglycerol acyltransferase (DGAT) 1 and 2, enzymes required for TG synthesis, were increased in LPS-treated animals, which could contribute to the increase in TG levels in the kidney (19). Furthermore, recent studies by our laboratory have shown that the expression of RXRα, RXRβ, LXRα, and FXR is decreased in the kidney following LPS administration (20, 21). Given that our previous studies have suggested that the changes in FA and TG metabolism in the liver, heart, and adipose tissue during the acute-phase response were in part mediated by changes in the expression of PPARs, TRs, and RXR, we hypothesized that LPS treatment would decrease the expression of PPARα, PGC1, and TRs in mouse kidney, leading to a decrease in FA oxidation, which could then contribute to the renal TG accumulation that occurs with infection and inflammation.
MATERIALS AND METHODS
Materials
LPS (Escherichia coli 55:B5) was obtained from Difco and freshly diluted to the desired concentration in pyrogen-free 0.9% saline. Tri-Reagent was obtained from Sigma. Effectene transfection reagent was purchased from Qiagen. [α-32P]dCTP (3,000 Ci/mmol) and [1-14C]oleic acid (51.0 mCi/mmol) were purchased from PerkinElmer Life Sciences. Oligo(dT)-cellulose type 77F was from Amersham Biosciences. iScript™cDNA Synthesis Kit and iQ™SYBR Green Supermix were purchased from BioRad, Hercules, CA.
Animals
Eight week-old female and male C57BL/6 mice and PPARα-deficient mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were maintained in a barrier room with a normal 12 h light cycle and were provided with Harlan standard rodent chow and water ad libitum. For a typical experiment, mice were injected intraperitoneally with 100 μg of LPS in saline or with saline alone. The dose of LPS used in this study has significant effects on TG and cholesterol metabolism, but is nonlethal because the half-maximally lethal dose (LD50) for LPS in rodents is ∼30–50 mg/kg body weight (3, 22). Food was withdrawn immediately after the injection of LPS because LPS induces anorexia in rodents. At the indicated time points, mice were euthanized with an overdose of halothane and kidneys were excised and stored at −80°C. All experiments were performed according to protocols approved by the Animal Studies Subcommittee of the San Francisco Veterans Affairs Medical Center.
Lipid analysis
Lipids were extracted with CHCl3-CH3OH-H2O (4:2:3; by volume) from homogenized kidney lysate. Lipid composition was examined by high-performance TLC.
FA oxidation
Fresh kidneys were removed from control and 16 h LPS-treated animals and placed in cold 0.9% saline solution. Slices (0.5 mm) were then prepared with a McIlwain tissue slicer, and 100 mg portions were placed in center well 25 ml Erlenmeyer flasks. Each flask contained 2 ml Krebs-Ringer phosphate buffer (pH 7.4), 1 μCi 0.4 mM [14C]oleic acid, and 0.15 mM BSA (FA-free). Blank samples contained no tissue. Flasks were then sealed with septum caps and incubated in a shaking water bath for 2 h at 37°C. After the 2 h incubation, 0.9 ml 1 N NaOH was added to each center well through the stopper cap, and 1 ml 1 N H2SO4 was also added to the incubation mix through the stopper cap. Flasks were allowed to incubate an additional 15 min, after which time, flasks were removed from the water bath, caps were removed, and 450 ml aliquots of NaOH (containing trapped [14C]CO2) were removed from center wells and added to counting vials containing 12 ml of ScintiSafe 30% LSC-counting cocktail from Fisher Scientific.
Isolation of RNA and Northern blot analysis
Total RNA from mouse kidney was isolated from 100 mg of snap-frozen tissue using Tri-Reagent. Poly(A)+ RNA was purified using oligo(dT) cellulose and quantified by measuring absorption at 260 nm. Ten micrograms of poly(A)+ RNA was denatured and electrophoresed on a 1% agarose/formaldehyde gel. The uniformity of sample loading was checked by ultraviolet visualization of the ethidium bromide-stained gel before electrotransfer to Nytran membrane (Schleicher and Schuell, Keene, NH). Prehybrization, hybridization, and washing procedures were performed as described previously (9). Membranes were probed with [α-32P]dCTP-labeled cDNAs using Rediprime™ II random priming labeling system (Amersham, UK). mRNA levels were detected by autoradiography or by Personal FX phosphoimager (BioRad) and quantified using Quantity One software (BioRad). FACS cDNA was a gift from Dr. P. Smith (Abbott Laboratory, Columbus, OH). The mouse cDNA probes for Northern blotting were generated by reverse transcriptase PCR, starting from total RNA from mouse liver (FATP-2, ME, TRβ) and heart (TRα, CPT1α, and CPT1β) using the primers shown in Table 1.
TABLE 1.
