Abstract
Motor neuron diseases (MND) are a heterogeneous group of disorders that includes amyotrophic lateral sclerosis (ALS) and result in death of motor neurons. These diseases may produce characteristic perturbations of the metabolome, the collection of small-molecules (metabolites) present in a cell, tissue, or organism. To test this hypothesis, we used high performance liquid chromatography followed by electrochemical detection to profile blood plasma from 28 patients with MND and 30 healthy controls. Of 317 metabolites, 50 were elevated in MNDpatients and more than 70 were decreased (p < 0.05). Among the compounds elevated, 12 were associated with the drug Riluzole. In a subsequent study of 19 subjects with MND who were not taking Riluzole and 33 healthy control subjects, six compounds were significantly elevated in MND, while the number of compounds with decreased concentration was similar to study 1. Our data also revealed a distinctive signature of highly correlated metabolites in a set of four patients, three of whom had lower motor neuron (LMN) disease. In both datasets we were able to separate MND patients from controls using multivariate regression techniques. These results suggest that metabolomic studies can be used to ascertain metabolic signatures of disease in a non-invasive fashion. Elucidation of the structures of signature molecules in ALS and other forms of MND should provide insight into aberrant biochemical pathways and may provide diagnostic markers and targets for drug design.
Keywords: amyotrophic lateral sclerosis, motor neuron disease, coulometric array, metabolic profiling
1. Introduction
Motor neuron diseases are a heterogeneous group of disorders with signs and symptoms that affect motor neurons. The causes for these disorders are largely unknown (Rowland and Shneider, 2001). Amyotrophic lateral sclerosis (ALS) is the most common form of motor neuron diseases (MND) in adults, affecting both anterior horn cells and corticospinal tracts. The median age of ALS onset is 55 years. Fifty percent of patients die within three years of onset of symptoms, and 90% die within five years (Kurtzke and Kurland, 1989). The incidence of ALS is approximately 2 per 100,000 per year (McGuire et al., 1996; Mitsumoto et al., 1998) and may be increasing (Lilienfeld et al., 1989). The lifetime risk of ALS is 1 in 600 to 1 in 1000. The prevalence is between 4 and 6 per 100,000. The majority of ALS cases are sporadic (SALS); 10% are familial (FALS), some of which arise from mutations in SOD1 (Rosen et al., 1993), ALS2 (Hadano et al., 2001; Yang et al., 2001), dynactin (Puls et al., 2003) and senataxin (Lambrechts et al., 2003). Whether ALS is a single disease entity or a syndrome caused by different conditions remains unknown. Also, it is not clear if MNDs are a spectrum of the same disease. The involvement of both upper and lower motor neurons in ALS sets it apart from motor neuron diseases that involve purely upper or lower motor neurons (UMN, LMN). No treatment prevents, halts or reverses ALS, although a delay of up to three months in mortality occurs with the use of the drug Riluzole (Bensimon et al., 1994; Lacomblez et al., 1996).
Multiple mechanisms have been implicated in the pathogenesis of motor neuron death in ALS. These include glutamate toxicity, mitochondrial dysfunction, protein misfolding and apoptosis (Cleveland and Rothstein, 2001). This complexity suggests that a global, metabolomic approach might simultaneously survey most or all implicated pathways, thereby revealing a biochemical signature for the disease and providing new insights into disease mechanisms.
Metabolomics is the comprehensive study of the repertoire of small molecules present in cells, tissues, or other biological samples (Harrigan and Goodacre, 2003). This approach attempts to capture global changes and overall physiological status in biochemical networks and pathways in order to elucidate sites of perturbations (Nicholson and Wilson, 2003; Kell, 2004; Lindon et al., 2004; van der Greef et al., 2004). It is currently being investigated as a tool for drug safety assessment and disease diagnosis (Lindon et al., 2004). Metabolic changes have been reported in patients with hypertension and coronary heart disease (Brindle et al., 2002; 2003). Additionally, there is great interest in integrating metabolomic, transcriptomic and proteomic data in a systems biology approach to understanding global changes in biological states (van der Greef et al., 2004).
We therefore investigated whether MNDs such as ALS are associated with detectable metabolic changes. Biochemical signatures in ALS could provide biomarkers for the disease and its progression, tools for better stratification of patients, and novel therapeutic targets. At present, no single biochemical abnormality has the sensitivity and specificity needed for diagnosing ALS (Rothstein et al., 1990; Camu et al., 1993; Beal et al., 1997; Smith et al., 1998; Beuche et al., 2000; Bogdanov et al., 2000; Simpson et al., 2003). We looked for global biochemical differences that might distinguish ALS from healthy controls and for biochemical signatures that would enable further sub-classification of MNDs.
