Abstract
The four serotypes of dengue virus (DENV1-4) are causative agents of dengue fever and dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS). Previous DENV infection is a risk factor for DHF/DSS during subsequent infection by a different serotype. Nonetheless, most primary and secondary DENV infections are asymptomatic. To investigate the possible mechanisms of immune protection in vivo, 129/Pas mice lacking IFN-α/β and -γ receptors (AG129) were used to model secondary infection using both DENV1-DENV2 and DENV2-DENV4 sequences. At intervals between sequential infections of 4 to 52 weeks, protection against secondary heterologous DENV infection was observed. Passive transfer of DENV-immune serum was protective against replication of heterologous challenge virus in all tissues tested, whereas adoptive transfer of DENV-immune cells significantly protected mice from replication of the challenge virus only when a lower inoculum was administered. These findings are relevant for understanding both natural and vaccine-induced immunity to DENV.
Keywords: flavivirus, heterologous, protection, dengue virus, antibody, cellular immunity, serotype-specific, serotype-cross-reactive, AG129
INTRODUCTION
The four serotypes of dengue virus (DENV1-4), in the genus Flavivirus, family Flaviviridae, are the causative agents of the mosquito-borne illness dengue fever (DF), which affects over 50 million people annually. In some cases, DF progresses to dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS), which is a severe, life-threatening disease characterized by increased vascular permeability responsible for 250,000-500,000 hospitalizations each year in tropical and sub-tropical regions worldwide (Gubler, 1998). Although a number of dengue vaccine candidates are in various stages of clinical trials, no specific therapies or vaccines are currently available (Hombach, 2007; Whitehead et al., 2007). A successful tetravalent dengue vaccine must confer robust protection against all four serotypes of DENV, as previous infection by one serotype is a recognized risk factor for DHF/DSS following a heterologous (cross-serotype-reactive) DENV infection (Halstead, 1981). Nonetheless, most primary and secondary DENV infections are asymptomatic (Balmaseda et al., 2006; Burke et al., 1988; Endy et al., 2002), and the mechanistic basis of protection versus enhancement in sequential DENV infections is not well-defined. Epidemiologic studies in humans yield invaluable information; however, animal models of DENV infection allow characterization of protective as well as pathologic immune responses during a secondary DENV infection in vivo.
Studies of heterologous DENV infections have been conducted in non-human primates and to a limited extent in mice. In monkeys, sequential heterologous DENV infections resulted in either protection (Kochel et al., 2005), partial protection (Koraka et al., 2007; Scherer et al., 1972) or enhancement (Marchette et al., 1973). Enhanced infection after transfer of either pooled human cord blood from DENV-immune mothers (Halstead, 1979) or a chimeric human-chimpanzee anti-DENV neutralizing monoclonal antibody (mAb) (Goncalvez et al., 2007) resulted in increased viremia in rhesus monkeys that is postulated to represent antibody-dependent enhancement (ADE). In mice, protection from death using a secondary, homologous (serotype-specific) DENV infection has been demonstrated (Johnson and Roehrig, 1999; Price and Thind, 1972); while reports of heterologous sequential DENV infections in mice noted an increase in thrombocytopenia (Sarkar et al., 1976) or documented heterologous immune responses (Beaumier et al., 2008). To the best of our knowledge, no published study in mice has documented the occurrence of either protection or enhancement of DENV titers in sequential, heterologous infections.
Wildtype mice are susceptible to DENV infection only after high doses and do not manifest robust viral replication (Chen et al., 2004; Huang et al., 2000; Paes et al., 2005; Shresta et al., 2004a). Thus, various immunocompromised mice have been used to test responses to DENV in vivo (An et al., 1999; An et al., 2004; Bente et al., 2005; Blaney et al., 2002; Johnson and Roehrig, 1999; Lin et al., 1998; Shresta et al., 2004b; Wu et al., 1995). In this study, mice of the 129 strain lacking receptors for interferon (IFN)-α/β and IFN-γ (AG129) were used to model sequential DENV infections. AG129 mice have a number of relevant attributes; for instance, they support robust replication of clinical isolates of all four DENV serotypes ((Kyle et al., 2007; Shresta et al., 2004b), S. Balsitis and E. Harris, unpublished data), display cellular tropism similar to that seen in humans (Durbin et al., 2008; Kyle et al., 2007; Neves-Souza et al., 2005), exhibit thrombocytopenia that is inversely related to viral load (S. Balsitis and E. Harris, unpublished data), develop high levels of soluble non-structural 1 (NS1) protein during DENV infection comparable to levels seen in humans (Schul et al., 2007; S. Balsitis and E. Harris, unpublished data), and experience increased vascular permeability upon infection with certain DENV strains (Shresta et al., 2006). In addition, AG129 mice are currently used to test anti-DENV compounds (Schul et al., 2007) and vaccines (Calvert et al., 2006; Huang et al., 2003; Johnson and Roehrig, 1999). An important advantage of the AG129 mouse strain is that it allows investigation of the adaptive immune response to DENV infection, since the adaptive immune system appears to be intact (van den Broek et al., 1995), with the caveat that IFN-mediated interactions between the innate and adaptive arms of immunity are lacking. Specifically, AG129 mice were found to have normal numbers of major lymphocyte subsets and normal levels of constitutive MHC expression.
To dissect the role of humoral versus cellular immune responses in secondary DENV infection, we conducted two types of experiments in AG129 mice: (1) sequential infection with two distinct serotypes or (2) passive transfer of DENV-immune serum or adoptive transfer of DENV-immune cells to naïve recipients, followed by challenge with a heterologous serotype. Serotype cross-protective immunity, rather than enhancement, was observed up to one year after infection with two different serotype sequences. Passive and adoptive transfer experiments suggested that protection was primarily mediated by heterologous antibodies, but also revealed a potential contribution by serotype cross-reactive immune cells.
