Summary
While the signals that control neutrophil migration from the blood to sites of infection have been well characterized, little is known about their migration patterns within lymph nodes, or the strategies that neutrophils use to find their local sites of action. To address these questions, we used two-photon scanning laser microscopy (TPSLM) to examine neutrophil migration in intact lymph nodes during infection with an intracellular parasite, Toxoplasma gondii. We find that neutrophils form both small, transient or large, persistent swarms via a strikingly coordinated migration pattern. We provide evidence that cooperative action of neutrophils and parasite egress from host cells can trigger swarm formation. Neutrophil swarm formation coincides in space and time with the removal of macrophages that line the subcapsular sinus of the lymph node. Our data provide insights into the cellular mechanisms underlying neutrophil swarming and suggest new roles for neutrophils in shaping immune responses.
Keywords: neutrophil, parasite, multiphoton microscopy
Introduction
Neutrophils are the most abundant nucleated cell in the blood, and play a crucial role in immune responses to pathogens. Neutrophils are best known for their role in phagocytosis and killing of extracellular bacteria, however they can provide protection against a diverse set of pathogens, and do so by performing a variety of different functions (reviewed in (Appelberg, 2007; Nathan, 2006)). These functions include tissue remodeling, antigen presentation, recruiting other blood cells, and polarizing T cell responses (Beauvillain et al., 2007; Megiovanni et al., 2006; Pesce et al., 2008; Tvinnereim et al., 2004). For example, neutrophils play an important protective role during infection with the intracellular protozoan parasite, Toxoplasma gondii (Bennouna et al., 2003; Bliss et al., 2001; Denkers et al., 2004; Sayles and Johnson, 1996) in spite of the fact that the parasite is relatively resistant to killing by neutrophils (Channon et al., 2000; Nakao and Konishi, 1991b). There is evidence that the protective effect of neutrophils during Toxoplasma infection is due in part to the production of IL12 by neutrophils and the subsequent shaping of the adaptive immune response (Bennouna et al., 2003). However, the precise roles of neutrophils during infection remain poorly understood.
Neutrophils are produced in the bone marrow, circulate in the blood, and are rapidly recruited to sites of infection in response to a variety of chemoattractants produced by inflamed tissues (reviewed in (Baggiolini, 1998; Scapini et al., 2000). Recently a number of studies have shown that neutrophils can also traffic to lymph nodes in response to infection (Abadie et al., 2005; Maletto et al., 2006; Pesce et al., 2008), raising the possibility that neutrophils may modulate immune responses within lymph nodes. One powerful tool for investigating the function of cells within lymph nodes is two-photon scanning laser microscopy (TPSLM), an imaging method that provides dynamic information about cell migration and interactions within tissue samples (reviewed in (Bousso and Robey, 2004; Germain et al., 2006; Sumen et al., 2004; Cahalan and Parker, 2008)). Thus far, this approach has been primarily used to examine responses to model antigens by T and B cells, and is just beginning to be applied in the setting of infection and to examine immune responses of non-lymphoid cells (Egen et al., 2008; Zinselmeyer et al., 2008).
In spite of their abundance, physiological importance, and clear indications that they can traffic to lymph nodes during infection, we know very little about what neutrophils do in the lymph node. Do neutrophils work individually, or in groups? What are the cues that guide neutrophil migration within lymph nodes? What impact do neutrophils have on other cell types in lymph nodes? Here we address these questions using TPSLM and a Toxoplasma gondii - mouse infection model. We find that neutrophils accumulate in the subcapsular sinus of the draining lymph node following infection, and form both small, transient and large, persistent swarms via a highly coordinated migration pattern. We provide evidence that cooperative action of neutrophils and parasite egress from host cells can trigger swarm formation, and that neutrophil swarms lead to the removal of macrophages that line the subcapsular sinus of the lymph node. These results provide insight into the cellular mechanisms that lead to neutrophil swarms and suggest new potential functions for neutrophils in lymph nodes.
Results
An experimental model to examine neutrophil migration in lymph nodes
In order to examine the behavior of neutrophils in lymph nodes during infection, we infected mice with the intracellular protozoan parasite, Toxoplasma gondii. Following injection of live fluorescent parasites into the earflap, parasites can be found within one hour in the subcapsular sinus of the draining lymph node in association with LYVE1+ lymphatic vessels (Fig 1A). Many parasites are found within the CD169+ macrophages that line the subcapsular sinus (Fig 1Bi), a distribution similar to that seen with other particulate antigens, such as viruses and immune complexes (Carrasco and Batista, 2007; Junt et al., 2007; Phan et al., 2007).
Fig. 1.
Location of T. gondii relative to lymphatics, CD169 macrophages, and neutrophils in draining lymph nodes following earflap infection. (A) 20 um frozen section of a draining lymph node 4 hours after an earflap injection with RFP (red) parasites. Middle panels show staining with LYVE-1 (blue) used to visualize the lymphatic system. Right hand panel shows a high magnification image. (B) RFP parasites (red) are found inside CD169+ subcapsular sinus macrophages (blue) close to LysGFP reporter bright (green) neutrophils. Middle panel (i) shows an enlarged area with parasites inside CD169+ cells. Right panel (ii) shows an enlarged area with intact parasites inside neutrophils. (C) Same samples as in (A) showing signal from the LysGFP reporter (green). (D) Clusters of LysGFP high cells (green) (left panel) stain positive for the neutrophil marker Ly6G (blue). Right panel shows the same image without the LysGFP signal.
In order to track neutrophils relative to Red Fluorescent Protein (RFP)-labeled parasites we used reporter mice in which GFP is under the control of the lysozyme M promoter (lysGFP) (Faust et al., 2000). While this reporter is also expressed by macrophages and a subset of dendritic cells, the vast majority of the GFPhigh cells in the lymph nodes at 1-5 hours after infection are neutrophils, based on their cell surface phenotype (Ly6Ghigh, CD11bhigh, CD11clow) (Rydstrom and Wick, 2007; Sasmono et al., 2007) (Fig S1A). Neutrophil recruitment to the draining lymph nodes occurred rapidly and was dependent on the TLR/IL1R adaptor protein, MyD88 (Fig. S1A). Interestingly, many neutrophils were associated with LYVE1+ lymphatic vessels (Figure 1C). This is consistent with the possibility that neutrophils entered the lymph node via lymphatics, as has been reported previously (Abadie et al., 2005; Maletto et al., 2006). We also detected neutrophils within blood vessels of infected lymph nodes (data not shown), suggesting that neutrophils may enter lymph nodes via both blood and lymph.