2. Materials and methods
2.1. Clinical samples: MND and control subjects
Clinical samples were obtained from the MGH Neurology Clinical Trial Unit. Research participants provided informed consent. Data on gender, age, weight, medications, including experimental agents and antioxidants, and tobacco use were collected. For participants with MND, date and site of symptom onset, date of diagnosis, and family history of MND were recorded. Controls were healthy subjects unrelated by blood to MND patients. Table 1 summarizes the characteristics of subjects. For Study 1: twenty three had sporadic ALS and five had pure LMN disease (Supporting table 1). For Study 2: Seventeen subjects had ALS and two had pure UMN disease (Supporting table 2). Four subjects had familial ALS (FALS), one of whom had an SOD1 mutation. Three of the FALS subjects exhibited only LMN signs. Supporting table 3 lists subjects in common between studies 1 and 2. Supporting tables 4 and 5 detail medications taken.
Table 1.
Summary of research participants
| Study 1 MND vs Control
|
Study 2 MND (non-Riluzole) vs Control
|
|||
|---|---|---|---|---|
| MND (n = 28) | Controls (n =3 0) | MND (n = 19) | Controls (n = 33) | |
| Age in Years ± SD | 54.5 ± 12.4 | 55.6 ± 13.0 | 52.3 ± 14.8 | 56.2 ± 13.5 |
| Caucasian (%) | 100 | 100 | 89 | 100 |
| Male (%) | 62 | 55 | 68 | 67 |
| Non-smokers (%) | 93 | 90 | 82 | 88 |
| Using antioxidantsa (%) | 89 | 31 | 84 | 29 |
| Average number of medicationsb | 9 | 2 | 7 | 3 |
| Disease duration in days from onset to death ± SD | 1856.6 ± 980.9 | 1485.2±763.3 | ||
In both studies, the proportion of participants taking antioxidants was greater for patients than for controls (p < 8.8 × 10−6 and p < 3.4 × 10−4, respectively, by Fisher’s exact test, two sided).
In both studies, the average number of medications was significantly higher for patients than for controls (p < 2.1 × 10−6 and p < 9.4 × 10−3, respectively, by t-test).
2.2. Sample preparation and analysis
Plasma was obtained from blood drawn into two 10- mL green top tubes containing sodium heparin. All samples were centrifuged at 1800 × g for 10 min within 30 min of collection. The supernatants were aliquoted in multiple cryovials and frozen at −80°C.
Plasma was prepared for analysis by extraction in acidified acetonitrile and analyzed by high performance liquid chromatography (HPLC) coupled with coulometric array detection as previously described (Matson et al., 1984; Milbury, 1997; Kristal et al., 1998; 2002; Vigneau-Callahan et al., 2001; Shi et al., 2002). During the preparation of samples, pools were created from equal-volume sub-aliquots of all samples. Thus, these pools comprised the aggregate analytical complexity of all the samples in the study, and, in principle, contained every significant peak present in any of the samples. Aliquots of the pool were analyzed after every seven samples in the run sequence. The pooling served several purposes. First, the periodic re-analysis of the pool served as a control on the overall performance of the analytical instruments, since replicate analyses should produce similar chromatograms. Second, the pools provided a benchmark for aligning chromatograms from different samples in the temporal dimension, since all peaks in the samples should be in the pools. Third, the concentration of each peak was expressed as a percentage of the concentration of that peak in the middle pool run. Fourth, the replicate analyses of the pool provided an estimate of the coefficient of variation associated with each peak, that is, the standard deviation of the peak height across pool replicates normalized to mean peak height. Peaks considered in the analysis were those that had good precision in the replicate analyses of the pool. Of the ~1500 peaks detected in the pooled samples, 317 were judged to meet this criterion in study1 and 289 in study 2.
2.3. Mass spectrometric analysis
LC-MS analysis was conducted on an HP1090 LC (Wilmington, DE) equipped with a Thermo Betasil C18 125 ×2.1 mm, 5 μm reversed phased column (San Jose, CA) with a binary isocratic composition of 65/35% B/A (A=95/5 and B=20/80 of 10 mM ammonium formate/methanol) at a flow rate of 0.2 mL/min. The LC was coupled to a Finnigan LCQ classic ion trap (San Jose, CA) or Micromass Q-Tof mass spectrometer (Manchester, UK) fitted with an electrospray ionization source and operated in full scan mode in the range from 50 to 500 Da.
