Abstract
Study Objectives:
To contrast the effects of total sleep deprivation (TSD) on executive and non-executive function in volunteers homozygous for either the short or long variant of a variable number tandem repeat polymorphism in PERIOD3, which is a genetic marker for susceptibility to the negative effect of sleep loss on waking performance.
Design:
Following two laboratory nights of baseline sleep, both groups underwent an approximately 40-hour constant routine, performing brief tests of executive, memory, attention, and motor function every 2 hours.
Setting:
Clinical Research Centre
Participants:
Fourteen PER34/4 (homozygotes for shorter variant of the gene) and 10 PER35/5 (homozygotes for longer variant) healthy, young adults (mean 25.0 ± 1.0 years).
Interventions:
Total sleep deprivation (∼ 40 hours) following baseline sleep.
Measurements and Results:
Hormonal assays established that melatonin levels, which reflect circadian phase, reached their midpoint around 04:00 in both genotypes. Cognitive performance deteriorated across the night, and was similar for both genotypes throughout, except 2–4 h after the midpoint of the melatonin rhythm. Only at this time-point and only on tests of executive function (e.g., 3-back, paced visual serial addition task) did PER35/5 participants perform reliably worse. Covariance analyses controlling for genotype dependent differences in homeostatic sleep pressure derived from principal component analysis of baseline sleep latency, slow wave sleep and wake after sleep onset largely removed these early morning differences in executive function.
Conclusions:
This PER3 polymorphism differentially influences the effects of sleep deprivation on executive and non-executive function in the early morning. These effects appear to be mediated through homeostatic sleep pressure.
Citation:
Groeger JA; Viola AU; Lo JCY; von Schantz M; Archer SN; Dijk DJ. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. SLEEP 2008;31(8):1159-1167.
Keywords: Executive function, sleep deprivation, genetic polymorphism
TASKS DIFFER QUALITATIVELY, IN TERMS OF COGNITIVE PROCESSES REQUIRED FOR THEIR EXECUTION, AND QUANTITATIVELY, IN TERMS OF THE DEMAND imposed on these processes. Some individuals respond to these qualitative and quantitative task demands more successfully than others. Studies have repeatedly shown that cognitive performance deteriorates when people remain continuously awake for long periods of time. Executive functions, which rely on frontal areas of the brain, are thought to be especially compromised by sleep deprivation,1–3 although other authors suggest the effects of sleep deprivation extend beyond the prefrontal cortex.4–6 Sleep deprivation-induced performance deterioration is more marked in some individuals than in others. These inter-individual differences in response to sleep deprivation have trait-like characteristics consistent with a genetic basis.7 The study described here compared the effects of remaining awake overnight on the performance of genotypically characterized participants who repeatedly performed tasks which relied to different extents on various cognitive processes.
The effects of sleep deprivation on performance have been shown to depend both on the length of time awake and on when in the subject's biological day the waking period begins. The interplay of the time awake and time of day, which reflect homeostatic sleep pressure and circadian rhythmicity respectively, typically results in effects of sleep loss which are most pronounced in the early morning, just after the melatonin midpoint, close to the core body temperature nadir.8,9
The preferred timing of sleep and waking activity varies between individuals.10 These differences in diurnal preference are thought to be related to the timing of circadian rhythms11 and the intrinsic period of the biological clock.12 The period of the biological clock is, in turn, determined by a group of genes, commonly referred to as clock genes.13,14
Extreme diurnal preference has been associated with a variable number tandem repeat (VNTR) polymorphism within the coding region of the clock gene PERIOD3 (PER3). Archer and colleagues15 found that people inheriting the longer VNTR from both parents (i.e., homozygotes for the 5-repeat allele, PER35/5) were more prevalent among those with extreme morning preference. Those inheriting the shorter allele (i.e., PER34/4) were more likely to have extreme evening preference. The PER3 4-repeat allele was also found to be more prevalent among delayed sleep phase disorder patients. The association between extreme diurnal preference and the PER3 VNTR polymorphism has been confirmed in subsequent studies,16,17 but reliable though it is, the association does not imply that the people defined by morningness-eveningness are equally well described by their PER3 VNTR genotype. That is, these phenotypes and genotypes are related, but not isomorphic.
We have recently reported the effects of extended waking on participants who were selected for being homozygous for the long or short alleles of the PER3 length polymorphism, but with no preselection in terms of any behavioral phenotype.18 In that prospective study, from which further data are reported below, carefully matched PER35/5 and PER34/4 participants, who remained awake for approximately 40 h in a constant routine (CR) protocol, were shown to differ in EEG activity in the theta range, as recorded during the Karolinska Drowsiness Test throughout the CR, and in their incidence of slow eye movements after 24 h of wakefulness. These markers of homeostatic sleep pressure increased more rapidly with time awake in PER35/5 than in PER34/4 individuals. Consistent with the suggestion that PER35/5 homozygotes (i.e., those statistically more likely to report extreme “morningness”) typically operate under greater sleep pressure, they fell asleep more readily and spent a greater proportion of time in slow wave sleep (SWS) before the CR began. Although the earlier report focussed on more physiological aspects of the data, we also reported that on a composite measure of performance, the PER35/5 subjects were more affected by sleep loss, in particular during the biological night. We hypothesized that the effects of the PER3 VNTR polymorphism on performance are mediated through its effects on homeostatic sleep pressure. We address this hypothesis below by statistically equating variability in baseline sleep initiation and continuity across participants, and by investigating whether the difference in performance between the genotypes is primarily observed for tasks that are usually thought to be sensitive to sleep loss.