Primers for cDNA probes
| CPT1α | TCGGTACTCTCTGAAGATGGC |
| GAGCAGAGTGGAATCGTGGGA | |
| CPT1β | CGGAAGCACACCAGGCAGT |
| GACGTTTGGAAGCTGTAGAGCA | |
| FATP2 | GGAATATTCAGAGCTTCAGAGTTT |
| TGAATGTGTATGGCGTGCCTGTGCC | |
| ME | CCACCAGCGCGGCTACCTGCTGACGCGGGA |
| CCTCTGACTCGCCGGTGCCGCAGCCCGATG | |
| TRα | ATGGAACAGAAGCCAAGCAAGGTGGAG |
| CTGCAGCAGAGCCACTTCCGTGTCA | |
| TRβ | GCCTGG GACAAGCAGAAGCCCCGT |
| AGCGACATT CCTGGCACTGGTTGCG |
Quantitative real-time PCR
First-strand cDNA was synthesized from 1 μg of total RNA with the iScript™cDNA Synthesis Kit (BioRad). The real-time PCR contained in a final volume 20 μl, 2 μl of cDNA, 450 nM forward and reverse primers, and 10 μl of 2 × SYBR Green PCR Master Mix (BioRad). PCR was carried out in 96-well plates using the Mx3000P™ Real-time PCR System (Stratagene, La Jolla, CA). The relative amount of all mRNAs was calculated using the comparative CT method. 36B4 mRNA was used as the invariant control for all experiments. Quantitative PCR (QPCR) primers are listed in Table 2.
TABLE 2.
Primers for quantitative PCR
| 36B4 | GCGACCTGGAAGTCCAACTAC |
| ATCTGCTGCATCTGCTTGG | |
| ACC | CTGTGCCTGGGATCTTTTGTGTC |
| CCAGGAACACTCCCAGCATGG | |
| AQP3 | TGTGTGTACTGGCCATCGTT |
| GTTGACGGCATAGCCAGAAT | |
| AtP5g1 | AGTTGGTGTGGCTGGATCA |
| GCTGCTTGAGAGATGGGTTC | |
| Cox5a | GGGTCACACGAGACAGATGA |
| GGAACCAGATCATAGCCAACA | |
| CPT1β | CCCATGTGCTCCTACCAGAT |
| CCTTGAAGAAGCGACCTTTG | |
| Cypt4a10 | CTCATTCCTGCCCTTCTCAG |
| GTAGTTCGAAGCGGAGCAGT | |
| FAS | GCTGCGGAAACTTCAGGAAAT |
| AGAGACGTGTCACTCCTGGACTT | |
| FATP 2 | ATGCCGTGTCCGTCTTTTAC |
| CTTCAGACCTCCACGACTCC | |
| GR | GCAAGTGGAAACCTGCTATGC |
| CATACATGCAGGGTAGAGTCATTCTT | |
| HNF 4a | ACCAAGAGGTCCATGGTGTTT |
| GTGCCGAGGGACGATGTAG | |
| Idh3a | CCTCCTGCTTAGTGCTGTGA |
| CGTTGCCTCCCAGATCTTT | |
| LIPIN 1 | CGCCAAAGAATAACCTGGAA |
| TGAAGACTCGCTGTGAATGG | |
| MCAD | ATGCCCTGGATAGGAAGACA |
| CATAGCCTCCGAAAATCTGC | |
| Ndufs8 | TGGCGGCAACGTACAAGTAT |
| CCTCGGATGAGTTCTGTCCA | |
| PPARα | CCTGAACATCGAGTGTCGAATAT |
| GTTCTTCTTCTGAATCTTGCAGCT | |
| PPARβ/δ | CCACGAGTTCTTGCGAAGTC |
| AACTTGGGCTCAATGATGTCA | |
| PPARγ | CCACCAACTTCGGAATCA |
| TTTGTGGATCCGGCAGTTA | |
| PGC1α | TAGGCCCAGGTACGACAGC |
| GCTCTTTGCGGTATTCATCC | |
| PGC1β | CAAGCTCTGACGCTCTGAAGG |
| TTGGGGAGCAGGCTTTCAC | |
| RXRγ | GCCACCCTGGAGGCCTATA |
| AGCAGAAGCTTGGCAAACCT | |
| SRC1 | TGGGTACCAGTCACCAGACA |
| GAATGTTTGCGTTTCCACCT | |
| SRC2 | ACAGAACCAGCCAAACCAAC |
| TGGTTGAGGATTTCCCTCTG | |
| TRAP | CCTTCTTTCTCCGCAGTCAC |
| GGAAGAGCAGCGTAAAATCG | |
| TRα | GGATGGAATTGAAGTGAATGGAA |
| CCGTTCTTTCTTTTTCGCTTTC | |
| TRβ | AACCAGTGCCAGGAATGTC |
| CCTCTTCTCACGGTTCTCCTC |
Statistical analysis
Data are expressed as the mean ± SE of experiments from four to five animals. The difference between the two experimental groups was analyzed using the Student's t-test. The difference between multiple groups was analyzed by ANOVA using the Bonferroni test.