GC-MS analysis was carried out on a Thermo PolarisQ gas chromatograph coupled with an ion-trap mass spectrometer (GC-MS). The system was fitted with a 30 m long, 0.25 mm I.D. RTX-5 with a film thickness of 0.25 μm 0.1 μm (Restek Corp. PA). After an initial hold time of five min, the GC column temperature was programmed from 40°C to a final temperature of 340°C at a rate of 20°C/min. The sample was prepared for GC-MS analysis by dissolving in 0.5 mL of N, O-Bis(trimethylsilyl)- trifluoroacetamide (BSTFA) containing 1% Trimethylchlorosilane (TMCS) (Pierce, IL) catalyst and heating in a sealed vial to 60°C for 15 min to form trimethylsilyl derivatives. The derivatized sample was then injected without further preparation into the GC-MS for characterization by electron impact ionization and chemical ionization (CI; CH4 reagent gas).
2.4. Data mining
To determine which metabolites were significantly elevated or reduced in MND: We used three measures of class association, the t-statistic, Pearson’s correlation coefficient, and the ‘relative class association’ measure (Golub et al., 1999). All three measures produced similar rankings of the metabolites by their level of association with MND versus control. We assessed statistical significance by permutation testing as described (Golub et al., 1999), and all measures showed similar numbers of metabolites to have significantly higher or lower concentrations in MND compared to controls. Figures 2 and 4 were based on the relative class association measure (Golub et al., 1999). For multivariate regression (Figures 3 and 5): Partial least squares discriminant analysis (PLS-DA) was performed using SIMCAP, v10.5 (Umetrics, Kinnelon, NJ) as described (Manley, 1994; Kennedy et al., 1997; Eriksson et al., 2001).
Figure 2.
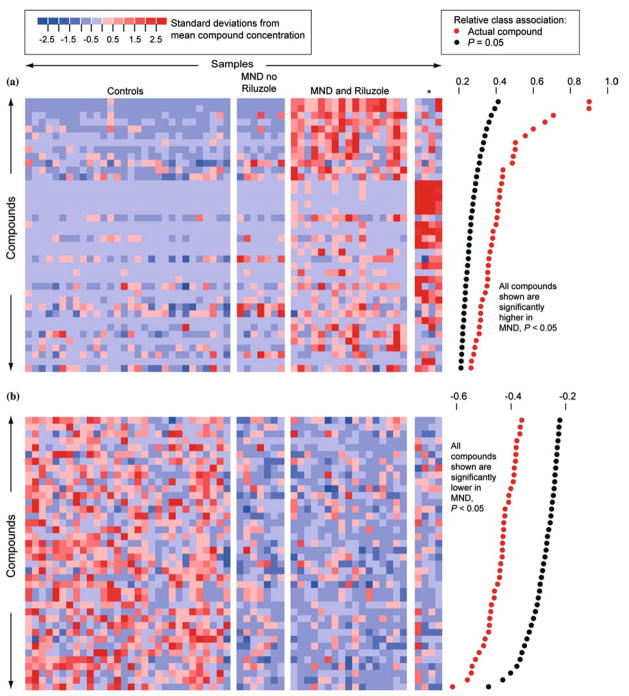
Metabolites with significantly different concentrations in normal and MND plasmas in study 1. (a) Metabolites higher in MND patients than in controls. Each row represents a metabolite, each column represents a healthy control or a patient, and each colored square represents the relative concentration of a single metabolite in a single person. Compounds are sorted by decreasing association with MND. Significant association measures at p = 0.05 are indicated by black dots at the right. The association measures for the actual data are indicated by red dots. A metabolite is significantly associated with MND if its relative class association (indicated by the red dot) is > than the relative class association in 95% of the randomly permuted replicates (indicated by the black dot). The metabolites that are high in MND define three subgroups. These consist of patients not taking Riluzole, patients taking Riluzole, and four patients with a distinctive signature (indicated by an asterisk). Three of these patients had LMN disease. Supporting figure 1 provides identifiers for metabolite peaks and study participants. (b) Metabolites lower in MND patients. Supporting figure 2 provides identifiers for metabolite peaks and study participants.
Figure 4.