The composite measure of performance reported by Viola et al.,18 which was intended to provide a global summary of performance across the 40-h CR, was derived by normalizing and averaging participants' scores for 12 tasks. The tasks performed ranged from simple and serial reaction time and pursuit tracking, through measures of sustained attention (i.e., sustained attention response task (SART), digit symbol substitution), verbal and spatial working memory (1-, 2-, and 3-backs), and the paced visual serial addition task (PVSAT). Particularly when they have been repeated many times (at least 10 times before contributing to the data reported below), several of these tests (e.g., simple reaction, pursuit tracking, verbal and spatial 1-backs) would be expected to place minimal load on those executive processes that are typically associated with reliance on the prefrontal cortex. By contrast, others (e.g., verbal and spatial 3-back, PVSAT) require repeated operation of shifting, updating and inhibitory processes, which have become paradigmatic examples of executive functions.19,20 Intermediate loadings on prefrontal and posterior parietal systems would be anticipated during verbal and spatial 2-back tasks, given the parametric increase in involvement, which occurs with increasing memory load.21 Inhibition of proponent responses is also associated with increased executive demand (e.g., SART22). Because of this, in the context of a CR, even highly repetitive tasks, such as sequence learning or digit-symbol coding, when configured appropriately may invoke executive functions or the brain structures they are presumed to rely upon. For example, repeatedly performing a serial reaction task will result in explicit learning of the sequenced component, and an expectation that a block of random trials will be encountered. Brain imaging studies show that when a sequence has become highly learned, a subtle change in the nature of the sequence resulted in altered blood flow in a number of regions including the left anterior cingulate, right dorsolateral prefrontal, and parietal areas.23 The anterior cingulated and mesial prefrontal cortex has also been shown to be “exclusively and specifically correlated with the explicit component of performance during recollection of a learned sequence”.24 Configuring a digit-symbol substitution task so that elements of the same digit and symbol sets are randomly mapped to each other each time the task is undertaken should result in increased interference from prior learning, and thus an increased requirement for inhibitory processing. Therefore, even tasks which are less typically associated with executive functioning, should increase involvement of the prefrontal cortex when well-learned responses must be avoided as the tasks are repeatedly encountered, as would be the case in a CR protocol.
This paper characterizes the effects of genotype, circadian phase, and sleep deprivation on particular aspects of cognitive function. Specifically, we investigate the prediction that individuals homozygous for the longer variant VNTR polymorphism should perform worse in the early hours of their biological day when they have remained awake continuously across the preceding night, particularly on tests that have been shown previously to be sensitive to sleep loss, i.e., tests of executive function, such as more demanding N-back working memory tasks and paced visual serial addition. A supplementary exploratory hypothesis is also tested: Statistically controlling sleep pressure on study entry (as indicated by proportion of the baseline night spent in SWS, latency to sleep onset, waking after sleep onset and total sleep time) removes any genotype difference in cognitive performance across the biological night.
METHODS
Full details of the experimental protocol have been described elsewhere.18 Those germane to this paper are reproduced below.
Participants
All participants provided written, informed consent after having received a detailed explanation of the study procedures, and received financial compensation for the inconvenience of participating in each phase of the study. The study protocol was agreed by the University of Surrey Ethics Committee, and was conducted in accordance with the principles of the Declaration of Helsinki, with full medical cover on-hand throughout the course of the laboratory study. Fourteen PER34/4 and 10 PER35/5 homozygotes completed the experiment. All participants were healthy and under 30 years of age. Genotype groups did not differ statistically in terms of age, gender, body-mass index or their subjective assessments of morningness-eveningness, daytime sleepiness or sleep quality, but were different in terms of objectively assessed baseline sleep measures (Table 1). The two genotypes did not differ significantly with respect to circadian phase. The point at which plasma melatonin concentrations first reached 25% of their peak value was defined as the start of the biological night; the first point after the peak when melatonin fell to 25% of their peak level was defined as its end. The midpoint of these values occurred, on average, at 04:10 (SD = 26 min), and was similar for both groups.18
Table 1.
Participant and Baseline Sleep Characteristics
| PER3 Genotype | PER35/5(N=10) | PER34/4(N=14) | P |
|---|---|---|---|
| Gender | 4 females, 6 males | 6 females, 8 males | |
| Age (years) | 25.2 ± 1.1 | 24.8 ± 1.0 | 0.8 |
| BMI (kg/m2) | 22.6 ± 0.6 | 21.5 ± 0.4 | 0.2 |
| PSQI | 3.4 ± 0.5 | 3.6 ± 0.3 | 0.9 |
| ESS | 8.8 ± 1.5 | 7.4 ± 1.5 | 0.6 |
| HÖ | 47.6 ± 3.2 | 49.6 ± 2.7 | 0.9 |
| Baseline latency to stage 2 (min) | 8.60 ± 1.30 | 18.10 ± 2.60 | <0.01 |
| Baseline SWS as % TST | 22.69 ± 2.13 | 15.69 ± 1.57 | <0.01 |
| Baseline WASO (min) | 1.90 ± 0.97 | 10.27 ± 5.17 | 0.1 |
| Baseline sleep pressure | 0.69 ± 0.23 | -0.53 ± 0.24 | <0.001 |
The following abbreviations are used: body-mass index (BMI); Pittsburgh Sleep Quality Index (PSQI); Epworth Sleepiness Scale (ESS); and Horne-Östberg questionnaire (HÖ); time awake after sleep onset (WASO). Mean ± standard error of the mean is shown; P values are for the comparison of the two genotypes; baseline sleep pressure as derived from principal components analysis.