At early times post infection, neutrophils contained proportionally more parasites compared to macrophages and dendritic cells (Fig. S1B). Although phagocytic destruction of pathogens is one protective mechanism used by neutrophils, the parasites inside neutrophils appear intact (Fig 1Bii). This is consistent with evidence that T. gondii can divide in vitro in human neutrophils (Channon et al., 2000; Nakao and Konishi, 1991b), and indications that the protective effects of neutrophils during T. gondii infection are due to immunoregulation rather than direct killing mechanisms (Bennouna et al., 2003; Bliss et al., 2001; Denkers et al., 2004; Sayles and Johnson, 1996).
Neutrophils form dynamic swarms in the subcapsulary sinus of the lymph node
Analysis of tissue sections of lymph nodes from infected mice showed that reporter positive cells formed large clusters along the subcapsular sinus that generally coincided with the location of parasites (Fig. 1 C, D). Virtually all of the reporter bright cells in the subcapsular sinus (SCS) region of the lymph node express Ly6G (Figure 1D), consistent with flow cytometric analysis (Fig. S1A) and confirming their identity as neutrophils.
To examine the dynamics of neutrophil cluster formation and the relationship of cluster formation to infection, we examined intact draining lymph nodes of lysGFP reporter mice infected with RFP parasites using 2-photon scanning laser microscopy (TPSLM). Visual inspection of time-lapse imaging data revealed the striking dynamics of neutrophil swarm formation (Fig 2A and Videos 1-3). Some swarms, which we term transient swarms, grew by rapid, large-scale, coordinated migration of neutrophils into the swarm and then quickly dissolved as neutrophils migrated out to join nearby growing swarms (Fig 2A and Suppl Video 1,2). Transient swarms formed and dispersed over a period of 10-40 minutes (mean duration 20 minutes) and remained relatively small (<4 × 104 microns3, corresponding to approximately 150 neutrophils). We also noted a distinct type of swarm, which we term persistent swarms, that grew throughout the imaging period (up to 38 minutes) both by continued migration of neutrophils into the swarm and by merging with nearby smaller swarms (Figure 2A and Suppl Video 3). Persistent swarms tended to be large and occasionally grew to fill the entire imaging volume (>6 × 105 microns3). The relationship between the size and persistence of the swarms is consistent with the notion that neutrophils themselves generate signals that induce swarming and that, once swarms reach a certain size they produce a large signaling center that can overwhelm competing nearby signals.
Fig. 2.
Neutrophil migration and swarm formation in infected lymph nodes. Mice expressing a macrophage/neutrophil transgenic reporter (LysGFP) (Faust et al., 2000) were infected in the ear flap and draining lymph nodes removed at 2 to 5 h post-infection were imaged using TPSLM. (A) Neutrophils form transient (left panel) and persistent (right panel) swarms in intact lymph nodes. The volumes of individual swarms were plotted versus elapsed imaging time. Transient swarms were all <4 × 104 microns3, corresponding to approximately 150 cells based on average volume of a single neutrophil from these runs (275 μm3). (B) Quantitation of neutrophil motility. Each point represents the average speed for an individual track of a neutrophil outside of a swarm. The tracks of neutrophils containing signal from red fluorescent parasites were plotted on the right. (C) Distance to swarm center versus time. Each line represents an individual track. Solid black lines correspond to neutrophils that eventually enter a swarm. Grey lines correspond to tracks of neutrophils that do not enter the swarm. (D) Plot of the mean “directionality angle” or psi for a run in which swarms formed. Psi is defined as the angle between the migration vector and the direction vector from the initial position of the migrating cell to the center of the swarm (see inset). Psi values were binned based on the distance of the cell to the swarm center, and the mean values are plotted versus the distance to swarm center. Dashed line indicates the value expected for random migration (90 degrees). Values less than 90 degrees indicate directed migration toward the swarm center. Values were expressed as mean ± standard error. The confidence interval over all distances is 73-77 degrees, and the p value for the probability that the mean distribution is 90 degrees is 10-40. (E) Coordinated migration during swarm formation. The “coordination angle” alpha is defined as the angle between the migration vectors for pairs of time points within 2 individual tracks (see inset). Alpha values were binned based on the distance between cell pairs and plotted versus the distance between pairs. Solid black line represents the values for a run with a swarm (Corresponds to Suppl Video 1). Grey line represents the values for a run with no obvious swarms. Values were expressed as mean ± standard error. The confidence interval for the run with a swarm over all distances is 77-83 degrees, and the p value for the probability that the mean distribution is 90 degrees is 10-5. The confidence interval for the run without a swarm over all distances is 88-93 degrees, and the p value for the probability that the mean distribution is 90 degrees is 0.3. (Corresponds to Suppl Video 5).
Out of a total of 40 runs representing ∼20 hours of cumulative imaging time, we observed 30 swarms, of which 16 were transient. We also observed neutrophil recruitment and swarming behavior in lymph nodes following earflap infection with Listeria monocytogenes (data not shown). This indicates that swarming is not unique to T. gondii infection, but may be a more general response to infection. While we occasionally observed neutrophil swarms in uninfected lymph nodes (2 persistent and 0 transient swarms seen in 14 runs), these were infrequent compared to infected lymph nodes. This, together with the clear correlation between neutrophil recruitment and infection seen from analysis of tissue sections and flow cytometry (Fig. 1 and S1), indicates that most neutrophil swarms form as a consequence of infection.
Quantitation of directed, coordinated migration by neutrophils
To relate neutrophil migration patterns to swarm formation, we tracked individual neutrophils before they entered swarms, or during migration without swarming (Fig. 2BE and Suppl. Video 4, 5). Neutrophils that were not in swarms migrated with average speeds of 11.9 micron/min, substantially faster than a recent report of neutrophil migration in the footpad (Zinselmeyer et al., 2008), but similar to that of naive T cells in intact lymph nodes ((Miller et al., 2002) and data not shown). Neutrophils containing parasites were only slightly slower that those that did not contain parasites (Fig. 2B).
