Abstract
While methamphetamine-induced changes in brain neurotransmitters, their receptors and transporters are well studied; the means by which methamphetamine abuse results in cognitive and behavioral abnormalities is unknown. Here we administered methamphetamine chronically, in doses relevant to recreational usage patterns, to nonhuman primates. Neurostructural analysis revealed decreased dendritic material and loss of spines in frontal lobe neurons. Molecular examination demonstrated that type I interferons (interferon-alpha and -beta) increased in the frontal lobe in response to chronic methamphetamine treatment, in correlation with the neuronal changes. Chronic methamphetamine thus results in significant changes in the primate brain, inducing cytokines and altering neuronal structure, both of which can contribute to functional abnormalities.
INTRODUCTION
D-methamphetamine hydrochloride (methamphetamine, METH) is a strong CNS stimulant. METH is similar in structure to dopamine, and is closely related to two similar stimulants, amphetamine and methylenedioxymethamphetamine (MDMA). METH is a popular selection among the drug abusing population due to its price, availability, and psychological effects, which including feelings of euphoria, increased performance, and enhancement of sexual pleasure. METH is a substrate for the dopamine transporter (DAT), and thus gains access to dopamine (DA) system neurons, where it inhibits the vesicular monoamine transporter (VMAT2), leading to increased DA in the synaptic cleft (Volz et al., 2007).
Although a number of studies have examined the effects of METH on neurotransmitters and transporters, less information is available concerning other effects on neurons. Studies in young gerbils showed that METH induces morphological alteration of mesocortical dopamine nerve fibers and their postsynaptic structures in the frontal cortex (Wahnschaffe and Esslen, 1985), whereas in adult gerbils, an increase in dendritic spines was present in the prefrontal, but not parietal, cortex, and degrading axon terminals were found in layers III and V of the anterior cingulate (Dawirs et al., 1991). Other experiments in METH-treated mice revealed shrinkage and degeneration of pyramidal cells in the frontal cortex and hippocampus (Kuczenski et al., 2007).
Although a great amount has been learned from studies on METH in rodents, less information is available concerning its effects on humans. An autopsy study revealed that human chronic METH users had decreased levels of DA, DAT, and the dopamine synthetic enzyme tyrosine hydroxylase in the striatum (Wilson et al., 1996), consistent with studies in rodents. Imaging studies have also revealed decreased DAT and VMAT2 as well as dopamine receptors in the striatum of living human METH abusers (Chang et al., 2007). High-resolution MRI has been used to examine the brains of chronic METH abusers. Gray matter cortical deficits were found, most significantly in the cingulate; smaller hippocampal volumes and a hypertrophy of the white matter were also present in METH abusers (Thompson et al., 2004). The exact mechanisms by which METH produces damage to the human CNS remain mostly hypothetical. Such mechanisms may involve toxic monoamine metabolites, glutamate-induced excitotoxic neurotoxicity, oxidative free-radical chemistry, and metabolic stress (Cadet et al., 2003).
Nonhuman primates provide excellent animal models to study the effects of drugs of abuse on the brain. We have reported a METH dose-escalation protocol in nonhuman primates, resulting in administration of levels of METH similar to that seen in recreational users, which results in both behavioral and physiological effects (Madden et al., 2005). A similar dose escalation protocol was recently used in vervet monkeys revealing changes in striatal dopaminergic systems and behavior (Melega et al., 2007). Here, we used this protocol of chronic METH treatment on rhesus monkeys to first examine whether METH induced changes in neuronal structure, and second to assess whether the chronic METH treatment produced changes in brain gene expression.
METHODS
Animals
Rhesus monkeys were free of SIV, SRV-type D, STLV-I and Cercopithecine herpesvirus 1. Three animals were treated with METH using our previously described dose escalation protocol (Madden et al., 2005). Animals received the maintenance dose of METH (0.75 mg/kg/dose, twice a day Monday through Thursday, once on Friday, none on weekends) for 31-32 weeks before sacrifice per experimental protocol. Three untreated animals were used for the Golgi analysis, and four for the gene expression analysis. At necropsy, performed after terminal anesthesia, animals were intracardially perfused with sterile PBS containing 1 U/ml heparin. Tissue samples for RNA isolation were frozen, and those for Golgi staining were immersion fixed in 10% neutral buffered formalin. All animal experiments were performed with approval from the TSRI Institutional Animal Care and Use Committee following NIH guidelines.