PROCEDURES
Study
Compliance by volunteers with the instruction to maintain habitual sleep schedules was verified by analysis of the sleep diary and actigraphy data prior to the start of the 5-day laboratory study. Following admission, volunteers slept and woke at their scheduled times during a first (habituation) night in the sleep laboratory. For familiarization purposes, participants performed the test battery on five occasions over the course of the baseline day before sleeping again according to their individual habitual bedtimes. Upon awakening from the baseline sleep episode participants were kept awake in bed under CR conditions,25 with sleep being prevented by research assistants and investigators who remained with the volunteer throughout. Volunteers stayed in bed in a semi-recumbent position for approximately 40 hours in constant environmental conditions (i.e., light <5 lux at eye level and temperature 18 ± 0.5°C) with no information on clock time throughout the CR. An indwelling cannula was inserted into a forearm vein at the beginning of the 40-h period for hourly blood sampling (including melatonin measurement). The participants were not allowed to leave the bed for the duration of the protocol, and wakefulness was verified by continuous electroencephalogram (EEG) and electrooculogram (EOG) recordings. During the CR, the subjects received hourly nutritional drinks (Fortisip, Koninklijke Numico, Amsterdam, Netherlands) as a substitute to main meals in order to meet their caloric demand, which was calculated for each subject using the Harris-Benedict formula with an activity factor of 1.3.26 During the 40-h CR period, the subjects completed the performance test battery every 2 h, lasting approximately 20 min to evaluate their cognitive performance.
Materials
The test battery consisted of 12 individual performance tests. Tests were presented on identical portable computers, running Active X, C#, and Exactics code to control presentation timing, stimulus presentation, and response detection and timing, with screen refresh rates of 60 Hz. All of the tests were run at 2-h intervals in one of two orders fixed for each participant. Spatial and verbal tests27 of working memory performance comprised verbal and spatial 1-, 2-, and 3-back tests (i.e., V1-, V2-, V3-, S1-, S2-, S3-back), with one of nine single white dots or consonants presented on a black background for 500 ms, with inter-item delays of 1,500 ms. Attention and throughput were assessed using sustained attention response task (SART28) and paced visual serial addition task (PVSAT29), in which single digits were presented every 950 or 2,000 ms, for a duration of 250 or 1,000 ms, with mouse-based responses being made to all but one of the digits (SART), or one of the 18 possible sums of consecutive digit-pairs being selected from an array (PVSAT); and digit symbol substitution test (DSST30), which required selection of a number from a randomly associated array of eight symbols and digits, with digit-symbol relationships randomized on each occasion the test was administered. Simple reaction time (SIMRT31) was also assessed using a mouse-based response reflecting both the latency between signal onset and initial movement (i.e., detection), and the time taken to complete the movement between a resting and target location (i.e., movement time). Mouse-based serial reaction time (SERRT32) was measured using three repetitions of a 13-term sequence of four on-screen positions, followed by 13 randomly occurring arrangements of the same four positions, and three further repetitions of the 13-term sequence. pursuit tracking (PTT33) skill was assessed by having participants move the mouse so as to decrease the onscreen distance between a cursor and a moving target circle, whose movement was controlled by separate sinusoidal functions governing vertical and horizontal target motion.
The dependent variables comprised reaction time data (SIMRT and SERRT), Euclidean distance measured in pixels (PTT) and correct response frequency (N-back, SART, PVSAT, and DSST). Several of these yielded two performance measures. Simple reaction time distinguished between SIMRTdet (i.e., latency between stimulus onset and removal of finger pressure on mouse button) and SIMRTres (i.e., latency between removing finger pressure from mouse button resting position and completion of movement to target location). Serial reaction time performance was quantified in terms of SERRTcost (i.e., difference between stimulus response times for correct responses to stimuli in random trial blocks and correct stimulus response times within the correct sequence performed before the random block) and SERRTrep (i.e., difference between stimulus response times within correctly performed sequences before and after interruption by a random block of trials). Digit symbol performance was assessed in terms of the number of translations that were attempted (DSSTatt) and the number of these which were accurate (DSSTacc). Sustained attention response task performance was characterized in terms of SARTomission (i.e., errors where a required response was omitted) and SARTcommission (i.e., errors where a response was included when none was required).
Where tests allowed (DSST, N-backs, PVSAT, SART), stimulus presentations were randomized afresh for each use of each test. Tests were administered in the same order for each participant in one of 2 pseudo-random orders (i.e., S2, SERRT, S1, V1, DSST, SIMRT, S3, V3, V2, PTT, SART, PVSAT, or its mirror image). In this study, executive functioning is indexed primarily by performance on S3-back, V3-back and PVSAT, although V2-back, S2-back might also be expected to show similar effects albeit more weakly. Because some aspects of DSST, SERRT, and SART also engage inhibitory processes we thought it possible that these might also load executive capacity more than the remaining tests.
Data Treatment and Statistical Analysis
Twenty-four subjects completed the CR protocol (10 PER35/5 and 14 PER34/4) and were included in the analysis set. For three PER34/4 homozygotes, performance on one task (SIMRT) was sufficiently erratic for their data to be excluded.
Data were analyzed with a correlated errors model using PROC MIXED (SAS, version 9.1.3). Tests of goodness of fit showed that an unstructured covariance structure offered the best means of comparing across tasks, and this solution was used to evaluate genotype group differences, treating time into night as a fixed factor repeated measure.
A second set of analyses was carried out in order to assess the effects of sleep pressure for those measures that showed main effects of genotype or interactions involving genotype. For this purpose, a mixed model with an unstructured covariance matrix was also used, while controlling for individual differences in homeostatic sleep pressure, operationalized as a single factor derived using Principal Components Analysis (SPSS, version 15.0.0) combining sleep latency (stage 2), SWS expressed as a percentage of total sleep time, and wake after sleep onset.
Two-sided significance tests were used for all analyses with α equal to 0.05. Comparisons were made between genotypes at each of the seven time points, using Bonferroni corrected post hoc contrasts. When statistical comparisons are being described, the values reported in the text are least square means generated by the models for the full analysis set (unless noted otherwise). Error estimates indicated by ± refers to the standard error for least square means. In Tables 1 and 2 and Figures 1 to 3 unadjusted (observed) means and standard errors are presented for convenience.
Table 2.