In principle, swarms could form either by directed migration, or by random migration and retention of cells at sites where swarms were growing, two possibilities that can be distinguished by dynamic imaging. Visual inspection of time-lapse runs in which neutrophil swarms were forming showed large-scale directed migration of neutrophils into swarm center (Suppl Videos 1-3). To quantitate this directional migration during swarm formation we measured the distance of neutrophils to the swarm center, and plotted the changes in this distance for individual tracks over time. These analyses confirmed that individual neutrophils moved persistently toward the swarm over time, indicative of directed migration (Fig. 2C). Persistent movement toward the swarm centers could be seen even when neutrophils were >70 microns away from the swarm center. As an alternative method for quantitating directed migration, we also calculated the angle (psi) defined by the migration trajectory vector and the vector between initial neutrophil position and swarm center, and plotted the average psi as function of distance to swarm center (Fig. 2D). The mean value for psi should be 90 degrees for random movement, and less than 90 degrees for directed migration into swarms. We find that mean psi is significantly less than 90 degrees even for cells at distances >75 microns away from the swarm center. Together these analyses indicate that the growth of swarms occurs by large-scale directed migration of neutrophils toward swarm centers.
We noted that neutrophils tended to migrate in “streams” with multiple neutrophils following parallel paths while entering or leaving swarms (Suppl Videos 1 and 6, 7). To quantitate this phenomenon, we calculated the angle between the migration trajectories of pairs of cells (alpha) and plotted mean alpha as a function of spatial distance between cells in the pair (Fig. 2E). For runs in which swarming was not observed, the mean alpha was close to 90 degrees, consistent with lack of coordinated movement. In contrast, for runs in which swarms formed, alpha was significantly less than 90 degrees, a trend that could be seen even for cell pairs that were >100 microns apart. This streaming behavior could reflect communication between migrating cells, as been described for swarm formation in Dictyostelium (Kriebel et al., 2003) and/or may reflect a common response to competing attractive signals from fluctuating swarm centers.
Neutrophils swarms are initiated by pioneer neutrophils and parasite egress
To obtain clues about the signals that initiate swarm formation, we carefully examined parasite and neutrophil behavior during each recorded example of swarm formation. In some cases swarms formed in regions in which no parasites were visible and in absence of any obvious initiating event. However, in 40% (9/22) of swarm initiation events examined, swarm formation occurred in 2 distinct temporal stages (Fig. 3, Fig S2, and Suppl Videos 6-9). In these examples, a few “pioneer” neutrophils formed a small cluster, followed a few minutes later by large-scale migration of cells into the cluster. Importantly, during the initial phase of swarm formation, some neutrophils can be seen migrating randomly past the swarm center and do not begin their direction migration toward the swarm until several minutes after the arrest of the pioneer neutrophils (Fig. 3, Fig S2, and Suppl Videos 6 and 8). This suggests that only the pioneer neutrophils responded to the initial signal, and that the late-arriving neutrophils were responding to an amplified signal generated by the pioneers.
Fig. 3.
Neutrophil swarm formation can occur in 2 temporal stages. Corresponds to Suppl Video 8. Two-phase swarm formation was observed in ∼40% (9/22) initiation events examined. Additional examples are in Suppl Videos 6, 7, 9. (A) Left panel shows a time point during stage 1 with the tracks of early arriving neutrophils depicted as white lines. Right panel shows a time point during stage 2 with the tracks of late-arriving neutrophils depicted as white lines. (B) Distance to swarm center versus time for the tracks of neutrophils that enter the swarm. The tracks in red correspond to early arriving neutrophils (stage 1), and the tracks in black correspond to late arriving neutrophils (stage 2). Note that tracks end when neutrophils arrive at the edge of the swarm, due to difficulty in identifying individual cells once they enter the swarm.
Another clue to the initiation of swarm formation came from examination of runs with particularly heavily infected lymph nodes. In these samples, we occasionally observed groups of closely apposed non-motile parasites that suddenly became motile and migrated rapidly away from the group (Fig. 4A, Fig S2A, and Suppl Videos 9-11). Such behavior is typical for parasite egress from infected cells leading to cell lysis and invasion of neighboring cells (Black and Boothroyd, 2000). Interestingly, egress coincided closely in space and time with the initiation of a swarm in most (4/5) examples observed. The response of neutrophils to parasite egress was extremely rapid, with directed migration detectable at the same time points or even seconds before increased parasite motility was first detectable (Fig. 4B, Fig. S2B).
Fig. 4.
Parasite egress coincides with neutrophil clustering. Parasite egress is indicated by close apposition of non-motile parasites followed by the sudden acquisition of parasite motility. Corresponds to Suppl Video 10. Neutrophil clustering was associated with four out of the five instances of parasite egress observed. Additional examples are shown in Suppl Videos 9 and 11. (A) Neutrophils (lysGFP reporter) are in green and T. gondii (RFP) are in red. The tracks of neutrophils that enter cluster are indicated as yellow lines. Left panel shows a time point before parasite egress. The non-motile group of parasites is indicated by a dashed white circle. Middle panel show the time point when parasite motility is first detected. Newly motile parasites are indicated by arrowheads. Right panel show a time point after egress. (B) Distance from neutrophil to site of parasite egress versus time. Black lines correspond to the tracks of individual cells that migrate toward the site of parasite egress. Shaded lines indicate tracks that do not join the cluster.