RESULTS
Primary DENV infection in mice confers protection against a heterologous DENV serotype in sequential infections
We and others have previously demonstrated that AG129 mice are highly susceptible to multiple DENV serotypes and strains (Johnson and Roehrig, 1999; Schul et al., 2007; Shresta et al., 2004b) and that the cellular tropism of DENV in AG129 mice is similar to that seen in humans (Kyle et al., 2007). Using the AG129 model system, we tested two different combinations of DENV sequential infections. Mice were first infected with DENV1 and then challenged with 105 PFU of DENV2 4, 15, 33, or 52 weeks later. Each DENV2 challenge experiment included an equal number of naïve, age-matched controls. Spleen, lymph nodes, blood and bone marrow cells were collected from all mice at day 3 p.i., which is the day of peak replication (Kyle et al., 2007), and viral load in each tissue was determined by plaque assay. Reduced or absent replication of the DENV2 challenge serotype was observed in DENV1-immune mice as compared to naïve controls at 4-, 15- and 33-week intervals between infections (Figure 1). Of note, protection against DENV2 replication in the bone marrow at the earliest timepoint (4 weeks post-DENV1 infection) is incomplete. This may correlate with lower initial levels of cross-serotype protective antibodies, as discussed below. At the longest time interval tested (52 weeks), heterologous protection against DENV2 had begun to wane in DENV1-immune mice, although DENV2 titers were still below the level of replication observed in naïve mice. Statistics were not performed based on the low numbers of mice in each group, but the near complete lack of viral replication after DENV2 challenge in DENV1-immune mice up to 33 weeks post infection is suggestive of extended cross-serotype protection in sequential infections.
Fig. 1.
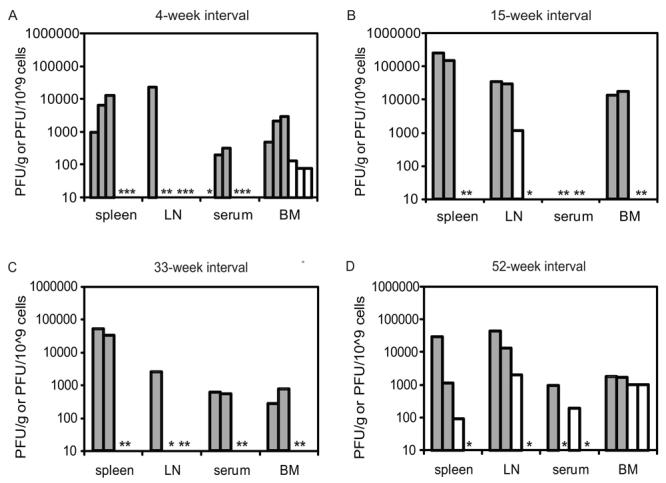
Previous DENV1 infection protects against DENV2 challenge. Mice were infected with DENV1 98J or Mochizuki (□), and age-matched naïve mice were used as controls (■).Mice were challenged at (A) 4, (B) 15, (C) 33, or (D) 52 weeks post-DENV1 infection with DENV2 PL046. At day three post-DENV2 infection, plaque assays were performed on tissue from the spleen, lymph nodes, serum, and bone marrow cells. Results from 2-3 mice per group are shown, and each bar represents an individual mouse (*; values below the limit of detection).
To confirm that protection in this heterologous DENV infection model was not restricted to one sequence of serotypes, we also infected AG129 mice with DENV2, followed by a secondary heterologous infection with 104 PFU of DENV4. Lymph nodes and bone marrow cells were collected from all mice at day 4-5 p.i., which are the days of peak replication (data not shown). Secondary DENV4 infections were performed after intervals of 4, 15 and 52 weeks, and DENV2-immune mice were protected against heterologous DENV4 at each time interval up to and including 52 weeks after infection (Figure 2).
Fig. 2.

Prior DENV2 infection protects against DENV4 challenge. Mice were infected with DENV2 PL046 (□), and age-matched naïve mice were used as controls (■). Micewere challenged at (A) 4, (B) 15, or (C) 52 weeks post-DENV2 infection with 104 PFU of DENV4 664. At day 4 or 5 post-DENV4 infection, plaque assays were performed on tissue from the lymph nodes and bone marrow cells. Since titers of DENV4 in serum and spleen of naïve mice are not sufficiently robust to detect a difference between groups, they were not included in the assessment. Results from 2-3 mice per group are shown, and each bar represents an individual mouse (*; values below the limit of detection).
Cross-neutralizing DENV-specific antibodies are present for at least 20 weeks post-infection
In humans, long-term, serotype-specific neutralizing antibodies have been found 40 years post-primary infection (Innis, 1997), and the presence of both serotype-specific and serotype-cross reactive antibodies have been documented (Guzman et al., 2007; Kochel et al., 2002). We tested for the presence of both serotype-specific and serotype-cross-reactive neutralizing antibodies in mice that had received a primary DENV1 or DENV2 infection. AG129 mice were infected with either DENV1 or DENV2, and 50% plaque reduction neutralization tests (PRNT50) were performed (Figure 3). The limit of detection for PRNT50 titers was <1:10, and naïve AG129 sera had a neutralizing titer of <1:10 against all DENV strains tested.
Fig. 3.

Cross-reactive neutralizing antibodies are present up to 20 weeks after primary DENV1 and DENV2 infection. Mice were infected with either (A) 102 PFU of DENV1 strain Mochizuki or (B) 105 PFU of DENV2 strain PL046. Results are shown for DENV1-immune serum (A) using DENV1 (◆) or DENV2 (◇) in the PRNT assay; and results for DENV2-immune serum (B) are shown using DENV2 (●) or DENV4 (○). The limit of detection for PRNT values is <1:10. Normal AG129 serum had a neutralizing value of <1:10 against all three DENV strains.
