Abstract
Prion diseases have a significant inflammatory component. Glia activation, which is associated with increased production of cytokines and chemokines, may play an important role in disease development. Among the chemokines upregulated highly and early upregulated during scrapie infections are ligands of CXCR3. To gain more insight into the role of CXCR3 in a prion model, CXCR3-deficient (CXCR3−/−) mice were infected intracerebrally with scrapie strain 139A and characterized in comparison to similarly infected wild-type controls. CXCR3−/− mice showed significantly prolonged survival times of up to 30 days on average. Surprisingly, however, they displayed accelerated accumulation of misfolded proteinase K-resistant prion protein PrPSc and 20 times higher infectious prion titers than wild-type mice at the asymptomatic stage of the disease, indicating that these PrP isoforms may not be critical determinants of survival times. As demonstrated by immunohistochemistry, Western blotting, and gene expression analysis, CXCR3-deficient animals develop an excessive astrocytosis. However, microglia activation is reduced. Quantitative analysis of gliosis-associated gene expression alterations demonstrated reduced mRNA levels for a number of proinflammatory factors in CXCR3−/− compared to wild-type mice, indicating a weaker inflammatory response in the knockout mice. Taken together, this murine prion model identifies CXCR3 as disease-modifying host factor and indicates that inflammatory glial responses may act in concert with PrPSc in disease development. Moreover, the results indicate that targeting CXCR3 for treatment of prion infections could prolong survival times, but the results also raise the concern that impairment of microglial migration by ablation or inhibition of CXCR3 could result in increased accumulation of misfolded PrPSc.
Transmissible spongiform encephalopathies or prion infections of the central nervous system (CNS) cause a progressive and ultimately lethal degeneration of neuronal tissue, but the underlying pathomechanisms are still elusive (13, 51, 57, 67). Activation of astro- and microglia precedes neuronal death and is a general hallmark of neurodegenerative protein misfolding diseases (44, 59, 68, 69). Glial activation in prion diseases is most likely a consequence of the accumulation of a disease-associated isoform(s) of the prion protein (termed PrPSc or PrPTSE). The reactive gliosis is characterized by increased expression levels of proinflammatory factors, such as components of the complement system, acute-phase proteins, cytokines, and chemokines (5, 15, 58, 59, 73). Many of these factors are thought to contribute to neuronal dysfunction and degeneration, suggesting that anti-inflammatory therapeutic approaches may help to fight deleterious effects of the disease-associated gliosis. However, the actual role of prion-induced glia activation and subsequent chemokine secretion in disease development is still far from clear.
Elevated chemokine expression levels have been observed in numerous pathologies of the brain, ranging from viral and bacterial infections to multiple sclerosis (35, 63, 65), Alzheimer's disease (18, 71), and stroke (66), indicating important roles in acute and chronic neurodegeneration (for further reviews see references 54 and 60). During prion infections of the CNS, chemokines CCL2 (20), CCL3 (41, 58), CCL5 (37, 43), and CCL6, CCL9, and CCL12 (73) have been found to be upregulated. Moreover, induction of the chemokines CXCL9 (61) and CXCL10 and CXCL13 (59) is seen at the early, asymptomatic stages of scrapie infection and is sustained at high levels until the end, possibly indicating an involvement in disease progression. In the periphery these chemokines are potent chemoattractants for T and B cells, respectively (38, 64). However, a significant increase in trafficking of these cell types into prion-infected brain tissue has never been reported. Furthermore, upon intracerebral prion inoculation, mice deficient for T and B cells develop disease identical to wild-type controls, suggesting that these lymphocytes, and adaptive immune responses in general, play no major role in prion pathogenesis in the CNS (36, 39).
The chemokine receptor CXCR3 is widely expressed in brain tissue and has been found on astrocytes (3, 24), microglia (3), neurons (72), and oligodendrocytes (49). Established CXCR3 ligands are CXCL9, CXCL10, and CXCL11 (30). Further chemokines, namely, CCL21 and CXCL13, which are regular ligands of receptors CCR7 and CXCR5, respectively, were suggested to recognize CXCR3 as well (33, 56). In vitro and in vivo disease models have shown that astrocytes, microglia, and neurons may produce the various CXCR3 ligands. The wide range of cells expressing CXCR3 and/or ligands thereof points toward a complex function of this system in glial-glial and glial-neuronal interactions within the CNS. So far, CXCR3 has been shown in vitro and in vivo to govern migration but not proliferation of microglia (4, 24, 55, 56).
We characterized the prion infection of CXCR3−/− mice in comparison to wild-type controls to determine the consequences of the impairment of microglial migration on disease development.
MATERIALS AND METHODS
Animals and scrapie infections.
Generation of CXCR3−/− mice has been described previously (28). CXCR3−/− mice and C57BL/6 wild-type controls, which were outbred from CXCR+/− crosses, were kept in the local animal facilities. Five- to 6-week-old mice were intracerebrally (i.c.) inoculated with 20 μl of a 10−3- or 10−4-diluted 10% brain homogenate prepared from a terminally diseased C57BL/6 wild-type mouse infected with the scrapie strain 139A (courtesy of R. H. Kimberlin, Edinburgh, United Kingdom) as previously described (61). Mock infections were performed using similarly diluted brain homogenates obtained from uninfected, healthy wild-type mice. Infected animals were monitored thrice weekly for clinical signs until the symptomatic stage was reached (9) and daily once disease onset was diagnosed. Mice were sacrificed at 125 days postinfection (dpi) or at the terminal stage of disease, at which animals would naturally succumb to the disease within the next 48 h. Analysis and comparisons at the terminal stage were performed for mice, which were sacrificed on the same day or less than 5 days apart. The animal experiments and care protocols were approved by the institutional review committee Landesamt für Gesundheit und Soziales (Berlin, Germany). Survival times in all groups were statistically analyzed using the unpaired t test and the log rank test.
