Abstract
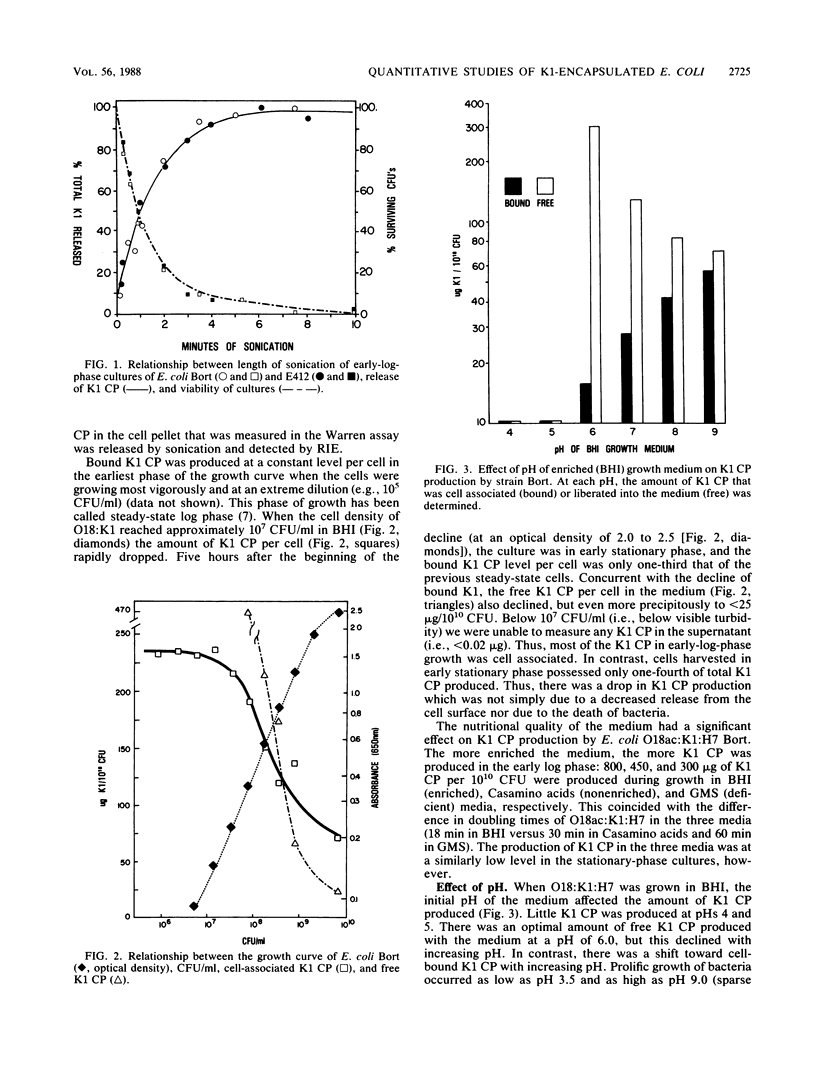
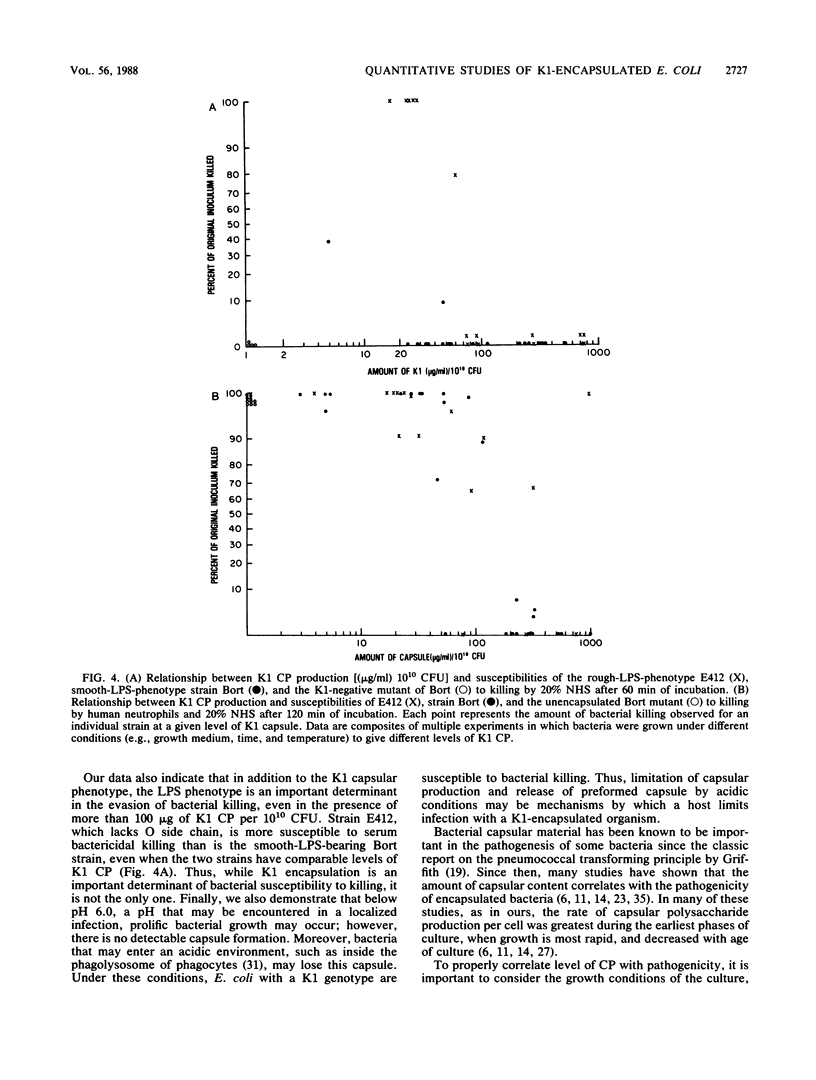
Since there are conflicting reports in the literature on a possible relationship between the K1 capsular polysaccharide (CP) content of Escherichia coli and its susceptibility to killing, we reexamined this issue in a strain that had a smooth lipopolysaccharide (LPS) phenotype (E. coli O18:K1:H7 Bort) and in a strain with a deep rough LPS phenotype (E412, spontaneously agglutinable: K1:H-). When cell-associated K1 capsular content was greater than 90 micrograms of K1 polysaccharide per 10(10) CFU, neither strain was lysed by 20% normal human serum. In contrast, at equivalent but lower levels of K1 CP content, E412 but not strain Bort was lysed by normal human serum. Thus, LPS phenotype is an additional surface determinant that affects bacterial susceptibility to killing. Organisms obtained from very early log phase, when cell-associated K1 CP is greatest, were significantly more virulent for mice than were bacteria harvested in stationary phase, when cell-associated K1 polysaccharide is lowest. We conclude that (i) there is a threshold level of K1 CP needed to confer protection from lysis by serum, and this is usually exceeded under standard growth conditions; (ii) at a given level of K1 CP the LPS phenotype is an important determinant of bacterial killing; and (iii) the loss of capsule at low pH may be an additional mechanism by which hosts defend against invasive infection by K1-encapsulated E. coli.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAER H., EHRENWORTH L. The pathogenicity of Klebsiella pneumoniae for mice: the relationship to the quantity and rate of production of type-specific capsular polysaccharide. J Bacteriol. 1956 Nov;72(5):713–717. doi: 10.1128/jb.72.5.713-717.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith J. R., Signer E. R., Epstein W. Transposition of the Lac region of E. coli. Cold Spring Harb Symp Quant Biol. 1966;31:393–401. doi: 10.1101/sqb.1966.031.01.051. [DOI] [PubMed] [Google Scholar]
