Abstract
Chemotherapy-induced pain is the most common treatment-limiting complication encountered by cancer patients receiving taxane-, vinca alkaloid- or platin-based chemotherapy. Several lines of evidence indicate that activation of pro-inflammatory cascades involving the release of cytokines including tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β) and interleukin-6 (IL-6) as well as various growth factors are key events in the pathogenesis of many types of nerve-injury related pain. Similar mechanisms might also be involved in the etiology of chemotherapy-induced pain. Thalidomide and minocycline have profound immunomodulatory actions in addition to their originally intended pharmacological actions. These compounds were evaluated here for effects in preventing the development of taxol-induced mechanical and thermal hyperalgesia in rats. Thalidomide (50.0 mg/kg) reduced taxol-induced mechanical allodynia and hyperalgesia whereas minocycline (20.0 mg/kg) reduced taxol-induced mechanical hyperalgesia and allodynia as well as taxol-induced thermal hyperalgesia. These results suggest that immunomodulatory agents may provide a treatment option for the protection or reversal of chemotherapy-related pain.
Keywords: Cancer, Chemotherapy, Neuropathy, Neuropathic pain
1. Introduction
Chemotherapy-induced pain is the most common treatment-limiting complication encountered by cancer patients receiving taxane-, vinca alkaloid-, or platin-based chemotherapy (Forman, 1990; Forsyth et al, 1997; Quasthoff and Hartung, 2002; Verstappen et al, 2003). Symptoms occur in a stocking and glove distribution often early in treatment and the final overall incidence and severity is directly related to the initial and cumulative drug doses, the co-administration of other chemotherapeutic agents and the presence of other diseases like diabetes or alcoholism (Chaudhry et al, 2003; Connelly et al, 1996; New et al, 1996; Rowinsky et al, 1993; Rowinsky and Donehower, 1995; Tuxen and Hansen, 1994). Chemotherapy-induced pain improves or completely resolves in 51% of patients, but becomes chronic in the remainder, affecting the quality of life and return to productivity in cancer survivors (Connelly et al, 1996). Psychophysical tests in patients with taxol-induced pain reveal marked impairment of Aβ function, less severe impairment of Aδ function, but preservation of C-fiber function and paradoxical burning pain to skin cooling (Dougherty et al, 2004; Dougherty et al, 2006).
The mechanism by which chemotherapy drugs produce pain remains unclear. Hypothetically, drugs like taxol or vincristine might damage nerves through their antineoplastic mechanism, binding to microtubules in axons, presumably altering axonal transport and subsequently inducing neuropathology (Tanner et al, 1998). However, no direct evidence supports this contention and such a mechanism is difficult to reconcile with the preferential impairment of nerve fiber function shown in psychophysical tests. Changes in spinal neuron processing of sensory information in chemotherapy-treated rats, cells that are protected from chemotherapy drugs by the blood brain barrier, suggests that other effects of these drugs might contribute to the generation of pain (Weng et al, 2003). For example, paclitaxel up-regulates multiple transcription factors (Zaks-Zilberman et al, 2001) enzymes, and cytokines, such as nuclear factor kappa-B (NF-κB) and tumor necrosis factor-alpha (TNF-α) that contribute to both neuropathic and inflammatory pain (Bao et al, 2001; Empl et al, 2001; Manni and Aloe, 1998; Opree and Kress, 2000; Sommer et al, 1998b). These effects suggest the possible involvement of alterations in glial functions in taxol pain like previously demonstrated for vincristine-induced pain (Sweitzer et al, 2006).
Two drugs, minocycline and thalidomide, each of which have profound immunomodulatory effects (Raghavendra et al, 2003) are evaluated for efficacy in protecting rats from taxol-induced pain in this study. Each drug has a well known primary pharmacologic effect, minocycline is an antimicrobial agent structurally related to tetracycline (Kriz et al, 2002; Wells et al, 2003; Yrjanheikki et al, 1999), and thalidomide was introduced as a sedative-hypnotic (Calabrese and Fleischer, 2000). However, minocycline also decreases the release of multiple pro-inflammatory cytokines (Amin et al, 1996; Kriz et al, 2002; Wells et al, 2003; Yrjanheikki et al, 1999), and thalidomide both suppresses the synthesis and release of pro-inflammatory cytokines as well as increases the release of anti-inflammatory cytokines (Ribeiro et al, 2000; Sommer et al, 1998a; Sommer et al, 2001b; Wagner et al, 1998). Each have previously also shown efficacy in the treatment of neuropathic pain in rats and/or mice (Bennett, 1999; Ledeboer et al, 2005; Narita et al, 2006).
2. Results
A total of 50 rats were used in the study. Among these, the mortality rate with taxol chemotherapy was zero. Moreover, none of the animals showed any failure to groom and all gained weight, albeit some more slowly than in untreated rats (see below). No animals showed any gross neurological abnormalities or any sign of self-mutilating behavior or autotomy. Animals were divided into five treatment groups, vehicle (n= 10); taxol (n=8); thalidomide (n=8); taxol-thalidomide (n=8); minocycline (n=8); and taxol-minocycline (n=8).
2.1 Taxol induces mechanical allodynia and hyperalgesia
The administration of taxol caused mechanical allodynia and hyperalgesia by day 7 of chemotherapy as evidenced by changes in both the responses to the complete vonFrey filament series as well as by changes in the 50% withdrawal threshold (Figures 1 and 2). Taxol-induced hyperalgesia lasted through day 29 of the experiment while the allodynia persisted through day 45 of the experiment (Polomano et al, 2001). For example, at day 7 the taxol-vehicle rats showed a nociceptive response rate of 32.0 ± 7.0% to the 1.14g vonFrey filament whereas the vehicle-treated rats showed a 1.0 ± 1.1 % response rate (p < 0.05) indicative of allodynia. Mechanical hyperalgesia is evidenced by the reduction in the 50% mechanical withdrawal threshold over this time frame. Whereas the vehicle-treated rats had a 50% withdrawal threshold of 12.3 ± 0.9g that in the taxol-treated rats had dropped to 2.7 ± 0.8g (p < 0.001). By day 50 the responses of the taxol-vehicle treated rats to all vonFrey filaments showed recovery to levels that were not statistically different from the levels of the vehicle-treated rats.
Figure 1.