Golgi analysis
Blocks were cut from the fixed tissue containing frontal cortex and caudate, and stained using the rapid Golgi method. Briefly, blocks were impregnated with an osmium tetroxide and potassium dichromate mixture, followed by immersion in silver nitrate; then dehydrated and embedded in nitrocellulose; coronal sections were cut at 120 μm. For analysis, camera lucida drawings were made of the basilar dendritic tree of neurons in the indicated layer/structure using a 40× long-working-distance oil-immersion lens, a 1.6× intermediate lens and a 10× ocular viewer. For each control animal in each indicated area 9 neurons were randomly taken for examination, in the METH-treated animals 8 neurons were randomly taken for examination. The extent and distribution of neuronal dendritic branching were evaluated by Sholl analysis, whereby a transparent overlay of increasingly larger concentric circles at 10 μm intervals is superimposed on the camera lucida drawings. The number of dendritic branch intersections with each progressively larger circle is counted from the soma of each neuron. The spines were also analyzed. Visible flanking spines were counted along 30 μm terminal-tip segments of four total segments for each neuron at a magnification of 1,200×.
Quantitative real-time PCR
RNA was isolated from frontal lobe and caudate samples, taken following necropsy, using TRIzol reagent (Invitrogen, Carlsbad, CA), and then further purified utilizing the RNeasy mini kit (Qiagen, Valencia, CA). cDNA was synthesized and real-time PCR performed using dual-labeled hydrolysis probes as described (Marcondes et al., 2007). Sequences for the IFNα primers were GATGATCCAGCAGACCTTCA and TAGGAGGGTCTCATCCCAAG, and the probe TCTTCAGCACAAAGGACTCATCTGCTG; for IFNβ the primers were CGCTCTGGCACAACAGGTAGT and GAGCAATTTGGAGGAGACACTTG, and the probe AGGCGACACTGTTCCTGTTCTCA; and for IFNγ the primers were GCAGATAATGGAACTCTTTTCTTAGACA and AGGAGACAATTTGGCTCTGCAT, and the probe TTTCTGTCACTCTCCTCTTTCCAATTCCTCAA. The sequences of the control primers/probes were as described (Marcondes et al., 2007); the sequences for the other genes are available upon request. To compute the relative amounts of specific mRNA in the samples, the average cycle threshold (Ct) of the primary signals for the TATA-box binding protein, glyceraldehyde-3-phosphate dehydrogenase, and 18S ribosomal RNA (as controls) was subtracted from that of the specified genes to give the change in Ct (dCt), which are log2 values.
Statistical analysis
Statistical analysis for the Sholl dendritic analysis was carried out with the Wilcoxon signed-rank test, for the spine analysis Student’s unpaired t-test, and for gene expression (using the log2 dCt values) two-way ANOVA followed by Bonferroni post-hoc tests, all using Prism software (GraphPad Software, San Diego, CA). For all tests, alpha was set at 0.05 to determine significance.
RESULTS
In order to examine the effect of this chronic METH-treatment protocol on the brain, we first performed a Golgi-impregnation study on selected neuronal populations. Layer V neurons were examined in two regions of the frontal lobe (anterior cingulate gyrus and middle frontal gyrus) and spiny neurons were examined in the caudate nucleus. The neurons were analyzed for dendritic branches and spines, allowing a structural examination of the functional parts of neurons. Sholl analysis was used to characterize the amount of dendritic material and its distribution. Significant loss of dendrites was found in the anterior cingulate and middle frontal gyri, but not in the caudate (Figure 1A). Examination of spines on the terminal dendritic tips revealed significant loss (17% less in the METH group) only in the middle frontal gyrus (Figure 1B). These data are consistent with METH-induced damage in the brain, and reveals that frontal lobe neurons are targets of the direct or indirect actions of chronic METH administration.