- Benjamin W. H., Jr, Posey B. S., Briles D. E. Effects of in vitro growth phase on the pathogenesis of Salmonella typhimurium in mice. J Gen Microbiol. 1986 May;132(5):1283–1295. doi: 10.1099/00221287-132-5-1283. [DOI] [PubMed] [Google Scholar]
- Bortolussi R., Ferrieri P., Björkstén B., Quie P. G. Capsular K1 polysaccharide of Escherichia coli: relationship to virulence in newborn rats and resistance to phagocytosis. Infect Immun. 1979 Jul;25(1):293–298. doi: 10.1128/iai.25.1.293-298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolussi R., Ferrieri P., Quie P. G. Influence of growth temperature of Escherichia coli on K1 capsular antigen production and resistance to opsonization. Infect Immun. 1983 Mar;39(3):1136–1141. doi: 10.1128/iai.39.3.1136-1141.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. R., Williams P. The influence of environment on envelope properties affecting survival of bacteria in infections. Annu Rev Microbiol. 1985;39:527–556. doi: 10.1146/annurev.mi.39.100185.002523. [DOI] [PubMed] [Google Scholar]
- Bukantz S. C., Cooper A., Bullowa J. G. The Elaboration of Soluble Capsular Polysaccharide by Pneumococcus III In Relation to Growth Phases in vitro. J Bacteriol. 1941 Jul;42(1):29–49. doi: 10.1128/jb.42.1.29-49.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLINS F. M. THE EFFECT OF THE GROWTH RATE ON THE COMPOSITION OF S. ENTERITIDIS CELL WALLS. Aust J Exp Biol Med Sci. 1964 Apr;42:255–262. doi: 10.1038/icb.1964.27. [DOI] [PubMed] [Google Scholar]
- Cho S., Gasparich G., Sledjeski D., Ezzell C., Vermeulen C. W. The realm of the steady state in Escherichia coli. Biochem Biophys Res Commun. 1984 Oct 30;124(2):625–628. doi: 10.1016/0006-291x(84)91600-0. [DOI] [PubMed] [Google Scholar]