Taxol produces mechanical hyperalgesia that is blocked by thalidomide. The scatter and line plots show the time course of change in the 50% mechanical withdrawal threshold to the application of vonFrey filaments to the mid-plantar surface of the hindpaws (vehicle, open circles; taxol alone, filled circles; filled squares, taxol-thalidomide; open squares, thalidomide alone); whereas the bar graphs (bottom of figure) show the mean percentage of withdrawal responses shown by each treatment group (vehicle, open bars; taxol alone, grey bars; taxol-thalidomide, cross-hatched bars; thalidomide alone, black bars) to the lowest force vonFrey filament that was tested (1.14g). Taxol produced a significant decrease in 50% withdrawal threshold by day 7 of the experiment when compared to vehicle treatment. This was due to a significantly increased withdrawal frequency to both low and high strength filaments. Thalidomide attenuated taxol-induced hyperalgesia such that the 50% withdrawal threshold was not different from vehicle. Thalidomide alone had no effect on mechanical withdrawal threshold. Stars indicate statistical difference from vehicle whereas the diamonds indicate statistical difference from taxol. One symbol, p<0.05; two symbols, p<0.01; three symbols, p<0.001.
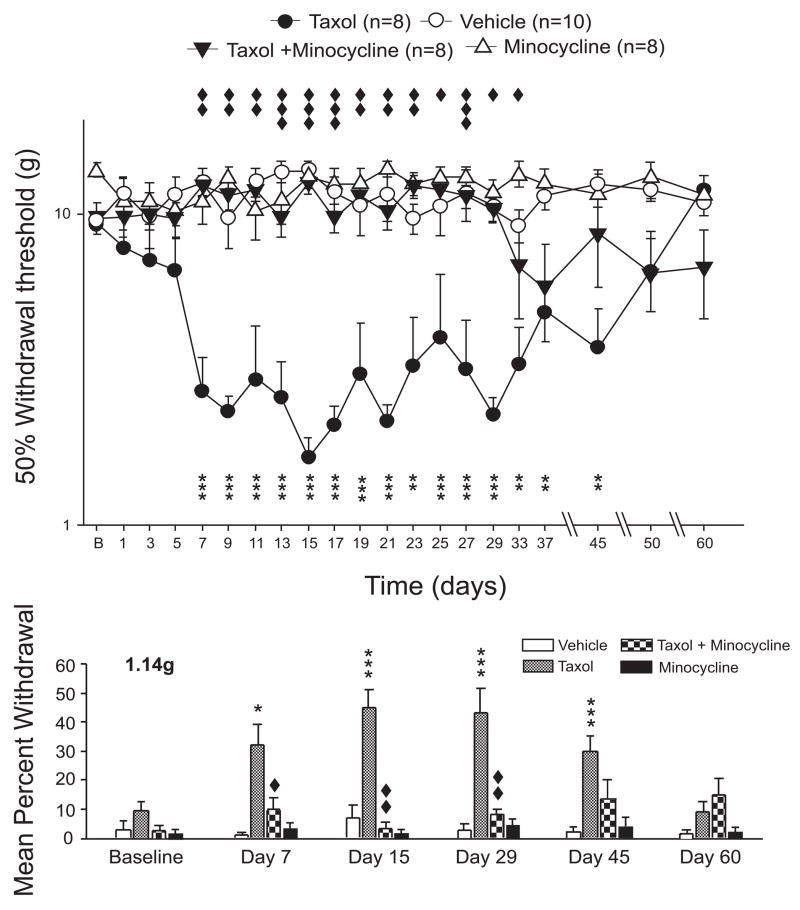
Figure 2.
Taxol produces mechanical hyperalgesia that is blocked by minocycline. The scatter and line plots (top of figure) show the time course of change in the 50% mechanical withdrawal threshold to the application of vonFrey filaments to the mid-plantar surface of the hindpaws (vehicle, open circles; taxol alone, filled circles; taxol-minocycline, filled triangles, minocycline alone, open triangles); whereas the bar graphs (bottom of figure) show the mean percentage of withdrawal responses shown by each treatment group (vehicle, open bars; taxol, grey bars; taxol-minocycline, checkered bars; minocycline alone, black bars) to the lowest vonFrey filaments (1.14g) tested. Minocycline prevented taxol-induced hyperalgesia such that the 50% withdrawal threshold was never different from vehicle until well after the drug was discontinued. As shown in the bar graphs, the effects of minocycline were evident in preventing taxol-induced changes in the responses of both low and high strength von Frey filaments. Minocycline alone had no effect on mechanical withdrawal. Stars indicate statistical difference from vehicle whereas the diamonds indicate statistical difference from taxol. One symbol, p<0.05; two symbols, p<0.01; three symbols, p<0.001.
2.2 The effects of thalidomide and minocycline on taxol-induced mechanical hyperalgesia
Thalidomide and minocycline reduced the development of both taxol-induced mechanical allodynia and hyperalgesia but each drug showed unique patterns in their effects. For example, thalidomide-treated rats showed an early onset of both mechanical allodynia and hyperalgesia at day 7 of chemotherapy with taxol treatment. But, these signs quickly resolved in the thalidomide-taxol rats such that by day 15 no changes in response frequencies compared to vehicle were evident. Minocycline on the other hand had a potent short term effect in preventing taxol-induced hyperalgesia, but over the longer-term seemed to lose antihyperalgesic effects with drug discontinuation (Figure 2). Thus, minocycline-treated rats showed no change in response to any von Frey filaments compared to vehicle-treated rats through day 50 of the experiment, and the response rates of the minocycline-taxol treated rats were significantly less than those of the thalidomide-vehicle treated rats. However, the 50% withdrawal threshold in the minocycline-taxol treated rats was noted to fall on day 29 from a value of 9.82 ± 0.37g to 4.88 ± 0.44g and then maintained this value for the remainder of the experiment. Neither thalidomide or minocycline alone had any significant effect on responsiveness to mechanical stimuli (Figures 1 and 2).
2.3 Taxol induces thermal hyperalgesia
Taxol treated rats showed significantly reduced paw withdrawal latencies to radiant heat indicating the development of thermal hyperalgesia by day 7 of chemotherapy (Figure 3). For example, by day 9 of chemotherapy the taxol rats showed a mean decrease in withdrawal latency of −3.8 ± 1.5 s whereas the vehicle treated rats showed a mean paw withdrawal latency that was +0.8 ± 1.3s of baseline. Thermal hyperalgesia remained evident through day 50 of the experiment on the taxol-treated rats.
Figure 3.
Minocycline prevents taxol-induced thermal hyperalgesia but thalidomide does not. The scatter and line graphs show the mean difference in mean paw withdrawal latency for the vehicle (open circles), taxol-vehicle (filled circles), thalidomide-taxol (filled squares, upper panel), and minocycline-taxol (filled triangles, lower panel) rats for each day of the experiment in comparison to the baseline mean paw withdrawal latency. Taxol produced thermal hyperalgesia that was significant from day 7 of the experiment. Similarly, the thalidomide-taxol treated rats also showed thermal hyperalgesia that was significant from day 7 of the experiment (upper panel). In contrast, minocycline-taxol treated rats did not develop any change in mean paw withdrawal latency that was significantly different from baseline at any time point in the study. Stars indicate points of significant difference between vehicle and vehicle, diamonds indicate points of significant difference between thalidomide-taxol and vehicle, and crosses indicate points of significant difference between minocycline-taxol and taxol.
2.4 The effects of thalidomide and minocycline on taxol-induced thermal hyperalgesia
Thalidomide and minocycline showed differential effects on taxol-induced thermal hyperalgesia (Figure 3). The thermal withdrawal thresholds of the thalidomide-taxol treated rats were reduced in the same fashion as the taxol treated rats showing a mean decrease of −3.01 ± 0.12 s from the baseline level by day 9 of the experiment that was significantly different from the mean change in latency shown by the vehicle (p < 0.05). The minocycline-taxol rats showed far better maintenance of thermal withdrawal threshold over the course of the experiment, with no data points that showed a significant change from the vehicle-treated rats, and data points at days 9, 11, 21, 23, 29, 33, 37 and 45 that were significantly above those of the taxol-vehicle treated rats. Neither thalidomide or minocycline had any effects on thermal withdrawal thresholds (Figure 3).
2.5 The effects of taxol on body weight
As noted above the administration of taxol did not cause early mortality in any experimental rats. Moreover, there was no apparent morbidity. Thus, taxol-vehicle treated rats showed an increase in body weight that was not statistically different from the vehicle treated rats through the experiment (Data not shown).
2.6 The effects of thalidomide and minocycline on body weight during chemotherapy
As noted above the administration of taxol did not alter the body weight of the rats when compared with saline treated rats throughout the experiment. However, minocycline-taxol treated animals gained weight significantly more slowly than vehicle-treated rats from day 5 to day 17 of the study. At day 5 minocycline-taxol treated rats had gained 4.22 ± 0.62% body weight from baseline versus a 12.42 ± 1.79% gain in the vehicle-treated rats (p <0.001). Thalidomide did not affect the body weight of the animals in comparison to vehicle or taxol-vehicle treated rats (Data not shown).
2.7 The effects of taxol on motor performance
The administration of taxol was associated with a small, non-statistically significant, drop in time spent by chemotherapy-treated rats on the rotating rod s (Figure 4). After this transient episode, taxol treated rats showed the same pattern of motor learning manifested by vehicle treated rats. This data suggests that taxol, at the dose used in the present study, induces a purely sensory neuropathy.
Figure 4.
The scatter and line plots show the mean duration that animals in each treatment group remained on the rotarod over the time course of the experiment. Rats in the vehicle-taxol group showed a lower mean rotarod time at days 1 and 3 of the experiment but these differences were not statistically different from the vehicle group times. The rotarod times for all groups became essentially identical by day 5 and remained so for the remainder of the experiment. Open circles, vehicle; filled circles, taxol; filled squares, thalidomide-taxol; filled triangles, minocycline-taxol.
2.8 The effects of thalidomide and minocycline on motor performance
Rats receiving thalidomide or minocycline during chemotherapy with taxol showed no change in rotarod performance compared to vehicle-treated controls (Figure 4). Similarly, single drug therapy with thalidomide or minocycline had no effects on rotarod performance (Data not shown).
3. Discussion
Thalidomide is shown here to reduce taxol-induced mechanical hyperalgesia whereas minocycline reduces taxol-induced mechanical as well as thermal hyperalgesia. Thalidomide is currently used due to its immunomodulatory properties in the treatment of several diseases including erythemia leprosun nodosum, Bechet’s syndrome, aphtous stomatitis and graft-versus-host disease (Calabrese and Fleischer, 2000). The exact mechanism of action is not clear, but modulation of the production and release of TNF-α is thought to be especially important. Thalidomide reduces synthesis of TNF-α, IL-12 and IL-8 in culture of LPS-stimulated human monocytes and microglial cells (Lokensgard et al, 2000; Moller et al, 1997; Sampaio et al, 1991; Sampaio et al, 1993). Thalidomide also accelerates the degradation of pro-inflammatory cytokines (Moreira et al, 1993); and dampens their intracellular signaling by inhibiting the translocation of NF-κB into the nucleus (Keifer et al, 2001). These alterations of cytokine trafficking and intracellular signaling result in altered cellular immunity such that patterns of lymphocyte activity shift from Th1 to Th2 dominant, levels of IL-4 increase in the short term and those of IFN-γ decrease over the long term (Mchugh et al, 2003). Thalidomide also alters the expression of adhesion molecules (ICAM and L-selectin) in endothelial cells and in polymorphonuclear leucocytes, thus influencing tissue migration of inflammatory cells, and reduces phagocytic activity in monocytes (Barnhill et al, 1984; Geitz et al, 1996).
Minocycline is an antibiotic structurally related to tetracycline that has neuroprotective pharmacologic actions in several models of nervous system injury in addition to its antimicrobial activity. Minocycline improves locomotor rating and histopathology scores in mice with spinal cord lesion compared to non-treated controls (Wells et al, 2003) and reduces glutamate and kainate-induced exitotoxicity (Tikka et al, 2001). Like thalidomide, minocycline also inhibits LPS-induction of TNF-α in vivo and the induction by LPS of both TNF-α and IL-1β from human monocytes in vitro (Shapira et al, 1996). Finally, minocycline improves survival rate, delays onset of muscle weakness and decreases microglia activation in mice with experimentally-induced amyotrophic lateral sclerosis (Kriz et al, 2002).
Several lines of evidence suggest that the immunomodulatory effects of thalidomide and minocycline underlie their protective effects on taxol-induced neuropathic pain shown here. Tumor necrosis factor-alpha (TNF-α) and other functionally-related pro-inflammatory cytokines such as interleukin-1 beta (IL-1β) and interleukin-6 (IL-6) are becoming well recognized as a key mediators in the pathogenesis of many types of neuropathic pain (Bennett, 1999; Bennett, 2000; DeLeo and Yezierski, 2001). TNF-α, IL-1β, and IL-6 are increased in the nerves of rats with chronic constriction injury (CCI) of the sciatic nerve (Okamoto et al, 2001; Wagner and Myers, 1996a); and administration of anti-TNF or anti-IL-6 antibodies reduce the mechanical allodynia and thermal hyperalgesia of CCI neuropathy (Arruda et al, 2000; Schafers et al, 2001; Sommer et al, 1998b; Sommer et al, 2001a). Antagonists to TNF-α, IL-6 or IL-1 also prevent and reverse hyperalgesia in rats with sciatic inflammatory neuropathy (Milligan et al, 2003); and experimental autoimmune neuritis is similarly associated with TNF-α positive macrophages infiltrating nerve sheaths and again anti-TNF-α antibodies are protective in this model of autoimmune demyelination (Stoll et al, 1993). Injection of TNF-α around or into peripheral nerves or injection of either TNF-α, IL-1β or Il-8 into the hindpaws produces hyperalgesia in awake behaving rats (Ferreira et al, 1988; Sachs et al, 2002; Sorkin and Doom, 2000; Wagner and Myers, 1996a) and also produce increases in evoked-activity, decreases in mechanical activation thresholds, and increased spontaneous ongoing activity in Aδ and C fibers (Fukuoka et al, 1994; Ozaktay et al, 2002; Sorkin et al, 1997). Thalidomide reverses and prevents the hyperalgesia in CCI and spinal nerve ligation neuropathies, respectively (Bennett, 1999; Bennett, 2000), an effect shown in CCI that correlates to decreases in TNF-α levels in sciatic nerves (George et al, 2000).
TNF-α and pro-inflammatory cytokines may similarly mediate taxol-induced peripheral neuropathy and hyperalgesia. Taxol has LPS-mimetic activity inducing the synthesis of several proinflammatory cytokines and chemokines, including both TNF-α and IL-1β, in human monocytes, T-lymphocytes and breast cancer cell lines and in murine macrophages and breast cancer cell lines (Ding et al, 2002; Manthey et al, 1992; Moos et al, 1999; White et al, 1998; Zaks-Zilberman et al, 2001). The increased synthesis and release of TNF-α and IL-1β is independent of the effects of taxol on microtubules (Moos and Fitzpatrick, 1998), but instead is linked in rodents to an up-regluation of activity of NF-κB and microtubule-associated protein kinases that in turn is driven by activation of the toll-like 4 receptor (Byrd-Leifer et al, 2001). In humans, taxol may require an additional immune stimulus to evoked elevated levels of TNF-α and IL-1β(Allen et al, 1993), and does not appear to be linked via the toll-4 receptor (Byrd-Leifer et al, 2001; Kawasaki et al, 2001). Nevertheless, this particular species difference likely has little overall functional significance for cytokine induction during chemotherapy with taxol in humans given the abundance of antigenic stimuli present in cancer patients. More directly, minocycline reduces pro-inflammatory cytokine responses and hyperalgesia in sciatic nerve inflammation hyperalgesia (Ledeboer et al, 2005) and pro-inflammatory cascades induced by taxol treatment can be suppressed using interleukin-10 gene therapy. This manipulation results in loss of chemotherapy-induced hyperalgesia (Ledeboer et al, 2007). Finally, the observation that propentofylline, a glial modulating agent, reduces vincristine-induced pain, suggests this cell group as important in generating the pro-inflammatory cytokine responses (Sweitzer et al, 2006).
Still to be reconciled with the idea that taxol-induced neuropathic pain is caused by the induction of pro-inflammatory cytokines around nerve endings is the long-standing view that chemotherapy-induced neuropathy is caused by the well known anti-microtubule effects of these drugs. The thinking has been that chemotherapy drugs like taxol cause alterations in microtubule organization and cytoskeletal ultrastructure of axons that leads to dysfunction and hence pain (Bradley et al, 1970; Green et al, 1977; Shelanski and Wisniewski, 1969; Tanner et al, 1998; Theiss and Meller, 2000). Sciatic nerves of rats treated with very high doses of taxol (25 mg/kg) do indeed show signs of mild axonal degeneration and activated Schwann cells (Cavaletti et al, 2000); and similarly nerves in rats treated with a somewhat lower dose of taxol (15 mg/kg) also show decreased mean axonal caliber and degenerated myelin profiles in dorsal but not ventral roots (Cliffer et al, 1998). Nerve histopathology in a patient with severe taxol-induced peripheral neuropathy that occurred following 17 cycles (6603 mg) of treatment similarly showed axonal degeneration, a diminished number of myelinated fibers and Schwann cells with myelin degradation products (Sahenk et al, 1994). However, histopathology is not prerequisite for the induction of pain by chemotherapy. For example, nerves from rats with chemotherapy-induced hyperalgesia following less aggressive taxol treatment (1 mg/kg/day × 3) only showed endoneurial edema without signs of overt damage to either myelinated or unmyelinated axons (Polomano et al, 2001). Moreover, histopathology can be produced by administration of many compounds around nerves and thus does not necessarily imply that microtubules and ultrastructure was damaged by taxol. For example, in addition to hyperalgesia, intra- or epi-neural injection of TNF-α also induces leukocyte margination and migration in endothelial cells and increased number of macrophages in both the epi- and endoneurium followed by demyelination and axonal degeneration like observed with CCI and focally induced sciatic neuritis (Bennett, 1999; Bennett, 2000; Redford et al, 1995; Wagner and Myers, 1996a). Given that Schwann cells are a source of TNF-α as well as Il-1β(Skundric D S et al, 2002; Wagner and Myers, 1996b) we propose that exposure of these cells to either pro-inflammatory cytokines released from tissue macrophages or possibly to taxol itself promotes cytokine release and subsequently pain and neuropathology.
Finally, it must be also be considered that pharmacological properties of thalidomide and minocycline aside from their immunomodulatory capacity could explain the effects observed here on taxol-evoked hyperalgesia. Thalidomide first received notoriety as a nearly ideal sedative hypnotic that produced comfortable sleep without producing hangover effects {Lasagna, 1960 19821 /id}. This effect was suggested as mediated via interruption or modulation of serotonin’s actions within the CNS {Youdim, 1985 19820 /id}.
In conclusion, thalidomide and minocycline ameliorate pain-related behavior normally seen in the animals following treatment with taxol. We hypothesize that taxol causes hyperalgesia by its ability to alter the synthesis of the proinflammatory cytokines that excite sensory nerves.
4. Materials and Methods
Thirty-four male Sprague-Dawley rats were used. They were housed singly on a 12 / 12 h light/dark cycle, at a room temperature of 22° C with free access to food and water. Rats were tested by the same experimenter throughout who was blinded to drug treatment. Each rat was followed and tested for a length of 60 days.
4.1 Drugs
All rats received taxol (Sigma) 1 mg/kg (i.p) in four alternate day injections reaching a cumulative dose of 4 mg/kg per rat (Polomano et al, 2001). Taxol was dissolved in 5% DMSO and stored in a stock solution of 1 mg/ml and then diluted in physiological saline before each injection, having a final concentration of 1% DMSO.
Thalidomide (Sigma) was injected at a dose of 50 mg/kg (i.p) daily beginning with the first dose of Taxol. Animals receiving thalidomide alone received the same dose over the same interval but without taxol treatment. Each rat received a total of seven thalidomide injections, ending in a cumulative dose of 350 mg/kg per animal. This drug was stored in 10% DMSO solution at a concentration of 50 mg/ml and diluted in physiological saline to 2% DMSO before each injection.
Minocycline (Sigma) was given daily at a dose of 10 mg/kg (i.p) starting with the first dose of taxol. Animals receiving minocycline alone received the same dose over the same interval but without taxol treatment. Each rat received a total of seven minocycline injections, reaching a final cumulative dose of 70 mg/kg per animal. Minocycline was dissolved in physiological saline and stored in solution at 10 mg/ml and then diluted in saline to a final volume of 1 ml before the administration.
All drug injections were made in the evening following the conclusion of all daily behavioral tests.
4.2 Mechanical Stimulation
Withdrawal thresholds to mechanical stimulation were determined by using calibrated von Frey filaments (0.002 – 14.6g). Briefly, rats were loosely restrained below plexiglass boxes (12 × 20 × 15 cm) placed on a wire mesh and allowed to acclimate for 15 minutes. Each von Frey hair was applied five times from underneath the mesh to the mid-plantar surface of each hind paw. A positive response was defined as a brisk withdrawal lifting or flinching of the paw in response to filament application. The responses to all filaments for both paws were tabulated as a single value. The 50 percent withdrawal threshold was defined, as the lowest force that produced 5 or more responses. The filament force value for each animal was averaged across all animals in each group to get a mean value for each treatment group (Weng et al, 2003).
4.3 Thermal Stimulation
Thermal responsiveness was assessed by quantification of withdrawal latency to radiant heat (Hargreaves et al, 1988). Briefly, the rats were placed on a glass surface loosely restrained below plexiglass boxes and allowed to acclimate for fifteen minutes. A radiant heat source (diameter 5 mm) was then applied to the mid-plantar surface of the hind paws and the time to paw withdrawal was measured. A total of three stimuli were applied alternately to each foot with an inter-stimulus interval of 5 minutes between stimuli (10 minutes per stimulus per paw). A cut-off limit of 24 seconds was established to avoid skin damage.
4.4 Rotarod Performance
Rotarod performance was used to assess sensory-motor coordination (Norreel et al, 2003; O’Connor et al, 2003). The rotation velocity was set to increase from 4 to 40 rpm over 2 minutes. The period of time in seconds at which the animal fell from the rod was recorded. For those animals which did not fall down, a cut-off limit of 120 seconds was used, after which they were removed from the rotating wheel. The mean time for each treatment group of animals was taken for statistical analysis.
4.5 Data analysis
Data are presented as mean ± standard error. Drug effects for all data were determined using the Mann-Whitney test. P < 0.05 was considered significant.
Acknowledgments
This work was supported by NIH grant number NS39933, and NS46606.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen JN, Moore SA, Wewers MD. Taxol enhances but does not induce interleukin-1 beta and tumor necrosis factor-alpha production. J Lab Clin Med. 1993;122:374–381. [PubMed] [Google Scholar]
- 2.Amin AR, Attur MG, Thakker GD, Patel PD, Vyas PR, Patel RN, Patel IR, Abramson SB. A novel mechanism of action of tetracyclines: Effects on nitricáoxideásynthases. Proc Natl Acad Sci USA. 1996;93:14014–14019. doi: 10.1073/pnas.93.24.14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arruda JL, Sweitzer S, Rutkowski MD, DeLeo JA. Intrathecal anti-IL-6 antibody and IgG attenuates peripheral nerve injury-induced mechanical allodynia in the rat: possible immune modulation in neuropathic pain1. Brain Res. 2000;879:216–225. doi: 10.1016/s0006-8993(00)02807-9. [DOI] [PubMed] [Google Scholar]