A serotype-specific antibody response of AG129 mice to DENV1 infection was detected by 2 weeks post-infection (p.i.), and high levels of DENV1-neutralizing antibodies were present through at least 20 weeks p.i. (Figure 3A). Heterologous neutralizing antibodies against DENV2 were just at the level of detection at 4 weeks p.i., which may explain in part the lower level of cross-protection seen at this early timepoint (Figure 1A), as compared to later timepoints when the titers were higher (Figure 3A). Cross-serotype protective antibodies began to decline after 16 weeks p.i., but were still present until at least 20 weeks p.i.. Similarly, DENV2 infection induced robust neutralizing titers against DENV2 as detected by PRNT50 beginning 2 weeks p.i.. Serotype-cross-reactive neutralizing antibodies against DENV4 serotype were also present, beginning 2 weeks p.i. and lasting until at least 16 weeks p.i. (Figure 3B). In a separate experiment, neutralizing antibodies were measured at 61 weeks after infection with either DENV1 (n=2) or DENV2 (n=2). In DENV1-immune mice, the PRNT50 titers against DENV1 were 1:1792 and 1:870, and the PRNT50 titers against the heterologous DENV2 were 1:117 and 1:100. In DENV2-immune mice, the PRNT50 titers against DENV2 were 1:586 and 1:337, whereas the PRNT50 titers against DENV4 were 1:29 and 1:21. Thus, DENV-immune mouse serum contains both homologous and heterologous neutralizing antibodies over an extended time period after primary infection.
Passive transfer of DENV-immune serum confers protection against homologous and heterologous DENV challenge
To examine the capacity of antibodies to protect against DENV challenge in AG129 mice, we first passively transferred mAbs or polyclonal sera, followed by a homologous challenge. In all passive transfer experiments, antiserum was delivered via an intraperitoneal (ip) route 24 hours prior to infection with 105 PFU DENV2 strain PL046. For experiments involving transfer of polyclonal antiserum, pre-challenge sera were tested by PRNT in a subset of mice. Effective neutralizing titers in recipient mice demonstrated a 1:10 dilution of transferred sera (data not shown). Non-parametric statistics (the Wilcoxon Rank Sum test) were used for analysis of both passive and adoptive transfer studies, in order to avoid statistical assumptions about the data analyzed. With this test, p-values are not derived from the magnitude of the difference between groups, but rather from the extent of overlap in the values for the two datasets being compared. In these experiments, we have defined protection against viral challenge as a significant reduction in viral titers in the tissues tested.
A neutralizing mAb directed against the DENV2 envelope (E) protein (3H5) resulted in reduced titers of the DENV2 challenge (Figure 4A), with a 3-log reduction of viral titers in the spleen (p=0.0495) and a 1-log reduction in the lymph nodes (p=0.0495) at day 3 p.i. Passive transfer of of polyclonal 300μL anti-DENV2 sera (PRNT50 titer against DENV2 = 1:1225) completely protected mice from DENV2 replication in spleen (p=0.0369) and serum and substantially reduced levels of viral replication in the lymph nodes (Figure 4B). Next, to test the capacity of DENV1-immune serum to provide heterologous protection against a DENV2 challenge, we passively transferred 400μL of DENV1-immune sera (PRNT50 titer against DENV1 = 1:663 and against DENV2 = 1:276) and subsequently challenged mice sc with DENV2. This resulted in a 1-2 log reduction in viral titers in the spleen (p=0.0209), lymph nodes (p=0.0433), and serum (p=0.0455) of infected mice (Figure 4C). As was observed in the sequential infection studies, reduction of viral titers in the lymph nodes was less complete than that observed in spleen tissue.
Fig. 4.
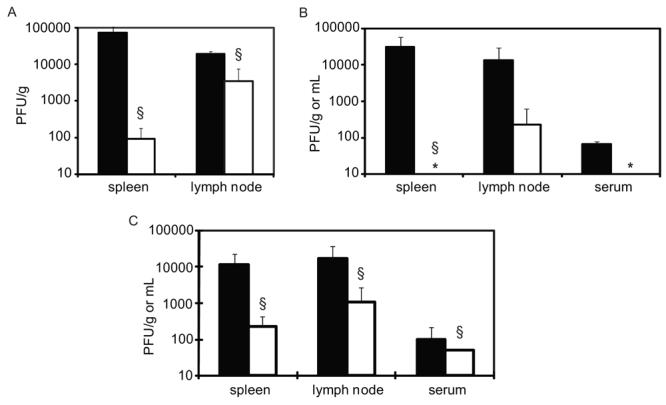
Passive transfer of both monoclonal and polyclonal antibodies protects against homologous as well as heterologous DENV2 challenge. AG129 mice received passive transfer of DENV-immune serum via an intraperitoneal injection 24 hours prior to homologous or heterologous challenge. All mice were challenged with 105 PFU of DENV2 strain PL046. Three days post-infection, plaque assays were performed on tissue from the blood, spleen and lymph nodes. (A) Mice received 250μg of the neutralizing anti-DENV2 monoclonal the antibody 3H5 (□) or 250μg of the anti-DENV1 monoclonal anti antibody 15F3 as an isotype control (■); n=3/group under each condition. (B) Mice received 300μL of DENV2-immune serum (□); or normal mouse serum (■); n=3/group under each condition. (C) Mice received 400μL of DENV1-immune serum (□) (n=4) and controls received either 400 μL of normal mouse serum (n=2) or no serum (n=2) (■). (*; value below the limit of detection; §, p=value of <0.05 by Wilcoxon Rank Sum test).
Adoptive transfer of DENV-immune cells confers partial protection against both homologous and heterologous DENV infection
Previously, the presence of serotype-specific and serotype-cross-reactive DENV-specific T cells in mice after infection with all four DENV serotypes was demonstrated two weeks p.i. and was still detectable one year p.i. (Rothman et al., 1996; Rothman et al., 1989). Even though neutralizing antibodies appear capable of mediating homologous and heterologous protection in vivo, this does not discount a complementary and/or synergistic means of protection by the cellular immune response. Therefore, we tested whether adoptive transfer of DENV-immune spleen cells could mediate a reduction in viral titers after homologous and/or heterologous challenge.
Immune spleen cells were transferred into naive mice via iv tail-vein injection performed 24 hours before sc infection with DENV2 PL046. The ability to successfully transfer total spleen cells after iv injection was confirmed in a subset of mice by detection of CFSE-stained cells in recipient mice 24-36 hours following transfer of 1.5 × 107 cells/mouse (data not shown). Moreover, donor spleen cells from DENV2-immune mice were capable of responding to stimulation with DENV2 cellular antigen by secretion of IFN-γ as detected by ELISA Figure 5A). In contrast, neither naïve spleen cells nor DENV1-immune cells produced IFN-γ after stimulation with DENV2 cellular antigen. All donor spleen cells produced IFN-γ in response to concanavalin A, confirming their capacity to produce IFN-γ upon stimulation (data not shown).
