Abstract
A gap junction is composed of two hemichannels and possesses a relatively large pore size (~ 10–15 Å), allowing passage of ions and molecules up to 1 kDa. Here, we report that connexin hemichannels and gap junctions in the guinea pig cochlea had significant charge selectivity among permeating molecules. In coincubation with anionic and cationic fluorescent dyes, hemichannel permeability in isolated cochlear supporting cells showed significant charge selectivity; 31% of cells had only cationic dye influx and 6% of cells had only anionic dye influx. Charge-selective influx contrary to dye size was also found, indicating charge as a dominant determinant in permeability. The cell–cell gap junctional permeability was consistent with hemichannel permeability and also showed strong charge selectivity; the permeation of anionic dyes was slower than that of cationic probes in the cochlear sensory epithelium. With a combination of immunofluorescent staining for connexin26 (Cx26) and Cx30, which are the predominant connexin isoforms in the cochlea, Cx26 was demonstrated to correlate with anionic permeability. The data indicated that cochlear gap junctions have strong charge selectivity in molecular permeability and metabolic communication. Cx26 mutation may induce specific, irreparable impairment in intercellular signalling and energy and nutrient supplies in the cochlea, causing cell degeneration and hearing loss, given that many important cell-signalling and nutrient and energy molecules (e.g. IP3, ATP, cAMP and cGMP) are anions.
Keywords: deafness, gap junction, hemichannel, inner ear, metabolism, selectivity
Introduction
A gap junctional channel is composed of two hemichannels that connect two adjacent cells allowing direct exchange of ions and small molecules (Bennett et al., 1991; Harris, 2001; Evans & Martin, 2002). Six newly synthesized connexin monomers assemble to form a hexagonal connexon (hemichannel) with a relatively large aqueous pore (~ 10–15 Å in diameter), through which can pass ions and molecules up to 1 kDa. It has thus been generally assumed that connexin hemichannels and gap junctions impose little selectivity on the movement of permeants.
As more and more connexins have been identified and their biophysical properties have been defined in transfectants, it has been revealed that gap junctional channels composed of different connexins have preferences in permeability to ions and small molecules (Veenstra et al., 1994; Elfgang et al., 1995; Beblo & Veenstra, 1997; Wang & Veenstra, 1997; Bevans et al., 1998; Cao et al., 1998; Nicholson et al., 2000). Most connexin channels exhibit a preference for cations, consistent with a slight fixed negative charge in the pore. It has also been found that unpaired hemichannels in nonjunctional plasma membranes can open under certain physiological and pathological conditions, such as reduction in extracellular Ca++, depolarization or hyperpolarization, and metabolic inhibition (Paul et al., 1991; DeVries & Schwartz, 1992; Trexler et al., 1996; John et al., 1999; Kondo et al., 2000; Contreras et al., 2002). However, the connexin selectivity and function still remain largely unexplored and undefined, especially in native cells.
Gap junctions play an important role in hearing. Connexin26 (Cx26) mutations are associated with a high incidence of hearing loss (Kelsell et al., 1997; Denoyelle et al., 1998). In the cochlea, gap junctional coupling or connexin expression only exists in supporting cells, not in hair cells. Cx26 and Cx30 are the predominant isoforms. (Kikuchi et al., 1995; Lautermann et al., 1998; Forge et al., 1999, 2003; Zhao & Santos-Sacchi, 1999; Zhao, 2000). Targeted ablation of Cx26 in cochlear supporting cells causes hair cell and supporting cell degeneration (Cohen-Salmon et al., 2002). However, the functions of gap junctional coupling and connexin channels in the cochlea remain unclear. This also limits our understanding of the pathology of connexin mutation-induced hearing loss. Previous studies revealed that gap junctional coupling between supporting cells was sensitive to Ca++, pH, membrane tension and voltage (Santos-Sacchi, 1985; Sato & Santos-Sacchi, 1994; Zhao & Santos-Sacchi, 1998, 2000). Patch-clamp recording also demonstrated that connexins could form various heterotypic and heteromeric channels between supporting cells with asymmetric voltage gating (Zhao & Santos-Sacchi, 2000) to induce directional transjunctional transfer (Zhao, 2000). In this experiment, we used multiple fluorescent probes with patch-clamp recording to assess connexin permeability and selectivity in cochlear supporting cells. Both hemichannels and gap junctions demonstrated significant charge selectivity to molecular permeants. With a combination of immunofluorescent staining, Cx26 was demonstrated to be mainly responsible for the permeability to anionic molecules in the cochlea.
Preliminary reports of this work have been presented in abstract forms (Zhao, 2003a,b).
Materials and methods
Isolation of cochlear cells
Cochlear supporting cells were freshly isolated from the inner ears of adult guinea pigs (250–450 g) using microdissection (Zhao & Santos-Sacchi, 1998, 2000). Temporal bones were removed after decapitation under over-dose pentobarbital anaesthesia and dissected in a modified Leibovitz medium to reveal the organ of Corti. After removal of the tectorial membrane and stria vascularis, the basilar membrane with the organ of Corti (auditory sensory epithelium) was picked away with a sharpened needle. Isolated pieces of the sensory epithelium were further digested with 1 mg/mL trypsin and shaken for 5–10 min. Dissociated cochlear cells were transferred to a recording dish and continuously perfused with a standard extracellular solution (in mM: NaCl, 142; KCl, 5.37; MgCl2, 1.47; CaCl2, 2; and HEPES, 10; 300 mOsm, pH 7.2) to remove residual enzymes. Experiments were performed within 4 h of isolation at room temperature (23 °C).
Fluorescent dye uptake assay
Membrane-impermeable fluorescent dyes EAM-1 (MW 139, charge +1), ethidium bromide (EB; MW 314, charge +1), Alexa Fluor 350 (MW 326, charge −1), Lucifer Yellow (LY; MW 444, charge −2), and propidium iodide (PI; MW 414, charge +2) were used to assess connexin channel permeability and selectivity. Each dye was individually dissolved in a nominally Ca++-free extracellular solution (the standard extracellular solution without CaCl2 and MgCl2), at a concentration of 2.5 mM. Before the experiments an anionic dye and a cationic dye were equally mixed and diluted to a final concentration of 1.25 mM for coapplication to cells. After an incubation of 20 min, residual dyes were washed away by perfusion with a standard extracellular solution that contained 2 mM Ca++ and 1.47 mM Mg++. Cellular dye uptake was examined under a microscope (Nikon, TE300) with corresponding fluorescent filters. Fluorescent images were captured by a CCD camera and presented by Photoshop 5.5.
Fluorescent dyes were purchased from Molecular Probes (Eugene, OR, USA) except dye EAM-1, which was purchased from Macrocyclics Co. (Dallas, TX, USA).
Pharmacological agents and applications
Gap junctional channel blockers 18α-glycyrrhetinic acid (18-AGA) and Proadifen hydrochloride (SKF-525A) were dissolved in dimethyl sulfoxide and a Ca++-free extracellular solution, respectively, at a concentration of 10 mM. Ten minutes before dye incubation, 35 μM 18-AGA or 100 μM SKF-525A was applied to cells.