Effects of Time into the Night on Cognitive Performance: Mean ± Standard Error in Relation to the Midpoint of the Melatonin Rhythm
| −6 h | −4 h | −2 h | Melatonin Midpoint | +2 h | +4 h | +6 h | |
|---|---|---|---|---|---|---|---|
| #SERRTrep (ms) | 24 ± 26 | 45 ± 22 | 45 ± 28 | 1 ± 17 | −11 ± 21 | 37 ± 29 | 13 ± 18 |
| SERRTcost (ms) | 41 ± 15 | 29 ± 13 | 46 ± 18 | 52 ± 21 | 35 ± 19 | 47 ± 17 | 28 ± 17 |
| SARTomission | 1.38 ± 0.80 | 6.46 ± 3.69 | 7.13 ± 3.82 | 6.83 ± 3.06 | 10.00 ± 4.07 | 13.25 ± 3.40 | 12.21 ± 3.29 |
| SARTcommission | 4.25 ± 0.56 | 4.58 ± 0.72 | 3.79 ± 0.64 | 3.50 ± 0.57 | 4.21 ± 0.49 | 4.17 ± 0.48 | 3.92 ± 0.58 |
| SIMRTdet (ms) | 275 ± 16 | 293 ± 22 | 285 ± 18 | 290 ± 17 | 312 ± 22 | 311 ± 18 | 309 ± 19 |
| SIMRTmov (ms) | 679 ± 42 | 716 ± 38 | 703 ± 27 | 734 ± 36 | 778 ± 40 | 788 ± 30 | 788 ± 40 |
| #V1BK | 46.00 ± 0.54 | 45.88 ± 0.57 | 42.92 ± 2.20 | 40.13 ± 2.74 | 38.75 ± 1.95 | 37.67 ± 2.05 | 35.92 ± 2.37 |
| #V2BK | 42.08 ± 1.04 | 40.50 ± 1.49 | 34.46 ± 2.62 | 34.21 ± 3.03 | 32.96 ± 2.03 | 32.08 ± 2.36 | 34.42 ± 2.26 |
| #V3BK | 36.79 ± 1.44 | 34.96 ± 1.74 | 32.96 ± 2.53 | 29.71 ± 2.76 | 26.63 ± 2.51 | 28.29 ± 2.37 | 29.42 ± 2.03 |
| #S1BK | 42.88 ± 0.69 | 39.13 ± 1.33 | 39.29 ± 2.19 | 37.13 ± 2.56 | 35.29 ± 2.05 | 30.92 ± 2.30 | 30.79 ± 2.30 |
| #S2BK | 37.63 ± 1.28 | 35.42 ± 1.64 | 34.38 ± 1.94 | 34.83 ± 1.80 | 32.50 ± 2.13 | 28.54 ± 2.21 | 31.88 ± 1.76 |
| #S3BK | 34.08 ± 1.57 | 32.04 ± 1.71 | 30.88 ± 2.22 | 29.08 ± 2.58 | 25.75 ± 2.51 | 26.04 ± 2.07 | 25.38 ± 1.98 |
| #PVSAT | 19.46 ± 1.14 | 17.88 ± 1.11 | 16.83 ± 1.40 | 16.67 ± 1.56 | 15.08 ± 1.40 | 12.63 ± 1.47 | 16.21 ± 1.31 |
| PTT (pixel error) | 188 ± 11 | 207 ± 16 | 218 ± 16 | 234 ± 19 | 279 ± 27 | 308 ± 29 | 321 ± 29 |
| #DSSTacc | 15.96 ± 0.58 | 15.83 ± 0.49 | 15.54 ± 0.48 | 14.83 ± 0.68 | 13.46 ± 0.97 | 13.25 ± 0.96 | 12.92 ± 0.86 |
| #DSSTatt | 17.50 ± 0.51 | 17.21 ± 0.49 | 16.46 ± 0.47 | 15.92 ± 0.66 | 14.79 ± 1.00 | 14.58 ± 0.95 | 13.88 ± 0.81 |
The following abbreviations are used: SERRTrep (msec): serial reaction task difference between stimulus response times within correctly performed sequences before and after interruption by a random block of trials; SERRTcost (msec): serial reaction task difference stimulus response times for correct responses to stimuli in random trial blocks and correct stimulus response times within the correct sequence performed before the random block; SARTomission: sustained attention to response task performance where a required response was omitted; SARTcommission: sustained attention to response task performance where a response was included when none was required; SIMRTdet (msec): simple reaction time latency between stimulus onset and removal of finger pressure on mouse button; SIMRTmov (msec): simple reaction time latency between removing finger pressure from mouse button resting position and completion of movement to target location; V1BK: verbal 1-back working memory task; V2BK: verbal 2-back working memory task; V3BK: verbal 3-back working memory task; S1BK: spatial 1-back working memory task; S2BK: spatial 2-back working memory task; S3BK: spatial 3-back working memory task; PVSAT: paced visual serial addition task; PTT (pixel error): pursuit tracking task; DSSTacc: digit symbol substitution task, number correct; DSSTatt: digit symbol substitution task, number attempted. N=24, except for DSST, where n=21 (10 PER35/5)
Measures on which higher numbers reflect better performance.
Figure 1.
Overnight performance on the paced visual serial addition task in PER35/5 and PER34/4 participants. Mean numbers of correct responses (SE) are plotted relative to the midpoint of the melatonin rhythm. *P < 0.05, Bonferroni corrected
Figure 2.
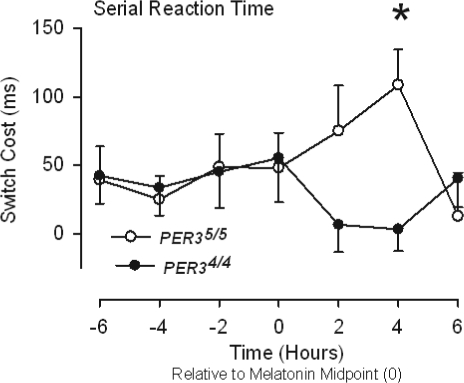
Overnight performance on the serial reaction time task in PER35/5 and PER34/4 participants. Mean switch costs, i.e., increase in time to respond to random rather learned sequences of stimuli (SE), are plotted relative to the melatonin midpoint. Higher values indicate poorer performance. *P < 0.05, Bonferroni corrected.