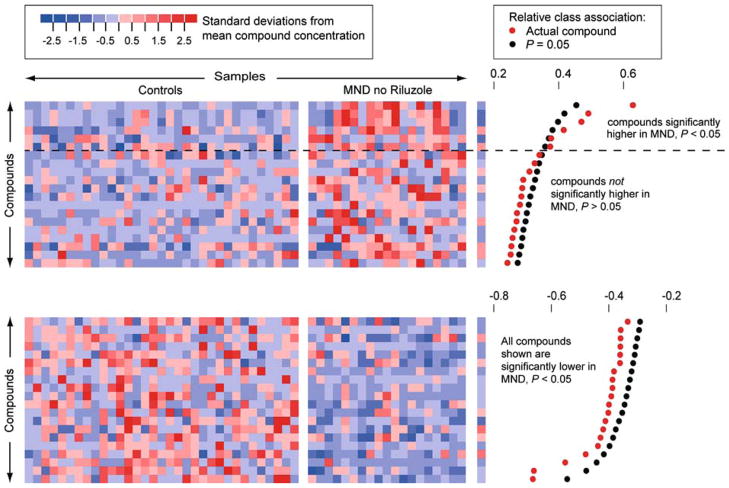
Metabolites with significantly different concentrations in normal controls and in MND patients not taking Riluzole. Six metabolites were significantly higher in MND patients not taking Riluzole than in normal controls. Over 70 metabolites were significantly lower in MND patients. Supporting figure 3 provides identifiers for metabolite peaks and study participants.
Figure 3.
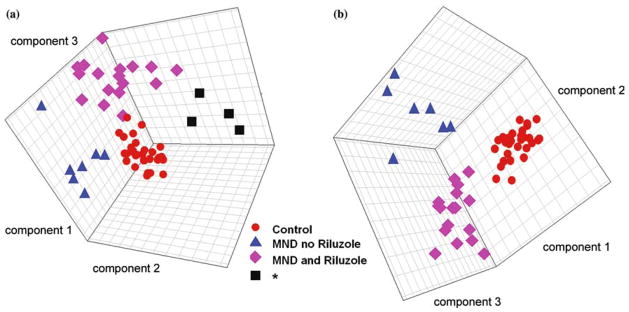
PLS-DA distinguished subgroups of MND in study 1. Models using projections into three dimensions provided statistically significant separations between subgroups (p < 0.01 by permutation test –random assignment of samples to subgroups). (a) Model including the four patients with a distinctive signature, three of whom had LMN disease, indicated by an asterisk. (b) Model excluding these patients.
Figure 5.
PLS-DA distinguished MND from controls in study 2. A model using a projection into two dimensions provided a statistically significant separation (p < 0.01 by permutation test) between MND and controls.
3. Results
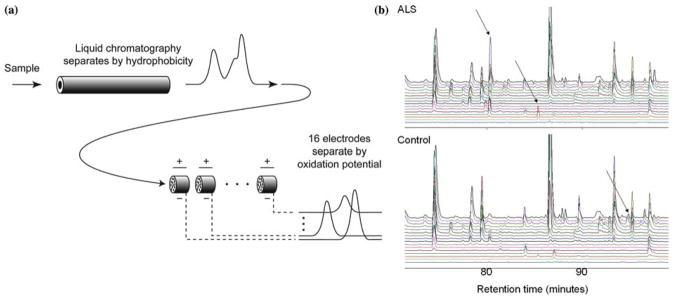
Our first study was designed to assess whether there are systematic differences between redox-active metabolites in the blood of patients with MND and healthy controls (table 1). We analyzed the blood plasma of 30 healthy controls and of 28 individuals with MND using electrochemical detection, which consists of high performance liquid chromatography separation followed by with coulometric array detection. This technique separates compounds in two dimensions: by hydrophobicity and oxidation potential (figure 1). Chromatographic data were reduced to tabular form by initial signal processing, which included aligning the chromatograms in the temporal dimension, followed by the use of peak heights as estimators of compound concentration. We obtained relative concentrations for 317 metabolites (Supporting Data Set 1).
Figure 1.
Metabolic profiling using coulometric array detection, a form of electrochemical detection. (a) Coulometric array detection separates molecules in two dimensions: hydrophobicity and oxidation potential. Samples are injected into a high performance liquid chromatography (HPLC) column, where they are fractionated by hydrophobicity. The eluent of the column flows through porous electrodes representing 16 different electrical potentials – the coulometric array. These electrodes detect redox-active metabolites by measuring their oxidation potentials. Each electrode generates one chromatogram. Thus, the output consists of 16 parallel chromatograms corresponding to 16 different oxidation potentials. The height of a peak in one of the output chromatograms provides the concentration of a metabolite with a particular hyrdophobicity and oxidation potential. As indicated schematically in this figure, this method is able to use oxidation potential to separate peaks that still overlap after separation by hydrophobicity. (b) Coulometric array chromatograms from plasmas of an ALS patient and a healthy control. Arrows indicate three differences between the chromatograms. Chromatograms are arranged from the lowest oxidation potential at the bottom to highest at the top.