Neutrophil swarms lead to removal of subcapsulary sinus macrophages
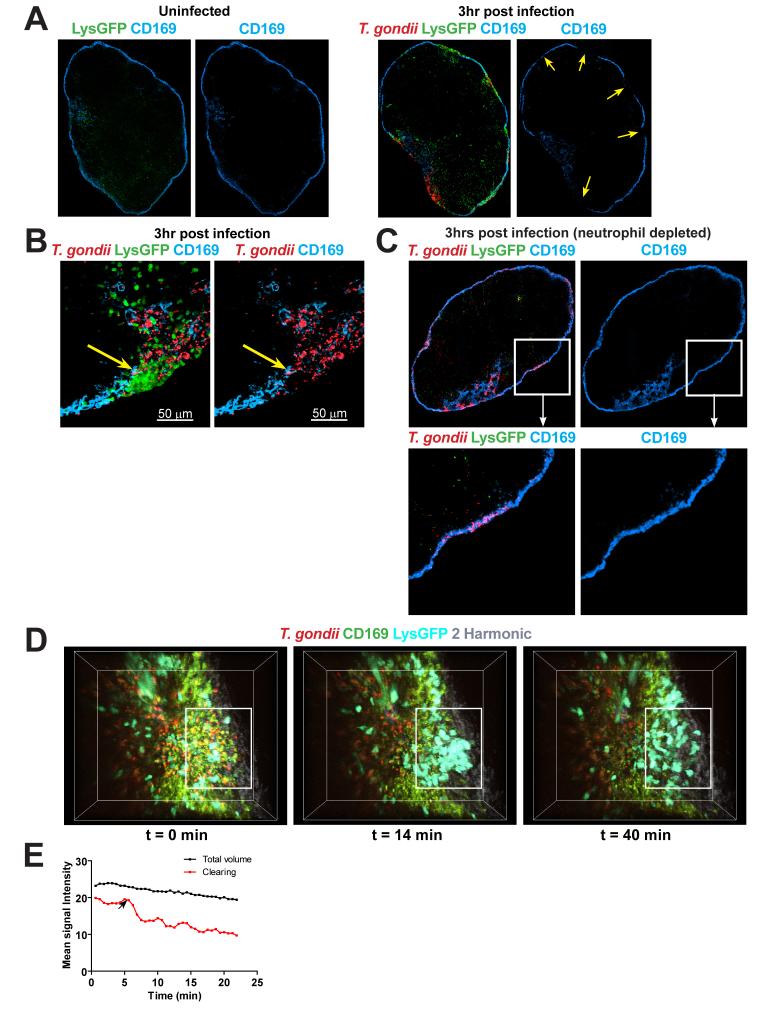
Because neutrophils are known to degrade tissues by releasing matrix metalloproteinases, the appearance of swarms in the subcapsular sinus of lymph nodes raised the possibility that neutrophil recruitment to lymph nodes during infection could alter lymph node structure. Indeed we find that, while uninfected lymph nodes have a continuous layer of CD169+ macrophages along the lymph node sinus (Fig. 5A left panels), infected lymph nodes show gaps in the CD169 staining that often coincides with the location of neutrophil clusters (Fig 5A, B right panels, arrows). We also see a loss of CD169+ cells by flow cytometric analysis of infected lymph nodes (Supplemental Figure 3), suggesting that SCS macrophage did not migrate elsewhere in the lymph node. In vivo depletion of neutrophils prior to infection largely prevented the appearance of gaps in the layer of CD169+ macrophages, even in regions of the subcapsular sinus that were heavily infected (Figure 5C). Thus although parasite egress also produces some cell lysis, neutrophil swarms, rather than infection per se, were primarily responsible for the gaps in the layer of SCS macrophages reported here. To examine the temporal relationship between swarming and the removal of subcapsular sinus macrophages we visualized neutrophil migration in real time in samples from lysGFP reporter mice that had been injected with fluorescent CD169+ antibodies along with RFP-labeled parasites (Fig. 5D and Suppl Video 12). At the beginning of the run, a continuous region of CD169 staining is visible, and after a few minutes a transient swarm appears and dissolves, leaving behind a gap in the CD169 staining in the same location as the transient swarm. Because spectral overlap between the GFP from neutrophils can obscure the loss of CD169 staining, we also examined lymph nodes from infected mice in which CD169 cells were labeled but neutrophils were not (Fig. 5E and Suppl. Video 13). In these samples we also observed regions of CD169 clearing with a size and rate of appearance that suggested that they coincide with neutrophil swarms. Together these data indicate that neutrophil swarms lead to the removal of subcapsular sinus macrophages.
Fig. 5.
Neutrophil clearing of CD169+ macrophages. (A) Low magnification confocal images of a whole lymph node of LysGFP reporter (green) stained with CD169 antibody (blue). The left panels show an uninfected lymph node while the right panels show a draining lymph node 3 hours after infection with RFP parasites (red). Right hand panels show CD169 staining only. Yellow arrows indicate location of gaps in the CD169 staining. (B) Higher magnification confocal image of a lymph node section showing a neutrophil cluster (green) in an area free of parasites (red) and CD169 staining (blue). Right panel shows CD169 and parasite signal only. The position of swarm is indicated by yellow arrows. (C) Confocal image of a draining lymph node from a mouse that was depleted of neutrophils just prior to infection, and analyzed 3 hours after infection with RFP parasites. The lower panels show an enlarged view of the boxed area indicating an area of high infection within a continuous CD169 layer. Right panel shows CD169 staining only. (D) Two-photon images of a lymph node showing CD169 staining (green), neutrophils (cyan), parasites (red) and lymph node capsule (2nd harmonic signal: grey). Left panel shows a time point just before a swarm forms. Center panel shows a time point during swarming. Cleared area is indicated by a white rectangle. Right panel shows a time point just after the swarm breaks up. Corresponds to Suppl Video 12. (E) Progressive disappearance of CD169 signal in local region of lymph node sinus. Fluorescence intensity of CD169 label in an area where clearing was observed by twophoton imaging and plotted against time (red line). The change in fluorescence intensity for the whole imaging volume is shown as a control for fluorophore bleaching (black line). Arrow indicates the approximate time when CD169 clearing was first observed. Corresponds to Suppl Video 13.
Neutrophil swarming and subcapsulary sinus macrophage removal also occur in mesenteric lymph nodes following oral infection
To confirm our observations using a more physiological route of infection, we infected LysGFP reporter mice orally with cysts generated from RFP-expressing parasites, and examined neutrophil recruitment, localization, and migration in mesenteric lymph nodes. While few neutrophils were detected in mesenteric lymph nodes of uninfected mice, neutrophils (GFPhigh Ly6G+) were readily detectable by day 4-5 following oral infection (Fig. 6A, B). Neutrophils form clusters throughout the lymph nodes of infected mice including some near the subcapsular sinus (Fig. 6B, white arrows). TPSLM of intact mesenteric lymph nodes revealed migration dynamics of reporter positive cells similar to that observed in draining lymph nodes following earflap infection (Suppl Video 14). Moreover, we also detected gaps in the layer of CD169+ SCS macrophage in mesenteric lymph nodes of infected mice, and these often co-incided with the location of clusters of reporter positive cells (Fig. 6C, white arrow). It is noteworthy that, while parasites and neutrophils are concentrated at the SCS of draining lymph nodes following earflap infection, parasites and neutrophils are found both in the SCS and in deeper regions of the mesenteric lymph node following oral infection. This is likely to reflect the less synchronous arrival of parasites in mesenteric lymph nodes following oral infection compared to earflap infection. Nevertheless, these results confirm that the recruitment and behavior of neutrophil in the SCS of the lymph node described here are not unique to the earflap infection model, but also occur following oral infection with T. gondii.