RESULTS
Effect of LPS treatment on serum parameters
As seen in previous experiments, LPS administration increased serum TG (control 59.2 ± 1.4 mg/dl vs. LPS 94.4 ± 9.7 mg/dl, P < 0.01) and serum amyloid A levels (control 57.6 ± mg/dl vs. LPS 792.0 ± 4.9 mg/dl, P < 0.01). Of note, LPS treatment did not affect serum creatinine levels (control 0.77 ± 0.07 mg/dl vs. LPS 0.83 ± 0.07 mg/dl, not significant).
Effect of LPS treatment on lipid composition of kidney
Our initial experiment determined the lipid composition of mouse kidney 16 h after the administration of 100 μg LPS. As previously reported by other investigators (18), TG mass increased by 4-fold with LPS treatment (Fig. 1). Additionally, LPS treatment resulted in a 2-fold increase in total FFA levels in the kidney, whereas cholesteryl ester mass showed a moderate increase (about 30%) (Fig. 1). In contrast, phosphatidylcholine, phosphatidyl ethanolamine, and cholesterol mass were not affected by LPS treatment. These data suggest that LPS administration leads to a specific increase in FFA, TG, and cholesteryl ester levels in the kidney.
Fig. 1.
Lipid composition of female mouse kidney 16 h after the administration of lipopolysaccharide (LPS). A: High-performance TLC plate showing lipid composition of mouse kidney. CE, cholesteryl ester; TG, triglyceride; Chol, cholesterol; PE, phosphatidylethanolamine; PC, phosphatidylcholine. B: Quantitative analysis of the various lipid products seen in A. The plate was scanned using the BioRad Chemidoc XRS system, and data were analyzed using Quantity One software, also from BioRad. Data (means ± SE, n = 5) are expressed as μg/mg protein. * P < 0.05; ** P < 0.01; *** P < 0.001 vs. control.
Effect of LPS treatment on FA oxidation in the kidney
When the FA [1-14C]oleic acid was used as a substrate for measuring renal FA oxidation, the production of 14CO2 was decreased by more than 40% from kidneys obtained from LPS-treated animals, compared with controls (Fig. 2A). This indicates that FA oxidation in mouse kidney is markedly reduced by LPS treatment.
Fig. 2.
Effect of LPS on β-oxidation and the expression of genes that mediate fatty acid uptake, esterification, transport, and oxidation. A: Female animals were injected with either 100 μg LPS or vehicle (0.9% sterile saline). After 16 h, FA oxidation was measured as described in detail in Materials and Methods. Data (means ± SE, n = 5) are expressed as a percentage of controls. *** P < 0.001 vs. control. B, C: Poly(A)+ RNA was isolated from frozen kidney samples, and Northern blot analysis was performed as described in Materials and Methods. Ten micrograms was loaded per lane. For MCAD, AQP3, and Cyp4a10, mRNA levels were determined by quantitative PCR (QPCR). First-strand cDNA was synthesized from mouse kidney total RNA, and real-time PCR was carried out as described in Materials and Methods section. The cycle of threshold (Ct) for MCAD was 18, for AQP3 23, and for Cyp4a10 18. Data (means ± SE, n = 5) are expressed as a percentage of controls. *** P < 0.001. FATP, FA transport protein; FACS, fatty acyl-CoA synthase; CPT1, carnitine palmitoyltransferase 1; ACO, acyl-CoA oxidase; MCAD, medium-chain acyl CoA dehydrogenase; AQP, aquaporin.
Effect of LPS treatment on genes that regulate FA metabolism in the kidney
Decreased FA oxidation following LPS treatment may result from reduced expression of several proteins required for FA oxidation. Therefore, we next determined the effect of LPS treatment on renal expression of genes that mediate FA uptake, esterification, transport, and oxidation. As shown in Fig. 2B, C, at 16 h after LPS treatment, mRNA levels of FATP-2, a dominant form of FA transporter in kidney, were decreased by 77%. However, two other FA transporters, FATP-1 and FATP-4, were not affected by LPS treatment (data not shown). LPS treatment also dramatically decreased expression of FACS, an enzyme responsible for converting FAs to fatty acyl-CoA, by 83%. Moreover, mRNA levels for two enzymes that transport FA moieties across the mitochondrial membranes, CPT1α and CPT1β, were decreased by 22% and 74%, respectively. Finally, expression of ACO and MCAD, key enzymes that mediate FA oxidation, was decreased by 36% and 68%, respectively. These data clearly indicate that the expression of a number of genes that play important roles in FA oxidation is downregulated in the kidney during the acute-phase response.