- Cross A. S., Gemski P., Sadoff J. C., Orskov F., Orskov I. The importance of the K1 capsule in invasive infections caused by Escherichia coli. J Infect Dis. 1984 Feb;149(2):184–193. doi: 10.1093/infdis/149.2.184. [DOI] [PubMed] [Google Scholar]
- Cross A. S., Kim K. S., Wright D. C., Sadoff J. C., Gemski P. Role of lipopolysaccharide and capsule in the serum resistance of bacteremic strains of Escherichia coli. J Infect Dis. 1986 Sep;154(3):497–503. doi: 10.1093/infdis/154.3.497. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., DAWES E. A. Factors influencing the polysaccharide content of Escherichia coli. Biochem J. 1949;45(3):331–337. doi: 10.1042/bj0450331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGUID J. P., WILKINSON J. F. The influence of cultural conditions on polysaccharide production by Aerobacter aerogenes. J Gen Microbiol. 1953 Oct;9(2):174–189. doi: 10.1099/00221287-9-2-174. [DOI] [PubMed] [Google Scholar]
- Dennis P. P., Bremer H. Macromolecular composition during steady-state growth of Escherichia coli B-r. J Bacteriol. 1974 Jul;119(1):270–281. doi: 10.1128/jb.119.1.270-281.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A. A., Howard C. J. The sensitivity to complement of strains of Escherichia coli related to their K antigens. Immunology. 1970 Mar;18(3):331–346. [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Gross R. J., Cheasty T., Rowe B. Isolation of bacteriophages specific for the K1 polysaccharide antigen of Escherichia coli. J Clin Microbiol. 1977 Dec;6(6):548–550. doi: 10.1128/jcm.6.6.548-550.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Glynn A. A. The virulence for mice of strains of Escherichia coli related to the effects of K antigens on their resistance to phagocytosis and killing by complement. Immunology. 1971 May;20(5):767–777. [PMC free article] [PubMed] [Google Scholar]
- Jacques Y. V., