Abstract
We purified and characterized an extracellular phospholipase produced by Listeria monocytogenes. This enzyme was separated as a homogeneous protein of 29 kDa by chromatography on DEAE-52 cellulose and Bio-Gel P100 columns. It is a zinc-dependent phospholipase C (PLC) that is mainly active at pH 6 to 7 and expresses lecithinase activity and a weaker sphingomyelinase activity. The exoenzyme also hydrolyzed phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and sphingomyelin but not phosphatidylinositol. It was distinct from the 36-kDa phosphatidylinositol PLC produced by L. monocytogenes and from the L. ivanovii sphingomyelinase. The pure protein expressed a weak, calcium-independent hemolytic activity and was not toxic in mice. Western immunoblot analysis using a rabbit immune serum raised against the enzyme showed that all virulent strains of L. monocytogenes tested produced in the culture supernatant a 29-kDa PLC. In contrast, no proteins antigenically related to the 29-kDa PLC were detected in supernatants of L. ivanovii, L. seeligeri, L. innocua, or L. welshimeri. The role in virulence of the 29-kDa PLC specifically produced by L. monocytogenes remains to be established.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauw G., Van Damme J., Puype M., Vandekerckhove J., Gesser B., Ratz G. P., Lauridsen J. B., Celis J. E. Protein-electroblotting and -microsequencing strategies in generating protein data bases from two-dimensional gels. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7701–7705. doi: 10.1073/pnas.86.20.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielecki J., Youngman P., Connelly P., Portnoy D. A. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 1990 May 10;345(6271):175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Langman R. E. Cell-mediated immunity to bacterial infection in the mouse. Thymus-derived cells as effectors of acquired resistance to Listeria monocytogenes. Scand J Immunol. 1972;1(4):379–391. doi: 10.1111/j.1365-3083.1972.tb03304.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cossart P., Vicente M. F., Mengaud J., Baquero F., Perez-Diaz J. C., Berche P. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun. 1989 Nov;57(11):3629–3636. doi: 10.1128/iai.57.11.3629-3636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domann E., Leimeister-Wächter M., Goebel W., Chakraborty T. Molecular cloning, sequencing, and identification of a metalloprotease gene from Listeria monocytogenes that is species specific and physically linked to the listeriolysin gene. Infect Immun. 1991 Jan;59(1):65–72. doi: 10.1128/iai.59.1.65-72.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARD K. F., SBARRA A. J., BARDAWIL W. A. Serology of Listeria monocytogenes. I. Characteristics of the soluble hemolysin. J Bacteriol. 1963 Feb;85:349–355. doi: 10.1128/jb.85.2.349-355.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986 Apr;52(1):50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy C., Gaillard J. L., Alouf J. E., Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987 Jul;55(7):1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A. Synthesis and secretion of interferon by murine fibroblasts in response to intracellular Listeria monocytogenes. Infect Immun. 