Initial exploration of these 317 metabolites in 58 individuals suggested that some metabolites had different concentrations in normal versus MND plasma. The concentrations of some of these metabolites are shown graphically in figure 2. Each column in the grid represents an individual, and each row represents a single metabolite. The color of each cell indicates the concentration of one metabolite in one person, relative to the concentrations of that metabolite in all the samples. For a given metabolite, blue shades indicate lower-than-average concentrations, red shades higher-than-average concentrations. Panel A shows metabolites with generally higher concentrations in MND patients than in controls, and panel B shows metabolites with generally lower concentrations in MND patients than in controls. We analyzed these data to determine if these differences were statistically significant after taking into consideration the multiple hypothesis testing implicit in the examination of 317 metabolites. We used permutation tests that randomly reshuffled diagnostic categories to assess which metabolites had statistically significant differences between controls and MND patients. These tests confirmed that over 100 metabolites showed significantly different concentrations in normal versus MND plasmas (p < 0.05 by permutation test; figure 2). Inspection of the profiles of metabolites that were elevated in MND patients revealed three apparent subgroups of patients (figure 2a). Examination of clinical data showed that these subgroups corresponded to: (i) ALS patients not taking the drug Riluzole; (ii) ALS patients taking Riluzole; and (iii) a set of four patients (subjects 20, 43, 44, and 48 in Supporting Table 1) in which LMN disease was over-represented and that was characterized by a distinctive signature of highly correlated metabolites. As shorthand we will refer to this last subgroup as the subgroup ‘enriched for LMN’. The distinctive signature of this group apparently does not stem from any medication specific to this subgroup. Only vitamin E was common to all members of this subgroup, and vitamin E is also taken by a large portion of subjects who do not show this signature (Supporting table 4).
We next analyzed these data to determine if the metabolites were capable of distinguishing four subgroups (normal controls, MND not taking Riluzole, MND taking Riluzole, and the subgroup enriched for LMN) using the 317 metabolite concentrations. Using a supervised projection technique, PLS-DA, we found a three-dimensional projection in which these four subgroups were significantly separate (p < 0.01 by permutation test; figure 3a) (Manley, 1994; Kennedy et al., 1997; Eriksson et al., 2001). Distinctions between subgroups were heightened when the subgroup enriched for LMN was removed (figure 3b).
It was clear that many metabolites strongly associated with MND in the first experiment were found primarily in patients taking Riluzole (figure 2a). This raised the question of whether it would be possible to distinguish MND from control without the metabolites associated with Riluzole use. As few patients were not taking Riluzole, we addressed this question in a second study of 19 MND patients, none of whom were taking Riluzole, and 33 healthy controls. For each subject, we measured the concentrations of 289 metabolites (Supporting Data Set 2). Permutation tests carried out as on the first data set showed six metabolites for which high concentrations were significantly associated with MND (figure 4). The lower number of metabolites that were significantly elevated in MND in this study compared to study 1 appeared to be due to the absence of Riluzole-associated compounds and to a smaller sample size. Over 70 metabolites had concentrations significantly lower in MND plasma. We also found a projection into two dimensions that clearly and significantly separated the normal controls from the MND patients (p < 0.01 by permutation test; figure 5). This demonstrated that separation of MND from controls could be achieved even without the strong metabolic signal induced by Riluzole.
In study 1, the signature for the drug Riluzole was strong and could be used to identify an MND patient on the drug with 100% confidence in blinded studies (data not shown). In study 1, a major peak at 80.2 min on channel 5 (peak 1) and a minor peak at 78.9 min on channel 5 (peak 2) were uniquely associated with patients taking Riluzole. The electrochemical signatures of these peaks were typical of a hydroxyl group on a conjugated multiple ring structure. Neither peak corresponded to the retention time and electrical potential of the parent Riluzole compound (72.5 min, channel 8), which was not observed.