Although a decrease in the expression of any one of the aforementioned genes may diminish FA oxidation activity, it is likely that the LPS-induced impairment of FA oxidation activity is a result of a coordinate downregulation of several genes that mediate different steps along the FA oxidation pathway. In contrast to the effects of LPS on the genes involved in FA oxidation, the mRNA levels of genes involved in FA synthesis, acetyl CoA carboxylase and FA synthase, were not altered in the kidney following LPS treatment (data not shown). However, other genes regulated by PPARα in the kidney were also downregulated following LPS administration (AQP3 and Cyp4a10) (Fig. 2C).
Effect of LPS treatment on PPAR expression in the kidney
PPARα stimulates the expression of the genes in the FA oxidation pathway that were decreased in the kidney after LPS administration (11, 12). Therefore, we next determined the effect of LPS administration on the mRNA levels of the three PPAR isoforms in mouse kidney. As shown in Fig. 3A, PPARα mRNA levels were decreased by 75% in the kidney of LPS-treated animals. In contrast, the mRNA levels of PPARβ/δ and PPARγ were not altered in the kidney of LPS-treated animals. Unfortunately, due to the presence of numerous nonspecific bands, we were unable to measure PPARα protein levels in the kidney by Western blotting using several commercially available antibodies (Cayman Chemical, R and D Systems, and Santa Cruz Biotechnology). These data indicate that LPS treatment specifically decreases the expression of PPARα in the kidney. Previously, we found a 50% decrease in RXRα, which is an obligate partner for PPARα in the kidney (2, 21).
Fig. 3.
Effect of LPS on peroxisome proliferator-activated receptor (PPAR) and thyroid hormone receptor (TR) mRNA levels. A: Effect of 16 h LPS administration in female mice on PPAR mRNA levels utilizing QPCR. First-strand cDNA was synthesized from mouse kidney total RNA, and real-time PCR was carried out as described in Materials and Methods. The Ct values were 24 for PPARα, 24 for PPARβ/δ, and 28 for PPARγ. Data (means ± SE, n = 4) are expressed as a percentage of controls. *** P < 0.001. B, C: Effect of 16 h LPS administration in female mice on levels of TR and malic enzyme (ME). Poly(A)+ RNA was isolated from frozen kidney samples, and Northern blot analysis was performed as described in Materials and Methods. Ten micrograms was loaded per lane. Data (means ± SE, n = 5) are expressed as a percentage of controls. *** P < 0.001.
Effect of LPS treatment on the expression of TR and other nuclear hormone receptors in the kidney
In addition to PPARα, activation of TR also stimulates the expression of genes involved in FA metabolism, such as CPT1 and malic enzyme (13–15, 23). We therefore next determined the effect of LPS administration on mRNA levels of TR in the kidney 16 h after LPS treatment (Fig. 3B). We found that both TRα and TRβ mRNA levels were decreased, by 92% and 84%, respectively (similar results were seen using QPCR; TRα decreased by 75% and TRβ decreased by 71%). Consistent with the suppression of TRs, mRNA levels of malic enzyme were also decreased by 53% (Fig. 3B). In addition, we also assessed the effect of LPS treatment on the expression of other nuclear hormone receptors in the kidney. We found that the mRNA levels of HNF4 (60%), glucocorticoid receptor (54%), vitamin D receptor (83%), PXR (83%), RXRγ (78%), and ERRα (83%) all decrease with LPS treatment. In contrast, LXRβ did not change with LPS treatment.
Effect of LPS treatment on coactivators in the kidney
Recent studies by our laboratory have shown that the acute-phase response not only decreases the expression of key nuclear hormone receptors that regulate lipid metabolism but also decreases coactivators that are required for the increase in gene transcription that occurs with activation of nuclear hormone receptors (16, 17, 24). Figure 4A shows the effect of LPS treatment on coactivator mRNA levels in the kidney. Both PGC1α and -β were markedly decreased following LPS treatment (75.5% and 65.8% decrease, respectively). In contrast, mRNA levels of SRC1, SRC2, TRAP, and Lipin 1 were not affected by LPS treatment. These results demonstrate that the acute-phase response, in addition to decreasing the expression of specific nuclear hormone receptors, also decreases the expression of a subset of coactivators that regulate gene transcription.
Fig. 4.
Effect of LPS on coactivators and mitochondrial genes. A: Effect of 16 h LPS administration in female mice on levels of coactivators that are required for the increase in gene transcription that occurs with activation of nuclear hormone receptors utilizing QPCR. Ct values were 23 for PGC1α, 26 for PGC1β, 24 for SRC1, 24 for SRC2, 25 for thyroid hormone receptor-associated protein (TRAP), and 22 for Lipin 1. Data (means ± SE, n = 4) are expressed as a percentage of controls. ** P < 0.01. B: Effect of 16 h LPS administration in female mice on levels of mitochondrial genes that are required for β-oxidation utilizing QPCR. The Ct values were 20 for Atp5g1, 19 for COX5a, 21 for Idh3a, and 19 for Ndufs8. Data (means ± SE, n = 4) are expressed as a percentage of controls. ** P < 0.01.