To determine prion titers an end point titration was carried out in which serial dilutions of a brain homogenate prepared from a terminally ill wild-type mouse were inoculated i.c. into tga20 mice (n = 4 per dilution) (22). The 50% lethal doses (LD50s) were calculated according to the Spearman-Kaerber method and plotted against survival times. The resulting curve could be described by the equation y = −0.361x3 + 5.057x2 − 28.67x + 123.83, where y is the survival time (in days postinfection) and x the log LD50 (R2 = 0.9979). Next, tga20 mice (n = 4 per sample) were i.c. infected with 20 μl of 10−2-diluted 10% brain homogenates prepared from CXCR3−/− or wild-type control mice sacrificed at 125 dpi or at the terminal stage of the disease. Prion titers (LD50/ml of 10% brain homogenate) in these samples were then calculated using the survival time/LD50 relationship described above (52).
Histology and immunohistochemistry.
Immunohistochemistry was performed according to previously published procedures (31, 61). Serial sagittal paraffin sections (6 μm) for a minimum of three samples/group and time point were examined histologically by hematoxylin and eosin staining for spongiform changes (data not shown). For detection of activated astrocytes, sections were stained for glial fibrillary acidic protein (GFAP; 1:1,000; Dako, Glostrup, Denmark). Serial sagittal cryo sections (8 μm) were examined for microglia activation with an anti-Mac-1 (CD11b) monoclonal antibody (1:200; kindly provided by B. Engelhardt, Munster, Germany). The total microglia population was stained with an antibody against ionized calcium binding adapter molecule 1 (Iba-1; 1:100; Dako, Glostrup, Denmark). Stainings were visualized using the ABC method and diaminobenzidine or 3-amino-9-ethylcarbazol as chromogens. Differences between CXCR3−/− and wild-type mice were evaluated by independent scoring of tissue samples by three investigators without previous knowledge of the group to which the mice belonged.
Paraffin-embeded tissue (PET) blot analysis.
Paraffin sections of formalin-fixed brain tissues were transferred onto a nitrocellulose membrane and stained as previously described (62). The immunodetection of PrPSc was performed with the anti-PrP antibody 6H4 (1:10,000; Prionics, Zürich, Switzerland) followed by incubation with an alkaline phosphatase-linked anti-mouse immunoglobulin antiserum (1:2,000; Dako, Glostrup, Denmark). The final staining was performed with nitro blue tetrazolium-5-bromo-4-chloro-3-indolyl phosphate.
Western blot analysis.
For Western blot analysis of proteinase K (PK)-resistant PrPSc, 10% brain homogenates were made in homogenization puffer (phosphate-buffered saline, 0.5% Triton X-100, and 0.05% sodium dodecyl sulfate) and subjected to PK digestion at a final concentration of 100 μg/ml for 1 h at 37°C (1). After sodium dodecyl sulfate-polyacrylamide gel electrophoresis and protein transfer, PrPSc bands were detected using the 6H4 antibody (1:5,000; Prionics, Schlieren-Zurich, Switzerland). For Western blot analysis of differences in GFAP expression, anti-GFAP antibody (1:5,000; Dako, Glostrup, Denmark) was used. To control for amounts of loaded protein, membranes were stripped by incubation in 0.2 M glycine-HCl (pH 2.5), 0.05% Tween 20 for 30 min at room temperature and reprobed with anti-β-actin antibody (1:5,000; Sigma, Hamburg, Germany). Blots were quantified and analyzed using Quantity One software following the manufacturer's instructions (Bio-Rad, Munich, Germany). Immunoprecipitations with the PrPSc-specific antibody 15B3 (kindly provided by A. Raeber, Prionics) prior to Western blot analysis were performed as previously described (47).
Quantitative real-time PCR.
Brains from scrapie-infected CXCR3−/− and wild-type mice as well as from mock-infected and uninfected CXCR3−/− and wild-type mice were removed 125 dpi and at the terminal stage of disease, frozen in liquid nitrogen, and stored at −80°C. Total RNA from whole brains was prepared with Trizol reagent (Invitrogen, Karlsruhe, Germany) according to the manufacturer's protocol. After digestion with DNase I for 45 min at 37°C, total RNA was purified with the RNeasy protect mini kit (Qiagen, Hilden, Germany) and 1.25 μg was reverse transcribed using the First-Strand cDNA synthesis kit (Amersham, Freiburg, Germany). Gene expression levels from each group of mice were subsequently determined by real-time PCR by employing a GeneAmp 5700 sequence detection system (Perkin-Elmer, Boston, MA) and the Sybr green PCR kit (Qiagen, Hilden Germany), following data analysis using the ΔΔCt method. To compensate for variations in amounts of input RNA and efficiencies of reverse transcription, an endogenous housekeeping gene (glyceraldehyde-3-phosphate dehydrogenase) was quantified and results were normalized to these values. Quantification of signal was achieved by setting thresholds within the logarithmic phase of PCR and determined by the cycle number at which this threshold was reached (Ct). The Ct obtained for glyceraldehyde-3-phosphate dehydrogenase was subtracted from the Ct of the target gene to yield ΔCt. The increase of target gene expression level was calculated according to the formula 2(ΔCt1 − ΔCt2), where ΔCt1 is the normalized test sample value and ΔCt2 the normalized mock-infected control sample value.
Primers.
The following primers were used: for GFAP (primer A, GCG GGA GTC GGC CAG TTA CC; primer B, GAC CTC ACC ATC CC GCA TCT); 2′,5′-oligoadenylate synthetase (OAS) (primer A, GGT CTC TGA GCT TCA AGC TGA G; primer B, TAC TGT GGA GGC AAT GGC TTC AA); Spi-2 (primer A, ATC TGC CCT GCT GTC CTC TG; primer B, GCG CTG GCA TTT CCT GTG TA); CXCL10 (primer A, GCA ACT GCA TCC ATA TCG ATG AC; primer B, TGT GCG TGG CTT CAC TCC A); CCL2 (primer A, CCC CAC TCA CCT GCT GCT AC; primer B, ACG GGT CAA CTT CAC ATT CA); CCL3 (primer A, ACT GCC CTT GCT GTT CTT CT; primer B, CTG CCG GTT TCT CTT AGT CA); CCL5 (primer A, TGC CCA CGT CAA GGA GTA TT; primer B, CAG GAC CGG AGT GGG AGT A).
RESULTS
Accelerated accumulation of PrPSc and infectivity but prolonged survival times in the absence of CXCR3.