These unknown peaks were isolated from pools and concentrated 100-fold by sequential column preparations to allow detection by mass spectrometry (MS). The retention times and electrochemical responses were then cross verified in an MS-compatible HPLC formate buffer system. The retention times of peaks 1 ([M + H]+=357.048) and 2 ([M + H]+=359.068) were 4.57 and 10.04 min, respectively. That of Riluzole was 19.55 min. Inclusion of the heteroatoms of Riluzole (F-3, O; S; N-2) in the consideration of these exact masses, yielded no reasonable elemental combination indicative of the presence of a Riluzole moiety on either of the two protonated species. MS/MS fragmentation data obtained with both Q-Tof and ion trap mass spectrometers produced the following fragment ions: m/z 340, 322, 282, and 264 for the parent of m/z 357 and the series: m/z 341, 267, 251, and 249 for the parent of m/z 359. By comparison, MS/MS of protonated Riluzole yielded mostly low abundance fragments of m/z 208, 193 166, and 149. This would suggest that the two peaks are not directly derived metabolites of the drug. However, due to the limited scan range (50–500 Da) in which the LC-MS data were acquired, this does not exclude the possibility of a conjugate or a fragment or an induced metabolite which may lead to a species of molecular mass outside of our scan range.
When the isolated fraction was subsequently examined by GC-MS electron impact ionization, two peaks were observed with spectra consistent with those for the TMS esters of hexadecanoic acid and octadecanoic acid. Further analysis of the sample using methane chemical ionization revealed molecular ions at 641 and 697, inconsistent with hexadecanoic or octadecanoic acid (which had already been eliminated on the grounds of retention time). When MS/MS was performed on the molecular ions, each yielded two fragments: 313 + 331 and 341 + 359, respectively. MS3 on the 313 and 341 ions yielded a 131 ion. This suggested the two molecules may be fatty acid-containing cellular components whose exact identity is still to be determined.
4. Discussion
We report that: (i) metabolic profiles based on approximately 300 metabolites permit mathematical separation of MND and control subjects; (ii) MND is associated more with a general down-regulation than elevation of metabolites; (iii) a set of highly correlated metabolites is characteristic of a subset of MND patients that was significantly enriched for LMN disease; (iv) 12 compounds are significantly up-regulated in MND patients taking Riluzole.
The metabolites that are significantly up and down regulated in MND constitute a preliminary biochemical signature for the disease. The specificity of these signatures will be further validated by comparison to metabolic profiles in other neurodegenerative diseases, in neuromuscular disorders, and in additional cohorts of MND patients and healthy controls. If these signatures can be confirmed in a large population of patients just after diagnosis or through retrospective analysis of pre-existing banked sera, these signatures would become diagnostic markers for the disease. Such biomarkers, once their chemical identities are determined and mapped to biochemical pathways, may point to new hypotheses about the pathogenesis of MNDs. Additionally, they could enable early detection and serve as surrogate indices of disease activity that would enhance the monitoring of therapeutic effects in treatment trials. Longitudinal studies are needed to achieve this goal.
The drug Riluzole induces a strong metabolic signature. The two most strongly induced metabolites seem not to derive chemically from the drug, but may reflect modified metabolic processes. Elucidating the structures of these molecules could identify biochemical pathways that are subject to perturbations by the drug, some of which could contribute to its efficacy and some to its side effects. Although Riluzole is believed to act through glutaminergic-related pathways, its full mechanisms of action and side effects remain poorly understood.
It remains unclear whether pure LMN disorders, pure UMN disease and ALS share a common underlying pathophysiologic process. Nonetheless, our data suggest that metabolomics might contribute to the classification of MNDs, which will be crucial for basic research and clinical trials. Our data revealed a set of highly correlated metabolites found in a subset of four of the MND patients, all but one of which had LMN disease. Full metabolic analysis of MNDs could result in a better stratification and understanding of these seemingly related diseases.
The initial biochemical signatures for LMN disease and ALS, once confirmed in larger patient populations and studied longitudinally, could lead to identification of diagnostic and surrogate markers for the disease. Such signatures could also enable better stratification of MND subtypes and provide insight into aberrant biochemical pathways and targets for drug design. Metabolomics offers a potentially powerful new tool for a more comprehensive understanding of disease states.
Acknowledgments
This work was supported in part by the Al-Athel ALS Research Foundation, the ALS Therapy Alliance, the Angel Fund, the DeBourgknect ALS Research Fund, the National Institutes of Health (NINDS and NIA), Project ALS, and the U.S. Department of Defense. We thank L. Bruijn of the ALS Foundation, D. W. Cleveland, J. Rebek Jr., J.D. Rothstein, and P. Schimmel for scientific discussions.
Footnotes
S.R., M.E.C. and M.B. contributed equally to this work. W.R.M. and B.S.K. contributed equally to this work. S.R., M.B., W.R.M., B.S.K., C.B., S.H., P.V., M.F.B., and R.K-D. have financial interests in Metabolon Inc., a company engaged in metabolic profiling.