It is well known that PGC1 regulates the expression of mitochondrial genes that play an important role in β-oxidation (25, 26). Therefore, we next determined the effect of LPS treatment on the expression of selected mitochondrial genes. As shown in Fig. 4B, the mRNA levels of Atp5g1, COX5a, Idh3a, and Ndufs8 all decreased in the kidney of LPS-treated animals.
Effect of LPS treatment on mRNA levels in males
The above studies were all carried out in female mice. Therefore, we next determined whether similar changes occur in male mice. In males, 16 h after treatment with 100 μg LPS, there was a 45% decrease in PPARα, a 68% decrease in PGC1α, a 56% decrease in PGC1β, a 64% decrease in TRα, a 54% decrease in TRβ, and a 54% decrease in FATP-2 mRNA levels in the kidney. Thus, similar decreases in kidney gene expression are induced by LPS treatment in male and female mice.
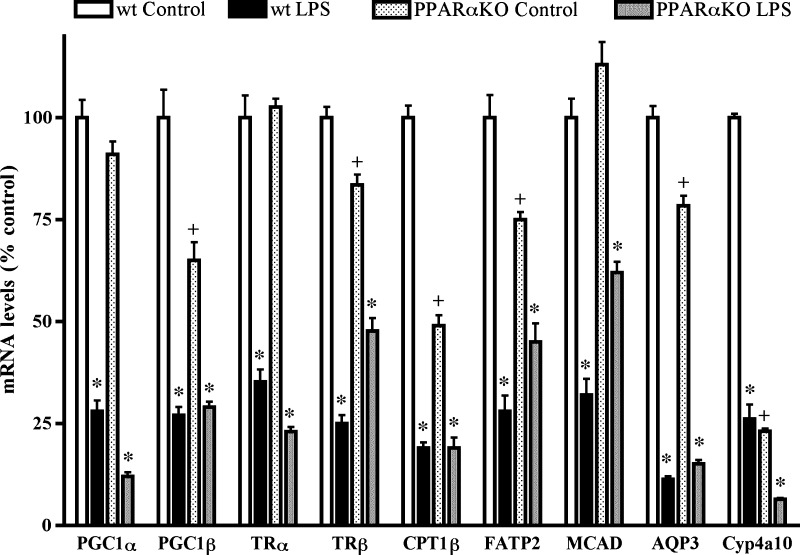
Effect of LPS treatment on mRNA levels in PPARα-deficient mice
As shown in Fig. 5, the mRNA levels of CPT1β and Cyp4a10 were markedly decreased in PPARα knock-out mice, compared with wild-type controls, whereas the levels of PGC1β, FATP2, and AQP3 were modestly decreased. In contrast, the mRNA levels of PGC1α and MCAD were similar in PPARα knock-out and wild-type mice. Following LPS treatment, the mRNA levels of PGC1α, PGC1β, CPT1β, FATP2, MCAD, AQP3, and Cyp4a10 all decreased, indicating LPS regulation of the expression of these genes independent of PPARα.
Fig. 5.
Effect of LPS in PPARα-deficient mice. Effect of 16 h LPS administration in PPARα-deficient and wild-type mice on mRNA levels utilizing QPCR. First-strand cDNA was synthesized from mouse kidney total RNA, and real-time PCR was carried out as described in Materials and Methods. Data (means ± SE, n = 5) are expressed as a percentage of controls. * P < 0.001 LPS vs. its respective control; + P < 0.01 control knock-out vs. control wild type.
DISCUSSION
Infection and inflammation lead to an increase in serum TG levels due to both an increase in VLDL production and a decrease in clearance (2). The dose of LPS that produces hypertriglyceridemia is similar to that which produces fever, anorexia, and changes in acute-phase proteins, suggesting that the increase in serum TG levels is a very sensitive physiologic response to infection rather than a toxic effect (2). In adipose tissue, lipolysis increases and the FFAs are delivered to the liver, where they are reesterified into TG (2, 3). Hepatic de novo FA synthesis is increased, and FA oxidation is decreased, facilitating the increased incorporation of FAs into TG (2, 3, 5, 6). If the production of TG is greater than the ability to secrete TG in VLDL, fatty liver occurs (27). FA binding protein and FATPs are also decreased in heart, muscle, and adipose tissue after LPS treatment (16, 28, 29). Moreover, studies have found a decrease in the uptake and oxidation of FAs and an increase in the accumulation of TG in the heart with sepsis and LPS administration (7, 8).