Fig. 5.

Adoptive transfer of DENV-immune cells confers partial homologous and heterologous protection. DENV-immune spleen cells were adoptively transferred into AG129 mice via intravenous injection 24 hours prior to homologous or heterologous challenge. Three days post-infection, plaque assays were performed on tissue from the blood, spleen and lymph nodes. (A) Interferon-γ responses from donor cells used used for adoptive transfer experiments in response to stimulation with control antigen (■) or DENV2 antigen (□). (B) Each mouse received 3 × 107 naïve spleen cells (■), DENV1-immune spleen cells (  ) or DENV2-immune spleen cells (□), followed by challenge with 105 PFU of DENV2 PL046 (n=3 mice/group). (C) Each mouse received 3 × 107 naïve spleen cells (■), DENV1-immune spleen cells (
) or DENV2-immune spleen cells (□), followed by challenge with 105 PFU of DENV2 PL046 (n=3 mice/group). (C) Each mouse received 3 × 107 naïve spleen cells (■), DENV1-immune spleen cells (  ) or DENV2-immune spleen cells (□), followed by challenge with 3 × 104 PFU of DENV2 PL046 (n=6-7 mice/group). (*; value below the limit of detection; §, p=value of <0.05 by Wilcoxon Rank Sum test as compared to control mice receiving naïve cells).
) or DENV2-immune spleen cells (□), followed by challenge with 3 × 104 PFU of DENV2 PL046 (n=6-7 mice/group). (*; value below the limit of detection; §, p=value of <0.05 by Wilcoxon Rank Sum test as compared to control mice receiving naïve cells).
After transfer of 3 × 107 cells/mouse, mice were infected 24 hours later with 105 PFU DENV2, and spleen, lymph nodes and blood were collected on day 3 p.i. for plaque assay analysis. A reduction of viral titers was observed in spleen tissue in mice that received DENV2-immune spleen cells (p=0.0495) (Figure 5B). No reduction was observed in the lymph nodes and serum, nor was protection observed in any tissue after iv transfer of either naïve or DENV1-immune cells under these conditions. We performed additional adoptive transfer studies involving a lower challenge dose (3 x 104 PFU of DENV2) to determine if a lower viral inoculum could reveal a protective role for immune cells in heterologous infection. A statistically significant reduction of viral titers was observed in the spleen after both homologous (p=0.0127) and heterologous (p=0.0027) challenge, but this reduction was not observed in the lymph nodes or serum even following challenge with the lower viral dose (Figure 5C).
DISCUSSION
For up to one year after primary infection, protection against heterologous DENV challenge was observed in AG129 mice using two separate viral sequences (DENV1—DENV2 and DENV2—DENV4), as evidenced by a reduction in viral titers at early timepoints p.i. To test which arm of the immune response (humoral versus cellular) was responsible, either DENV-immune serum or DENV-immune spleen cells were transferred into naïve recipients, followed by challenge with a heterologous DENV serotype. Passive transfer of antibodies was found to significantly decrease levels of viral replication after both homologous and heterologous challenge. Consistent with this, high titers of homologous neutralizing antibodies and lower, but detectable, levels of cross-serotype-reactive neutralizing antibodies were found up to over one year after primary infection. In comparison, adoptive transfer of DENV-immune cells followed by DENV2 challenge conferred significant homologous and heterologous protection only at a lower challenge dose. These results imply that heterologous neutralizing antibodies alone could be responsible for the reduction of viral titers observed in the heterologous sequential infection model, but that DENV-specific immune cells may also provide an important contribution.
Previous evidence from studies in mice had shown that passive transfer of either monoclonal or polyclonal antibodies can be protective against homologous DENV challenge (Henchal et al., 1988; Kaufman et al., 1989; Kaufman et al., 1987). The first study using AG129 mice with DENV found that passive transfer of either homologous neutralizing monoclonal or polyclonal antibodies was poorly protective against neurovirulent death due to DENV2 challenge with a mouse-passaged DENV2 (Johnson and Roehrig, 1999). However, we observed an antibody-mediated reduction of viral titers of a homologous DENV2 clinical isolate in peripheral tissues following passive transfer of both monoclonal and polyclonal anti-DENV2 antibodies. Of particular interest, we also observed heterologous protection provided by anti-DENV1 sera, followed by DENV2 challenge. One recent study reported adoptive transfer of DENV-immune cells followed by heterologous challenge, but as DENV replicates poorly in immunocompetent BALB/c mice, it was not possible to measure reduction or enhancement of viral replication in this study (Beaumier et al., 2008). A study involving adoptive transfer of a CD8+ T cell clone specific for DENV2 in HepG2-grafted scid-mice reported equivocal results, as both protective (prolonged survival) and pathogenic (shortened average survival time) effects were observed (An et al., 2004). Of note, the adoptive transfer studies performed in the current study involving DENV-immune cells did not differentiate between T cells and B cells. Thus, the possibility exists that adoptively transferred DENV-immune B cells could have produced DENV-specific antibodies in response to heterologous DENV challenge, and these antibodies might be able to account for the protection observed in spleen tissue during the adoptive transfer studies.
Although AG129 mice have proven to be a tractable system in which to evaluate the mechanism of protection against DENV replication in vivo, the immunocompromised nature of this strain remains a limitation in translating these results directly to humans. Nonetheless, an advantage of this model is that the early cellular tropism of DENV in AG129 mice does remain restricted to the cell types infected in humans (Kyle et al., 2007), unlike the widespread changes in tropism observed after infection of IFN-receptor-deficient mice with other viruses (Fiette et al., 1995; Garcia-Sastre et al., 1998; Mrkic et al., 1998; Ryman et al., 2000; Samuel and Diamond, 2005; Steinhoff et al., 1995). AG129 mice have been shown to have a normal repertoire of T cells (van den Broek et al., 1995), and immune spleen cells used for adoptive transfer in this study were found to respond to stimulation by DENV2 antigen as measured by IFN-γ secretion, confirming that antigen-specific recognition by immune cells antigen can develop in AG129 mice, even in the absence of IFN receptors.