Figure 1. Neurostructural analysis using Golgi staining in the Anterior Cingulate Gyrus (ACG) Middle Frontal Gyrus (MFG) and Caudate Nucleus.
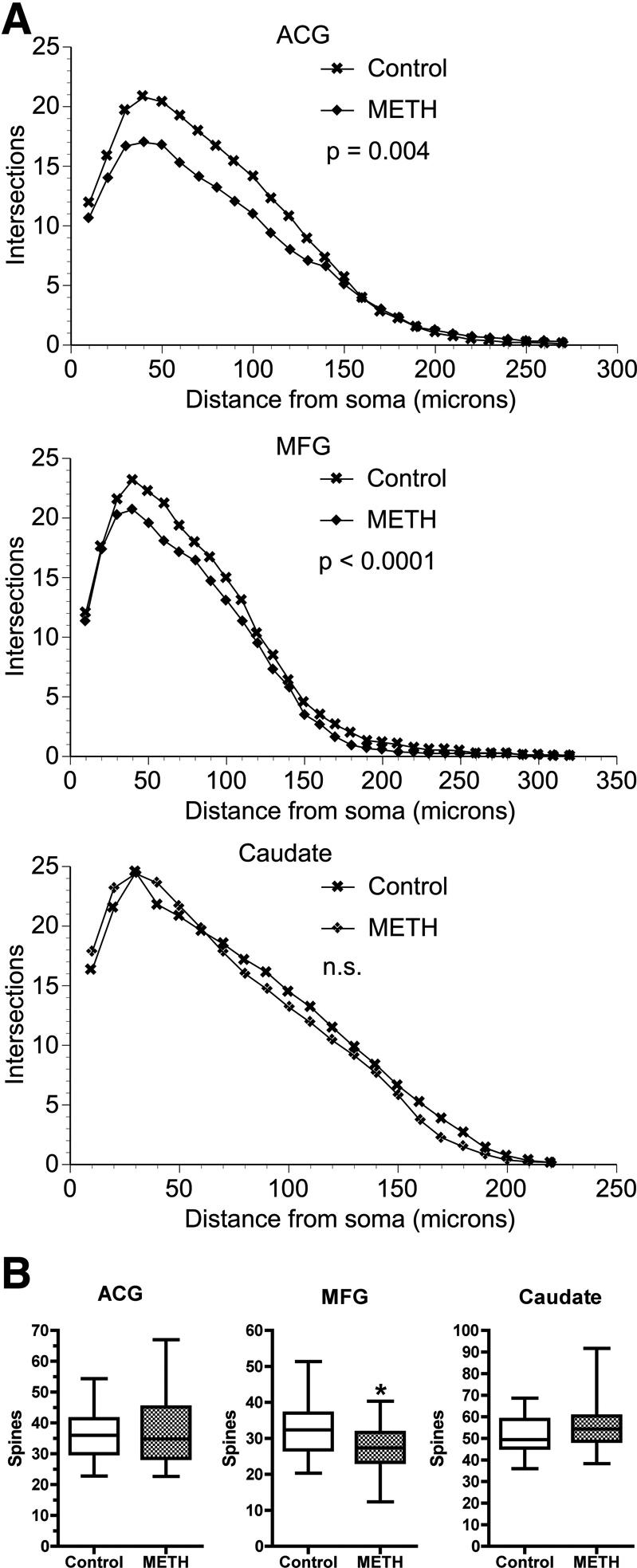
A) Sholl analysis of dendrites. For each region, the number of dendritic intersections in concentric circles of the indicated distance from the soma was determined to assess the amount and distribution of dendritic material in Control and METH-treated brains. The Wilcoxon signed-rank test was used to determine the p-values, n.s. = not significant). B) The number of spines in a 30 μm length on the terminal dendritic tips is indicated by box and whisker plots for each region in Control and METH-treated animals. Student’s t-test revealed a significant (p = 0.0064) difference in the MFG, indicated by an asterisk.