P2X receptor blockers adenosine 5′-triphosphate-2′, 3′-dialdehyde (oxidized ATP, oATP) and pyridoxalphosphate-6-azophenyl-2′, 4′-disulphonic acid (PPADS) were freshly prepared before experiments. Cells were preincubated with oATP (300 μM) for 30–60 min or PPADS (50 μM) for 5–10 min before dye administration.
For metabolic inhibition a stock solution of 2.5 mg/mL of antimycin A, an inhibitor of electron transfer in the respiratory chain, was prepared in ethanol and diluted to a final concentration of 5 ng/mL, and cells were preincubated for 45 min.
All chemicals (apart from dyes) were purchased from Sigma (St Louis, USA).
Dye injection with patch-clamp recording in the cochlear sensory epithelium
Pieces of isolated cochlear sensory epithelia were transferred to the recording dish without trypsin enzyming and perfused with a standard extracellular solution. A fluorescent dye mixture of either Alexa 350 and EB or LY and PI was filled into the patch pipette with an intracellular solution (in mM: KCl, 140; MgCl2, 2; EGTA, 5; and HEPES, 10; pH 7.2, 300 mOsm). The concentration of each dye was 1.25 mM. The pipette tip diameter was 1.5–2 μm. After the pipette tip formed a GΩ seal with the cell membrane, the cell membrane was ruptured by a brief suction to form a whole-cell configuration. Fluorescent dyes flowed into the patched cell via the patch pipette. The holding voltage of voltage clamp was set at −40 mV. The series resistance, seal resistance and input capacitance were measured with 10-mV test pulses using the windows-based patch-clamp software Jclamp (SciSoft, CT, USA) to monitor seal leakage and cell coupling (Zhao & Santos-Sacchi, 1998). The dye diffusion was captured by time-lapse fluorescent microscopy with a CCD camera.
Double immunofluorescent staining for Cx26 and Cx30
Isolated cochlear sensory epithelia were fixed with 4% paraformaldehyde in 0.1 M PBS (pH 7.4) for 30 min. After three washes with PBS, the epithelia were incubated in a blocking solution (10% goat serum and 1% BSA in the PBS) with 0.1% Triton X-100 for 20 min at room temperature. Then, the epithelia were incubated with primary antibodies to Cx26 (Cat. 33–5800; Zymed, CA, USA; 1 : 400) and to Cx30 (Cat. 71–2200; Zymed; 1 : 400) in the blocking solution at 4 °C overnight. After three washes with PBS, reaction to the corresponding secondary antibodies conjugated with Alexa Fluor® 488 or Alexa Fluor® 568 (1 : 400; Molecular Probes) in the blocking solution was followed at room temperature for 1 h to visualize binding of the primary antibodies. The staining images were photographed with a CCD camera.
Results
Hemichannel dye influx in cochlear supporting cells
Connexins in the mammalian cochlea are only expressed in nonsensory supporting cells, which include Deiters cells, Hensen cells, Claudius cells and pillar cells. Connexin hemichannels can open under lowered extracellular Ca++ concentration. Figure 1 shows that cochlear supporting cells had dye influx during incubation with membrane-impermeable fluorescent dyes in a nominally Ca++-free extracellular solution. The cytoplasm and cell nuclei of Deiters cells, Hensen cells, pillar cells and Claudius cells had EAM-1, Alexa 350 and EB labelling (columns A–D in Fig. 1), whereas outer hair cells, which lack connexin expression, had no dye influx in the same incubation (column E in Fig. 1). Also, there was no dye influx in incubation with a relatively large, hemichannel-impermeable dye FITC–dextran (4.4 kDa) under the same nominally Ca++-free condition (data not shown).
Fig. 1.
Isolated cochlear supporting cells and hemichannel dye influx. Isolated cochlear cells were incubated with a mixture of membrane-impermeable dyes (EAM-1 and Alexa 350 or EB and Alexa 350) in a nominally Ca++-free extracellular solution. Images in the first row represent phase-contrast micrographs of isolated cochlear supporting cells and outer hair cells. The second and third rows are their corresponding fluorescent images. EAM-1 and Alexa 350 can be seen distributed in cytoplasm and cell nuclei. EB bound to DNA shows bright red fluorescence in cell nuclei and is still visible under fluorescent illumination for Alexa 350 in columns C and D. (Column E) Outer hair cells in the same incubation had no dye labelling. Scale bars, 20 μm (A and C), 15 μm (B, D and E).
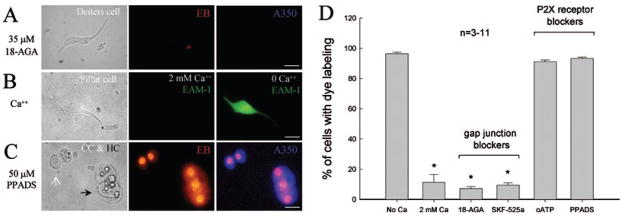
Gap junctional blockers were able to block dye influx. Figure 2A shows that application of a gap junctional channel blocker, 18-AGA (35 μM), blocked dye influx in a Deiters cell; no dye influx was visible in coincubation with EB and Alexa 350 in the nominally Ca++-free extracellular solution. Ca++ was also able to block dye influx. Figure 2B shows that a pillar cell had no dye influx in incubation at 2 mM Ca++, but then had dye labelling after reduction in extracellular Ca++ by perfusion with nominally Ca++-free extracellular solution. In addition, blockage of P2X receptors did not abolish such dye influx. Figure 2C shows that application of a purinergic antagonist, PPADS (50 μM), had no effect on dye influx in supporting cells. Quantitative analyses (Fig. 2D) show that 2 mM Ca++ could reduce dye uptake of supporting cells from 96.4% of cells to 11.3% (n = 5; 262 cells). Application of 35 μM 18-AGA almost completely blocked dye influx and reduced cellular dye uptake to 7.16% (n = 3; 250 cells). Another gap junctional blocker, SKF-525A (100 μM), which can block gap junctional coupling in the cochlea (Spiess et al., 2002), also reduced dye uptake in supporting cells to 9.5% (n = 3; 113 cells). However, irreversible blockage of P2X7 receptors with preincubation of 300 μM oATP for 60 min did not affect dye influx in cochlear supporting cells. With administration of oATP or PPADS, > 90% of supporting cells still had dye uptake (n = 3 for each blocker; 93 and 236 cells, respectively).
Fig. 2.
Blockade of dye influx by gap junction blockers but not P2X receptor blockers. (Row A) A gap junctional channel blocker 18-AGA blocked dye influx in supporting cells. No dye influx was visible in a Deiters cell in incubation with a mixture of EB and Alexa 350 dyes in the nominally Ca++-free extracellular solution. Note that there is a visible red EB-stained detritus spot in the middle panel as an internal control. (Row B) Extracellular Ca++ blocked dye influx. No dye influx was visible in a pillar cell in incubation with EAM-1 at 2 mM extracellular Ca++ concentration for 10 min. Dye influx appeared after perfusion of the nominally Ca++-free extracellular solution. (Row C) A P2X receptor inhibitor, PPADS, did not block low Ca++-induced dye influx. A white arrow and a black arrow indicate a Claudius cell pair and a Hensen cell group, respectively, which still had dye influx under application of 50 μM PPADS. (D) Quantitative analysis of the effects of extracellular Ca++, gap junctional blockers and P2X receptor inhibitors on dye influx in cochlear supporting cells. The concentrations of 18-AGA, SKF-525A, oATP and PPADS were 35, 100, 300 and 50 μM, respectively. Dye-labelled cells were counted and normalized to the total number of examined cells in each experiment, and then averaged. Error bars represent SEM. *P < 0.001 compared to cellular dye uptake at 0 mM Ca++ (ANOVA). Scale bars, 20 μm (A), 10 μm (B and C).
Charge selectivity in hemichannel permeability
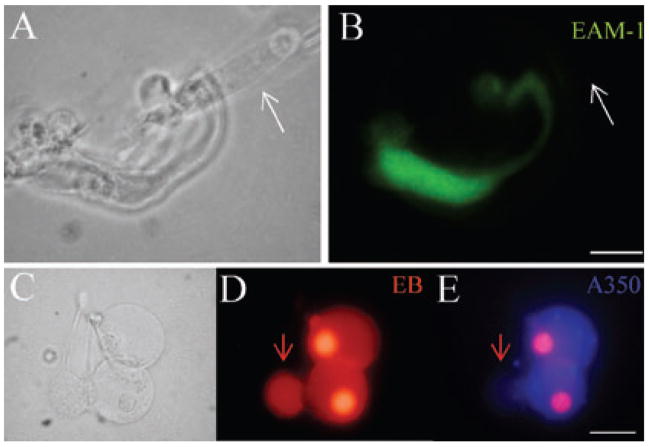
Dye influx of connexin hemichannels in cochlear supporting cells had distinct selectivity for cationic and anionic dyes (Figs 3–6). Figure 3A–F shows a Deiters cell and a Claudius cell that had influx of cationic EB but did not have influx of anionic Alexa 350 in a coincubation of these two dyes. Hemichannels in these two cells preferred the uptake of cationic EB. When we used another cationic dye, EAM-1, to replace EB, charge-selective permeability was still seen. Figure 3G–I shows one Hensen cell that had cationic EAM-1 labelling but no anionic Alexa 350 labelling. Selective permeability to anionic dyes was also visible. Figure 3J–L shows a Hensen cell that had anionic Alexa 350 labelling but no cationic EAM-1 labelling. In this case, selective dye influx was also contrary to molecular weight, because EAM-1 (MW 139) has less than half the molecular weight of Alexa 350 (MW 326).
Fig. 3.
Charge selectivity in hemichannel dye influx in cochlear supporting cells. Isolated cochlear supporting cells were incubated with either a mixture of EB (charge +1) and Alexa 350 (charge −1) or a mixture of EAM-1 (charge +1) and Alexa 350 in a nominally Ca++-free extracellular solution. Panels A–C and D–F represent a Deiters cell and a Claudius cell, respectively, that (B and E) had cationic EB labelling, but (C and F) had no anionic Alexa 350 labelling in coincubation. (F) Bright red EB fluorescence in cell nuclei was still visible under fluorescent illumination for Alexa 350. Panels G–I represent a Hensen cell that (H) had cationic EAM-1 labelling but (I) had no anionic Alexa 350 labelling in coincubation. Panels J–L represent a Hensen cell that (K) had no small cationic EAM-1 (139 Da) influx, but (L) had larger anionic Alexa 350 (326 Da) influx. Scale bars, 20 μm (A–C), 15 μm (G–L).
Fig. 6.
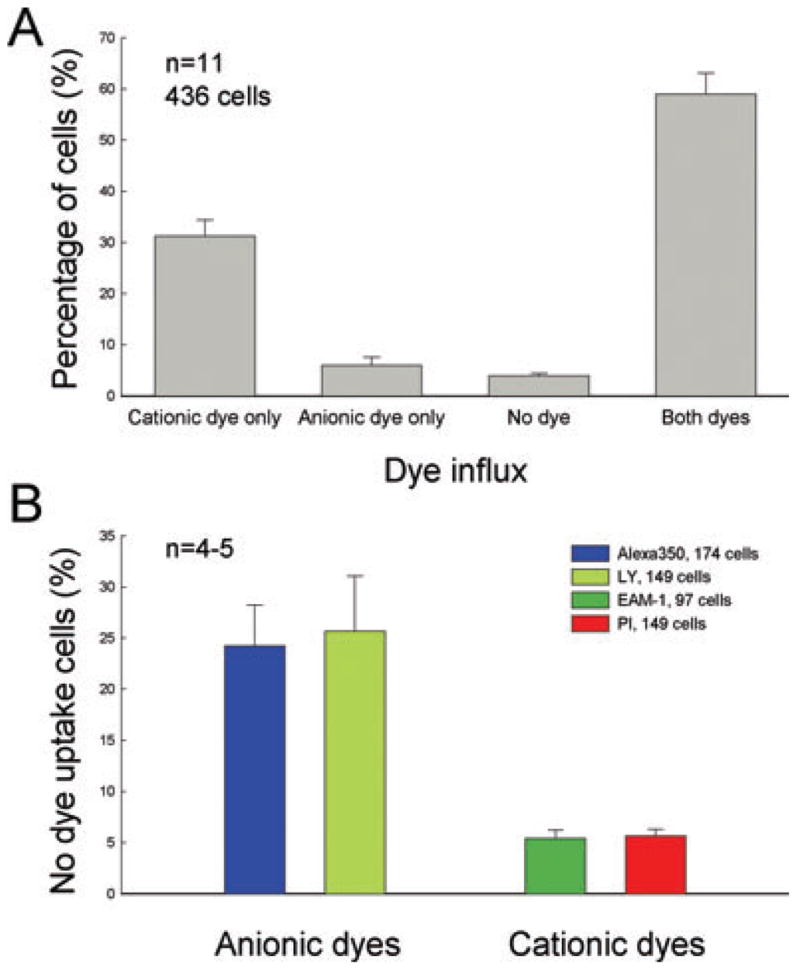
Quantitative analysis of selective permeability of connexin hemichannels in cochlear supporting cells. (A) Percentage of cochlear supporting cells for selective dye influx. Single cells that had cationic dye influx but no anionic dye influx (cationic dye only) or had anionic dye influx but no cationic dye influx (anionic dye only) were counted and normalized to the total number of examined cells in each experiment. ‘No dye’ means cells that had no dye influx in incubation. Data were averaged from 11 experiments. Error bars represent SEM. (B) Percentage of supporting cells with selective influx to different cationic and anionic dyes. For each dye, nonlabelled cells were counted and normalized to the total number of examined cells in each experiment. Data were averaged from 4 or 5 experiments per dye. The numbers in dye legend represent the total cell numbers. Error bars represent SEM. There are significant differences between permeabilities to cationic and anionic dyes (P < 0.001, ANOVA), but there is no significant difference between dyes of different molecular weights (P > 0.1, ANOVA).