Studies of the long-term immune response in humans have provided some interesting parallels for the data we report here. In 1950, Sabin (1950) reported that complete cross-protective immunity from heterologous challenge was present in human volunteers for 1-2 months after a primary DENV infection, with partial immunity present up to 9 months resulting in milder disease of shorter duration upon reinfection, and that complete serotype-specific immunity against symptomatic dengue was present up to 18 months post-infection (Sabin, 1950). Long-term, serotype-specific neutralizing antibodies have been reported to be present 40 years after a single dengue epidemic (Innis, 1997), and Cuban investigators have recorded the long-term presence of both DENV-specific T cells and antibodies up to 20 years after natural infection (Guzman et al., 2007; Sierra et al., 2002).
Further studies of the heterologous neutralizing ability of anti-DENV antibodies raised in AG129 mice will include investigation of strain and serotype differences in neutralizing capacity, as well as identification of the DENV epitopes that correspond to this neutralizing ability. In parallel studies, we have also found that ADE can be experimentally induced in the AG129 mouse under different conditions and are currently further characterizing this phenomenon (S. Balsitis, J.L. Kyle, P.R Beatty, E. Harris, unpublished data). The results of this and future studies may thus contribute to a better understanding of the factors that differentiate protection versus enhancement during DENV infection, as well as an improved ability to evaluate candidate dengue vaccines.
MATERIALS AND METHODS
Viruses and cell lines
DENV was propagated in C6/36 Aedes albopictus cells (American Type Culture Collection [ATCC]) as described previously (Shresta et al., 2004a). DENV1 Mochizuki (mouse-passaged; passage number unknown) was obtained from R. Tesh (University of Texas Medical Branch at Galveston, Texas); DENV1 98J (C6/36-passaged; used at passage 7) is a clinical isolate from Guyana (Holden et al., 2006); DENV2 PL046 (C6/36-passaged; passage number unknown) is a Taiwanese clinical isolate (received from H.-Y. Lei, National Cheng Kung University, Taiwan); and DENV4 664 (C6/36-passaged; used at passage 4) is a Thai clinical isolate and a gift of S. Kliks (Pediatric Dengue Vaccine Initiative, Berkeley, CA). Virus titers were obtained by plaque assay on baby hamster kidney (BHK-21 clone 15; BHK) cells (Shresta et al., 2004a). Before injection into mice, virus was diluted in phosphate-buffered saline (PBS). Viral load in mice was determined in tissue samples obtained from blood, lymph nodes, spleen and bone marrow by plaque assay. Tissue samples were processed and quantitated as described previously (Shresta et al., 2004a). Bone marrow cells were collected from both femur and tibia bones directly into PBS, and red blood cells in bone marrow were lysed using red cell lysis buffer (eBioscience).
Infection of AG129 mice
AG129 mice (van den Broek et al., 1995) were originally obtained from M. Aguet (Swiss Institute for Experimental Cancer Research, Epalinges, Switzerland) and were bred in the University of California (UC) Berkeley Northwest Animal Facility. All experimental procedures were pre-approved and were performed according to the guidelines of the UC Berkeley Animal Care and Use Committee. Experiments were initiated with mice 5-8 weeks of age. Subcutaneous (sc) injection was performed under the ventral skin of the hindlimbs, in a total volume of 200μl divided equally between both right and left limbs, and intravenous injection was performed the tail vein in a total volume of 200μl. Sequential DENV1-DENV2 infections were performed using either 102 or 104 PFU of DENV1 Mochizuki or 105 PFU of DENV1 98J as a primary infection, and 105 or 107 PFU of DENV2 PL046 as a secondary infection. Sequential DENV2-DENV4 infections consisted of 105 PFU of DENV2 PL046 followed by 104 PFU of DENV4 664.
Plaque reduction neutralization test (PRNT)
AG129 mice were infected with either 102 plaque forming units (PFU) of DENV1 strain Mochizuki or 105 PFU of DENV2 strain PL046, and monthly bleeds were staggered across two groups of mice, such that serum were collected from 3-4 mice in the total cohort every two weeks. Serum was collected from mice by retro-orbital bleed. PRNT assays were performed in triplicate based on the original protocol described by Russell et al (Russell et al., 1967). Briefly, complement was inactivated by incubating serum in a 56°C water bath for 30 minutes, then 5 serial 3-fold or 4-fold dilutions of serum were prepared, starting at 1:10, in α-MEM (Invitrogen) with 5% fetal bovine serum (FBS; Hyclone), 10mM HEPES (Invitrogen), and 100U penicillin/100μg streptomycin (P/S; Invitrogen). Working stocks of virus were prepared that yielded 20-60 plaques/well in a 12-well tissue culture plate. Viruses used for PRNT tests were DENV1 98J; DENV2 PL046; and DENV4 664. Thirty μL of each serum dilution was combined with 30μL of virus and incubated for 90 minutes at 37°C with 5% CO2. After incubation, 50μL of the virus-serum mixture was transferred to 80% confluent BHK cells and processed as in a standard plaque assay. Percent neutralization for each well was calculated as [1 — (number of plaques in test wells/number of plaques in control wells containing only virus)]*100. Fifty percent neutralization titer (PRNT50) values were calculated for each sample by fitting a variable sigmoidal response curve in GraphPad Prism 5.00 (GraphPad Software), using fixed constraints at the top and bottom of <100% and >0%, respectively. Normal mouse serum collected from naïve AG129 mice (negative control) and the neutralizing anti-flavivirus antibody 4G2 (ATCC; positive control) were included in each assay, as well as a back-titration prepared as a 1:10 dilution of each virus working stock. Serum dilution values are expressed as the reciprocal of the original serum dilution, but not including the 1:2 dilution factor introduced during the neutralization step.