In order to assess potential mediators of these neuronal changes, we examined the expression of interferons (IFN), since we had previously found that in METH-using HIV-infected people compared to HIV-infected individuals who did not use METH, IFN-induced genes were elevated in the brain (Everall et al., 2005). Indeed, the administration of METH in rhesus monkeys caused a significant change in IFN gene expression. Quantitative real-time PCR showed a significant up-regulation of IFNα (12.6-fold) and IFNβ (16-fold) in the frontal lobe of the METH group (Figure 2A) but not in the caudate (Figure 2B). No expression of IFNγ could be detected in either control or METH-administered animals. Levels of transcripts of a number of other genes were also assessed in the frontal lobe, however no significant changes were found for the individual genes (Figure 2C).
Figure 2. Assessment of gene expression using quantitative real-time PCR.

A) Frontal lobe. Two-way ANOVA revealed increased expression of type I interferons in METH-treated animals compared to controls (p = 0.0003), and Bonferroni post-hoc test indicated both IFNα and IFNβ to be significantly increased (p < 0.01 for both). B) Caudate nucleus. Two-way ANOVA revealed that the increase in type I interferons did not reach statistical significance (p = 0.0558). C) Frontal lobe. Assessment of other genes: brain derived neurotropic factor (BNDF), cellular oncogene c-Fos (FOS), interleukin 1α (IL1α), interleukin 1β (IL1β), interleukin 6 (IL6), monocyte chemoattractant protein 1 (MCP-1), regulator of G-protein signaling 5 (RGS5), and tumor necrosis factor α (TNFα) revealed that although the Control and METH groups differed by two-way ANOVA (p = 0.0233), Bonferroni post-hoc tests could not identify genes that significantly differed between the groups.
DISCUSSION
In the Golgi-impregnation experiment we found that METH administrations caused significant loss of dendrites in both areas of the frontal cortex examined: the anterior cingulate and middle frontal gyri, but not in the caudate. With further examination of spines on the terminal dendritic tips, we found significant loss only in the middle frontal gyrus. Molecular analysis revealed an increase in type I interferons (IFNα and IFNβ) in the frontal lobe, correlating with these neuronal changes.
The overall branching pattern and distribution of dendrites are important determinants of neuronal function, but the functional effect resulting from this apparent plasticity depends on the exact changes in neuronal connectivity. In this regard, we found that in addition to the decrease in dendrites, the number of dendritic spines, the major sites of synaptic contacts, were also decreased in the middle frontal gyrus. Decreased dendritic branching and spines indicates substantial damage to neurons as a result of chronic METH in primates.
As previously mentioned, high-resolution MRI has revealed significant changes in the cingulate gyrus and hippocampus of human METH abusers (Thompson et al., 2004). Our Golgi analysis may be a reflection of such changes in the cingulate, however we found no evidence for hippocampal changes by Golgi analysis (data not shown).
Molecular analysis revealed an increase in both IFNα and IFNβ in the frontal lobe. These type I IFNs are essential in coping with viral infections in CNS (Haller et al., 2006). However this protective effect can come with a significant price. In vivo studies of transgenic mice producing IFNα chronically from astrocytes have shown that overproduction on IFNα can lead to severe pathology in the brain, including neurodegeneration, calcification of the basal ganglia, progressive inflammatory encephalopathy, and meningoencephalitis (Akwa et al., 1998). Although we found that the increased IFN correlates with the neurostructual changes, the mechanisms by which IFN may lead to these changes are not clear.
One of the untoward features of METH abuse is an increased risk for other unhealthy actions. Due to the risk-induced behaviors posed by METH and enhanced sexual pleasure; METH users are more likely to have several sexual partners, leading to higher risk of HIV infection (Gibson et al., 2002), resulting in a distinct overlap between METH users and the HIV+ population. Interestingly, we had found increased expression of IFN-induced genes in the brains of those with HIV-induced CNS disease who also used METH (Everall et al., 2005). The increase in IFN we find in the brain following METH administration may be the cause of these findings, and indicate a significant interaction of METH and HIV in the CNS. Certainly, our experiment reveals that METH administration results in significant changes in neuronal structure as well as gene expression in the primate CNS.