The mechanism by which infection and inflammation alter FA and TG metabolism is not completely understood, but recent studies have demonstrated that LPS administration decreases the levels of RXRs, PPARs, and TRs in liver, heart, and adipose tissue (9, 10, 16, 17). Moreover, several coactivators, including PGC1α and -β, that enhance the transcriptional activity of RXRs, PPARs, and TRs also decrease in liver, heart, and adipose tissue after LPS treatment (16, 17, 24). In the liver and heart, a decrease in RXRs, TRs, and PPARα and their coactivators could contribute to the decreased FA oxidation, whereas in adipose tissue, the decrease in RXRs and PPARγ and their coactivators could contribute to the increase in lipolysis (9, 10, 16, 17, 24).
Studies by other investigators have shown an increase in renal TG and cholesteryl ester levels in mice treated with LPS (18). In the present manuscript, we confirm these findings. Furthermore, in the present manuscript, we demonstrate that FA oxidation is decreased in the kidney of LPS-treated animals and this metabolic change is associated with a reduction in the expression of a number of key proteins that are important in FA oxidation. As previously reported by others, we find a decrease in FATP2 mRNA levels (19). Additionally, we also demonstrate a decrease in the expression ACS, CPT-1α and -β, MCAD, and ACO. In contrast, the expression of the enzymes required for FA synthesis, acetyl CoA carboxylase and FA synthase, is not altered in the kidney following LPS treatment. We would speculate that the decreased ability to oxidize FAs results in the increased accumulation of FAs, which provides increased substrate for the synthesis of TG and cholesteryl esters. Moreover, this hypothesis is supported by the studies by other investigators, which have shown that LPS treatment increases mRNA levels of DGAT1 and DGAT2, two enzymes that would enhance TG synthesis from FAs (19).
The expression of many of the genes involved in FA oxidation and metabolism is regulated by PPARα and TR. We have previously shown that the levels of LXRα, FXR, and RXRα, the obligate heterodimers partner for both PPARs and TRs, are downregulated in the kidney following LPS administration (20, 21). In the present study, we show that LPS administration markedly suppresses PPARα, TRα, and TRβ expression in mouse kidney. In addition, the expression of two key cofactors, PGC1α and PGC1β, that regulate the ability of PPARs to increase gene transcription, is also decreased in the kidneys of LPS-treated animals. These effects are specific, because the expression of other PPARs (PPARβ/δ and PPARγ) and other coactivators (SRC1, SRC2, TRAP, and Lipin 1) is not altered in the kidneys of LPS-treated animals. The decrease in PPARα, TRα, TRβ, PGC1α, and PGC1β mRNA levels could contribute to the decrease in mRNA levels of FATP-2, FACS, CPT1α, CPT1β, and ACO and therefore account for the reduction in FA oxidation in the kidney during infection and inflammation. Thus, the changes in the regulation of FA oxidation and the increase in TG accumulation in the kidney parallel the changes observed in the liver and heart and are probably part of a coordinated metabolic response during infection and inflammation.
Although PPARα is clearly an important transcription factor in regulating the expression of genes involved in FA oxidation (11, 12), our studies in PPARα knock-out mice clearly demonstrate that multiple factors regulate the expression of genes involved in FA oxidation. For example, CPT1β mRNA levels in the kidney are reduced by approximately 50% in PPARα knock-out mice, indicating an important role for PPARα in regulating the expression of this gene. Nevertheless, LPS treatment resulted in a further reduction in CPT1β mRNA levels. It is known that CPT1 expression is regulated by TR (15), and LPS decreases TR in both wild-type and PPARα knock-out mice. Thus, the decrease in the expression of a number of different transcription factors following LPS treatment is likely to regulate gene expression. Additionally, the LPS-induced decrease in key transcription factor coactivators such as PGC1α and PGC1β is likely to also affect the expression of many genes.
One can only speculate on the potential beneficial effects of increasing serum TG levels and decreasing FA oxidation in key organs such as the heart and kidney during infection and inflammation. However, during infection and inflammation, there is an increase in serum TG-rich lipoproteins, which could provide lipid substrates for other cells that play a crucial role in host defense or tissue repair (2). Studies by our laboratory have shown that LPS stimulates the uptake of TG by macrophages (30). Moreover, studies have shown that TG-rich lipoproteins directly bind toxic bacterial products such as endotoxin and lipotechoic acid and thereby reduce their harmful effects (2). A decrease in the uptake and oxidation of FAs in heart and kidney could be part of a coordinated systemic metabolic response to make more TG available for host defense and/or tissue repair.
In addition to the potential beneficial effects, it is likely that the decrease in FA uptake and oxidation in organs such as the heart and kidney could have detrimental effects. The oxidation of FA produces more energy per molecule, as compared with the oxidation of glucose or lactate; therefore, ATP generated from FA oxidation is an important energy source for these metabolically active tissues. The kidney requires considerable energy to support the transport of numerous electrolytes and small compounds in the tubules. During severe sepsis, multi-organ failure, including renal failure, often occurs (31), and one can speculate that the inability to generate energy via FA oxidation might contribute to the development of this abnormality.