Preparation of antibodies and spleen cells for passive or adoptive transfer
MAbs 15F3 and 3H5 (ATCC) were purified by Protein G affinity chromatography (Pierce). Polyclonal anti-DENV serum used for passive transfer and DEN-immune spleen cells used for adoptive transfer were obtained from AG129 mice 6-11 weeks after infection with 105 PFU of either DENV1 98J or DENV2 PL046, or from age-matched controls. To prepare polyclonal serum, blood was collected via cardiac puncture, allowed to clot, and then serum was removed and pooled. To prepare immune cells for adoptive transfer, spleens were collected from mice and pressed through a 100μM cell filter, red blood cells were lysed, and cells were resuspended in “complete RPMI” (RPMI [Invitrogen] with 10% FBS, 10mM HEPES, 1X P/S) at a density of either 6 × 107 cells/mL or 1.5 × 108 cells/mL (1-3 × 107 cells/mouse).
Preparation of spleen cells for carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling and detection by flow cytometry
Spleens were collected from mice and prepared as above, and cells were resuspended in PBS with 0.5% bovine serum albumin (BSA, Fisher Scientific) and 5% FBS. Cells were adjusted to a density of 108 cells/ml, added to an equal volume of 20μM CFSE (AnaSpec), and incubated for 10 minutes at room temperature. Staining was quenched by addition of 10X volume of ice-cold complete RPMI, followed by two more washes in the same medium. Cells were resuspended at 1.5 × 108 cells/mL in Hank’s Balanced Salt Solution (HBSS; Invitrogen) for intravenous (iv) injection into mice (3 × 107 cells/mouse). After 36 hours, spleens from mice injected with CFSE-labeled cells were collected and resuspended in PBS-0.5% BSA-0.02% sodium azide (Sigma), fixed in 4% paraformaldehyde, and stored at 4°C until analysis on a Coulter EPICS XL (Beckman Coulter). Results were analyzed using FlowJo software, version 5.7.2 (TreeStar).
ELISA detection of IFN-γ in cell culture supernatants
Spleens were collected from mice and prepared as above, and cells were resuspended in complete RPMI. Antigen stimulation was performed with 20μg/mL of DENV2 PL046-infected C6/36 cell lysate (cellular antigen) prepared at day 7 after infection or control cellular lysate from uninfected C6/36 cells, both diluted in sterile PBS. Controls included PBS alone and 5μg/mL of Concanavalin A (Sigma) in PBS. Cells were processed according to manufacturer’s instructions for mouse IFN-γ ELISA (Mabtech). Supernatants for detection of secreted IFN-γ were collected from control wells after 3 days and from antigen-stimulated wells after 7 days. Optical density was read at 405nm on an ELX808 Ultra Microplate Reader (Bio-tek Instruments), and results are shown from an average of triplicate wells.
Statistical Analysis
Statistical analysis of the difference between means was performed with STATA 7.0 (STATA Corporation) using the Wilcoxon Rank Sum test. Non-parametric statistics were used for analysis, since they make no assumptions about the underlying populations analyzed.
ACKNOWLEDGEMENTS
The authors wish to thank Heidi Snider, Kristin Sharar, Katie Minor, Diana Flores and the staff of the Office of Laboratory Animal Care for careful animal husbandry; Kate Williams, Diana Flores, Deya Calahorrano, and Tim Martyak for technical assistance; and past and present members of the Harris laboratory for their continued support. This work was supported by grants from the Pediatric Dengue Vaccine Initiative (grant CRA-14) and the National Institutes of Health (grant AI65359).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- An J, Kimura-Kuroda J, Hirabayashi Y, Yasui K. Development of a novel mouse model for dengue virus infection. Virology. 1999;263(1):70–77. doi: 10.1006/viro.1999.9887. [DOI] [PubMed] [Google Scholar]
- An J, Zhou DS, Zhang JL, Morida H, Wang JL, Yasui K. Dengue-specific CD8+ T cells have both protective and pathogenic roles in dengue virus infection. Immunol. Lett. 2004;95(2):167–174. doi: 10.1016/j.imlet.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Balmaseda A, Hammond SN, Tellez Y, Imhoff L, Rodriguez Y, Saborio SI, Mercado JC, Perez L, Videa E, Almanza E, Kuan G, Reyes M, Saenz L, Amador JJ, Harris E. High seroprevalence of antibodies against dengue virus in a prospective study of schoolchildren in Managua, Nicaragua. Trop. Med. Int. Health. 2006;11(6):935–942. doi: 10.1111/j.1365-3156.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- Beaumier CM, Mathew A, Bashyam HS, Rothman AL. Cross-reactive memory CD8(+) T cells alter the immune response to heterologous secondary dengue virus infections in mice in a sequence-specific manner. J. Infect. Dis. 2008;197(4):608–617. doi: 10.1086/526790. [DOI] [PubMed] [Google Scholar]
- Bente DA, Melkus MW, Garcia JV, Rico-Hesse R. Dengue fever in humanized NOD/SCID mice. J. Virol. 2005;79(21):13797–13799. doi: 10.1128/JVI.79.21.13797-13799.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney JE, Jr., Johnson DH, Manipon GG, Firestone CY, Hanson CT, Murphy BR, Whitehead SS. Genetic basis of attenuation of dengue virus type 4 small plaque mutants with restricted replication in suckling mice and in SCID mice transplanted with human liver cells. Virology. 2002;300(1):125–139. doi: 10.1006/viro.2002.1528. [DOI] [PubMed] [Google Scholar]
- Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Amer. J. Trop. Med. Hyg. 1988;38(1):172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- Calvert AE, Huang CY, Kinney RM, Roehrig JT. Non-structural proteins of dengue 2 virus offer limited protection to interferon-deficient mice after dengue 2 virus challenge. J. Gen. Virol. 2006;87(Pt 2):339–346. doi: 10.1099/vir.0.81256-0. [DOI] [PubMed] [Google Scholar]