ACKNOWLEDGEMENTS
This is manuscript #19538 from The Scripps Research Institute. We thank Michelle Zandonatti for RNA purification. This work was supported by NIH grants DA024467, MH073490 and MH062261.
REFERENCES
- Akwa Y, Hassett DE, Eloranta ML, Sandberg K, Masliah E, Powell H, Whitton JL, Bloom FE, Campbell IL. Transgenic expression of IFN-alpha in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J Immunol. 1998;161:5016–5026. [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, Deng X. Speed kills: cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. Fased J. 2003;17:1775–1788. doi: 10.1096/fj.03-0073rev. [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Dawirs RR, Teuchert-Noodt G, Busse M. Single doses of methamphetamine cause changes in the density of dendritic spines in the prefrontal cortex of gerbils (Meriones unguiculatus) Neuropharmacology. 1991;30:275–282. doi: 10.1016/0028-3908(91)90155-5. [DOI] [PubMed] [Google Scholar]
- Everall I, Salaria S, Roberts E, Corbeil J, Sasik R, Fox H, Grant I, Masliah E. Methamphetamine stimulates interferon inducible genes in HIV infected brain. J Neuroimmunol. 2005;170:158–171. doi: 10.1016/j.jneuroim.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Gibson DR, Leamon MH, Flynn N. Epidemiology and public health Consequences of methamphetamine use in California’s Central Valley. J Psychoactive Drugs. 2002;34:313–319. doi: 10.1080/02791072.2002.10399969. [DOI] [PubMed] [Google Scholar]
- Haller O, Kochs G, Weber F. The interferon response circuit: induction and suppression by pathogenic viruses. Virology. 2006;344:119–130. doi: 10.1016/j.virol.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Everall IP, Crews L, Adame A, Grant I, Masliah E. Escalating dosemultiple binge methamphetamine exposure results in degeneration of the neocortex and limbic system in the rat. Exp Neurol. 2007;207:42–51. doi: 10.1016/j.expneurol.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden LJ, Flynn CT, Zandonatti MA, May M, Parsons LH, Katner SN, Henriksen SJ, Fox HS. Modeling human methamphetamine exposure in nonhuman primates: chronic dosing in the rhesus macaque leads to behavioral and physiological abnormalities. Neuropsychopharmacology. 2005;30:350–359. doi: 10.1038/sj.npp.1300575. [DOI] [PubMed] [Google Scholar]
- Marcondes MC, Burdo TH, Sopper S, Huitron-Resendiz S, Lanigan C, Watry D, Flynn C, Zandonatti M, Fox HS. Enrichment and persistence of virus-specific CTL in the brain of simian immunodeficiency virus-infected monkeys is associated with a unique cytokine environment. J Immunol. 2007;178:5812–5819. doi: 10.4049/jimmunol.178.9.5812. [DOI] [PubMed] [Google Scholar]
- Melega WP, Jorgensen MJ, Lacan G, Way BM, Pham J, Morton G, Cho AK, Fairbanks LA. Long-Term Methamphetamine Administration in the Vervet Monkey Models Aspects of a Human Exposure: Brain Neurotoxicity and Behavioral Profiles. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301502. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz TJ, Fleckenstein AE, Hanson GR. Methamphetamine-induced alterations in monoamine transport: implications for neurotoxicity, neuroprotection and treatment. Addiction. 2007;102(Suppl 1):44–48. doi: 10.1111/j.1360-0443.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- Wahnschaffe U, Esslen J. Structural evidence for the neurotoxicity of methylamphetamine in the frontal cortex of gerbils (Meriones unguiculatus): a light and electron microscopical study. Brain Res. 1985;337:299–310. doi: 10.1016/0006-8993(85)90067-8. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey Al, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]