1986 Dec;54(3):787–792. doi: 10.1128/iai.54.3.787-792.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins E. M., Watson B. B. Extracellular Antigens from Listeria monocytogenes I. Purification and Resolution of Hemolytic and Lipolytic Antigens from Culture Filtrates of Listeria monocytogenes. Infect Immun. 1971 Apr;3(4):589–594. doi: 10.1128/iai.3.4.589-594.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathariou S., Metz P., Hof H., Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987 Mar;169(3):1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathariou S., Pine L., George V., Carlone G. M., Holloway B. P. Nonhemolytic Listeria monocytogenes mutants that are also noninvasive for mammalian cells in culture: evidence for coordinate regulation of virulence. Infect Immun. 1990 Dec;58(12):3988–3995. doi: 10.1128/iai.58.12.3988-3995.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. A., Seaman T. A., Woodbine M. Listeria monocytogenes-haemolysin: lecithinase. Zentralbl Bakteriol Orig A. 1973 Oct;225(1):66–79. [PubMed] [Google Scholar]
- Kreft J., Funke D., Haas A., Lottspeich F., Goebel W. Production, purification and characterization of hemolysins from Listeria ivanovii and Listeria monocytogenes Sv4b. FEMS Microbiol Lett. 1989 Jan 15;48(2):197–202. doi: 10.1111/j.1574-6968.1989.tb03298.x. [DOI] [PubMed] [Google Scholar]
- Krug E. L., Kent C. Phospholipase C from Clostridium perfringens: preparation and characterization of homogeneous enzyme. Arch Biochem Biophys. 1984 Jun;231(2):400–410. doi: 10.1016/0003-9861(84)90403-x. [DOI] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimeister-Wächter M., Domann E., Chakraborty T. Detection of a gene encoding a phosphatidylinositol-specific phospholipase C that is co-ordinately expressed with listeriolysin in Listeria monocytogenes. Mol Microbiol. 1991 Feb;5(2):361–366. doi: 10.1111/j.1365-2958.1991.tb02117.x. [DOI] [PubMed] [Google Scholar]
- Little C., Otnåss A. B. The metal ion dependence of phospholipase C from Bacillus cereus. Biochim Biophys Acta. 1975 Jun 24;391(2):326–333. doi: 10.1016/0005-2744(75)90256-9. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonel J. L. Clostridium perfringens toxins (type A, B, C, D, E). Pharmacol Ther. 1980;10(3):617–655. doi: 10.1016/0163-7258(80)90031-5. [DOI] [PubMed] [Google Scholar]
- Mengaud J., Braun-Breton C., Cossart P. Identification of phosphatidylinositol-specific phospholipase C activity in Listeria monocytogenes: a novel type of virulence factor? Mol Microbiol. 1991 Feb;5(2):367–372. doi: 10.1111/j.1365-2958.1991.tb02118.x. [DOI] [PubMed] [Google Scholar]
- Mengaud J., Geoffroy C., Cossart P. Identification of a new operon involved in Listeria monocytogenes virulence: its first gene encodes a protein homologous to bacterial metalloproteases. Infect Immun. 1991 Mar;59(3):1043–1049. doi: 10.1128/iai.59.3.1043-1049.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
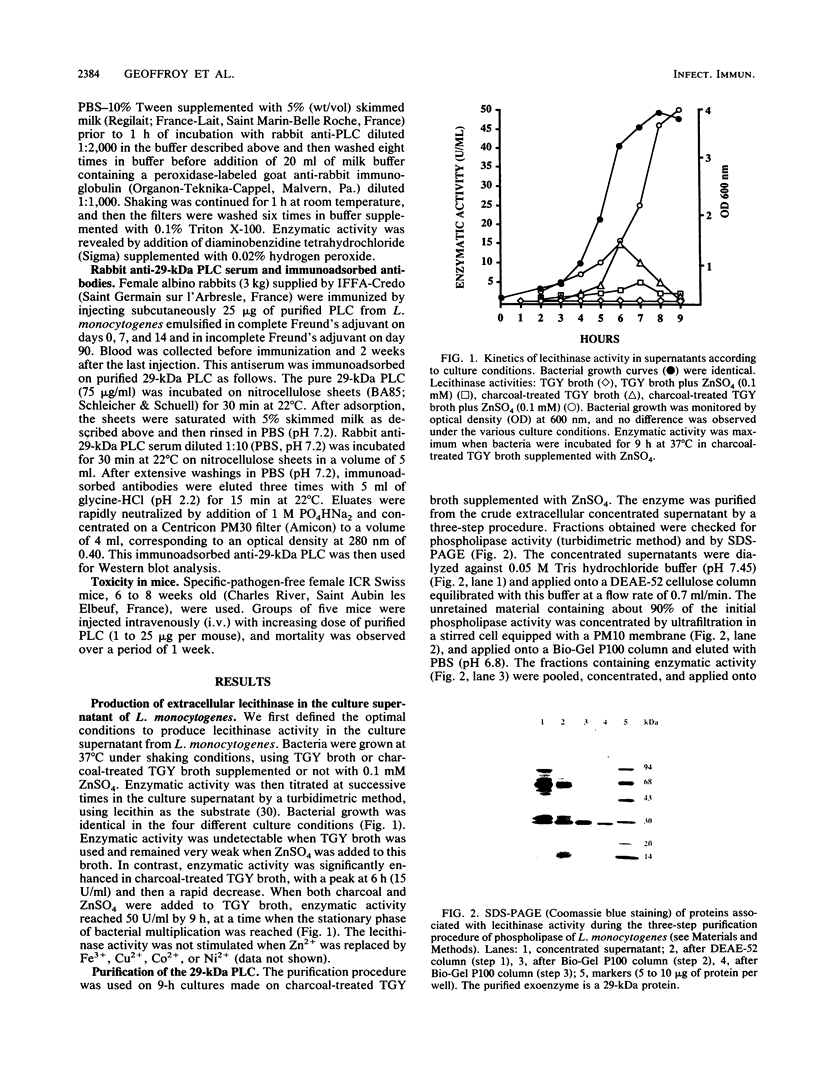