Figure 3.
Overnight performance on spatial N-Back performance in relation to memory load in PER35/5 and PER34/4 participants. Mean numbers of correct responses (SE), are plotted separately for the 1-, 2-, and 3-back, relative to the melatonin midpoint. * P < 0.05, Bonferroni corrected.
RESULTS
The primary focus in this paper is on the combined effects of genotype and sleep deprivation on cognitive performance, especially those tasks which rely heavily on the prefrontal cortex. A supplementary hypothesis is also addressed, that is, whether any genotype differences that emerge from the main analyses are reduced when genotype differences in sleep pressure are statistically controlled. The main effect of remaining awake overnight on the performance of each task, main effects of genotype on task performance, and the interaction of these two are addressed in turn, before considering the covariance analyses.
Effects of Time into the Night
Of the 16 indices of performance considered, 11 showed reliable deterioration in performance as time awake increased (see Tables 2 and 3). Sustained attention did not appear to suffer, as neither the number of errors of omission (SARTomission), nor errors of commission (SARTcommission) changed systematically across the observation period. The 2 indices derived from the serial reaction task, the extent of response slowing caused when random presentations replaced the learned sequence (SERRTcost) and the difference between response time for learned sequences performed before and after the intervening period of random presentations (SERRTrep), also showed no deterioration over the 12-h period considered here. Detection time within the simple reaction time (SIMRTdet) also did not increase significantly as time awake increased. In each case, the respondent is required to make a single, simple highly practiced motor response requiring little precision to an externally paced visual stimulus. When precise continuous motor responses were required, such as in the pursuit tracking task (PTT) or transport component of the simple reaction time (SIMRTmov), movement became significantly less precise and slower across the night. In contrast to these tasks, all of the other tasks require the brief retention, updating, and processing of visual stimuli, and in each case performance deteriorates overnight. In summary, statistically reliable effects of time into the night was also observed for DSST attempted and accuracy, PVSAT, Spatial 1-, 2- and 3-back and the Verbal 1-, 2-, and 3-back.
Table 3.
Effects of Genotype and Time into the Night on Cognitive Performance: Summary (F Values) of Mixed Effects Analysis Outcomes
| F -Time into night | F- Genotype | F- Time into night by Genotype | |
|---|---|---|---|
| DSSTatt | 4.07** | 0.89 | 0.96 |
| DSSTacc | 5.09*** | 1.18 | 1.41 |
| PTT | 5.94*** | 0.63 | 0.81 |
| PVSAT | 9.65*** | 2.4 | 2.06 |
| S1-back | 5.46*** | 2.47 | 0.95 |
| S2-back | 2.82* | 4.52* | 0.66 |
| S3-back | 5.90*** | 5.63* | 5.83** |
| SARTomission | 2.50 | 0.37 | 1.95 |
| SARTcommission | 0.62 | 2.79 | 1.51 |
| SERRTrep | 0.48 | 1.09 | 1.47 |
| SERRTcost | 0.89 | 0.85 | 0.84 |
| SIMRTdet# | 0.69 | 6.59* | 2.18 |
| SIMRTmov## | 3.85* | 0.67 | 2.33 |
| V1-back | 6.06*** | 1.32 | 2.44 |
| V2-back | 5.92*** | 2.21 | 2.67* |
| V3-back | 5.69*** | 5.51* | 2.06 |
See Table 2 for clarification of abbreviations.
All reliable genotype effects show PER34/4 outperforming PER35/5 *P < 0.05; **P < 0.01; ***P < 0.001
df for Time into night and interaction (6,14.2); df for the main effect of genotype (1,20.2)
df for Time into night and interaction (6,11.3); df for the main effect of genotype (1,14.7)
Effects of Genotype
Few statistically reliable effects of genotype were detected. However, PER35/5 participants were slower to detect stimulus onset in the simple reaction (SIMRTdet: 332 ms ± 19 (PER35/5) > 266 ms ± 18 (PER34/4), P < 0.05). They also performed worse on the more complex working memory tasks: spatial 2-back (30.24 ± 2.06 (PER35/5) > 35.99 ± 1.74 (PER34/4), P < 0.05) and both spatial 3-back (24.83 ± 2.32 (PER35/5) > 32.04 ± 1.96 (PER34/4), P < 0.05) and verbal 3-back (26.79 ± 2.49 (PER35/5) > 34.44 ± 2.10 (PER34/4), P < 0.05).
Genotype × Time into the Night Interactions
Performance on 2 of the more demanding working memory tests, Spatial 3-back and Verbal 2-back, was affected differently by time awake in the two genotypes (Table 3), such that PER35/5 participants performed worse, especially 2-4 h after the melatonin midpoint (Figs. 3 and 4). The most demanding verbal working memory task, verbal 3-back, shows a trend in this direction, but failed to reach conventional levels of significance. No other tests showed statistically reliable interactions between time awake and genotype, although many (e.g., PVSAT, SERRT-cost, SERRT-repeat, and SART-omission) show effects at the 10% level. Bonferroni corrected contrasts between the performances of each genotype at each time point resulted in a consistent pattern of differences for certain types of test. Four h after the melatonin midpoint, i.e., after approximately 24 h of wakefulness, PER35/5 participants were less accurate when adding serial pairs of single digit numbers (i.e., PVSAT: PER35/5 8.10 ms ± 1.95, PER34/4 15.86 ms ± 1.65; P < 0.001, Figure 1). At the same time point, PER35/5 participants were substantially more disrupted by the introduction of random presentations within the serial reaction task (i.e., SERRT-cost: PER35/5 108 ms ± 22, PER34/4 3 ms ± 19; P < 0.001, Figure 2). Performance was also worse on the most complex Spatial working memory task, this time at 2 h after the melatonin midpoint (i.e., S3-back: PER35/5 15.70 ± 2.81, PER34/4 32.93 ± 2.38; P < 0.005, Figure 3). A similar early morning performance impairment was evident for performance on the most complex verbal working memory task 2 h after the melatonin midpoint (i.e., V3-back: PER35/5 18.60 ± 3.29, PER34/4 32.36 ± 2.78; P < 0.005, Figure 4), and 2 hours later (i.e., S3-back: PER35/5 20.20 ± 2.99, PER34/4 34.07 ± 2.54; P < 0.005, Figure 4).