Electronic supporting figures, tables and datasets are available at the Journal’s website.
References
- Beal M, Ferrante R, Browne S, Matthews R, Kowall N, Brown R. Increased 3-Nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann Neurol. 1997;42:644–654. doi: 10.1002/ana.410420416. [DOI] [PubMed] [Google Scholar]
- Bensimon G, Lacomblez L, Meininger V. A controlled trial of Riluzole in amyotrophic lateral sclerosis. N Engl J Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- Beuche W, Yushchenko M, Mader M, Maliszewska M, Felgenhauer K, Weber F. Matrix metalloproteinase-9 is elevated in serum of patients with amyotrophic lateral sclerosis. Neuroreport. 2000;11:3419–3422. doi: 10.1097/00001756-200011090-00003. [DOI] [PubMed] [Google Scholar]
- Bogdanov M, Brown RH, Matson W, Smart R, Hayden D, O’Donnell H, Flint Beal M, Cudkowicz M. Increased oxidative damage to DNA in ALS patients. Free Radic Biol Med. 2000;29:652–658. doi: 10.1016/s0891-5849(00)00349-x. [DOI] [PubMed] [Google Scholar]
- Brindle J, Antti H, Holmes E, Traner G, Nicholson J, Bethell H, Clarke S, Schofield P, McKilling E, Mosedale D, Grainer D. Rapid and non-invasive diagnosis of the presence and severity of coronary hear disease using 1H-NMRbased metabonomics. Nat Med. 2002;8:1439–1444. doi: 10.1038/nm1202-802. [DOI] [PubMed] [Google Scholar]
- Brindle J, Nicholson J, Schofield P, Grainger D, Holmes E. Application of chemometrics to 1H NMR spectroscopic data to investigate a relationship between human serum metabolic profiles and hypertension. Analyst. 2003;128:32–36. doi: 10.1039/b209155k. [DOI] [PubMed] [Google Scholar]
- Camu W, Billiard M, Baldy-Moulinier M. Fasting plasma and CSF amino acid levels in amyotrophic lateral sclerosis: a subtype analysis. Acta Neurol Scand. 1993;88:51–55. doi: 10.1111/j.1600-0404.1993.tb04186.x. [DOI] [PubMed] [Google Scholar]
- Cleveland D, Rothstein J. From Charcot to Lou Gehrig: Deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- Eriksson L, Johansson E, Kettaneh-Wold N, Wold S. Multi and Megavariate Analysis: Principles and Applications. Umetrics; Umea: 2001. [Google Scholar]
- Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, Coller H, Loh ML, Downing JR, Caligiuri MA, Bloomfield CD, Lander ES. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- Hadano S, Hand C, Osuga H, Yanagisawa Y, Otomo A, Devon R, Miyamoto N, Showguchi-Miyata J, Okada Y, Singaraja R, Figlewicz D, Kwiatkowski T, Hosler B, Sagie T, Skaung J, Nasir J, Brown RJ, Scherer S, Rouleau G, Hayden M, Ikeda J. A gene encoding a putative GTPase regulator is mutated in familial amyotrophic lateral sclerosis 2. Nat Genet. 2001;29:166–173. doi: 10.1038/ng1001-166. [DOI] [PubMed] [Google Scholar]
- Harrigan GG, Goodacre R, editors. Metabolic Profiling: Its Role in Biomarker Discovery and Gene Function Analysis. Kluwer Academic Publishers; Boston: 2003. [Google Scholar]
- Kell DB. Metabolomics and system biology: making sense of the soup. Curr Opin Microbiol. 2004;7:296–307. doi: 10.1016/j.mib.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Kennedy R, Lee Y, vanRoy B, Redd C, Lippman R. Solving Data Mining Problems Through Pattern Recognition. Prentice Hall PTR; Indianapolis: 1997. [Google Scholar]
- Kristal B, Vigneeau-Callahan K, Matson W. Simultaneous analysis of the majority of low-molecular weight, redoxactive compounds from mitochondria. Anal Biochem. 1998;263:18–25. doi: 10.1006/abio.1998.2831. [DOI] [PubMed] [Google Scholar]
- Kristal BS, Vigneau-Callahan K, Matson WR. Simultaneous analysis of multiple redox-active metabolites from biological matrices. Meth Mol Biol. 2002;186:185–194. doi: 10.1385/1-59259-173-6:185. [DOI] [PubMed] [Google Scholar]
- Kurtzke J, Kurland L. The epidemiology of neurologic disease. In: Joynt R, editor. Clinical Neurology. J.B. Lippincot; Philadelphia: 1989. pp. 1–43. [Google Scholar]