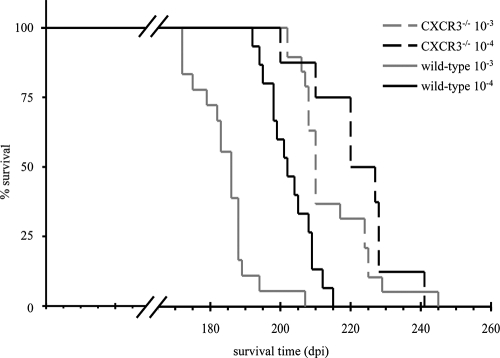
To assess the role of CXCR3 in a prion disease of the CNS, intracerebral scrapie infection of mice deficient for CXCR3 was compared to that in similarly infected wild-type mice. CXCR3−/− mice developed typical scrapie symptoms, including a characteristic reduction in mobility with progressive ataxia, progressive proprioceptive deficits, poor coat condition, and weight loss (61). The duration of this clinically overt symptomatic stage was similar to that in wild-type controls, but the onset of this stage was delayed (data not shown). CXCR3−/− mice survived prion infections on average 20 to 30 days longer than control mice (P < 0.001) (Fig. 1).
FIG. 1.
Significantly prolonged survival times of scrapie-infected CXCR3−/− mice compared to wild-type controls. CXCR3−/− and wild-type-mice survived i.c. prion inoculations of a 10−3-diluted brain homogenate for an average of 215.7 (n = 19) and 185.3 (n = 18) days, respectively (P < 0.0001) and inoculations of a 10−4 dilution for 222.8 (n = 8) and 203.7 (n = 15) days (P < 0.01).
The characteristic accumulation of PK-resistant PrPSc in scrapie infections of infected CXCR3−/− and control mice was analyzed by Western and PET blotting. For both methods, CXCR3−/− mice showed strongly increased PrPSc accumulation at 125 dpi, which appeared, however, to be equivalent at the terminal stage (Fig. 2 and 3). To assess PrPSc deposition and distribution, we examined scrapie-infected brains by PET blot analysis. Deposition started in the medulla oblongata and thalamus, followed by the hippocampus and cortex. Finally, typically diffuse PrPSc accumulations appeared in all brain areas, but staining was most intense in the cortex, hippocampus, and thalamus (Fig. 3). Overall, principal distribution and spread of PrPSc deposition was similar for both groups. To address the question of whether the more-rapid accumulation of PrPSc in receptor-deficient mice correlates with accumulation of prion infectivity, dilutions of 10% brain homogenates obtained from scrapie-infected CXCR3−/− and control mice were inoculated into tga20 indicator mice. tga20 mice receiving brain homogenates from control animals obtained at 125 dpi showed a mean survival time of 117 days. In contrast, tga20 mice exposed to brain homogenates from CXCR3−/− mice from the same stage (125 dpi) survived on average only 89 days (Table 1). This difference of 28 days corresponds to an at least 20 times higher titer of prion infectivity in the CXCR3−/− mice at 125 dpi and is in agreement with the increased PrPSc accumulation at this time point (Fig. 2 and 3; Table 1). tga20 mice infected with brain homogenate from terminally ill knockout and control mice showed no significant difference in survival times (Table 1). tga20 mice inoculated with brain material from healthy mock-infected mice never developed disease.
FIG. 2.
Western blot analysis of PrPSc accumulation in control and CXCR3−/− mice following i.c. inoculation. (A, upper half) Aliquots of 10% (wt/vol) brain homogenates obtained at 125 dpi or from terminally ill animals (two mice per time point and group) were treated with PK followed by Western blotting with the anti-PrP antibody 6H4 and enhanced chemiluminescence. (A, lower half) The same homogenates probed with antibody 6H4 before PK digestion for detection of total PrP. (B) Densitometric quantitation of PrPSc protein levels shown in panel A. (C) Detection of total PrPSc using the 15B3 antibody for immunoprecipitation.
FIG. 3.
PET blot analysis of PrPSc accumulation. Paraffin sections from wild-type control animals and CXCR3−/− mice obtained at 125 dpi, at the terminal stage of disease, and from a mock-infected control.
TABLE 1.
Determination of prion titers in wild-type and CXCR3−/− brain tissue in recipient tga20 mice
| Donor genotype | Time after inoculation of donor (dpi) | Survival of recipient tga20 mice (dpi) | Titer (LD50/ml of 10% brain homogenate) |
|---|---|---|---|
| Wild type | 125 | 117 | 9.0 ± 103 |
| Wild type | Terminal | 85 | 4.2 ± 105 |
| CXCR3−/− | 125 | 89 | 2.1 ± 105 |
| CXCR3−/− | Terminal | 83 | 5.8 ± 105 |
Massive astrocytosis but reduced microglia activation in CXCR3−/− mice.
To evaluate differences in the astrocytosis between CXCR3-deficient and wild-type mice, GFAP expression was determined by immunohistochemistry and Western blot analysis. CXCR3−/− mice showed two- to fourfold-increased GFAP expression in immunohistochemistry at the terminal stage of disease (Fig. 4A to F and I). At the asymptomatic stage (125 dpi), GFAP expression was found to be similar for CXCR3−/− and wild-type animals in the cerebellum, hippocampus, and cortex. However, in close correlation with brain areas of early PrPSc deposition, activation of astrocytes in CXCR3−/− mice was more pronounced in the medulla oblongata and thalamus (data not shown). Elevated GFAP expression in CXCR3−/− mice was confirmed by Western blot analysis, which showed, in agreement with the immunohistochemistry results, clear differences at the early time point and a sixfold augmentation at the terminal stage of the disease compared to the wild-type controls (Fig. 4G and H).
FIG. 4.