Figure 4.
Overnight performance on verbal N-Back performance in relation to memory load in PER35/5 and PER34/4 participants. Mean numbers of correct responses (SE), are plotted separately for the 1, 2, and 3 back, relative to the melatonin midpoint. * P < 0.05, Bonferroni corrected.
Controlling Effects of Sleep Pressure
Covariance analyses, which adjusted scores to take account of the genotype baseline difference in homeostatic sleep pressure (Table 1), were carried out for all measures of performance (Table 4). Out of the six significant effects involving genotype, 4 were no longer statistically reliable after the homeostatic sleep pressure covariate was entered in the model. Only the main effect of genotype on V3-back performance and the genotype × time awake interaction for S3-back performance remained statistically reliable at the 5% level, with PER34/4 still performing better. As might be expected, all 11 time-into-night effects remain reliable when individual differences in baseline sleep pressure are statistically controlled.
Table 4.
Effects of Controlling Individual Differences in Sleep Pressure on Genotype and Time into the Night on Cognitive Performance: Summary of Mixed Effects Covariance Analysis Outcomes
| Time into night |
Genotype |
Time into night by genotype |
Sleep pressure (covariate) |
|||||
|---|---|---|---|---|---|---|---|---|
| df (1,xx) | F | df (6,xx) | F | df (6,xx) | F | df | F | |
| DSSTatt | 25.7 | 4.07** | 17 | 2.67 | 17 | 0.96 | 21 | 2.6 |
| DSSTacc | 26.4 | 5.09** | 17 | 1.67 | 17 | 1.41 | 21 | 0.55 |
| PTT | 23.3 | 5.94** | 17 | 0.05 | 17 | 0.81 | 21 | 0.27 |
| PVSAT | 22.1 | 9.65*** | 17 | 2.06 | 17 | 2.06 | 21 | 0.23 |
| S1-back | 26.1 | 5.46** | 17 | 0.89 | 17 | 0.95 | 21 | 1.5 |
| S2-back | 24.6 | 2.82* | 17 | 1.31 | 17 | 0.66 | 21 | 0.9 |
| S3-back | 24.6 | 5.9** | 17 | 2.19 | 17 | 5.83** | 21 | 0.31 |
| SARTomission | 25 | 2.50 | 17 | 1.93 | 17 | 1.95 | 21 | 5.35* |
| SARTcommission | 25.1 | 0.62 | 17 | 1.01 | 17 | 1.51 | 21 | 0.18 |
| SERRTcost | 20.8 | 0.69 | 14.1 | 4.32 | 14.1 | 2.19 | 19.7 | 0.44 |
| SERRTrep | 16.8 | 4.02* | 11.6 | 1.61 | 11.6 | 2.22 | 12.3 | 0.96 |
| SIMRTdet# | 24.3 | 0.48 | 17 | 0.35 | 17 | 1.47 | 21 | 4.8* |
| SIMRTmov## | 26.6 | 0.89 | 17 | 0.96 | 17 | 0.84 | 21 | 0.16 |
| V1-back | 23.4 | 6.06** | 17 | 0.54 | 17 | 2.44 | 21 | 2.89 |
| V2-back | 26.9 | 5.92** | 17 | 1.89 | 17 | 2.67 | 21 | 0.04 |
| V3-back | 25.9 | 5.69** | 17 | 5.24* | 17 | 2.06 | 21 | 0.56 |
See Table 2 for clarification of abbreviations.
*P < 0.05; **P < 0.01; ***P < 0.001
DISCUSSION
This study tested the hypothesis that the effect of the PER3 VNTR polymorphism on the sleep deprivation-induced decline in performance is most pronounced for executive functions. We also tested the exploratory hypothesis that statistically controlling for sleep pressure on study entry, as indicated by the proportion of the baseline night spent in SWS, latency to sleep onset, and time awake after sleep onset, reduces differences in cognitive performance across the biological night. We found that performance at the beginning of the night was similar for both genotypes, but thereafter, on most tasks, performance deteriorated across the biological night. Only on executive tasks were participants homozygous for the longer repeat allele variant of PER3 significantly worse than for those homozygous for the shorter variant, and then only during the circadian alertness trough, 2 to 4 h after midpoint of plasma melatonin concentrations, (this corresponds to approximately 06:00 to 08:00 in the present sample). Some of these genotype differences in early morning executive functioning disappeared when baseline sleep pressure was equated, but remained evident for the most demanding tests of working memory. This suggests that PER35/5 homozygotes typically operate under a higher sleep pressure.