- Chen HC, Lai SY, Sung JM, Lee SH, Lin YC, Wang WK, Chen YC, Kao CL, King CC, Wu-Hsieh BA. Lymphocyte activation and hepatic cellular infiltration in immunocompetent mice infected by dengue virus. J. Med. Virol. 2004;73(3):419–431. doi: 10.1002/jmv.20108. [DOI] [PubMed] [Google Scholar]
- Durbin AP, Vargas MJ, Wanionek K, Hammond SN, Gordon A, Rocha C, Balmaseda A, Harris E. Phenotyping of peripheral blood mononuclear cells during acute dengue illness demonstrates infection and increased activation of monocytes in severe cases compared to classic dengue fever. Virology. 2008 doi: 10.1016/j.virol.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endy TP, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, Rothman AL, Vaughn DW, Ennis FA. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am. J. Epidemiol. 2002;156(1):40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- Fiette L, Aubert C, Muller U, Huang S, Aguet M, Brahic M, Bureau JF. Theiler's virus infection of 129Sv mice that lack the interferon alpha/beta or interferon gamma receptors. J. Exp. Med. 1995;181(6):2069–2076. doi: 10.1084/jem.181.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sastre A, Durbin RK, Zheng H, Palese P, Gertner R, Durbin JE. The role of interferon in influenza virus tissue tropism. J. Virol. 1998;72(11):8550–8558. doi: 10.1128/jvi.72.11.8550-8558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalvez AP, Engle RE, Claire M, Purcell RH, Lai CJ. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc. Natl. Acad. Sci. U. S. A. 2007;104(22):9422–9427. doi: 10.1073/pnas.0703498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler DJ. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998;11(3):480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman MG, Alvarez M, Rodriguez-Roche R, Bernardo L, Montes T, Vazquez S, Morier L, Alvarez A, Gould EA, Kouri G, Halstead SB. Neutralizing antibodies after infection with dengue 1 virus. Emerg. Infect. Dis. 2007;13(2):282–286. doi: 10.3201/eid1302.060539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J. Infect. Dis. 1979;140(4):527–533. doi: 10.1093/infdis/140.4.527. [DOI] [PubMed] [Google Scholar]
- Halstead SB. The Alexander D. Langmuir Lecture. The pathogenesis of dengue. Molecular epidemiology in infectious disease. Am. J. Epidemiol. 1981;114(5):632–648. doi: 10.1093/oxfordjournals.aje.a113235. [DOI] [PubMed] [Google Scholar]
- Henchal EA, Henchal LS, Schlesinger JJ. Synergistic interactions of anti-NS1 monoclonal antibodies protect passively immunized mice from lethal challenge with dengue 2 virus. J. Gen. Virol. 1988;69(Pt 8):2101–2107. doi: 10.1099/0022-1317-69-8-2101. [DOI] [PubMed] [Google Scholar]
- Holden KL, Stein DA, Pierson TC, Ahmed AA, Clyde K, Iversen PL, Harris E. Inhibition of dengue virus translation and RNA synthesis by a morpholino oligomer targeted to the top of the terminal 3′ stem-loop structure. Virology. 2006;344(2):439–452. doi: 10.1016/j.virol.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Hombach J. Vaccines against dengue: a review of current candidate vaccines at advanced development stages. Rev. Panam. Salud Publica. 2007;21(4):254–260. doi: 10.1590/s1020-49892007000300011. [DOI] [PubMed] [Google Scholar]
- Huang CY, Butrapet S, Tsuchiya KR, Bhamarapravati N, Gubler DJ, Kinney RM. Dengue 2 PDK-53 virus as a chimeric carrier for tetravalent dengue vaccine development. J. Virol. 2003;77(21):11436–11447. doi: 10.1128/JVI.77.21.11436-11447.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KJ, Li SY, Chen SC, Liu HS, Lin YS, Yeh TM, Liu CC, Lei HY. Manifestation of thrombocytopenia in dengue-2-virus-infected mice. J. Gen. Virol. 2000;81(Pt 9):2177–2182. doi: 10.1099/0022-1317-81-9-2177. [DOI] [PubMed] [Google Scholar]
- Innis BL. Antibody responses to dengue virus infection. In: Gubler DJ, Kuno G, editors. Dengue and Dengue Hemorrhagic Fever. CAB International, Wallingford: 1997. pp. 221–243. [Google Scholar]
- Johnson AJ, Roehrig JT. New mouse model for dengue virus vaccine testing. J. Virol. 1999;73(1):783–786. doi: 10.1128/jvi.73.1.783-786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman BM, Summers PL, Dubois DR, Cohen WH, Gentry MK, Timchak RL, Burke DS, Eckels KH. Monoclonal antibodies for dengue virus prM glycoprotein protect mice against lethal dengue infection. Amer. J. Trop. Med. Hyg. 1989;41(5):576–580. doi: 10.4269/ajtmh.1989.41.576. [DOI] [PubMed] [Google Scholar]
- Kaufman BM, Summers PL, Dubois DR, Eckels KH. Monoclonal antibodies against dengue 2 virus E-glycoprotein protect mice against lethal dengue infection. Amer. J. Trop. Med. Hyg. 1987;36(2):427–434. doi: 10.4269/ajtmh.1987.36.427. [DOI] [PubMed] [Google Scholar]
- Kochel TJ, Watts DM, Gozalo AS, Ewing DF, Porter KR, Russell KL. Cross-serotype neutralization of dengue virus in Aotus nancymae monkeys. J. Infect. Dis. 2005;191(6):1000–1004. doi: 10.1086/427511. [DOI] [PubMed] [Google Scholar]
- Kochel TJ, Watts DM, Halstead SB, Hayes CG, Espinoza A, Felices V, Caceda R, Bautista CT, Montoya Y, Douglas S, Russell KL. Effect of dengue-1 antibodies on American dengue-2 viral infection and dengue haemorrhagic fever. Lancet. 2002;360(9329):310–312. doi: 10.1016/S0140-6736(02)09522-3. [DOI] [PubMed] [Google Scholar]
- Koraka P, Benton S, van Amerongen G, Stittelaar KJ, Osterhaus AD. Characterization of humoral and cellular immune responses in cynomolgus macaques upon primary and subsequent heterologous infections with dengue viruses. Microbes Infect. 2007;9(8):940–946. doi: 10.1016/j.micinf.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Kyle JL, Beatty PR, Harris E. Dengue virus infects macrophages and dendritic cells in a mouse model of infection. J. Infect. Dis. 2007;195(12):1808–1817. doi: 10.1086/518007. [DOI] [PubMed] [Google Scholar]