Detection of astrocyte marker GFAP. (A to F) Immunohistochemical staining of GFAP-positive cells. (A, C, and D) Terminal stage of disease in wild-type mice; (B, E, and F) terminal stage in CXCR3−/− mice. Representative sections are shown for the cerebellum (A and B), cortex (C and E), and hippocampus (D and F). Magnification, ×400. (G) Semiquantitative analysis of GFAP staining intensities in various brain regions. CB, cerebellum; MO, medulla oblongata; MB/T, midbrain/thalamus; HC, hippocampus; Str, striatum; C, cortex. +, low expression level; ++, intermediate expression; +++, high expression. (H) Western blot analysis of GFAP expression in control and CXCR3−/− mice. Aliquots of 10% (wt/vol) brain homogenates obtained at 125 dpi or from terminally ill animals were analyzed using anti-GFAP antibody and enhanced chemiluminescence (two mice per time point and group). To control for equal amounts of loaded protein, anti-β-actin antibody was used to analyze the same homogenates. (I) Densitometric quantitation of the GFAP Western blot assay.
At the asymptomatic stage Mac-1-positive activated microglia were found in the midbrain, hippocampus, and striatum of infected control mice. CXCR3-deficient mice showed comparable Mac-1 staining patterns in the midbrain and striatum, whereas in the hippocampus numbers of Mac-1-expressing cells were clearly reduced in comparison to wild-type mice (data not shown). At the terminal stage controls showed up to threefold-increased Mac-1-positive cells in the whole brain, particularly in the hippocampus, striatum, and cortex, with strongest staining in the cerebellum and thalamus (Fig. 5).
FIG. 5.
Immunohistochemical staining for the microglia marker Mac-1. Sections are from animals in the terminal stage of disease in controls (A, C, and D) and CXCR3−/− mice (B, E, and F). Representative sections are shown for the cerebellum (A and B), cortex (C and E), and hippocampus (D and F). Magnification, ×400. (G) Semiquantitative analysis of Mac-1 staining intensities in various brain regions (symbols are as described for Fig. 4).
Another characteristic alteration in scrapie infection is the spongiform vacuolization of brain tissue. To confirm microvacuolization, brain sections of infected animals were subjected to hematoxylin and eosin staining. Interestingly, although there were obvious differences in survival time, PrPSc accumulation, astrocytosis, and microgliosis, no differences in the amount or distribution of vacuolization were found between CXCR3−/− and wild-type mice at any time point (data not shown).
For further analysis of disease-associated gliosis and its consequences, quantitative real-time PCR was performed to determine (inflammatory) marker gene mRNA levels in brains from prion-infected CXCR3−/− and wild-type mice as well as in mock-infected controls (Fig. 6).
FIG. 6.
Gene expression analysis by quantitative real-time PCR. Results shown are mRNA levels at 125 dpi and the terminal stage of the disease in C57BL/6 (gray bars) and CXCR3−/− (black bars) mice relative to the expression levels in uninfected control mice. Real-time PCR results represent the analysis of three mouse brains per group.
As expected, the strongly enhanced astrogliosis in CXCR3−/− mice was also reflected in 30- to 140-fold-elevated GFAP transcript levels. However, expression levels of all other markers analyzed were found to be reduced in brain tissue of CXCR3−/− mice at the asymptomatic stage (CXCL10, CCL5, and OAS) or even at both time points studied (CCL2, CCL3, and serine protease inhibitor 2 [Spi-2]), indicating a weaker inflammatory response in these animals.
Expression levels of these marker genes as well as prion protein and GFAP expression levels were similar for uninfected wild-type and CXCR3−/− mice (data not shown), demonstrating that ablation of CXCR3 per se does not cause a gliosis or a general imbalance of brain homeostasis, which is in agreement with a previous study with CXCR3−/− mice (55).
DISCUSSION
Overexpression of chemokines is one of the hallmarks of reactive gliosis in neurodegenerative diseases, including prion diseases. However, our knowledge concerning the contributions of such inflammatory reactions to prion diseases of the CNS is still very limited. We therefore characterized the scrapie infection of mice deficient for CXCR3 to learn more about a possible role of this chemokine receptor in chronic neurodegeneration.
Directed migration of microglia along concentration gradients of chemoattractants secreted by astrocytes or neurons is likely to be a prerequisite for establishing cell-cell interactions to promote further microglial activation and differentiation in vivo. Impaired migration toward stressed or injured neurons reduced the activation of CXCR3−/− microglia in an entorhinal cortex lesion model of acute neurodegeneration (55). However, although CXCR3 is required for microglial migration within brain tissue, it is eventually not critical for recruitment of peripheral macrophages into the CNS. It has been suggested that in the terminal stage of prion infections more than 50% of the Iba-1-positive microglial population is derived from peripheral macrophages which have migrated into the brain (50). When staining microglia for marker protein Iba-1 expression we found, however, no differences concerning Iba-1 expression of microglia between CXCR3−/− and control mice (data not shown). CXCR3 seems therefore not to be involved in directing monocyte migration across the blood-brain barrier, which is in agreement with a recent study of CXCR3−/− mice in an experimental autoimmune encephalomyelitis model (40).
Microglia activation was clearly reduced in prion-infected CXCR3−/− mice in comparison to wild-type controls (Fig. 5). The attenuated inflammatory response to the prion infection in CXCR3−/− mice was further reflected by lower mRNA expression levels of proinflammatory factors, which have already been shown to play a detrimental role in chronic neurodegeneration (Fig. 6). Ablation of CCL2 delayed disease onset and prolonged survival times in scrapie-infected mice (20). Furthermore, CCL3-deficient mice showed a substantial decrease of microglia-associated pathology, reduced neuronal apoptosis, and longer life spans in a murine Sandhoff disease model (70). Astrocytic overexpression of the human homolog of Spi-2, α1-antichymotrypsin, has been described to enhance Alzheimer-like pathology in amyloid protein precursor transgenic mice, and α1-antichymotrypsin polymorphisms were suggested to modulate age of disease onset and disease duration for Alzheimer's patients (26, 46). OAS activates RNase L, which is involved in apoptotic cell death via its nonspecific rRNA-degrading activity and may therefore participate in neuronal loss in scrapie (12, 59, 75). Early-stage overexpression of OAS was previously reported to correlate with shorter survival times in a murine scrapie model (9).