The contrast between effects of sleep deprivation on “executive” and “non-executive” tasks was motivated by the sleep and executive function hypothesis.2,3,35 That is, the effects of sleep deprivation are similar to the effects of ageing on cognition, in that impairment is most marked on executive prefrontal tasks (but see Salthouse36 for a contrasting position on ageing and executive function). Our data show, in a young, healthy, normally slept sample, that there are pervasive and substantial effects on cognition of remaining awake overnight. As presented in Table 3, 10 of the 12 tasks showed a statistically reliable decline in performance overnight. For 8 of the 16 measures of performance, these effects are highly reliable, with P-values of less than 0.001. In contradiction of the sleep and executive function hypothesis, there is no tendency evident for tasks which rely heavily on executive processing to show more reliable effects of remaining awake than non-executive tasks. A complex, but highly practiced, pursuit tracking task shows a similarly robust effect of remaining awake overnight. The absence of increased sensitivity to sleep loss among executive tasks is most clearly illustrated in the case of the N-back tasks, which typically show a parametric increase in prefrontal activity with increasing load (e.g.21). In the current study, time into the night shows highly robust effects for both modalities of the task in 1- and 3-back performance. Only on the most demanding of these is the genotype effect evident.
Although we question whether executive functions are particularly compromised by sleep loss, it is also clear from the present study that executive functions (i.e., 3-backs, PVSAT), are particularly vulnerable among those with the PER35/5 genotype. In addition, tasks that might not be regarded as typical executive tasks, DIGIT-SYMBOL substitution (DSSTacc) and serial reaction (SERRTcost), show a similar level of sensitivity to the joint effects of time into night and genotype. Arguably, in both cases the anticipatable, but nevertheless substantial cost of breaking from a highly learned sequence to track random presentations, increases the requirement to inhibit previous learning. However, these effects should only be attributed with caution to a lack of inhibitory control, given the absence of corroborating effects on the sustained attention response task. PER35/5 homozygotes tended to have more omission errors when performing SART, and sleep pressure correlated with number of SART omissions made overall. However, it was errors of commission, rather than errors of omission, that were expected to be sensitive to sleep loss, given the recent findings which showed less effective inhibitory processing in a go/no-go task after a night's sleep deprivation.1 One possibility is that the truncated version of SART used here, made up of some 100 rather than the usual 200 positive trials, but maintaining the usual ratio of go/no-go trials, may not have allowed responses to positive trials to become sufficiently strong to require substantial deployment of inhibitory processing (a possibility discounted by one of the task's originators, T. Manly, personal communication, 18 February, 2007). However, we also note that several recent studies have failed to find differential effects of the two types of SART error,38 or evidence of sensitivity to a decline in frontal functions, which SART is purported to measure.39 Our concerns over the sensitivity of the version of SART used here are somewhat heightened by the clear difference between genotypes on the simple reaction time task. Repeated simple reaction time trials obviously require a degree of alertness and sustained attention to enable rapid responding, but the main effect evident for Simple Reaction time was not present for SART. As described above, PER35/5 participants showed a longer delay after stimulus onset before they began to respond. Although this did not result in a statistically reliable interaction with time awake, perhaps because the three missing cases restricted statistical power.
As recent overviews make clear, there is considerable heterogeneity in the types of cognitive operations which are referred to as executive functions and in the tasks which have been used to index these functions.36 Typically associated with the frontal lobes, it is also clear there is also considerable heterogeneity in the brain systems, which are activated and deactivated when executive tasks are performed, and which are damaged in patients who perform poorly on executive tasks.19,40 Many of the tasks used in studies which support the “sleep loss as prefrontal deficit/ premature aging” view do indeed rely on operations such as updating, inhibition, and set-shifting. However, card sorting, problem solving, verbal fluency, creativity, random generation, go/no-go, and higher load N-back tasks are also very different from each other. The approach adopted here, where a broad range of tests are used, at least some of which have a dose-response like relationship with activity in the prefrontal cortex, has advantages. This is particularly so where repeated administration is required, and ensuring task repetitions are of equal difficulty, similar novelty, and neither become easier nor harder as the study progresses. In the current study, this allows us to be sure that it is not working memory per se that is impaired, since 1- and 2-back tasks are relatively unaffected. It is only when processing demands increase substantially, such as in 3-back or in PVSAT when each sequentially presented digit must be added to that which appeared immediately before, that the effects of genotype become evident. Perhaps surprisingly, early morning genotype-related impairment was also evident on the serial reaction task (but see23,24). However, the manner in which sequence learning is assessed, by measuring the time-cost of switching between a highly learned sequence and random trials, required the participant to anticipate that a change maybe about to occur, and requires a degree of response set switching typical of tests generally regarded as measures of executive function (e.g., card sorting, fluency).
The present study shares a methodological confound with many other studies which have assessed the effects of sleep deprivation. The profound, genotype-specific, deterioration of executive function we report here cannot be attributed unambiguously to the operation of circadian or homeostatic sleep processes because in the CR protocol both processes change simultaneously. The reduction in the scale of the genotype effect when baseline sleep is statistically equated across genotypes is suggestive of where the difference lies. For most people who experience normal sleep-wake and shiftwork schedules, sleep loss accumulation and circadian phase are not independent of each other. The pervasive deterioration in performance reported above may have profound practical consequences for early morning productivity and safety for us all, but particularly for those with the PER35/5 genotype.