- 4.Bao L, Zhu Y, Elhassan AM, Wu Q, Xiao B, Zhu J, Lindgren JU. Adjuvant-induced arthritis: IL-1 beta, IL-6 and TNF-alpha are up- regulated in the spinal cord. Neuroreport. 2001;12:3905–3908. doi: 10.1097/00001756-200112210-00010. [DOI] [PubMed] [Google Scholar]
- 5.Barnhill RL, Doll J, Millikan LE, Hastings RC. Studies on the anti-inflammatory properties of thalidomide: Effects on polymorphonuclear leukocytes and monocytes. J Amer Acad Dermatol. 1984;11:814–819. doi: 10.1016/s0190-9622(84)80458-2. [DOI] [PubMed] [Google Scholar]
- 6.Bennett GJ. Does a neuroimmune interaction contribute to the genesis of painful peripheral neuropathies? Proc Natl Acad Sci USA. 1999;96:7737–7738. doi: 10.1073/pnas.96.14.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett GJ. A neuroimmune interaction in painful peripheral neuropathy. Clin J Pain. 2000;16:S139–S143. doi: 10.1097/00002508-200009001-00005. [DOI] [PubMed] [Google Scholar]
- 8.Bradley WG, Lassman LP, Pearce GW, Walton JN. The neuromyopathy of vincristine in man. Clinical, electrophysiological and pathological studies. J Neurol Sci. 1970;10:107–131. doi: 10.1016/0022-510x(70)90013-4. [DOI] [PubMed] [Google Scholar]
- 9.Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of taxol. Eur J Immunol. 2001;31:2448–2457. doi: 10.1002/1521-4141(200108)31:8<2448::aid-immu2448>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Calabrese L, Fleischer AB. Thalidomide: Current and potential clinical applications. Am J Med. 2000;108:487–495. doi: 10.1016/s0002-9343(99)00408-8. [DOI] [PubMed] [Google Scholar]
- 11.Cavaletti G, Cavaletti E, Oggioni N, Sottani C, Minoia C, D’Incalci M, Zucchetti M, Marmiroli P, Tredici G. Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicol. 2000;21:389–394. [PubMed] [Google Scholar]
- 12.Chaudhry V, Chaudhry M, Crawford TO, Simmons-O’Brien E, Griffin JW. Toxic neuropathy in patients with pre-existing neuropathy. Neurology. 2003;60:337–340. doi: 10.1212/01.wnl.0000043691.53710.53. [DOI] [PubMed] [Google Scholar]
- 13.Cliffer KD, Siuciak JA, Carson SR, Radley HE, Park JS, Lewis DR, Zlotchenko E, Nguyen T, Garcia K, Tonra JR, Stambler N, Cedarbaum JM, Bodine SC, Lindsay RM, DiStefano PS. Physiological characterization of taxol-induced large fiber sensory neuropathy in the rat. Ann Neurol. 1998;43:46–55. doi: 10.1002/ana.410430111. [DOI] [PubMed] [Google Scholar]
- 14.Connelly E, Markman M, Kennedy A, Webster K, Kulp B, Peterson G, Belinson J. Paclitaxel delivered as 3-hr infusion with cisplatin in patients with gynecologic cancers: Unexpected incidence of neurotoxicity. Gynecol Onocol. 1996;62:166–168. doi: 10.1006/gyno.1996.0210. [DOI] [PubMed] [Google Scholar]
- 15.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 16.Ding AH, Porteu F, Sanchez E, Nathan CF. Shared actions of endotoxin and taxol on TNF receptors and TNF release. Science. 2002;248:370–372. doi: 10.1126/science.1970196. [DOI] [PubMed] [Google Scholar]
- 17.Dougherty PM, Cata JP, Burton AW, Vu K, Weng H-R. Dysfunction in multiple primary afferent subtypes revealed by quantitative sensory testing in patients with vincrsitine induced pain. J Pain Symptom Manag. 2006;33:166–179. doi: 10.1016/j.jpainsymman.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Dougherty PM, Cata JP, Cordella JV, Burton A, Weng H-R. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Empl M, Renaud S, Erne B, Fuhr P, Straube A, Schaeren-Wiemers N, Steck AJ. TNF-alpha expression in painful and nonpainful neuropathies. Neurology. 2001;56:1371–1377. doi: 10.1212/wnl.56.10.1371. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1β as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- 21.Forman A. Peripheral neuropathy in cancer patients: Clinical types, etiology and presentation. Oncology. 1990;4:85–89. [PubMed] [Google Scholar]
- 22.Forsyth PA, Balmaceda C, Peterson K, Seidman AD, Brasher P, DeAngelis LM. Prospective study of paclitaxel-induced peripheral neuropathy with quantitative sensory testing. J Neuro-Onocol. 1997;35:47–53. doi: 10.1023/a:1005805907311. [DOI] [PubMed] [Google Scholar]
- 23.Fukuoka H, Kawatani M, Hisamitsu T, Takeshige C. Cutaneous hyperalgesia induced by peripheral injection of interleukin-1 beta in the rat. Brain Res. 1994;657:133–140. doi: 10.1016/0006-8993(94)90960-1. [DOI] [PubMed] [Google Scholar]
- 24.Geitz H, Handt S, Zwingenberger K. Thalidomide selectively modulates the density of cell surface molecules involved in the adhesion cascade. Immunopharmacology. 1996;31:213–221. doi: 10.1016/0162-3109(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 25.George A, Marziniak M, Schafers M, Toyka KV, Sommer C. Thalidomide treatment in chronic constrictive neuropathy decreases endoneurial tumor necrosis factor-alpha, increases interleukin-10 and has long-term effects on spinal cord dorsal horn met-enkephalin. Pain. 2000;88:267–275. doi: 10.1016/S0304-3959(00)00333-X. [DOI] [PubMed] [Google Scholar]
- 26.Green SV, Donoso JA, Heller-Bettinger IE, Samson FE. Axonal transport disturbances in vincristine-induced peripheral neuropathy. Ann Neurol. 1977;1:255–262. doi: 10.1002/ana.410010311. [DOI] [PubMed] [Google Scholar]
- 27.Hargreaves KM, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 28.Kawasaki K, Gomi K, Nishijima M. Cutting edge:Gln22 of mouse MD-2 is essential for species-specific lipolysaccharide mimetic action of taxol. J Immunol. 2001;166:11–14. doi: 10.4049/jimmunol.166.1.11. [DOI] [PubMed] [Google Scholar]
- 29.Keifer JA, Guttridge DC, Ashburner BP, Baldwin AS. Inhibition of NF-κB activity by thalidomide through supression of IκB kinase activity. J Biol Chem. 2001;276:22382–22387. doi: 10.1074/jbc.M100938200. [DOI] [PubMed] [Google Scholar]
- 30.Kriz J, Nguyen MD, Julien JP. Minocycline Slows Disease Progression in a Mouse Model of Amyotrophic Lateral Sclerosis. Neurobiology of Disease. 2002;10:268–278. doi: 10.1006/nbdi.2002.0487. [DOI] [PubMed] [Google Scholar]