Impaired microglial migration in CXCR3−/− mice may well contribute to decreased phagocytosis and consequently less degradation of PrPSc deposits, because only migratory microglia can be expected to efficiently reach deposits of misfolded protein for subsequent engulfment. A role of phagocytes in uptake and degradation of PrPSc has been demonstrated in cell cultures (10, 11, 45) and in prion-infected animals (2, 42). Moreover, depletion of microglia from prion-infected organotypic brain slices was shown to promote dramatic increases in PK-resistant PrPSc and prion infectivity (19). We show here that amounts of PrPSc in the preclinical stage in brains of CXCR3−/− mice were significantly increased compared to the wild-type controls and infectious prion titers were at least 20-fold higher (Fig. 2 and 3 and Table 1). In combination, these data suggest that microglia negatively affect prion replication and PrPSc deposition in the CNS and that microglial migration driven by CXCR3 is critical for this activity.
Because folding intermediates of PrPSc may not necessarily be PK resistant, we used in addition the PrPSc-specific antibody 15B3 to detect PK-sensitive and -resistant forms of PrPSc simultaneously. However, as seen for PK-resistant PrPSc and infectious prions, this assay demonstrated similarly higher levels of total PrPSc in CXCR3−/− mice (Fig. 2). Surprisingly, neither preclinical PrPSc accumulation nor infectious prion titers were reflected by the survival times of CXCR3−/− mice, which survived on average up to 30 days longer than the wild-type controls. This observation is not necessarily in conflict with the described direct neurotoxicity of PK-resistant PrPSc in vitro (29) but calls into question whether this activity is a key determinant of survival times in vivo. Similarly, the vastly higher prion titers in brains of CXCR3−/− mice do not argue for a pronounced direct toxicity of infectious PrPSc conformers. Instead, these results may favor a scenario in which inflammatory glial responses act in concert with PrPSc to cause neurodegeneration (6-8, 17).
The other major alteration in brain pathology of prion-infected CXCR3−/− mice was the excessive increase in GFAP-positive astrocytes (Fig. 4), which may be directly linked to the more pronounced prion accumulation in these animals. Numerous studies have demonstrated that astrocytes are host cells for prion replication in vitro and in vivo (14, 16, 32, 34, 48, 53, 74). Moreover, in vitro astrocyte activation and proliferation were previously shown to result directly from exposure to PrPSc (21, 23, 25). PrPSc additionally renders astrocytes more responsive to mitogenic factors released by microglia (27, 61). Such a scenario, in which PrPSc may promote astrocyte proliferation, which in turn supports increased prion replication, was previously termed a “snowball effect” in a hamster prion model (72). It is important to note in this context that CXCR3−/− mice and wild-type mice showed a similar astrogliosis in a stab wound model of acute neurodegeneration (55). The overshooting proliferation of astrocytes in CXCR3−/− mice seen in our study is therefore a specific response to the prion infection and not an intrinsic property of these mice.
Taken together, we show here the development of a qualitatively and quantitatively different gliosis in the absence of CXCR3, which is characterized by (i) an attenuated microglial activation, (ii) excessive proliferation of GFAP-positive astrocytes, and (iii) reduced expression of inflammatory and potentially harmful factors. The experimental model identifies CXCR3 and its ligands as disease-modifying host factors affecting the complex and interlinked process of inflammatory glia activation and prion replication. Furthermore, our data suggest that targeting CXCR3 for palliative treatment of prion infections could prolong survival times but raise the concern that impairment of microglial migration by ablation or inhibition of CXCR3 may result in increased accumulation of misfolded PrPSc.
Acknowledgments
We thank K. Krohn, S. Lichy, and E. Westhäuser for excellent technical assistance, N. Holtkamp for helpful discussions, and A. Raeber (Prionics) for his support.
This work was supported in part by grant 01KO0515 from the Federal Ministry for Education and Research, Germany, and by funding under the Sixth Research Framework Programme of the European Union, Project AntePrion (LSHB-CT-2006-019090).
Footnotes
Published ahead of print on 8 October 2008.
REFERENCES
- 1.Baier, M., S. Norley, J. Schultz, M. Burwinkel, A. Schwarz, and C. Riemer. 2003. Prion diseases: infectious and lethal doses following oral challenge. J. Gen. Virol. 841927-1929. [DOI] [PubMed] [Google Scholar]
- 2.Beringue, V., M. Demoy, C. I. Lasmezas, B. Gouritin, C. Weingarten, J. P. Deslys, J. P. Andreux, P. Couvreur, and D. Dormont. 2000. Role of spleen macrophages in the clearance of scrapie agent early in pathogenesis. J. Pathol. 190495-502. [DOI] [PubMed] [Google Scholar]
- 3.Biber, K., I. Dijkstra, C. Trebst, C. J. De Groot, R. M. Ransohoff, and H. W. Boddeke. 2002. Functional expression of CXCR3 in cultured mouse and human astrocytes and microglia. Neuroscience 112487-497. [DOI] [PubMed] [Google Scholar]
- 4.Biber, K., A. Sauter, N. Brouwer, S. C. Copray, and H. W. Boddeke. 2001. Ischemia-induced neuronal expression of the microglia attracting chemokine secondary lymphoid-tissue chemokine (SLC). Glia 34121-133. [DOI] [PubMed] [Google Scholar]