Selective dye influx was also independent of energy-dependent active mechanisms (Fig. 4). After preincubation with antimycin A to inhibit cell metabolism, dye influx and selectivity still occurred. Figure 4A and B shows dye influx in a Deiters cell but not in an outer hair cell (indicated by a white arrow) in incubation with EAM-1 after preincubation with antimycin A. Figure 4C–E shows a selective dye influx in a supporting cell that had EB influx but no Alexa 350 influx in coincubation after pretreatment with antimycin A. Metabolic inhibition did not block dye influx and selectivity in cochlear supporting cells.
Fig. 4.
Ineffectiveness of metabolic inhibition on dye influx and selective permeability. Cells were preincubated with a metabolic inhibitor (antimycin A, 5 ng/mL) for 45 min before dye incubation. (A and B) A Deiters cell had dye influx but its connected outer hair cell (indicated by a white arrow) did not have dye labelling. (C–E) Metabolic inhibition did not abolish dye-selective permeability. A red arrow in panels D and E indicates one cell that had EB but no Alexa 350 influx. Scale bars, 20 μm (A and B), 30 μm (C–E).
Figure 5 shows the permeability and selectivity of connexin hemichannels in cochlear supporting cells to LY (charge −2) and PI (charge +2). The permeability of hemichannels to these fluorescent probes also demonstrated charge-sensitive selectivity. Figure 5A–C shows a Hensen cell that had both LY and PI influxes, while Fig. 5D–G shows a pillar cell and a Claudius cell that were selectively permeable to anionic LY but not to cationic PI in coincubation. A cation-selective permeability was also visible. Figure 5J–L shows a Hensen cell that had cationic PI influx but no anionic LY influx.
Fig. 5.
Hemichannel permeability and selectivity to bivalent fluorescent dyes LY and PI in cochlear supporting cells. A mixture of LY and PI was applied to the isolated cells in a nominally Ca++-free extracellular solution. The first row is the phase-contrast image and the second and third rows represent the corresponding fluorescent images. (A–C) A Hensen cell that had both LY and PI uptake. (B) LY was uniformly distributed in cytoplasm and nucleus, and (C) PI mainly labelled the nucleus. (D–I) Supporting cells had anionic LY influx but no cationic PI influx. (J–L) A Hensen cells that had cationic PI influx but no anionic LY influx. Note that the small LY-labelled detritus spots attached to the cell surface are visible in the centre of (K). Scale bar, 10 μm (A–C), 20 μm (D–L).
Figure 6 shows quantitative analysis of charge selectivity in cochlear supporting cells. In experiments (n = 11), 31.23% of examined supporting cells (n = 426) showed cationic dye influx but no anionic dye influx, 5.95% of cells had anionic dye influx but no cationic dye influx, and 3.94% of cells did not have dye influx (Fig. 6A). Other cells (58.88%) showed labelling with both cationic and anionic dyes. For each dye, percentages of cells without influx of anionic Alexa 350 and LY were 24.21% and 25.67%, respectively, and percentages of cells without influx of cationic EAM-1 and PI were 5.41% and 5.63%, respectively (Fig. 6B). Cationic EAM-1 has less than half the molecular weight of PI, but the percentages of influx to both dyes were almost the same. On the other hand, anionic LY and cationic PI have the same molecular weight, but there was a significant difference between cellular influx of these two dyes (25.7% vs. 5.63%; P < 0.001, ANOVA). Hemichannel permeability in cochlear supporting cells demonstrated significant charge selectivity.
Charge-selective permeability of cell–cell gap junctions in cochlear sensory epithelia
Charge selectivity was also found in the permeability of cell–cell gap junctions. Figure 7 shows significant differences in transjunctional transfers of anionic Alexa 350 and cationic EB in the cochlear sensory epithelium. A mixture of Alexa 350 and EB was injected into a Hensen cell in the sensory epithelium via a patch pipette. The cationic dye EB diffused broadly over 30 cells during 30 min whereas the coapplied anionic dye Alexa 350 only reached the first order of neighbouring cells and filled fewer than 3–4 cells during the same time.
Fig. 7.
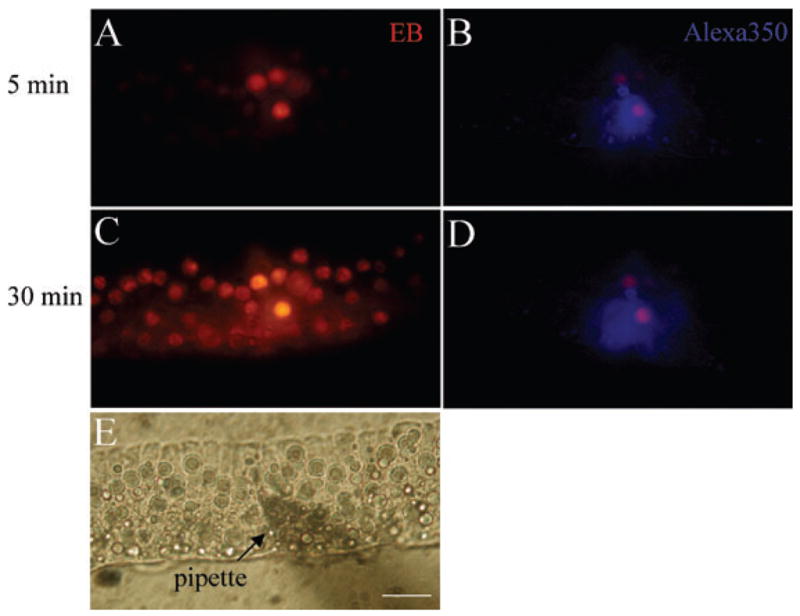
Gap junction charge selectivity in the cochlear sensory epithelium. (A–E) A mixture of an anionic dye, Alexa 350, and a cationic dye, EB, was loaded into a Hensen cell at the middle turn of the epithelium via the patch pipette under whole-cell configuration. The clamp voltage was set at −40 mV. A and B are fluorescent images under illumination for EB and Alexa 350, respectively, and were captured 5 min after the dye injection. Fluorescent images C and D were captured 30 min after the injection and show that cationic EB had broad diffusion in the epithelium whereas anionic Alexa 350 only had a very limited spread. (E) is a phase contrast image for the microinjection. Scale bar, 50 μm.
Figure 8 shows quantitative analysis of LY and PI transjunctional diffusion in the cochlear sensory epithelium. Dye-labelled cells were counted during LY and PI injection. Data fitting revealed that there were two phases in dye diffusion: the fast phase and the slow phase. The time constants of the fast phase were 1.91 and 2.34 min for cationic PI and anionic LY, respectively, representing dye flux through the patch pipette tip into the cell, cytoplasmic diffusion, and the permeability of gap junctions. Slow time constants for cationic PI and anionic LY were 14.6 and 65.8 min, respectively, mainly representing the transjunctional diffusion of the dyes. The slow time constant of PI diffusion was 4.5× faster than that of LY.
Fig. 8.
Transjunctional transfer of anionic LY and cationic PI in the cochlear sensory epithelium. The numbers of PI- and LY-filled cells were separately counted at the indicated time points. Data were averaged from five experiments and presented as mean ± SEM. Solid lines represent data fitting to a double exponential function: y = y1 × [1 − exp(−t/τ1)] + y2 × [1 −exp(−t/τ2)]. Fitting parameters are τ1 = 1.91 and 2.34 min, τ2 = 14.6 and 65.8 min, y1 = 4.77 and 2.24 and y2 = 3.85 and 4.89 for diffusion of cationic PI and anionic LY, respectively.
Selective dye influx vs. connexin expression in the cochlear sensory epithelium
Figure 9 shows hemichannel dye uptake in the cochlear sensory epithelium and immunofluorescent staining for Cx26 and Cx30. Double immunofluorescent staining for Cx26 and Cx30 shows strong Cx26-positive staining that formed a clear bend at the greater epithelial ridge (GER) in the outer edge of the epithelium (indicated by white arrows in Fig. 9B). Inside the GER, positive staining for both Cx26 and Cx30 was visible but the staining for Cx26 appeared weaker here than at the GER (Fig. 9B). In Fig. 9C and D, we used 50 μM of PI and 5 mM of LY to coincubate the epithelium. After 5 min incubation only LY influx appeared clearly and strongly at the GER, corresponding well with strong Cx26 staining (indicated by white arrows in Fig. 9D). At the inside of the epithelium where Cx26 and Cx30 expression occurred, both PI and LY influxes were visible (Fig. 9B and D). Corresponding to the relatively weak staining of Cx26 at the inside of the epithelium, the LY uptake was weaker than that at the GER.
Fig. 9.
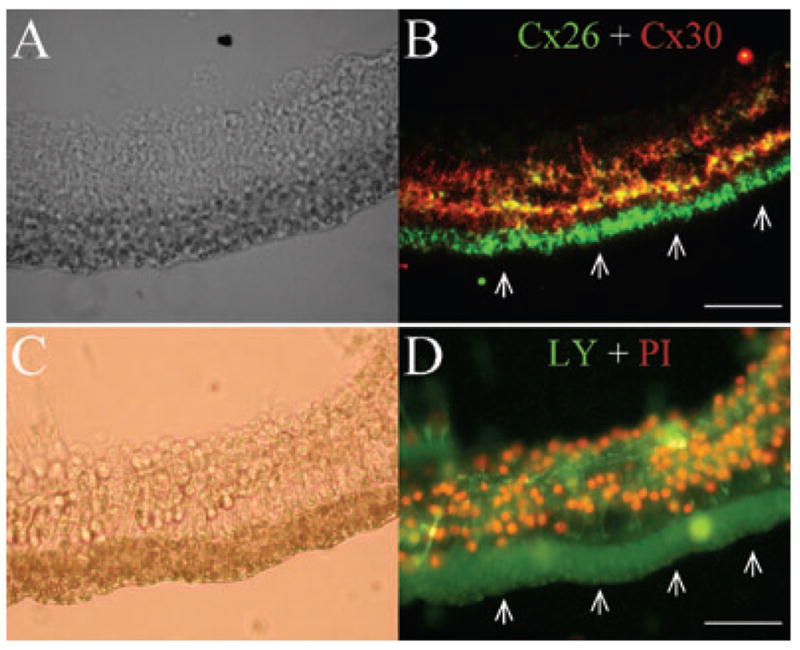
Hemichannel dye uptake and immunofluorescent staining for Cx26 and Cx30 in cochlear sensory epithelia. (A and B) Double-immunofluorescent staining of the epithelium for Cx26 (green) and Cx30 (red). (C and D) Dye uptake of hemichannels for LY and PI in the cochlear sensory epithelium. White arrows in panels B and D indicate the greater epithelial ridge in the epithelium, which had only LY uptake and Cx26 staining. Scale bar, 40 μm.
Discussion
In this experiment, we used multiple fluorescent probes to assess connexin channel permeability in the cochlea. Hemichannels and gap junctions in the cochlear supporting cells demonstrated significant charge selectivity in permeability. With a combination of double immunofluorescent staining for Cx26 and Cx30, the data revealed that permeability to negatively charged molecules was associated with Cx26 expression in supporting cells in the organ of Corti.
Hemichannel activity in cochlear supporting cells
The present experiment demonstrated that the functional connexin hemichannels that existed in cochlear supporting cells were permeable to membrane-impermeable dyes (Figs 1–6 and 9). However, many factors could also induce similar cellular dye influx. First, it has been reported that P2X receptors can alter the pore conformation to become permeable to cations and small molecules in the presence of ATP (Jacobson et al., 2002). P2X receptors have been found in cochlear supporting cells and also exist in hair cells (Chen & Bobbin, 1998; Housley et al., 1999), but no hair cell dye influx was found in the same incubation (Figs 1E and 4). P2X receptor blockers also were inefficient at blocking the dye influx (Fig. 2). These data argue that P2X receptors were not responsible for dye influx in the experiment.
Second, the isolation procedure itself can also induce cell membrane damage, allowing membrane-impermeable dye flux into cells. Membrane leakage-induced dye influx should be nonselective and insensitive to gap junctional blockers. This is inconsistent with our findings that there were significant charge selectivities in dye influx (Figs 3–6) and that gap junction blockers could block the dye influx (Fig. 2). However, membrane leakage-induced dye labelling could not be completely ruled out because some supporting cells still had dye labelling after application of gap junctional blockers (Fig. 2D). This residual cellular dye labelling could result from plasma membrane leakage but could not be > 10% in our counted cells (Fig. 2). Dye influxes therefore mainly resulted from hemichannel permeability.
Finally, the observed hemichannel activity was also unlikely to have been an artifact of cell dissociation. Electron microscopic studies demonstrated that, during cell dissociation, gap junctions were pulled off cells as membrane vesicles rather than splitting apart to form arrays of hemichannels (Severs, 1995; Gaietta et al., 2002). We also observed that hemichannel dye influx still occurred in intact sensory epithelia which were nondissociated (Fig. 9A and B), indicating that native hemichannels exist in the cochlear supporting cells.
It was formerly believed that the hemichannel could not or should not function as a membrane channel because of its lethal effect. However, more and more functional hemichannels have been found in both connexin transfectants and native cells (see reviews: Bennett et al., 2003; Goodenough & Paul, 2003). Hemichannel function has been found to be involved in propagation of an intercellular Ca++ wave (Cotrina et al., 1998), efflux of NAD+ and ATP (Bruzzone et al., 2001; Stout et al., 2002), release of glutamate in astrocytes (Ye et al., 2003), limitation of cell apoptosis (Plotkin & Bellido, 2001) and acceleration of cell death during metabolic inhibition or ischaemia (John et al., 1999; Contreras et al., 2002). Support for functional hemichannels in vivo comes from horizontal cells in the retina, where gap junctions mediate the lateral inhibition of excitatory stimuli in the horizontal cell layer of the outer retina (Kamermans et al., 2001). Connexin hemichannels may also play an important role in the cochlea for intercellular signalling and hearing function. Indeed, our subsequent experiments have shown that hemichannels in the cochlea could release ATP to mediate outer hair cell electromotility (H.-B. Zhao, N. Yu, C.R. Fleming, unpublished observations), which is an active cochlear amplifier required for hearing function in mammals.