Fig. 6.
Neutrophil recruitment and swarm formation in mesenteric lymph nodes after oral infection. (A) Neutrophils (LysGFPhigh Ly6G+) in the mesenteric lymph nodes of orally infected mice. Flow cytometry analysis of mesenteric lymph nodes of uninfected LysGFP mice, or LysGFP reporter mice infected 5 days earlier with 20 cysts. (B) 20 um frozen sections of mesenteric lymph nodes of LysGFP reporter (green) mice before infection (left panel) or 4 days after oral infection with 45 cysts of Pruniaud-RFP (red) stained with Ly6G (blue). Arrows indicate neutrophil cluster at the subcapsulary sinus. Bottom panels show a high magnification image of a lymph node from an infected mouse. Clusters of LysGFP high cells (green) (left panel) stain positive for the neutrophil marker Ly6G (blue). (C) Same samples as in (B) were stained to visualize CD169+ subcapsular sinus macrophages (blue). Right panels show a high magnification image with an arrow indicating a region of CD169 clearing.
Discussion
In addition to their well-characterized role in infected tissues, recent evidence indicates that neutrophils also play important roles in immune responses within lymph nodes. Here we provide the first report of the dynamics of neutrophil behavior in intact lymph nodes in the setting of Toxoplasma gondii infection. We show that neutrophils migrate in a distinctive, coordinated fashion that leads to the formation of swarms near sites of infection at the subcapsular sinus of draining lymph nodes. We show that neutrophil swarms can be initiated by the cooperative action of neutrophils, as well as by parasite egress from host cells, and that neutrophil swarms lead to the removal of macrophages that line the subcapsular sinus of the lymph nodes. Our data provide a new perspective for future studies on the role of neutrophils in lymph nodes.
Perhaps the most striking feature of neutrophil behavior reported here is the highly co-operative nature of their migration patterns. This is reflected in the “paparazzi-like” behavior of neutrophils in which the initial arrest of a small number of neutrophils is followed minutes later by a massive influx of cells. This behavior is likely to be mediated by multiple chemoattractants including CXCL8/IL8, CXCL1, and leukotriene B4, which are both produced by, and attractive to, neutrophils (Baggiolini, 1998; Scapini et al., 2000). The co-operative nature of neutrophil migration is also reflected in their streaming behavior during swarm formation, which is reminiscent of swarm formation by the slime mold Dictyostelium. In the case of Dictyostelium, evidence suggests that individual cells leave trails of chemoattractants that are sensed by neighboring cells, leading to a head-to-tail migration pattern as cells migrate toward a swarm (Kriebel et al., 2003). Neutrophils and Dictyostelium appear to use similar signal transduction systems for chemoattraction (Mahadeo et al., 2007) and it will be interesting to explore whether similar mechanisms also underlie their co-operative migration patterns.
Another indication of cooperative action of neutrophils in swarm formation is correlation between the size and persistence of neutrophil swarms. Swarms containing fewer than 150 neutrophils tended to be transient, with neutrophils migrating rapidly and directionally toward swarm center, and then migrating equally rapidly out of the swarm to nearby join neighboring growing swarm centers. In contrast, swarms with >300 neutrophils invariably kept growing over the time they were observed, often merging with nearby swarms. Although it is likely that persistent swarms would disappear eventually in vivo, this would likely result from neutrophil apoptosis and phagocytosis by macrophages, in contrast to the outward migration of neutrophils seen during the dissolution of transient swarms. The correlation between size and persistence strongly suggests that the neutrophils themselves contribute to the gradient of chemoattractants that generates a swarm, and that once the swarm reaches a certain size, these signals become sufficiently strong to generate a stable swarm center that can override other competing signals in the environment.
Another striking aspect of neutrophil behavior is their extremely rapid migration toward sites of parasite egress. Because this response occurs within seconds of parasite egress, it is unlikely to be related to the IL1-dependent neutrophil recruitment in response to dying cells has been characterized previously (Chen et al., 2007). While the nature of the neutrophil-attracting signal that correlates with parasite egress is unclear, it is possible that material from lysed cells could be directly attractive for neutrophils, a phenomenon termed “necrotaxis” that has been previously described in vitro (Debru, 1993). Alternatively, the released parasites themselves could attract neutrophils, either due to a direct response to parasite PAMPS (Debierre-Grockiego et al., 2007; Nakao and Konishi, 1991a; Yarovinsky et al., 2005), or due to limited complement activation by parasites (Fuhrman and Joiner, 1989). An in vitro system to recapitulate neutrophil swarming in response to Toxoplasma infection would help to address these possibilities.
Given the multitude of signals known to attract neutrophils, and the indications from our data that neutrophils are responding to multiple, competing signals, it seems likely that many different types of chemoattractants contribute to neutrophil swarming behavior. It is unlikely that adaptive immunity plays a major role in the neutrophil behavior that we report here, since these responses occur in naïve mice a few hours after exposure to the parasites, time points well before T or B cells would be expected to be activated. Moreover, we have not observed any significant differences in neutrophil behavior in immunized versus naïve mice (data not shown). One major challenge to identify these signals will be to selectively block candidate signals during the swarming phase of the response. For example, while TLR/MyD88 signals are good candidates for mediating swarm formation, our observation that this pathway is also required for neutrophils to be recruited to the lymph node in the first place, complicates testing this idea.