- 5.Brown, A. R., S. Rebus, C. S. McKimmie, K. Robertson, A. Williams, and J. K. Fazakerley. 2005. Gene expression profiling of the preclinical scrapie-infected hippocampus. Biochem. Biophys. Res. Commun. 33486-95. [DOI] [PubMed] [Google Scholar]
- 6.Brown, D. R. 1999. Prion protein peptide neurotoxicity can be mediated by astrocytes. J. Neurochem. 731105-1113. [DOI] [PubMed] [Google Scholar]
- 7.Brown, D. R., B. Schmidt, and H. A. Kretzschmar. 1996. Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature 380345-347. [DOI] [PubMed] [Google Scholar]
- 8.Burwinkel, M., C. Riemer, A. Schwarz, J. Schultz, S. Neidhold, T. Bamme, and M. Baier. 2004. Role of cytokines and chemokines in prion infections of the central nervous system. Int. J. Dev. Neurosci. 22497-505. [DOI] [PubMed] [Google Scholar]
- 9.Burwinkel, M., A. Schwarz, C. Riemer, J. Schultz, F. van Landeghem, and M. Baier. 2004. Rapid disease development in scrapie-infected mice deficient for CD40 ligand. EMBO Rep. 5527-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carp, R. I., and S. M. Callahan. 1982. Effect of mouse peritoneal macrophages on scrapie infectivity during extended in vitro incubation. Intervirology 17201-207. [DOI] [PubMed] [Google Scholar]
- 11.Carp, R. I., and S. M. Callahan. 1981. In vitro interaction of scrapie agent and mouse peritoneal macrophages. Intervirology 168-13. [DOI] [PubMed] [Google Scholar]
- 12.Castelli, J. C., B. A. Hassel, K. A. Wood, X. L. Li, K. Amemiya, M. C. Dalakas, P. F. Torrence, and R. J. Youle. 1997. A study of the interferon antiviral mechanism: apoptosis activation by the 2-5A system. J. Exp. Med. 186967-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chesebro, B. 1999. Prion protein and the transmissible spongiform encephalopathy diseases. Neuron 24503-506. [DOI] [PubMed] [Google Scholar]
- 14.Cronier, S., H. Laude, and J. M. Peyrin. 2004. Prions can infect primary cultured neurons and astrocytes and promote neuronal cell death. Proc. Natl. Acad. Sci. USA 10112271-12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dandoy-Dron, F., F. Guillo, L. Benboudjema, J. P. Deslys, C. Lasmezas, D. Dormont, M. G. Tovey, and M. Dron. 1998. Gene expression in scrapie. Cloning of a new scrapie-responsive gene and the identification of increased levels of seven other mRNA transcripts. J. Biol. Chem. 2737691-7697. [DOI] [PubMed] [Google Scholar]
- 16.Diedrich, J. F., P. E. Bendheim, Y. S. Kim, R. I. Carp, and A. T. Haase. 1991. Scrapie-associated prion protein accumulates in astrocytes during scrapie infection. Proc. Natl. Acad. Sci. USA 88375-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eikelenboom, P., C. Bate, W. A. Van Gool, J. J. Hoozemans, J. M. Rozemuller, R. Veerhuis, and A. Williams. 2002. Neuroinflammation in Alzheimer's disease and prion disease. Glia 40232-239. [DOI] [PubMed] [Google Scholar]
- 18.Eikelenboom, P., R. Veerhuis, W. Scheper, A. J. Rozemuller, W. A. van Gool, and J. J. Hoozemans. 2006. The significance of neuroinflammation in understanding Alzheimer's disease. J. Neural Transm. 1131685-1695. [DOI] [PubMed] [Google Scholar]
- 19.Falsig, J., C. Julius, I. Margalith, P. Schwarz, F. L. Heppner, and A. Aguzzi. 2008. A versatile prion replication assay in organotypic brain slices. Nat. Neurosci. 11109-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felton, L. M., C. Cunningham, E. L. Rankine, S. Waters, D. Boche, and V. H. Perry. 2005. MCP-1 and murine prion disease: separation of early behavioural dysfunction from overt clinical disease. Neurobiol. Dis. 20283-295. [DOI] [PubMed] [Google Scholar]
- 21.Fioriti, L., N. Angeretti, L. Colombo, A. De Luigi, A. Colombo, C. Manzoni, M. Morbin, F. Tagliavini, M. Salmona, R. Chiesa, and G. Forloni. 2007. Neurotoxic and gliotrophic activity of a synthetic peptide homologous to Gerstmann-Straussler-Scheinker disease amyloid protein. J. Neurosci. 271576-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer, M., T. Rulicke, A. Raeber, A. Sailer, M. Moser, B. Oesch, S. Brandner, A. Aguzzi, and C. Weissmann. 1996. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 151255-1264. [PMC free article] [PubMed] [Google Scholar]
- 23.Florio, T., M. Grimaldi, A. Scorziello, M. Salmona, O. Bugiani, F. Tagliavini, G. Forloni, and G. Schettini. 1996. Intracellular calcium rise through L-type calcium channels, as molecular mechanism for prion protein fragment 106-126-induced astroglial proliferation. Biochem. Biophys. Res. Commun. 228397-405. [DOI] [PubMed] [Google Scholar]
- 24.Flynn, G., S. Maru, J. Loughlin, I. A. Romero, and D. Male. 2003. Regulation of chemokine receptor expression in human microglia and astrocytes. J. Neuroimmunol. 13684-93. [DOI] [PubMed] [Google Scholar]
- 25.Forloni, G., R. Del Bo, N. Angeretti, R. Chiesa, S. Smiroldo, R. Doni, E. Ghibaudi, M. Salmona, M. Porro, L. Verga, et al. 1994. A neurotoxic prion protein fragment induces rat astroglial proliferation and hypertrophy. Eur. J. Neurosci. 61415-1422. [DOI] [PubMed] [Google Scholar]
- 26.Gopalan, S. M., K. M. Wilczynska, B. S. Konik, L. Bryan, and T. Kordula. 2006. Astrocyte-specific expression of the α1-antichymotrypsin and glial fibrillary acidic protein genes requires activator protein-1. J. Biol. Chem. 2811956-1963. [DOI] [PubMed] [Google Scholar]
- 27.Hafiz, F. B., and D. R. Brown. 2000. A model for the mechanism of astrogliosis in prion disease. Mol. Cell. Neurosci. 16221-232. [DOI] [PubMed] [Google Scholar]