Connexin charge selectivity and connexin-specific permeability in the intercellular signalling and metabolic communications in the cochlea
In this experiment, both connexin hemichannels and cell–cell gap junctions in cochlear supporting cells demonstrated significant charge selectivity in permeability to molecules (Figs 3–8). Anionic dyes showed slower diffusion than cationic dyes in the cochlear sensory epithelium (Figs 7 and 8). Besides gap junctional permeability, slower cytoplasmic diffusion and dye binding to cytoplasmic molecules could also result in slow diffusion. These factors could contribute to the failure of the two diffusion curves to reach the same plateau in Fig. 8. However, given that most cytoplasmic proteins are negatively charged molecules, cationic dyes instead of anionic dyes should have greater affinity for cytoplasmic proteins and show slower diffusion than anionic dyes when their molecular weights are almost equal, as was the case in the experiment. Indeed, it has been reported that cationic dye has a strong affinity with anionic cytoplasmic components in oocytes, resulting in limitation of the dye diffusing through gap junctions (Cao et al., 1998). In this experiment, we also used cationic and anionic dyes in the same molar concentration for incubation and injection (see Materials and methods), except for the experiment illustrated in Fig. 9. This further excluded the possibility that the difference resulted from different amounts of cationic and anionic dyes used in the experiments. Thus, different permeabilities to cationic and anionic dyes are due to connexin charge-selective permeability.
In the cochlea, Cx26 and Cx30 are the predominant connexin isoforms in supporting cells. Previous studies in transfected cells suggest that Cx30 channels have a strong cationic preference and are impermeable to anionic LY (Manthey et al., 2001; Beltramello et al., 2003). Our findings are consistent with these reports. Cx26, rather than Cx30, was associated with LY uptake in the cochlear sensory epithelium (Fig. 9). In the transfectants, Cx26 channels showed permeability to cationic and anionic dyes (Elfgang et al., 1995; Manthey et al., 2001; Beltramello et al., 2003). In Fig. 9, we used a high concentration of anionic LY (5 mM) and reduced the concentration of cationic PI to 50 μM to coincubate the epithelium for 5 min. This might induce the PI uptake to become undetectable at the GER. However, clear PI influx was found in the Cx26- and Cx30-expressing area of the epithelium (Fig. 9). This could be due to either or both of the following: Cx26 and Cx30 are permeable to cationic PI, or there is a high permeability of Cx30 to PI. In any case, Cx26 expression correlated with anionic LY permeability in the cochlear sensory epithelium.
Charge-selective permeability may play an important role in intercellular signalling and metabolic communication in the cochlea, given that many cell-signalling molecules and metabolites, e.g. cAMP/cGMP, IP3, ATP and NAD+, are charged molecules. It has been reported that gap junctions have differential channel permeability for a range of signalling molecules, including cAMP (Bevans et al., 1998), NAD+ (Bruzzone et al., 2001), IP3 (Niessen et al., 2000) and adenosine and ATP (Goldberg et al., 2002). In the experiment, the transjunctional diffusion of anionic LY, which resembles IP3 in charge and size and has been used to mimic the permeability of cell signalling molecules (Elfgang et al., 1995; Valiunas et al., 2002), was shown to be 4.5-fold slower than that of cationic PI in the cochlear sensory epithelium (Fig. 8). In comparison with other ionic channels, this difference may be small. However, lifetimes of cell signaling molecules are usually short. For example, the lifetimes of IP3, cAMP and cGMP are between 1 and 60 s; the lifetime of free or cytosolically buffered Ca++ is even less than 1 s (Kasai & Petersen, 1994). So, a difference in the order of minutes can produce a ‘Yes’ or ‘No’ effect on permeations of these cell-signalling molecules through gap junctions. This may be crucial for intercellular signalling and nutrient supply to cochlear hair cells, because they have no direct blood vessel supply and thus must mainly rely on indirect gap junction-mediated intercellular transport for nutrition and metabolism.
Despite the fact that mixed cationic and anionic dye influx was found in 60% of supporting cells (Fig. 6), selective anionic dye permeability (i.e. impermeability to cationic dyes) was also found (Figs 3, 5 and 6). This is hard to explain by the permeability properties of Cx26 or Cx30 homomeric hemichannels because both of them are permeable to cations. It may result from heteromeric channel permeability. Previously, our patch-clamp recording revealed asymmetrical voltage-gating behaviours of heteromeric and heterotypic channels in cochlear supporting cells (Zhao & Santos-Sacchi, 2000; Zhao, 2003c). A recent experiment also documented that Cx26 and Cx30 can coassemble to form heteromeric hemichannels in liposomes (Locke et al., 2004). It has been reported that Cx26-and Cx32-coassembled heteromeric hemichannels demonstrated different permeabilities to cAMP and cGMP (Bevans et al., 1998; Locke et al., 2004). Cx26- and Cx30-composed heteromeric channels may also possess specific permeability to mediate molecular permeation.
The possible mechanisms of Cx26 mutation-induced cochlear cell degeneration and hearing loss
In this experiment, significant charge selectivity was found in the permeability of gap junctional coupling between cochlear supporting cells (Figs 7 and 8), which facilitated the transfer of cations. This is consistent with the proposed role of gap junctional coupling in the cochlea for K+ buffering. It has been long proposed that cochlear gap junctions involve transferring K+ released from hair cells and nerve endings at their extracellular space back to the endolymph. Connexin mutations may impair cochlear gap junctional communication to disrupt such K+-recycling and induce K+ accumulation near the hair cell extracellular space to produce ototoxic effects (Santos-Sacchi, 1991; Kikuchi et al., 1995). However, this K+-recycling impairment hypothesis lacks direct evidence and has been challenged by an increasing number of experiments. It has been found that many Cx26 mutants associated with deafness can still form functional gap junctional channels with good electrical coupling, implying retaining high permeability to K+ (Choung et al., 2002; Thonnissen et al., 2002; Bruzzone et al., 2003; Wang et al., 2003; Mese et al., 2004). It is also difficult to reconcile this K+-recycling model with available data that both Cx26 and Cx30 are permeable to K+ and cations. One connexin mutation would still leave another functional connexin available to achieve this task. Therefore, deafness associated with Cx26 mutations may not only depend on reduced K+ recycling in the cochlea, and may also impair other metabolic communications.
In this experiment, we found that Cx26 and Cx30 demonstrated charge selectivity in permeability to molecules in cochlear supporting cells; Cx26 is associated with anionic dye permeability (Figs 3–9). This may provide an additional or alternative explanation of metabolic disruption to potassium ototoxicity for pathological changes induced by Cx26 mutations in the cochlea. It has been reported that Cx26 mutations and ablation in cochlear supporting cells can induce a progressive degeneration of hair cells and supporting cells after the onset of hearing (Cohen-Salmon et al., 2002; Kudo et al., 2003), while Cx30 deficiency mainly causes significant decreases in the endocochlear potential and potassium concentration (Teubner et al., 2003). Because most cell signaling molecules and metabolites (e.g. cyclic nucleotides, IP3, ATP and glutamine) are anions, cochlear intercellular signalling and metabolic communications may mainly rely on the Cx26 channels. So, Cx26 mutations may induce irreparable impairment in intercellular signalling and energy and nutrient supplies in the cochlea to cause hair cell and supporting cell degeneration. This may also account for why Cx26 mutations are so serious and irreparable that they can induce a high incidence of hearing loss in the clinic even if Cx30 is coexpressed in the cochlea.