In summary, the present study demonstrates that LPS administration increases kidney TG, FFAs, and cholesteryl ester levels. These increases may in part be accounted for by a decrease in FA oxidation due to the decreased expression of key proteins required for the uptake and oxidation of FAs. Finally, key transcription factors that regulate FA oxidation and metabolism, RXRα, PPARα, TFα, and TRβ, and their key coactivators, PGC1α and PGC1β, are downregulated in the kidney by LPS treatment and might mediate some of the alterations in FA metabolism that occur during infection and inflammation.
Abbreviations
ACO, acyl-CoA oxidase
CPT, carnitine palmitoyltransferase
Ct, cycle of threshold
DGAT, diacylglycerol acyltransferase
FA, fatty acid
FACS, fatty acyl-CoA synthase
FATP, FA transport protein
LPS, lipopolysaccharide
MCAD, medium-chain acyl CoA dehydrogenase
PPAR, peroxisome proliferator-activated receptor
RXR, retinoid X receptor
TG, triglyceride
TR, thyroid hormone receptor
Published, JLR Papers in Press, June 23, 2008.
Footnotes
This work was supported by grants from the Research Service of the Department of Veterans Affairs and by National Institutes of Health Grant 5 RO1 AR-049932.
References
- 1.Gabay C., and I. Kushner. 1999. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340 448–454. [DOI] [PubMed] [Google Scholar]
- 2.Khovidhunkit W., M. S. Kim, R. A. Memon, J. K. Shigenaga, A. H. Moser, K. R. Feingold, and C. Grunfeld. 2004. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 45 1169–1196. [DOI] [PubMed] [Google Scholar]
- 3.Feingold K. R., I. Staprans, R. A. Memon, A. H. Moser, J. K. Shigenaga, W. Doerrler, C. A. Dinarello, and C. Grunfeld. 1992. Endotoxin rapidly induces changes in lipid metabolism that produce hypertriglyceridemia: low doses stimulate hepatic triglyceride production while high doses inhibit clearance. J. Lipid Res. 33 1765–1776. [PubMed] [Google Scholar]
- 4.Nonogaki K., A. H. Moser, X. M. Pan, I. Staprans, C. Grunfeld, and K. R. Feingold. 1995. Lipoteichoic acid stimulates lipolysis and hepatic triglyceride secretion in rats in vivo. J. Lipid Res. 36 1987–1995. [PubMed] [Google Scholar]
- 5.Beylot M., M. Guiraud, G. Grau, and P. Bouletreau. 1989. Regulation of ketone body flux in septic patients. Am. J. Physiol. 257 E665–E674. [DOI] [PubMed] [Google Scholar]
- 6.Takeyama N., Y. Itoh, Y. Kitazawa, and T. Tanaka. 1990. Altered hepatic mitochondrial fatty acid oxidation and ketogenesis in endotoxic rats. Am. J. Physiol. 259 E498–E505. [DOI] [PubMed] [Google Scholar]
- 7.Liu M. S., and J. J. Spitzer. 1977. In vitro effects of E. coli endotoxin on fatty acid and lactate oxidation in canine myocardium. Circ. Shock. 4 181–190. [PubMed] [Google Scholar]
- 8.Wang X., and R. D. Evans. 1997. Effect of endotoxin and platelet-activating factor on lipid oxidation in the rat heart. J. Mol. Cell. Cardiol. 29 1915–1926. [DOI] [PubMed] [Google Scholar]
- 9.Beigneux A. P., A. H. Moser, J. K. Shigenaga, C. Grunfeld, and K. R. Feingold. 2000. The acute phase response is associated with retinoid X receptor repression in rodent liver. J. Biol. Chem. 275 16390–16399. [DOI] [PubMed] [Google Scholar]
- 10.Beigneux A. P., A. H. Moser, J. K. Shigenaga, C. Grunfeld, and K. R. Feingold. 2003. Sick euthyroid syndrome is associated with decreased TR expression and DNA binding in mouse liver. Am. J. Physiol. Endocrinol. Metab. 284 E228–E236. [DOI] [PubMed] [Google Scholar]
- 11.Kliewer S. A., H. E. Xu, M. H. Lambert, and T. M. Willson. 2001. Peroxisome proliferator-activated receptors: from genes to physiology. Recent Prog. Horm. Res. 56 239–263. [DOI] [PubMed] [Google Scholar]
- 12.Schoonjans K., B. Staels, and J. Auwerx. 1996. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J. Lipid Res. 37 907–925. [PubMed] [Google Scholar]
- 13.Stakkestad J. A., and J. Bremer. 1982. The metabolism of fatty acids in hepatocytes isolated from triiodothyronine-treated rats. Biochim. Biophys. Acta. 711 90–100. [DOI] [PubMed] [Google Scholar]