- Lin YL, Liao CL, Chen LK, Yeh CT, Liu CI, Ma SH, Huang YY, Huang YL, Kao CL, King CC. Study of Dengue virus infection in SCID mice engrafted with human K562 cells. J. Virol. 1998;72(12):9729–9737. doi: 10.1128/jvi.72.12.9729-9737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchette NJ, Halstead SB, Falkler WA, Jr., Stenhouse A, Nash D. Studies on the pathogenesis of dengue infection in monkeys. 3. Sequential distribution of virus in primary and heterologous infections. J. Infect. Dis. 1973;128(1):23–30. doi: 10.1093/infdis/128.1.23. [DOI] [PubMed] [Google Scholar]
- Mrkic B, Pavlovic J, Rulicke T, Volpe P, Buchholz CJ, Hourcade D, Atkinson JP, Aguzzi A, Cattaneo R. Measles virus spread and pathogenesis in genetically modified mice. J. Virol. 1998;72(9):7420–7427. doi: 10.1128/jvi.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves-Souza PC, Azeredo EL, Zagne SM, Valls-de-Souza R, Reis SR, Cerqueira DI, Nogueira RM, Kubelka CF. Inducible nitric oxide synthase (iNOS) expression in monocytes during acute Dengue Fever in patients and during in vitro infection. BMC Infect. Dis. 2005;5:64. doi: 10.1186/1471-2334-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paes MV, Pinhao AT, Barreto DF, Costa SM, Oliveira MP, Nogueira AC, Takiya CM, Farias-Filho JC, Schatzmayr HG, Alves AM, Barth OM. Liver injury and viremia in mice infected with dengue-2 virus. Virology. 2005;338(2):236–246. doi: 10.1016/j.virol.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Price WH, Thind IS. The mechanism of cross-protection afforded by dengue virus against West Nile virus in hamsters. J. Hyg. (Lond) 1972;70(4):611–617. doi: 10.1017/s0022172400022476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman AL, Kurane I, Ennis FA. Multiple specificities in the murine CD4+ and CD8+ T-cell response to dengue virus. J. Virol. 1996;70(10):6540–6546. doi: 10.1128/jvi.70.10.6540-6546.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman AL, Kurane I, Zhang YM, Lai CJ, Ennis FA. Dengue virus-specific murine T-lymphocyte proliferation: serotype specificity and response to recombinant viral proteins. J. Virol. 1989;63(6):2486–2491. doi: 10.1128/jvi.63.6.2486-2491.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell PK, Nisalak A, Sukhavachana P, Vivona S. A plaque reduction test for dengue virus neutralizing antibodies. J. Immunol. 1967;99(2):285–290. [PubMed] [Google Scholar]
- Ryman KD, Klimstra WB, Nguyen KB, Biron CA, Johnston RE. Alpha/beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J. Virol. 2000;74(7):3366–3378. doi: 10.1128/jvi.74.7.3366-3378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin AB. The dengue group of viruses and its family relationships. Bacteriol. Rev. 1950;14(3):225–232. [PubMed] [Google Scholar]
- Samuel MA, Diamond MS. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 2005;79(21):13350–13361. doi: 10.1128/JVI.79.21.13350-13361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar JK, Das BC, Mukherjee KK, Chakrabarti SK. Observation on second heterotypic dengue virus infection in mice. Indian J. Med. Res. 1976;64(12):1713–1719. [PubMed] [Google Scholar]
- Scherer WF, Breakenridge FA, Dickerman RW. Cross-protection studies and search for subclinical disease in new world monkeys infected sequentially with different immunologic types of Dengue viruses. Am. J. Epidemiol. 1972;95(1):67–79. doi: 10.1093/oxfordjournals.aje.a121372. [DOI] [PubMed] [Google Scholar]
- Schul W, Liu W, Xu HY, Flamand M, Vasudevan SG. A dengue fever viremia model in mice shows reduction in viral replication and suppression of the inflammatory response after treatment with antiviral drugs. J. Infect. Dis. 2007;195(5):665–674. doi: 10.1086/511310. [DOI] [PubMed] [Google Scholar]
- Shresta S, Kyle JL, Robert eatty, P., Harris E. Early activation of natural killer and B cells in response to primary dengue virus infection in A/J mice. Virology. 2004a;319(2):262–273. doi: 10.1016/j.virol.2003.09.048. [DOI] [PubMed] [Google Scholar]
- Shresta S, Kyle JL, Snider HM, Basavapatna M, Beatty PR, Harris E. Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J. Virol. 2004b;78(6):2701–2710. doi: 10.1128/JVI.78.6.2701-2710.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shresta S, Sharar KL, Prigozhin DM, Beatty PR, Harris E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J. Virol. 2006;80(20):10208–10217. doi: 10.1128/JVI.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra B, Garcia G, Perez AB, Morier L, Rodriguez R, Alvarez M, Guzman MG. Long-term memory cellular immune response to dengue virus after a natural primary infection. Int. J. Infect. Dis. 2002;6(2):125–128. doi: 10.1016/s1201-9712(02)90073-1. [DOI] [PubMed] [Google Scholar]
- Steinhoff U, Muller U, Schertler A, Hengartner H, Aguet M, Zinkernagel RM. Antiviral protection by vesicular stomatitis virus-specific antibodies in alpha/beta interferon receptor-deficient mice. J. Virol. 1995;69(4):2153–2158. doi: 10.1128/jvi.69.4.2153-2158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek MF, Muller U, Huang S, Aguet M, Zinkernagel RM. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 1995;69(8):4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat. Rev. Microbiol. 2007;5(7):518–528. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- Wu SJ, Hayes CG, Dubois DR, Windheuser MG, Kang YH, Watts DM, Sieckmann DG. Evaluation of the severe combined immunodeficient (SCID) mouse as an animal model for dengue viral infection. Am. J. Trop. Med. Hyg. 1995;52(5):468–476. doi: 10.4269/ajtmh.1995.52.468. [DOI] [PubMed] [Google Scholar]