- Michel E., Reich K. A., Favier R., Berche P., Cossart P. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol Microbiol. 1990 Dec;4(12):2167–2178. doi: 10.1111/j.1365-2958.1990.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Minkes M., Stanford N., Chi M. M., Roth G. J., Raz A., Needleman P., Majerus P. W. Cyclic adenosine 3',5'-monophosphate inhibits the availability of arachidonate to prostaglandin synthetase in human platelet suspensions. J Clin Invest. 1977 Mar;59(3):449–454. doi: 10.1172/JCI108659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau H., Pieroni G., Jolivet-Reynaud C., Alouf J. E., Verger R. A new kinetic approach for studying phospholipase C (Clostridium perfringens alpha toxin) activity on phospholipid monolayers. Biochemistry. 1988 Apr 5;27(7):2319–2323. doi: 10.1021/bi00407a012. [DOI] [PubMed] [Google Scholar]
- Mounier J., Ryter A., Coquis-Rondon M., Sansonetti P. J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990 Apr;58(4):1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möllby R., Holme T., Nord C. E., Smyth C. J., Wadström T. Production of phospholipase C (alpha-toxin), haemolysins and lethal toxins by Clostridium perfringens types A to D. J Gen Microbiol. 1976 Sep;96(1):137–144. doi: 10.1099/00221287-96-1-137. [DOI] [PubMed] [Google Scholar]
- NJOKU-OBI A. N., JENKINS E. M., NJOKU-OBI J. C., ADAMS J., COVINGTON V. PRODUCTION AND NATURE OF LISTERIA MONOCYTOGENES HEMOLYSINS. J Bacteriol. 1963 Jul;86:1–8. doi: 10.1128/jb.86.1.1-8.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G. J. Lipid composition of erythrocytes in various mammalian species. Biochim Biophys Acta. 1967 Oct 2;144(2):221–232. doi: 10.1016/0005-2760(67)90152-x. [DOI] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Jacks P. S., Hinrichs D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988 Apr 1;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Joanis B., Garnier T., Cole S. T. Gene cloning shows the alpha-toxin of Clostridium perfringens to contain both sphingomyelinase and lecithinase activities. Mol Gen Genet. 1989 Nov;219(3):453–460. doi: 10.1007/BF00259619. [DOI] [PubMed] [Google Scholar]
- Siddique I. H., Lin I. F., Chung R. A. Purification and characterization of hemolysin produced by Listeria monocytogenes. Am J Vet Res. 1974 Feb;35(2):289–286. [PubMed] [Google Scholar]
- TAUBENECK U. Susceptibility of Proteus mirabilis and its stable L-forms to erythromycin and other macrolides. Nature. 1962 Oct 13;196:195–196. doi: 10.1038/196195b0. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso J. Y., Siebel C. Cloning and expression of the phospholipase C gene from Clostridium perfringens and Clostridium bifermentans. Infect Immun. 1989 Feb;57(2):468–476. doi: 10.1128/iai.57.2.468-476.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Boland J. A., Dominguez L., Rodriguez-Ferri E. F., Suarez G. Purification and characterization of two Listeria ivanovii cytolysins, a sphingomyelinase C and a thiol-activated toxin (ivanolysin O). Infect Immun. 1989 Dec;57(12):3928–3935. doi: 10.1128/iai.57.12.3928-3935.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson B. B., Lavizzo J. C. Extracellular antigens from Listeria monocytogenes. II. Cytotoxicity of hemolytic and lipolytic antigens of Listeria for cultured mouse macrophages. Infect Immun. 1973 May;7(5):753–758. doi: 10.1128/iai.7.5.753-758.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]