Acknowledgments
The author thanks Carrie R. Fleming and Patricia G. Wilson for technical support. This work was supported by NIH/NIDCD grants DC04618 and DC05989.
Abbreviations
- 18-AGA
18α-glycyrrhetinic acid
- Cx26
connexin26
- EB
ethidium bromide
- GER
greater epithelial ridge
- LY
Lucifer Yellow
- oATP
adenosine 5′-triphosphate-2′, 3′-dialdehyde (oxidized ATP)
- PI
propidium iodide
- PPADS
pyridoxalphosphate-6-azophenyl-2′, 4′-disulphonic acid
- SKF-525A
proadifen hydrochloride
References
- Beblo DA, Veenstra RD. Monovalent cation permeation through the connexin40 gap junction channel. Cs, Rb, K, Na, Li, TEA, TMA, TBA, and effects of anions Br, Cl, F, acetate, aspartate, glutamate, and NO3. J Gen Physiol. 1997;109:509–522. doi: 10.1085/jgp.109.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramello M, Bicego M, Piazza V, Ciubotaru CD, Mammano F, D’Andrea P. Permeability and gating properties of human connexins 26 and 30 expressed in HeLa cells. Biochem Biophys Res Commun. 2003;305:1024–1033. doi: 10.1016/s0006-291x(03)00868-4. [DOI] [PubMed] [Google Scholar]
- Bennett MVL, Barrio LC, Bargiello TA, Spray DC, Hertzberg E, Saez JC. Gap junctions: New tools, new answers, new questions. Neuron. 1991;6:305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Bennett MVL, Contreras JE, Bukauskas FF, Saez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevans CG, Kordel M, Rhee SK, Harris AL. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem. 1998;273:2808–2816. doi: 10.1074/jbc.273.5.2808. [DOI] [PubMed] [Google Scholar]
- Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001;15:10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Veronesi V, Gomes D, Bicego M, Duval N, Marlin S, Petit C, D’Andrea P, White TW. Loss-of-function and residual channel activity of connexin26 mutations associated with non-syndromic deafness. FEBS Lett. 2003;533:79–88. doi: 10.1016/s0014-5793(02)03755-9. [DOI] [PubMed] [Google Scholar]
- Cao F, Eckert R, Elfgang C, Nitsche JM, Snyder SA, Hulser DF, Willecke K, Nicholson BJ. A quantitative analysis of connexin-specific permeability differences of gap junctions expressed in HeLa transfectants and Xenopus oocytes. J Cell Sci. 1998;111:31–43. doi: 10.1242/jcs.111.1.31. [DOI] [PubMed] [Google Scholar]
- Chen C, Bobbin RP. P2X receptors in cochlear Deiters’ cells. Br J Pharmacol. 1998;124:337–344. doi: 10.1038/sj.bjp.0701848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choung YH, Moon SK, Park HJ. Functional study of GJB2 in hereditary hearing loss. Laryngoscope. 2002;112:1667–1671. doi: 10.1097/00005537-200209000-00026. [DOI] [PubMed] [Google Scholar]
- Cohen-Salmon M, Ott T, Michel V, Hardelin JP, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K, Petit C. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr Biol. 2002;12:1106–1111. doi: 10.1016/s0960-9822(02)00904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JE, Sanchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Saez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J Neurosci. 1998;18:8794–8804. doi: 10.1523/JNEUROSCI.18-21-08794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoyelle F, Lina-Granade G, Plauchu H, Bruzzone R, Chaïb H, Lévi-Acobas F, Weil D, Petit C. Connexin26 gene linked to a dominant deafness. Nature. 1998;393:319–320. doi: 10.1038/30639. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J Physiol (Lond) 1992;445:201–230. doi: 10.1113/jphysiol.1992.sp018920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein RA, Hulser DF, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WH, Martin PE. Gap junctions: structure and function (Review) Mol Membr Biol. 2002;19:121–136. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- Forge A, Becker D, Casalotti S, Edwards J, Evans WH, Lench N, Souter M. Gap junctions and connexin expression in the inner ear. Novartis Found Symp. 1999;219:134–150. doi: 10.1002/9780470515587.ch9. [DOI] [PubMed] [Google Scholar]
- Forge A, Becker D, Casalotti S, Edwards J, Marziano N, Nevill G. Gap junctions in the inner ear: comparison of distribution patterns in different vertebrates and assessement of connexin composition in mammals. J Comp Neurol. 2003;467:207–231. doi: 10.1002/cne.10916. [DOI] [PubMed] [Google Scholar]
- Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–507. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Moreno AP, Lampe PD. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J Biol Chem. 2002;277:36725–36730. doi: 10.1074/jbc.M109797200. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- Housley GD, Kanjhan R, Raybould NP, Greenwood D, Salih SG, Jarlebark L, Burton LD, Setz VC, Cannell MB, Soeller C, Christie DL, Usami S, Matsubara A, Yoshie H, Ryan AF, Thorne PR. Expression of the P2X (2) receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission. J Neurosci. 1999;19:8377–8388. doi: 10.1523/JNEUROSCI.19-19-08377.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Jarvis MF, Williams M. Purine and pyrimidine (P2) receptors as drug targets. J Med Chem. 2002;45:4057–4093. doi: 10.1021/jm020046y. [DOI] [PubMed] [Google Scholar]
- John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN. Connexin-43 hemichannels opened by metabolic inhibition. J Biol Chem. 1999;274:236–240. doi: 10.1074/jbc.274.1.236. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science. 2001;292:1178–1180. doi: 10.1126/science.1060101. [DOI] [PubMed] [Google Scholar]
- Kasai H, Petersen OH. Spatial dynamics of second messengers: IP3 and cAMP as long-range and associative messengers. Trends Neurosci. 1994;17:95–101. doi: 10.1016/0166-2236(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Kelsell DP, Dunlop J, Stevens HP, Lench NJ, Liang JN, Parry G, Mueller RF, Leigh IM. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature. 1997;387:80–83. doi: 10.1038/387080a0. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Kimura RS, Paul DL, Adams JC. Gap junctions in the rat cochlea: immunohistochemical and ultrastructural analysis. Anat Embryol. 1995;191:101–118. doi: 10.1007/BF00186783. [DOI] [PubMed] [Google Scholar]
- Kondo RP, Wang SY, John SA, Weiss JN, Goldhaber JI. Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J Mol Cell Cardiol. 2000;32:1859–1872. doi: 10.1006/jmcc.2000.1220. [DOI] [PubMed] [Google Scholar]