- 14.Stakkestad J. A., and J. Bremer. 1983. The outer carnitine palmitoyltransferase and regulation of fatty acid metabolism in rat liver in different thyroid states. Biochim. Biophys. Acta. 750 244–252. [DOI] [PubMed] [Google Scholar]
- 15.Jansen M. S., G. A. Cook, S. Song, and E. A. Park. 2000. Thyroid hormone regulates carnitine palmitoyltransferase Ialpha gene expression through elements in the promoter and first intron. J. Biol. Chem. 275 34989–34997. [DOI] [PubMed] [Google Scholar]
- 16.Feingold K., M. S. Kim, J. Shigenaga, A. Moser, and C. Grunfeld. 2004. Altered expression of nuclear hormone receptors and coactivators in mouse heart during the acute-phase response. Am. J. Physiol. Endocrinol. Metab. 286 E201–E207. [DOI] [PubMed] [Google Scholar]
- 17.Lu B., A. H. Moser, J. K. Shigenaga, K. R. Feingold, and C. Grunfeld. 2006. Type II nuclear hormone receptors, coactivator, and target gene repression in adipose tissue in the acute-phase response. J. Lipid Res. 47 2179–2190. [DOI] [PubMed] [Google Scholar]
- 18.Zager R. A., A. C. Johnson, and S. Y. Hanson. 2005. Renal tubular triglyercide accumulation following endotoxic, toxic, and ischemic injury. Kidney Int. 67 111–121. [DOI] [PubMed] [Google Scholar]
- 19.Johnson A. C., A. Stahl, and R. A. Zager. 2005. Triglyceride accumulation in injured renal tubular cells: alterations in both synthetic and catabolic pathways. Kidney Int. 67 2196–2209. [DOI] [PubMed] [Google Scholar]
- 20.Kim M. S., J. Shigenaga, A. Moser, K. Feingold, and C. Grunfeld. 2003. Repression of farnesoid X receptor during the acute phase response. J. Biol. Chem. 278 8988–8995. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y., A. H. Moser, J. K. Shigenaga, C. Grunfeld, and K. R. Feingold. 2005. Downregulation of liver X receptor-alpha in mouse kidney and HK-2 proximal tubular cells by LPS and cytokines. J. Lipid Res. 46 2377–2387. [DOI] [PubMed] [Google Scholar]
- 22.Faggioni R., G. Fantuzzi, C. Gabay, A. Moser, C. A. Dinarello, K. R. Feingold, and C. Grunfeld. 1999. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am. J. Physiol. 276 R136–R142. [DOI] [PubMed] [Google Scholar]
- 23.Heimberg M., J. O. Olubadewo, and H. G. Wilcox. 1985. Plasma lipoproteins and regulation of hepatic metabolism of fatty acids in altered thyroid states. Endocr. Rev. 6 590–607. [DOI] [PubMed] [Google Scholar]
- 24.Kim M. S., T. R. Sweeney, J. K. Shigenaga, L. G. Chui, A. Moser, C. Grunfeld, and K. R. Feingold. 2007. Tumor necrosis factor and interleukin 1 decrease RXRalpha, PPARalpha, PPARgamma, LXRalpha, and the coactivators SRC-1, PGC-1alpha, and PGC-1beta in liver cells. Metabolism. 56 267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Handschin C., and B. M. Spiegelman. 2006. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr. Rev. 27 728–735. [DOI] [PubMed] [Google Scholar]
- 26.Finck B. N., and D. P. Kelly. 2006. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J. Clin. Invest. 116 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanza-Jacoby S., and E. L. Rosato. 1994. Regulatory factors in the development of fatty infiltration of the liver during gram-negative sepsis. Metabolism. 43 691–696. [DOI] [PubMed] [Google Scholar]
- 28.Memon R. A., K. R. Feingold, A. H. Moser, J. Fuller, and C. Grunfeld. 1998. Regulation of fatty acid transport protein and fatty acid translocase mRNA levels by endotoxin and cytokines. Am. J. Physiol. 274 E210–E217. [DOI] [PubMed] [Google Scholar]
- 29.Memon R. A., N. M. Bass, A. H. Moser, J. Fuller, R. Appel, C. Grunfeld, and K. R. Feingold. 1999. Down-regulation of liver and heart specific fatty acid binding proteins by endotoxin and cytokines in vivo. Biochim. Biophys. Acta. 1440 118–126. [DOI] [PubMed] [Google Scholar]
- 30.Funk J. L., K. R. Feingold, A. H. Moser, and C. Grunfeld. 1993. Lipopolysaccharide stimulation of RAW 264.7 macrophages induces lipid accumulation and foam cell formation. Atherosclerosis. 98 67–82. [DOI] [PubMed] [Google Scholar]
- 31.Schrier R. W., and W. Wang. 2004. Acute renal failure and sepsis. N. Engl. J. Med. 351 159–169. [DOI] [PubMed] [Google Scholar]