- 28.Hancock, W. W., B. Lu, W. Gao, V. Csizmadia, K. Faia, J. A. King, S. T. Smiley, M. Ling, N. P. Gerard, and C. Gerard. 2000. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J. Exp. Med. 1921515-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hetz, C., M. Russelakis-Carneiro, K. Maundrell, J. Castilla, and C. Soto. 2003. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 225435-5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horuk, R. 2001. Chemokine receptors. Cytokine Growth Factor Rev. 12313-335. [DOI] [PubMed] [Google Scholar]
- 31.Jeffrey, M., C. M. Goodsir, A. Holliman, R. J. Higgins, M. E. Bruce, P. A. McBride, and J. R. Fraser. 1998. Determination of the frequency and distribution of vascular and parenchymal amyloid with polyclonal and N-terminal-specific PrP antibodies in scrapie-affected sheep and mice. Vet. Rec. 142534-537. [DOI] [PubMed] [Google Scholar]
- 32.Jeffrey, M., C. M. Goodsir, R. E. Race, and B. Chesebro. 2004. Scrapie-specific neuronal lesions are independent of neuronal PrP expression. Ann. Neurol. 55781-792. [DOI] [PubMed] [Google Scholar]
- 33.Jenh, C. H., M. A. Cox, W. Hipkin, T. Lu, C. Pugliese-Sivo, W. Gonsiorek, C. C. Chou, S. K. Narula, and P. J. Zavodny. 2001. Human B cell-attracting chemokine 1 (BCA-1; CXCL13) is an agonist for the human CXCR3 receptor. Cytokine 15113-121. [DOI] [PubMed] [Google Scholar]
- 34.Kercher, L., C. Favara, C. C. Chan, R. Race, and B. Chesebro. 2004. Differences in scrapie-induced pathology of the retina and brain in transgenic mice that express hamster prion protein in neurons, astrocytes, or multiple cell types. Am. J. Pathol. 1652055-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kielian, T. 2004. Microglia and chemokines in infectious diseases of the nervous system: views and reviews. Front. Biosci. 9732-750. [DOI] [PubMed] [Google Scholar]
- 36.Klein, M. A., R. Frigg, E. Flechsig, A. J. Raeber, U. Kalinke, H. Bluethmann, F. Bootz, M. Suter, R. M. Zinkernagel, and A. Aguzzi. 1997. A crucial role for B cells in neuroinvasive scrapie. Nature 390687-690. [DOI] [PubMed] [Google Scholar]
- 37.Lee, H. P., Y. C. Jun, J. K. Choi, J. I. Kim, R. I. Carp, and Y. S. Kim. 2005. The expression of RANTES and chemokine receptors in the brains of scrapie-infected mice. J. Neuroimmunol. 15826-33. [DOI] [PubMed] [Google Scholar]
- 38.Legler, D. F., M. Loetscher, R. S. Roos, I. Clark-Lewis, M. Baggiolini, and B. Moser. 1998. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J. Exp. Med. 187655-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewicki, H., A. Tishon, D. Homann, H. Mazarguil, F. Laval, V. C. Asensio, I. L. Campbell, S. DeArmond, B. Coon, C. Teng, J. E. Gairin, and M. B. Oldstone. 2003. T cells infiltrate the brain in murine and human transmissible spongiform encephalopathies. J. Virol. 773799-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu, L., D. Huang, M. Matsui, T. T. He, T. Hu, J. Demartino, B. Lu, C. Gerard, and R. M. Ransohoff. 2006. Severe disease, unaltered leukocyte migration, and reduced IFN-gamma production in CXCR3−/− mice with experimental autoimmune encephalomyelitis. J. Immunol. 1764399-4409. [DOI] [PubMed] [Google Scholar]
- 41.Lu, Z. Y., C. A. Baker, and L. Manuelidis. 2004. New molecular markers of early and progressive CJD brain infection. J. Cell. Biochem. 93644-652. [DOI] [PubMed] [Google Scholar]
- 42.Maignien, T., M. Shakweh, P. Calvo, D. Marce, N. Sales, E. Fattal, J. P. Deslys, P. Couvreur, and C. I. Lasmezas. 2005. Role of gut macrophages in mice orally contaminated with scrapie or BSE. Int. J. Pharm. 298293-304. [DOI] [PubMed] [Google Scholar]
- 43.Marella, M., and J. Chabry. 2004. Neurons and astrocytes respond to prion infection by inducing microglia recruitment. J. Neurosci. 24620-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meda, L., P. Baron, and G. Scarlato. 2001. Glial activation in Alzheimer's disease: the role of Aβ and its associated proteins. Neurobiol. Aging 22885-893. [DOI] [PubMed] [Google Scholar]
- 45.Mohan, J., J. Hopkins, and N. A. Mabbott. 2005. Skin-derived dendritic cells acquire and degrade the scrapie agent following in vitro exposure. Immunology 116122-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mucke, L., G. Q. Yu, L. McConlogue, E. M. Rockenstein, C. R. Abraham, and E. Masliah. 2000. Astroglial expression of human α1-antichymotrypsin enhances Alzheimer-like pathology in amyloid protein precursor transgenic mice. Am. J. Pathol. 1572003-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nazor, K. E., F. Kuhn, T. Seward, M. Green, D. Zwald, M. Purro, J. Schmid, K. Biffiger, A. M. Power, B. Oesch, A. J. Raeber, and G. C. Telling. 2005. Immunodetection of disease-associated mutant PrP, which accelerates disease in GSS transgenic mice. EMBO J. 242472-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ning, Z. Y., D. M. Zhao, H. X. Liu, J. M. Yang, C. X. Han, Y. L. Cui, L. P. Meng, C. D. Wu, M. L. Liu, and T. X. Zhang. 2005. Altered expression of the prion gene in rat astrocyte and neuron cultures treated with prion peptide 106-126. Cell. Mol. Neurobiol. 251171-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Omari, K. M., G. R. John, S. C. Sealfon, and C. S. Raine. 2005. CXC chemokine receptors on human oligodendrocytes: implications for multiple sclerosis. Brain 1281003-1015. [DOI] [PubMed] [Google Scholar]
- 50.Priller, J., M. Prinz, M. Heikenwalder, N. Zeller, P. Schwarz, F. L. Heppner, and A. Aguzzi. 2006. Early and rapid engraftment of bone marrow-derived microglia in scrapie. J. Neurosci. 2611753-11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. USA 9513363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prusiner, S. B., S. P. Cochran, D. F. Groth, D. E. Downey, K. A. Bowman, and H. M. Martinez. 1982. Measurement of the scrapie agent using an incubation time interval assay. Ann. Neurol. 11353-358. [DOI] [PubMed] [Google Scholar]
- 53.Raeber, A. J., R. E. Race, S. Brandner, S. A. Priola, A. Sailer, R. A. Bessen, L. Mucke, J. Manson, A. Aguzzi, M. B. Oldstone, C. Weissmann, and B. Chesebro. 1997. Astrocyte-specific expression of hamster prion protein (PrP) renders PrP knockout mice susceptible to hamster scrapie. EMBO J. 166057-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ransohoff, R. M., L. Liu, and A. E. Cardona. 2007. Chemokines and chemokine receptors: multipurpose players in neuroinflammation. Int. Rev. Neurobiol. 82187-204. [DOI] [PubMed] [Google Scholar]
- 55.Rappert, A., I. Bechmann, T. Pivneva, J. Mahlo, K. Biber, C. Nolte, A. D. Kovac, C. Gerard, H. W. Boddeke, R. Nitsch, and H. Kettenmann. 2004. CXCR3-dependent microglial recruitment is essential for dendrite loss after brain lesion. J. Neurosci. 248500-8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rappert, A., K. Biber, C. Nolte, M. Lipp, A. Schubel, B. Lu, N. P. Gerard, C. Gerard, H. W. Boddeke, and H. Kettenmann. 2002. Secondary lymphoid tissue chemokine (CCL21) activates CXCR3 to trigger a Cl− current and chemotaxis in murine microglia. J. Immunol. 1683221-3226. [DOI] [PubMed] [Google Scholar]
- 57.Rezaie, P., and P. L. Lantos. 2001. Microglia and the pathogenesis of spongiform encephalopathies. Brain Res. Brain Res. Rev. 3555-72. [DOI] [PubMed] [Google Scholar]
- 58.Riemer, C., S. Neidhold, M. Burwinkel, A. Schwarz, J. Schultz, J. Kratzschmar, U. Monning, and M. Baier. 2004. Gene expression profiling of scrapie-infected brain tissue. Biochem. Biophys. Res. Commun. 323556-564. [DOI] [PubMed] [Google Scholar]
- 59.Riemer, C., I. Queck, D. Simon, R. Kurth, and M. Baier. 2000. Identification of upregulated genes in scrapie-infected brain tissue. J. Virol. 7410245-10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Savarin-Vuaillat, C., and R. M. Ransohoff. 2007. Chemokines and chemokine receptors in neurological disease: raise, retain, or reduce? Neurotherapeutics 4590-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schultz, J., A. Schwarz, S. Neidhold, M. Burwinkel, C. Riemer, D. Simon, M. Kopf, M. Otto, and M. Baier. 2004. Role of interleukin-1 in prion disease-associated astrocyte activation. Am. J. Pathol. 165671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schulz-Schaeffer, W. J., S. Tschoke, N. Kranefuss, W. Drose, D. Hause-Reitner, A. Giese, M. H. Groschup, and H. A. Kretzschmar. 2000. The paraffin-embedded tissue blot detects PrPSc early in the incubation time in prion diseases. Am. J. Pathol. 15651-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Szczucinski, A., and J. Losy. 2007. Chemokines and chemokine receptors in multiple sclerosis. Potential targets for new therapies. Acta Neurol. Scand. 115137-146. [DOI] [PubMed] [Google Scholar]
- 64.Taub, D. D., A. R. Lloyd, K. Conlon, J. M. Wang, J. R. Ortaldo, A. Harada, K. Matsushima, D. J. Kelvin, and J. J. Oppenheim. 1993. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J. Exp. Med. 1771809-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsunoda, I., T. E. Lane, J. Blackett, and R. S. Fujinami. 2004. Distinct roles for IP-10/CXCL10 in three animal models, Theiler's virus infection, EAE, and MHV infection, for multiple sclerosis: implication of differing roles for IP-10. Mult. Scler. 1026-34. [DOI] [PubMed] [Google Scholar]
- 66.Wang, Q., X. N. Tang, and M. A. Yenari. 2007. The inflammatory response in stroke. J. Neuroimmunol. 18453-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weissmann, C. 2004. The state of the prion. Nat. Rev. Microbiol. 2861-871. [DOI] [PubMed] [Google Scholar]
- 68.Williams, A., A. M. Van Dam, D. Ritchie, P. Eikelenboom, and H. Fraser. 1997. Immunocytochemical appearance of cytokines, prostaglandin E2 and lipocortin-1 in the CNS during the incubation period of murine scrapie correlates with progressive PrP accumulations. Brain Res. 754171-180. [DOI] [PubMed] [Google Scholar]
- 69.Williams, A. E., L. J. Lawson, V. H. Perry, and H. Fraser. 1994. Characterization of the microglial response in murine scrapie. Neuropathol. Appl. Neurobiol 2047-55. [DOI] [PubMed] [Google Scholar]
- 70.Wu, Y. P., and R. L. Proia. 2004. Deletion of macrophage-inflammatory protein 1 alpha retards neurodegeneration in Sandhoff disease mice. Proc. Natl. Acad. Sci. USA 1018425-8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wyss-Coray, T. 2006. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat. Med. 121005-1015. [DOI] [PubMed] [Google Scholar]
- 72.Xia, M. Q., B. J. Bacskai, R. B. Knowles, S. X. Qin, and B. T. Hyman. 2000. Expression of the chemokine receptor CXCR3 on neurons and the elevated expression of its ligand IP-10 in reactive astrocytes: in vitro ERK1/2 activation and role in Alzheimer's disease. J. Neuroimmunol. 108227-235. [DOI] [PubMed] [Google Scholar]
- 73.Xiang, W., O. Windl, G. Wunsch, M. Dugas, A. Kohlmann, N. Dierkes, I. M. Westner, and H. A. Kretzschmar. 2004. Identification of differentially expressed genes in scrapie-infected mouse brains by using global gene expression technology. J. Virol. 7811051-11060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ye, X., A. C. Scallet, R. J. Kascsak, and R. I. Carp. 1998. Astrocytosis and amyloid deposition in scrapie-infected hamsters. Brain Res. 809277-287. [DOI] [PubMed] [Google Scholar]
- 75.Zhou, A., J. Paranjape, T. L. Brown, H. Nie, S. Naik, B. Dong, A. Chang, B. Trapp, R. Fairchild, C. Colmenares, and R. H. Silverman. 1997. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. EMBO J. 166355-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]