- Kudo T, Kure S, Ikeda K, Xia AP, Katori Y, Suzuki M, Kojima K, Ichinohe A, Suzuki Y, Aoki Y, Kobayashi T, Matsubara Y. Transgenic expression of a dominant-negative connexin26 causes degeneration of the organ of Corti and non-syndromic deafness. Hum Mol Genet. 2003;12:995–1004. doi: 10.1093/hmg/ddg116. [DOI] [PubMed] [Google Scholar]
- Lautermann J, ten Cate WJF, Altenhoff P, Grümmer R, Traub O, Frank HG, Jahnke K, Winterhager E. Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell Tissue Res. 1998;294:415–420. doi: 10.1007/s004410051192. [DOI] [PubMed] [Google Scholar]
- Locke D, Stein T, Davies C, Morris J, Harris AL, Evans WH, Monaghan P, Gusterson B. Altered permeability and modulatory character of connexin channels during mammary gland development. Exp Cell Res. 2004;298:643–660. doi: 10.1016/j.yexcr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Manthey D, Banach K, Desplantez T, Lee CG, Kozak CA, Traub O, Weingart R, Willecke K. Intracellular domains of mouse connexin26 and -30 affect diffusional and electrical properties of gap junction channels. J Membr Biol. 2001;18:137–148. doi: 10.1007/s00232-001-0017-1. [DOI] [PubMed] [Google Scholar]
- Mese G, Londin E, Mui R, Brink PR, White TW. Altered gating properties of functional Cx26 mutants associated with recessive non-syndromic hearing loss. Hum Genet. 2004;115:191–199. doi: 10.1007/s00439-004-1142-6. [DOI] [PubMed] [Google Scholar]
- Nicholson BJ, Weber PA, Cao F, Chang H, Lampe P, Goldberg G. The molecular basis of selective permeability of connexins is complex and includes both size and charge. Braz J Med Biol Res. 2000;33:369–378. doi: 10.1590/s0100-879x2000000400002. [DOI] [PubMed] [Google Scholar]
- Niessen H, Harz H, Bedner P, Kramer K, Willecke K. Selective permeability of different connexin channels to the second messenger inositol 1,4,5-trisphosphate. J Cell Sci. 2000;113:1365–1372. doi: 10.1242/jcs.113.8.1365. [DOI] [PubMed] [Google Scholar]
- Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI, Bellido T. Bisphosphonate-induced, hemichannel-mediated, anti-apoptosis through the Src/ERK pathway: a gap junction-independent action of connexin43. Cell Commun Adhes. 2001;8:377–382. doi: 10.3109/15419060109080757. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. The effects of cytoplasmic acidification upon electrical coupling in the organ of Corti. Hear Res. 1985;19:207–215. doi: 10.1016/0378-5955(85)90140-6. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. Isolated supporting cells from the organ of Corti: some whole cell electrical characteristics and estimates of gap junctional conductance. Hear Res. 1991;52:89–98. doi: 10.1016/0378-5955(91)90190-k. [DOI] [PubMed] [Google Scholar]
- Sato Y, Santos-Sacchi J. Cell coupling in the supporting cells of Corti’s organ: Sensivity to intracellular H+ and Ca++ Hear Res. 1994;80:21–24. doi: 10.1016/0378-5955(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Severs NJ. Microscopy of the gap junction: a historical perspective. Microsc Res Techn. 1995;31:338–346. doi: 10.1002/jemt.1070310503. [DOI] [PubMed] [Google Scholar]
- Spiess AC, Lang H, Schulte BA, Spicer SS, Schmiedt RA. Effects of gap junction uncoupling in the gerbil cochlea. Laryngoscope. 2002;112:1635–1641. doi: 10.1097/00005537-200209000-00020. [DOI] [PubMed] [Google Scholar]
- Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- Teubner B, Michel V, Pesch J, Lautermann J, Cohen-Salmon M, Sohl G, Jahnke K, Winterhager E, Herberhold C, Hardelin JP, Petit C, Willecke K. Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum Mol Genet. 2003;12:13–21. doi: 10.1093/hmg/ddg001. [DOI] [PubMed] [Google Scholar]
- Thonnissen E, Rabionet R, Arbones ML, Estivill X, Willecke K, Ott T. Human connexin26 (GJB2) deafness mutations affect the function of gap junction channels at different levels of protein expression. Hum Genet. 2002;111:190–197. doi: 10.1007/s00439-002-0750-2. [DOI] [PubMed] [Google Scholar]
- Trexler EB, Bennett MVL, Bargiello TA, Verselis VK. Voltage gating and permeation in a gap junction hemichannel. Proc Natl Acad Sci USA. 1996;93:5836–5841. doi: 10.1073/pnas.93.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiunas V, Beyer EC, Brink PR. Cardiac gap junction channels show quantitative differences in selectivity. Circ Res. 2002;91:104–111. doi: 10.1161/01.res.0000025638.24255.aa. [DOI] [PubMed] [Google Scholar]
- Veenstra RD, Wang HZ, Beyer EC, Brink PR. Selective dye and ionic permeability of gap junction channels formed by connexin45. Circ Res. 1994;75:483–490. doi: 10.1161/01.res.75.3.483. [DOI] [PubMed] [Google Scholar]
- Wang HL, Chang WT, Li AH, Yeh TH, Wu CY, Chen MS, Huang PC. Functional analysis of connexin-26 mutants associated with hereditary recessive deafness. J Neurochem. 2003;84:735–742. doi: 10.1046/j.1471-4159.2003.01555.x. [DOI] [PubMed] [Google Scholar]
- Wang HZ, Veenstra RD. Monovalent ion selectivity sequences of the rat connexin43 gap junction channel. J Gen Physiol. 1997;109:491–507. doi: 10.1085/jgp.109.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HB. Directional rectification of gap junctional voltage gating between Deiters cells in the inner ear of guinea pig. Neurosci Lett. 2000;296:105–108. doi: 10.1016/s0304-3940(00)01626-8. [DOI] [PubMed] [Google Scholar]
- Zhao HB. Asymmetric gap junctional permeability and charge selectivity in the cochlear supporting cells. Assoc Res Otolaryngol 26th Annual Meeting; Daytona Beach, FL. February 22–27; 2003a. http://www.aro.org. [Google Scholar]
- Zhao HB. Hemichannel activities in native cochlear supporting cells. The 47th Biophysical Society Annual Meeting; San Antonio, Texas. March 1–5; 2003b. http://www.biophysic.org. [Google Scholar]
- Zhao HB. Biophysical properties and functional analysis of inner ear gap junctions for deafness mechanisms of nonsyndromic hearing loss. Proceedings of the 9th International Meeting on Gap Junctions; Cambridge, UK. August 23–28.2003c. [Google Scholar]
- Zhao HB, Santos-Sacchi J. Effect of membrane tension on gap junctional conductance of supporting cells in Corti’s organ. J Gen Physiol. 1998;112:447–455. doi: 10.1085/jgp.112.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HB, Santos-Sacchi J. Voltage gating of gap junctions in cochlear supporting cells: Evidence for nonhomotypic channels. J Membr Biol. 2000;175:17–24. doi: 10.1007/s002320001051. [DOI] [PubMed] [Google Scholar]
- Zhao HB, Santos-Sacchi J. Auditory collusion and a coupled couple of outer hair cells. Nature. 1999;399:359–362. doi: 10.1038/20686. [DOI] [PubMed] [Google Scholar]