Our data also add to the growing body of evidence for the importance of CD169+ subcapsular sinus macrophage of the lymph nodes (Carrasco and Batista, 2007; Hickman et al., 2008; Junt et al., 2007; Phan et al., 2007). Parasites are found predominantly within these macrophage a few hours after infection, and neutrophil swarms in infected regions of the subcapsular sinus lead to the appearance of gaps in the layer of CD169+ cells which normally form a continuous layer around an uninfected lymph node. While the fate of the SCS macrophages is not known, we suspect that they are removed by neutrophils based on several considerations. First, we have never observed any migrating CD169+ macrophages, even while imaging areas in which gaps were forming (Supplementary Videos 12, 13 and data not shown). Second, we are able to detect the loss of CD169+ cells by flow cytometric analysis, consistent with the removal of these cells and arguing against the possibility that they have migrated to a different part of the lymph node. Finally, a direct role for neutrophils is supported by the well-documented ability of neutrophils to produce matrix metalloproteases and participate in tissue remodeling. Regardless of the mechanism, given the emerging evidence for the importance of subcapsular sinus of the lymph node in trapping pathogens and initiating adaptive immune responses (Carrasco and Batista, 2007; Hickman et al., 2008; Junt et al., 2007; Phan et al., 2007), it will be important to examine whether the removal of SCS macrophage by neutrophils has an impact on subsequent immune responses.
In summary, our analysis of neutrophil migration in intact lymph nodes has provided evidence that signals released during parasite egress from host cells and cooperative action of neutrophils can induce the formation of dynamic neutrophil swarms in the SCS, leading to the removal of macrophages that reside there. In addition to direct phagocytic killing, neutrophils also use a variety of other non-cell autonomous mechanisms to protect against pathogens, including the release of neutrophil extracellular traps (NETs) (Brinkmann et al., 2004), containing DNA, microbiocidal products and tissue remodeling factors such as matrix metalloproteinase (Appelberg, 2007; Nathan, 2006). It is tempting to speculate that the swarming behavior of neutrophils reported here could increase the local concentration of these released products and improve the effectiveness of these mechanisms to induce inflammation and provide protection against pathogens.
Experimental Procedures
Mice
All mice were bred and housed in pathogen-free conditions at the AALAC-approved animal facility at Life Science Addition, University of California, Berkeley. All animal experiments were approved by the Animal Care and Use Committee of UC Berkeley. LysGFP reporter mice were a gift from Thomas Graf (Albert Einstein College of Medicine, Bronx, NY), and have been previously described (Faust et al., 2000). MyD88 gene KO mice (originally generated in Dr. S. Akira’s laboratory, Osaka University, Osaka, Japan) (Adachi et al., 1998) were backcrossed onto C57BL/6 background.
Parasites
To generate a T.gondii cell line expressing the tandem (td) Tomato variant of red fluorescent protein (RFP) (Shaner et al., 2004) we amplified tdTomato using the primers 5′-AGTCCCTAGGGTGAGCAAGGGCGAGGAG and 5′AGTCCCCGGGCTTGTACAGCTCGTCCATGC. We digested the resultant PCR product with AvrII and XmaI and ligated this into the corresponding restriction enzyme sites of the pCTG vector (GvD and R. Opperman, unpublished) to generate the vector pCTR2T, where expression of cytosol-localised tdTomato is driven from the constitutive α-tubulin promoter. We transfected this construct into the RHΔhxgprt strain of T. gondii and generated stable lines expressing tdTomato by chloramphenicol selection as previously described (Gubbels et al., 2005). Briefly, 1 × 107 parasites were resuspended in cytomix containing 50 μg of linearized pCTR2T vector. The parasites mixture was added to a 2 mm electroporation cuvette and electroporated using a BTX ECM 630 electroporator at 1500 V, 25 Ω and 25 μF. Transfected cells were passed onto fresh host cells. 16 hours post-transfection, chloramphenicol was added to a final concentration of 6.8 μg/mL. Cells were passaged in the presence of chloramphenicol until stable lines were generated. Clonal lines of the RH/tdTomato parasite strain were obtained by fluorescence-activated cell sorting. Briefly, parasites were filtered through a 3 μm filter, pelleted and resuspend in phosphate-buffered saline. Parasites were sorted using a MoFlo cytometer (Dako, Ft Collins, CO) with an Enterprise 631 laser tuned to 488 nm for excitation and an emission filter with a band pass of 570/40 nm. Bright parasites were sorted into 96 well plates, and wells containing single plaques after one week of growth were considered to consist of a clonal parasite line. All parasites were maintained in confluent human foreskin fibroblasts.
For mouse infections, parasites were prepared from almost fully lysed fibroblast cultures by first releasing the parasites from fibroblasts by passing them through 21g 1 ½ gauge and 23g1 needles 5-10 times. Parasites were then filtered through a 3 μm filter, pelleted and resuspended in phosphate-buffered saline. 5 ×105-1 × 107 parasites (typically 5 ×106) in 10 μl volume were injected into the earflap. No differences in the anatomical distribution of parasites or neutrophils in draining lymph nodes were observed over this range of infection. Some of the mice used for 2-photon experiments were injected with 106 parasites intraperitoneally at least 4 weeks prior to infection and imaging. No differences in neutrophil behaviour were observed between immunized and naïve animals. For neutrophil depletion experiments, mice were injected i.p. with 1 mg of Ly6G antibody (clone 1A8) two days prior to infection and also i.v. with 0.5 mg of Ly6G antibody one day prior to infection.
For oral infections, Prugnaud-RFP parasites were engineered essentially as described above for RH parasites, and cysts were isolated from brain homogenates of CBA/J mice (Jackson Laboratory, Bar Harbor, Maine) infected with 300-400 parasites i.p. 4-6 months prior. Cysts were counted after staining with Dolichos Biflorus Agglutinin (Vector Laboratories, Burlingame, CA). LysGFP reporter mice were infected with 20-45 cysts by gavage. Mesenteric lymph nodes were used for microscopy or analysed by flow cytometry.
Antibodies and flow cytometry
Lymph nodes were dissociated by collagenase digestion. Cell suspensions were filtered, stained and analyzed by flow cytometry. The following antibodies were from eBioscience: phycoerythrin-Cy5 conjugated anti-CD11b (clone M1/70), and phycoerythrin-Cy5 conjugated anti-CD11c (clone N418). Fluorescein isothiocyanate-conjugated anti-Ly6G (clone 1A8) was from BD Biosciences. Acquisitions were performed with a Coulter Epics XL-MCL flow cytometer (Beckman-Coulter) and data were analyzed with the FlowJo software (Tree Star, Ashland, Oreg.).