- Lacomblez L, Bensimon G, Leigh P, Guillett P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Lancet. 1996;347:1425–1431. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- Lambrechts D, Storkebaum E, Morimoto M, Del-Favero J, Desmet F, Marklund SSW, et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet. 2003;34:383–394. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- Lilienfeld DE, Chan E, Ehland J, Godbold J, Landrigan PJ, Marsh G, Perl DP. Rising mortality from motoneuron disease in the USA, 1962–84. Lancet. 1989;1:710–713. doi: 10.1016/s0140-6736(89)92218-6. [DOI] [PubMed] [Google Scholar]
- Lindon JC, Holmes E, Nicholson JK. Metabolomics and its role in drug development and disease diagnosis. Expert Rev Mo Diagn. 2004;4:189–199. doi: 10.1586/14737159.4.2.189. [DOI] [PubMed] [Google Scholar]
- Manley B. Multivariate Statistical Methods: A Primer. 2. CRC Press; Boca Raton: 1994. [Google Scholar]
- Matson W, Langials P, Volicer L, Gamache P, Bird E, Mark K. N-electrode three dimensional liquid chromatography with electrochemical detection for determination of neurotransmitters. Clin Chem. 1984;30:1477–1488. [PubMed] [Google Scholar]
- McGuire V, Longstreth W, Koepsell T, Belle G van. Incidence of ALS in three counties in western Washington state. Neurology. 1996;47:571–573. doi: 10.1212/wnl.47.2.571. [DOI] [PubMed] [Google Scholar]
- Milbury P. Coulometric Array Detectors for HPLC. V.S.P. International Science; Utrecht, the Netherlands: 1997. CEAS generation of large multiparameter databases for determining categorical process involvement of biomolecules; pp. 125–141. [Google Scholar]
- Mitsumoto H, Chad D, Pioro E. Amyotrophic Lateral Sclerosis. Oxford Press; New York: 1998. [Google Scholar]
- Nicholson JK, Wilson ID. Understanding ‘global’ systems biology: metabonomics and the continuum of metabolism. Nat Rev Drug Discov. 2003;2:668–676. doi: 10.1038/nrd1157. [DOI] [PubMed] [Google Scholar]
- Puls I, Jonnakuty C, LaMonte B, Holzbaur E, Tokito M, Mann E, Floeter M, Bidus K, Drayna D, Oh S, Brown R, Ludlow C, Fischbeck K. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33:455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- Rosen D, Siddique T, Patterson D, Figlewicz D, Sapp E, Hentati A, Donaldson D, Goto J, O’Reagan J, Deng H. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rothstein J, Tsai G, Kuncl R, et al. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann Neurol. 1990;28:18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- Rowland L, Shneider N. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- Shi H, Vigneau-Callahan K, Matson W, Kristal B. Attention to relative response across sequential electrodes improves quantitation of coulometric array. Anal Biochem. 2002;302:239–245. doi: 10.1006/abio.2001.5568. [DOI] [PubMed] [Google Scholar]
- Simpson E, Henkel J, Henry Y, Smith R, Appel S. Elevated levels of 4-HNE in the sera of patients wtih amyotrophic lateral sclerosis. Neurology. 2003;60:A242. [Google Scholar]
- Smith R, Henry Y, Mattson M, Appel S. Presence of 4-hydroxynonenal in cerebrospinal fluid of patients with sporadic amyotrophic lateral sclerosis. Ann Neurol. 1998;44:696–699. doi: 10.1002/ana.410440419. [DOI] [PubMed] [Google Scholar]
- Greef J van der, Stroobant P, Heijden R van der. The role of analytical sciences in medical systems biology. Curr Opin Chem Biol. 2004;8:559–565. doi: 10.1016/j.cbpa.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Vigneau-Callahan K, Shestopalov A, Milbury P, Matson W, Kristal B. Characterization of diet-dependent metabolic serotypes: I. Analytical and biological variability issues. J Nutr. 2001;131:924S–932S. doi: 10.1093/jn/131.3.924S. [DOI] [PubMed] [Google Scholar]
- Yang Y, Hentati A, Deng H, Dabbagh O, Sasaki T, Hirano M, Hung W, Ouahchi K, Yan J, Azim A, Cole N, Gascon G, Yagmour A, Ben-Hamida M, Pericak-Vance M, Hentati F, Siddique T. The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat Genet. 2001;29:160–165. doi: 10.1038/ng1001-160. [DOI] [PubMed] [Google Scholar]