ACKNOWLEDGMENTS
This research was supported by the BBSRC (BSS/B/08523). We thank the staff of the Surrey Clinical Research Centre for their help in conducting the study, and particularly Stuart Peters for his statistical support.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Groeger has received research support from GlaxoSmithKline, Lundbeck, and Merck and has consulted for Lundbeck and Merck. Dr. Dijk has received research support from BBSRC, Wellcome Trust, Philips Lighting, Lundbeck, and GlaxoSmithKline; has consulted for Philips Lighting, Lundbeck, Cephalon, Merck, GlaxoSmithKline, Sanofi-Aventis, Pfizer and Takeda; and has participated in a speaking engagement for Servier. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Chuah YML, Venkatraman V, Dinges DF, Chee MW. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci. 2006;26:7156–62. doi: 10.1523/JNEUROSCI.0906-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison Y, Horne JA, Rothwell A. Prefrontal neuropsychological effects of sleep deprivation in young adults- a model of healthy aging? Sleep. 2000;23:1067–73. [PubMed] [Google Scholar]
- 3.Horne JA. Human sleep, sleep loss and behaviour. Implications for the prefrontal cortex and psychiatric disorder. Br J Psychiatry. 1993;162:413–9. doi: 10.1192/bjp.162.3.413. [DOI] [PubMed] [Google Scholar]
- 4.Choo WC, Lee WW, Venkatraman V, Sheu FS, Chee MW. Dissociation of cortical regions modulated by both working memory load and sleep deprivation and by sleep deprivation alone. Neuroimage. 2005;25:579–87. doi: 10.1016/j.neuroimage.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 5.Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–7. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 6.Mu Q, Mishory A, Johnson KA, et al. Decreased brain activation during a working memory task at rested baseline is associated with vulnerability to sleep deprivation. Sleep. 2005;28:433–46. doi: 10.1093/sleep/28.4.433. [DOI] [PubMed] [Google Scholar]
- 7.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 8.Cajochen C, Khalsa SB, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277:R640–9. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 9.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–63. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 10.Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 11.Duffy JF, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med. 1999;47:141–50. [PMC free article] [PubMed] [Google Scholar]
- 12.Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115:895–9. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- 13.Hastings M. The brain, circadian rhythms, and clock genes. BMJ. 1998;317:1704–7. doi: 10.1136/bmj.317.7174.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–7. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 15.Archer SN, Robilliard D, Skene DJ, et al. A length polymorphism in the circadian clock gene PER3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–5. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 16.Jones KHS, Ellis J, von Schantz M, Skene DJ, Dijk DJ, Archer SN. Age-related change in the association between a polymorphism in the PER3 gene and preferred timing of sleep and waking activities. J Sleep Res. 2007;16:12–6. doi: 10.1111/j.1365-2869.2007.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira DS, Tufik S, Louzada FM, et al. Association of the length polymorphism in the human Per3 gene with the delayed sleep-phase syndrome: Does latitude have an influence upon it? Sleep. 2005;28:29–32. [PubMed] [Google Scholar]
- 18.Viola AU, Archer SN, James LM, et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 2007;17:613–8. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 19.Collette F, Hogge M, Salmon E, van der Linden M. Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience. 2006;139:209–21. doi: 10.1016/j.neuroscience.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 20.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cognit Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 21.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 22.Fassbender C, Murphy K, Foxe JJ, et al. A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Cogn Brain Res. 2004;20:132–43. doi: 10.1016/j.cogbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Berns GS, Cohen JD, Mintun MA. Brain regions responsive to novelty in the absence of awareness. Science. 1997;276:1272–5. doi: 10.1126/science.276.5316.1272. [DOI] [PubMed] [Google Scholar]
- 24.Destrebecqz A, Peigneux P, Laureys S, et al. Cerebral correlates of explicit sequence learning. Brain Res Cogn Brain Res. 2003;16:391–8. doi: 10.1016/s0926-6410(03)00053-3. [DOI] [PubMed] [Google Scholar]
- 25.Duffy JF, Dijk DJ. Getting through to circadian oscillators: Why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- 26.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci U S A. 1918;4:370–3. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubat-Silman AK, Dagenbach D, Absher JR. Patterns of impaired verbal, spatial, and object working memory after thalamic lesions. Brain Cogn. 2002;50:178–93. doi: 10.1016/s0278-2626(02)00502-x. [DOI] [PubMed] [Google Scholar]
- 28.Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. ‘Oops!’: Performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia. 1997;35:747–58. doi: 10.1016/s0028-3932(97)00015-8. [DOI] [PubMed] [Google Scholar]
- 29.Feinstein A, Brown R, Ron M. Effects of practice of serial tests of attention in healthy subjects. J Clin Exp Neuropsychol. 1994;16:436–47. doi: 10.1080/01688639408402654. [DOI] [PubMed] [Google Scholar]
- 30.Gilmore GC, Royer FL, Gruhn JJ. Age differences in symbol-digit substitution task performance. J Clin Psychol. 1983;39:114–24. doi: 10.1002/1097-4679(198301)39:1<114::aid-jclp2270390122>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Smith SJ, Groeger JA, Bowditch SC, Grose RI. Effects of electromagnetic fields emitted by communication systems on human cognition. Bioelectromagnetics. (in press) [Google Scholar]
- 32.Curran T, Keele SW. Attentional and nonattentional forms of sequence learning. J Exp Psychol Learn Mem Cogn. 1993;19:189–202. [Google Scholar]
- 33.Maquet P, Schwartz S, Passingham R, Frith C. Sleep-related consolidation of a visuomotor skill: Brain mechanisms as assessed by functional magnetic resonance imaging. J Neurosci. 2003;23:1432–40. doi: 10.1523/JNEUROSCI.23-04-01432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–93. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- 35.Harrison Y, Horne JA. Sleep loss impairs short and novel language tasks having a prefrontal focus. J Sleep Res. 1998;7:95–100. doi: 10.1046/j.1365-2869.1998.00104.x. [DOI] [PubMed] [Google Scholar]
- 36.Salthouse TA. Relations between cognitive abilities and measures of executive functioning. Neuropsychology. 2005;19:532–45. doi: 10.1037/0894-4105.19.4.532. [DOI] [PubMed] [Google Scholar]
- 37.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 38.Fronczek R, Middelkoop HA, van Dijk JG, Lammers GJ. Focusing on vigilance instead of sleepiness in the assessment of narcolepsy: High sensitivity of the sustained attention to response task (SART) Sleep. 2006;29:187–91. [PubMed] [Google Scholar]
- 39.Whyte J, Grieb-Neff P, Gantz C, Polansky M. Measuring sustained attention after traumatic brain injury: Differences in key findings from the sustained attention to response task (SART) Neuropsychologia. 2006;44:2007–14. doi: 10.1016/j.neuropsychologia.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;6:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]