- 31.Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED, Martin D, Maier SF, Johnson KW, Leinwand LA, Chavez RA, Watkins LR. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun. 2007;21:686–698. doi: 10.1016/j.bbi.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Lokensgard RJ, Hu S, van Fenema EM, Sheng WS, Peterson PK. Effect of thalidomide on chemokine production by human microglia. J Infect Dis. 2000;182:983–987. doi: 10.1086/315754. [DOI] [PubMed] [Google Scholar]
- 34.Manni L, Aloe L. Role of IL-1β and TNF-α in the regulation of NGF in experimentally induced arthritis in mice. Rheumatol Int. 1998;18:97–102. doi: 10.1007/s002960050065. [DOI] [PubMed] [Google Scholar]
- 35.Manthey CL, Brandes ME, Perera PY, Vogel SN. Taxol increases steady-state levels of lipopolysaccharide-inducible genes and protein-tyrosine phosphorylation in murine macrophages. J Immunol. 1992;149:2459–2465. [PubMed] [Google Scholar]
- 36.Mchugh SM, Rifkin IR, Deihton J, Wilson AB, Lachmann PJ, Lockwood CM, Ewan PW. The immunosuppresive drug thalidomide induces T helper cell type 2 (Th 2) and concomittantly inhibits Th 1 cytokine production in mitogen- and antigen-stimulated peripheral blood mononuclear cell cultures. Clin Exp Immunol. 2003;99:160–167. doi: 10.1111/j.1365-2249.1995.tb05527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milligan ED, Twining C, Chacur M, Biedenkapp J, O’Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moller DR, Wysocka M, Geenlee BM, Ma X, Wahl L, Flockhart DA, Trinchieri G, Karp CL. Inhibition of IL-12 production by thalidomide. J Immunol. 1997;159:5157–5161. [PubMed] [Google Scholar]
- 39.Moos PJ, Fitzpatrick FA. Taxane-mediated gene induction is independent of microtubule stabilization: Induction of trancription regulators and enzymes that modulate inflammation and apoptosis. Proc Natl Acad Sci USA. 1998;95:3896–3901. doi: 10.1073/pnas.95.7.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moos PJ, Muskardin DT, Fitzpatrick FA. Effect of taxol and taxotere on gene expression in macrophages: Induction of the prostaglandin H synthase-2 isoenzyme. J Immunol. 1999;162:467–473. [PubMed] [Google Scholar]
- 41.Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Smith KA, Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor α by enhancing mRNA degradation. J Exp Med. 1993;177:1675–1680. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narita M, Yoshida T, Nakajima M, Narita M, Miyatake M, Takagi T, Yajima Y, Suzuki T. Direct evidence for spinal cord microglia in the development of a neuropathic pain-like state in mice. J Neurochem. 2006;97:1337–1348. doi: 10.1111/j.1471-4159.2006.03808.x. [DOI] [PubMed] [Google Scholar]
- 43.New PZ, Jackson CE, Rinaldi D, Burris H, Barohn RJ. Peripheral neuropathy secondary to docetaxel (Taxotere) Neurology. 1996;46:108–111. doi: 10.1212/wnl.46.1.108. [DOI] [PubMed] [Google Scholar]
- 44.Norreel JC, Vinay L, Fontes M, Clarac F. Close relationship between motor impairments and loss of functional motoneurons in a Charcot-Marie-Tooth type 1A model. Neuroscience. 2003;116:695–703. doi: 10.1016/s0306-4522(02)00741-8. [DOI] [PubMed] [Google Scholar]
- 45.O’Connor C, Heath DL, Cernak I, Nimmo AJ, Vink R. Effects of daily versus weekly testing and pre-training on assessment of neurologic impairment following diffuse traumatic brain injury. J Neurotrauma. 2003;20:985–993. doi: 10.1089/089771503770195830. [DOI] [PubMed] [Google Scholar]
- 46.Okamoto K, Martin DP, Schmelzer JD, Mitsui Y, Low PA. Pro- and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp Neurol. 2001;169:386–391. doi: 10.1006/exnr.2001.7677. [DOI] [PubMed] [Google Scholar]
- 47.Opree A, Kress M. Involvement of the proinflammatory cytokines tumor necrosis factor-α, IL-1β, and IL-6 but not IL-8 in the development of heat hyperalgesia: Effects on heat-evoked calcitonin gene-related peptide release from rat skin. J Neurosci. 2000;20:6289–6293. doi: 10.1523/JNEUROSCI.20-16-06289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ozaktay AC, Cavanaugh JM, Asik I, DeLeo JA, Weinstein JN. Dorsal root sensitivity to interleukin-1 beta, interleukin-6 and tumor necrosis factor in rats. Eur Spine J. 2002;11:467–475. doi: 10.1007/s00586-002-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- 50.Quasthoff S, Hartung H-P. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 51.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 52.Redford EJ, Hall SM, Smith KJ. Vascular changes and demyelination induced by the intraneural injection of tumour necrosis factor. Brain. 1995;118(Pt 4):869–878. doi: 10.1093/brain/118.4.869. [DOI] [PubMed] [Google Scholar]
- 53.Ribeiro RA, Vale ML, Ferreira SH, Cunha FQ. Analgesic effect of thalidomide on inflammatory pain. Eur J Pharmacol. 2000;391:97–103. doi: 10.1016/s0014-2999(99)00918-8. [DOI] [PubMed] [Google Scholar]
- 54.Rowinsky EK, Donehower RC. Paclitaxel (Taxol) New Eng J Med. 1995;332:1004–1013. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 55.Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol) Sem Oncol. 1993;20:1–15. [PubMed] [Google Scholar]
- 56.Sachs D, Cunha FQ, Poole S, Ferreira SH. Tumour necrosis factor-α, interleukin-1β and interleukin-8 induce persistent mechanical nociceptor hypersensitivity. Pain. 2002;96:89–97. doi: 10.1016/s0304-3959(01)00433-x. [DOI] [PubMed] [Google Scholar]
- 57.Sahenk Z, Barohn R, New P, Mendell JR. Taxol neuropathy. Electrodiagnostic and sural nerve biopsy findings. Arch Neurol. 1994;51:726–729. doi: 10.1001/archneur.1994.00540190110024. [DOI] [PubMed] [Google Scholar]