Statistical analysis
Values were expressed as mean ± standard error (SD). Levels of significance were calculated by unpaired t tests using the GraphPad Prism program (San Diego, CA, US). Differences were considered significant at P<0.05.
2-Photon Imaging
One to five hours following ear flap injection, mice were sacrificed, and dorsal cervical (draining) lymph nodes were isolated and imaged by Two-Photon Laser Scanning Microscopy (TPLSM) while being perfused with warmed, oxygenated medium as described previously (Witt et al., 2005). Imaging was performed using either upright Zeiss NLO 510 with a Spectra-Physics MaiTai laser tuned to 900-920nm. In that case GFP and tdTomato emission light was separated using a 560 dichroic mirror and collected using non-descanned detectors. Alternatively, 2-photon imaging was performed using a custom-built microscope with a Spectra-Physics MaiTai laser tuned to 900-920nm, the emission light was separated using 495 and 560 dichroics and collected using 3 detectors. Bandpass filters HQ 450/80M and HQ 645/75M were used to minimize spectral overlap.
Typical imaging volumes (164 × 164 × 40 microns or 172×143×80 microns respectively) corresponded to regions of the lymph nodes extending up to 200 microns below the surface of the capsule and were scanned every 13-37 seconds for 20-40 minutes. Mesenteric lymph nodes were imaged under similar conditions 4-5 days after oral infection.
Data Analysis
The x,y,z co-ordinates of individual cells over time were obtained using Imaris Bitplane Software. Motility parameters were calculated using Matlab (code available upon request). Parameters reported here include Speed (defined as path length over time; microns/minute) and distance to swarm center (microns). To measure the volumes of individual neutrophil swarms, isosurfaces were generated in the GFP channel (threshold of 130, 6 μm Gaussian filter), and the volume of each isosurface was determined over time. The volume of a single neutrophil was estimated using the same method (using 1 μm Gaussian filter), and 15 individual cell volumes were averaged.
Immunofluorescence
Isolated lymph nodes were fixed with 4% formalin in PBS for 1 hr, sequentially submerged in 10, 20, 30% sucrose for 18-24 hrs each, and frozen over dry ice in OCT. 20 micron serial sections were generated by cryosectioning (MICROM H550,Microm GmbH, Walldorf, Germany) and stored at -80°C. Sections were brought to room temperature, fixed with cold acetone for 10 min, air dried and incubated with 10% mouse serum in Fc blocking reagent (2.4G2 culture supernatant) for 1hr. The tissue was stained with unconjugated anti-Ly6G (BD Biosciences), purified rat anti mouse CD169 (AbD Serotecor), or biotin-conjugated anti-LYVE-1 (R&D systems) overnight at 4°C. After primary staining slides were washed 4x in PBS and incubated with either anti-rat Alexa 647 or streptavidin Alexa 633 (Invitrogen) for 2 hrs at room temperature. Sections were then washed 4x in PBS and coverslipped using VectaShield (Vector Laboratories, Burlingame, CA) mounting medium. Stained lymph node sections were visualized on Zeiss 510 Axioplan META NLO upright microscope with a 10x air objective (Plan-Neofluar 10x/0,3) and a 40x oil objective (Plan-Neofluar 40x/1.3 oil WD=0.17mm) using 488nm, 543nm and 633nm laser lines. Images were analysed and assembled using Adobe Photoshop and Imaris Bitplane.
Supplementary Material
Acknowledgements
We thank Hector Nolla (UCB) and Julie Nelson (CTEGD) for assistance with flow cytometry, Mark Rivera for flow cytometry analysis, Thomas Graf for providing lysMGFP reporter mice, Ying Fang Liao for tracking neutrophils, Ena Ladi for assistance with quanitating neutrophil motility, Dan Portnoy, Matt Welsh, and members of the Robey lab for comments on the manuscript. This work was funded by the NIH (BS and ER), Human Frontier Science Program Fellowship (TC), and C.J. Martin Overseas Fellowship (400489) from the Australian National Health and Medical Research Council (GvD).
Abbreviations
- GFP
green fluorescent protein
- RFP
red fluorescent protein
- PAMP
pathogen associated molecular pattern
- TPSLM
2-photon scanning laser microscopy
- SCS
subcapsular sinus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abadie V, Badell E, Douillard P, Ensergueix D, Leenen PJ, Tanguy M, Fiette L, Saeland S, Gicquel B, Winter N. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood. 2005;106:1843–1850. doi: 10.1182/blood-2005-03-1281. [DOI] [PubMed] [Google Scholar]
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Appelberg R. Neutrophils and intracellular pathogens: beyond phagocytosis and killing. Trends in microbiology. 2007;15:87–92. doi: 10.1016/j.tim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Beauvillain C, Delneste Y, Scotet M, Peres A, Gascan H, Guermonprez P, Barnaba V, Jeannin P. Neutrophils efficiently cross-prime naive T cells in vivo. Blood. 2007;110:2965–2973. doi: 10.1182/blood-2006-12-063826. [DOI] [PubMed] [Google Scholar]
- Bennouna S, Bliss SK, Curiel TJ, Denkers EY. Cross-talk in the innate immune system: neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J Immunol. 2003;171:6052–6058. doi: 10.4049/jimmunol.171.11.6052. [DOI] [PubMed] [Google Scholar]
- Black MW, Boothroyd JC. Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev. 2000;64:607–623. doi: 10.1128/mmbr.64.3.607-623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss SK, Gavrilescu LC, Alcaraz A, Denkers EY. Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infect Immun. 2001;69:4898–4905. doi: 10.1128/IAI.69.8.4898-4905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousso P, Robey E. Dynamic behavior of T cells and thymocytes in lymphoid organs as revealed by 2-photon microscopy. Immunity. 2004;21:349–355. doi: 10.1016/j.immuni.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Cahalan MD, Parker I. Imaging the choreography of lymphocyte trafficking and the immune response. Curr Opin Immunol. 2006;18:476–482. doi: 10.1016/j.coi.2006.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD, Parker I. Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu Rev Immunol. 2008;26:585–626. doi: 10.1146/annurev.immunol.24.021605.090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Channon JY, Seguin RM, Kasper LH. Differential infectivity and division of Toxoplasma gondii in human peripheral blood leukocytes. Infect Immun. 2000;68:4822–4826. doi: 10.1128/iai.68.8.4822-4826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- Debierre-Grockiego F, Campos MA, Azzouz N, Schmidt J, Bieker U, Resende MG, Mansur DS, Weingart R, Schmidt RR, Golenbock DT, et al. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J Immunol. 2007;179:1129–1137. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- Debru C. A particular form of chemotaxis: necrotaxis. An historical view. Blood cells. 1993;19:5–19.; discussion 20-13.
- Denkers EY, Butcher BA, Del Rio L, Bennouna S. Neutrophils, dendritic cells and Toxoplasma. Int J Parasitol. 2004;34:411–421. doi: 10.1016/j.ijpara.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Egen JG, Rothfuchs AG, Feng CG, Winter N, Sher A, Germain RN. Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity. 2008;28:271–284. doi: 10.1016/j.immuni.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000;96:719–726. [PubMed] [Google Scholar]
- Fuhrman SA, Joiner KA. Toxoplasma gondii: mechanism of resistance to complement-mediated killing. J Immunol. 1989;142:940–947. [PubMed] [Google Scholar]
- Germain RN, Miller MJ, Dustin ML, Nussenzweig MC. Dynamic imaging of the immune system: progress, pitfalls and promise. Nat Rev Immunol. 2006;6:497–507. doi: 10.1038/nri1884. [DOI] [PubMed] [Google Scholar]
- Gubbels MJ, Striepen B, Shastri N, Turkoz M, Robey EA. Class I major histocompatibility complex presentation of antigens that escape from the parasitophorous vacuole of Toxoplasma gondii. Infect Immun. 2005;73:703–711. doi: 10.1128/IAI.73.2.703-711.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman HD, Takeda K, Skon CN, Murray FR, Hensley SE, Loomis J, Barber GN, Bennink JR, Yewdell JW. Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat Immunol. 2008;9:155–165. doi: 10.1038/ni1557. [DOI] [PubMed] [Google Scholar]
- Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Kriebel PW, Barr VA, Parent CA. Adenylyl cyclase localization regulates streaming during chemotaxis. Cell. 2003;112:549–560. doi: 10.1016/s0092-8674(03)00081-3. [DOI] [PubMed] [Google Scholar]
- Mahadeo DC, Janka-Junttila M, Smoot RL, Roselova P, Parent CA. A chemoattractant-mediated Gi-coupled pathway activates adenylyl cyclase in human neutrophils. Mol Biol Cell. 2007;18:512–522. doi: 10.1091/mbc.E06-05-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maletto BA, Ropolo AS, Alignani DO, Liscovsky MV, Ranocchia RP, Moron VG, Pistoresi-Palencia MC. Presence of neutrophil-bearing antigen in lymphoid organs of immune mice. Blood. 2006;108:3094–3102. doi: 10.1182/blood-2006-04-016659. [DOI] [PubMed] [Google Scholar]
- Megiovanni AM, Sanchez F, Robledo-Sarmiento M, Morel C, Gluckman JC, Boudaly S. Polymorphonuclear neutrophils deliver activation signals and antigenic molecules to dendritic cells: a new link between leukocytes upstream of T lymphocytes. J Leukoc Biol. 2006;79:977–988. doi: 10.1189/jlb.0905526. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science (Washington DC) 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- Nakao M, Konishi E. Neutrophil chemotactic factors secreted from Toxoplasma gondii. Parasitology. 1991a;103(Pt 1):29–34. doi: 10.1017/s0031182000059254. [DOI] [PubMed] [Google Scholar]
- Nakao M, Konishi E. Proliferation of Toxoplasma gondii in human neutrophils in vitro. Parasitology. 1991b;103(Pt 1):23–27. doi: 10.1017/s0031182000059242. [DOI] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Pesce JT, Liu Z, Hamed H, Alem F, Whitmire J, Lin H, Liu Q, Urban JF, Jr., Gause WC. Neutrophils clear bacteria associated with parasitic nematodes augmenting the development of an effective th2-type response. J Immunol. 2008;180:464–474. doi: 10.4049/jimmunol.180.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- Rydstrom A, Wick MJ. Monocyte recruitment, activation, and function in the gut-associated lymphoid tissue during oral Salmonella infection. J Immunol. 2007;178:5789–5801. doi: 10.4049/jimmunol.178.9.5789. [DOI] [PubMed] [Google Scholar]
- Sasmono RT, Ehrnsperger A, Cronau SL, Ravasi T, Kandane R, Hickey MJ, Cook AD, Himes SR, Hamilton JA, Hume DA. Mouse neutrophilic granulocytes express mRNA encoding the macrophage colony-stimulating factor receptor (CSF-1R) as well as many other macrophage-specific transcripts and can transdifferentiate into macrophages in vitro in response to CSF-1. J Leukoc Biol. 2007;82:111–123. doi: 10.1189/jlb.1206713. [DOI] [PubMed] [Google Scholar]
- Sayles PC, Johnson LL. Exacerbation of toxoplasmosis in neutrophil-depleted mice. Natural immunity. 1996;15:249–258. [PubMed] [Google Scholar]
- Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Sumen C, Mempel TR, Mazo IB, von Andrian UH. Intravital microscopy: visualizing immunity in context. Immunity. 2004;21:315–329. doi: 10.1016/j.immuni.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Tvinnereim AR, Hamilton SE, Harty JT. Neutrophil involvement in cross-priming CD8+ T cell responses to bacterial antigens. J Immunol. 2004;173:1994–2002. doi: 10.4049/jimmunol.173.3.1994. [DOI] [PubMed] [Google Scholar]
- Witt CM, Raychaudhuri S, Schaefer B, Chakraborty AK, Robey EA. Directed Migration of Positively Selected Thymocytes Visualized in Real Time. PLoS Biol. 2005;3:e160. doi: 10.1371/journal.pbio.0030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- Zinselmeyer BH, Lynch JN, Zhang X, Aoshi T, Miller MJ. Video-rate two-photon imaging of mouse footpad - a promising model for studying leukocyte recruitment dynamics during inflammation. Inflamm Res. 2008;57:93–96. doi: 10.1007/s00011-007-7195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.