- 58.Sampaio EP, Sarno EN, Galilly R, Cohn AZ, Kaplan G. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med. 1991;173:699–703. doi: 10.1084/jem.173.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sampaio EP, Sarno EN, Galilly R, Cohn AZ, Kaplan G. Thalidomide selectively inhibits tumor necrosis factor α production by stimulated human monocytes. J Exp Med. 1993;173:699–703. doi: 10.1084/jem.173.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schafers M, Brinkhoff J, Neukirchen S, Marziniak M, Sommer C. Combined epineurial therapy with neutralizing antibodies to tumor necrosis factor-alpha and interleukin-1 receptor has an additive effect in reducing neuropathic pain in mice. Neurosci Lett. 2001;310:113–116. doi: 10.1016/s0304-3940(01)02077-8. [DOI] [PubMed] [Google Scholar]
- 61.Shapira L, Soskolone WA, Houri Y, Barak V, Halabi A, Stabholz A. Protection against endotoxic shock and lipopolysaccharide-induced local inflammation by tetracyline: Correlation with inhibition of cytokine secretion. Infect Immun. 1996;64:825–828. doi: 10.1128/iai.64.3.825-828.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shelanski ML, Wisniewski K. Neurofibrillary degeneration. Arch Neurol. 1969;20:199–206. doi: 10.1001/archneur.1969.00480080099012. [DOI] [PubMed] [Google Scholar]
- 63.Skundric DS, Dai R, James J, Lisak R. Activation of IL-1 signaling pathway in schwann cells during diabetic neuropathy. Ann N Y Acad Sci. 2002;958:393–398. doi: 10.1111/j.1749-6632.2002.tb03011.x. [DOI] [PubMed] [Google Scholar]
- 64.Sommer C, Lindenlaub T, Teuteberg P, Schafers M, Hartung T, Toyka KV. Anti-TNF-antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res. 2001a;913:86–89. doi: 10.1016/s0006-8993(01)02743-3. [DOI] [PubMed] [Google Scholar]
- 65.Sommer C, Marziniak M, Myers RR. The effect of thalidomide treatment on vascular pathology and hyperalgesia caused by chronic constriction injury of rat nerve. Pain. 1998a;74:83–91. doi: 10.1016/S0304-3959(97)00154-1. [DOI] [PubMed] [Google Scholar]
- 66.Sommer C, Schafer M, Marziniak M, Toyka KV. Etanercept reduces hyperalgesia in experimental painful neuropathy. J Peripher Nerv Syst. 2001b;6:67–72. doi: 10.1046/j.1529-8027.2001.01010.x. [DOI] [PubMed] [Google Scholar]
- 67.Sommer C, Schmidt C, George A. Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp Neurol. 1998b;151:138–142. doi: 10.1006/exnr.1998.6797. [DOI] [PubMed] [Google Scholar]
- 68.Sorkin LS, Doom CM. Epineurial application of TNF elicits an acute mechanical hyperalgesia in the awake rat. J Peripher Nerv Syst. 2000;5:96–100. doi: 10.1046/j.1529-8027.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 69.Sorkin LS, Xaio WH, Wagner R, Myers RR. Tumour necrosis factor-α induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. 1997;81:255–262. doi: 10.1016/s0306-4522(97)00147-4. [DOI] [PubMed] [Google Scholar]
- 70.Stoll G, Jung S, Jander S, van der Meide P, Hartung HP. Tumor necrosis factor-α in immune-mediated demyelination and Wallerian degeneration of the rat peripheral nervous system. J Neuroimmunol. 1993;45:175–182. doi: 10.1016/0165-5728(93)90178-2. [DOI] [PubMed] [Google Scholar]
- 71.Sweitzer SM, Pahl JL, DeLeo JA. Propentofylline attenuates vincristine-induced peripheral neuropathy in the rat. Neurosci Lett. 2006;400:258–261. doi: 10.1016/j.neulet.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 72.Tanner KD, Levine JD, Topp KS. Microtubule disorientation and axonal swelling in unmyelinated sensory axons during vincristine-induced painful neuropathy in rat. J Comp Neurol. 1998;395:481–492. [PubMed] [Google Scholar]
- 73.Theiss C, Meller K. Taxol impairs anterograde axonal transport of microinjected horseradish peroxidase in dorsal root ganglia neurons in vitro. Cell Tissue Res. 2000;299:213–224. doi: 10.1007/s004419900120. [DOI] [PubMed] [Google Scholar]
- 74.Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tuxen MK, Hansen SW. Neurotoxicity secondary to antineoplastic drugs. Cancer Treat Rev. 1994;20:191–214. doi: 10.1016/0305-7372(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 76.Verstappen CCP, Heimans JJ, Hoekman K, Postma TJ. Neurotoxic complications of chemotherapy in patients with cancer. Drugs. 2003;63:1549–1563. doi: 10.2165/00003495-200363150-00003. [DOI] [PubMed] [Google Scholar]
- 77.Wagner R, Janjigian M, Myers RR. Anti-inflammatory interleukin-10 therapy in CCI neuropathy decreases thermal hyperalgesia, macrophage recruitment and endoneurial TNF-α expression. Pain. 1998;74:35–42. doi: 10.1016/S0304-3959(97)00148-6. [DOI] [PubMed] [Google Scholar]
- 78.Wagner R, Myers RR. Endoneurial injection of TNF-α produces neuropathic pain behaviors. Neuroreport. 1996a;7:2897–2901. doi: 10.1097/00001756-199611250-00018. [DOI] [PubMed] [Google Scholar]
- 79.Wagner R, Myers RR. Schwann cells produce tumor necrosis factor alpha: Expression in injured and non-injured nerves. Neuroscience. 1996b;73:625–629. doi: 10.1016/0306-4522(96)00127-3. [DOI] [PubMed] [Google Scholar]
- 80.Wells JE, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126:1628–1637. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- 81.Weng H-R, Cordella JV, Dougherty PM. Changes in sensory processing in the spinal dorsal horn accompany vincristine-induced hyperalgesia and allodynia. Pain. 2003;103:131–138. doi: 10.1016/s0304-3959(02)00445-1. [DOI] [PubMed] [Google Scholar]
- 82.White CM, Martin BK, Lee LF, Haskill JS, Ting JPY. Effects of paclitaxel on cytokine synthesis by unprimed human monocytes, T lymphocytes, and breast cancer cells. Cancer Immunol Immunother. 1998;46:104–112. doi: 10.1007/s002620050468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zaks-Zilberman M, Zaks TZ, Vogel SN. Induction of proinflammatory and chemokine genes by lipopolysaccharide and paclitaxel (Taxol) in murine and human breast cancer cell lines. Cytokine. 2001;15:156–165. doi: 10.1006/cyto.2001.0935. [DOI] [PubMed] [Google Scholar]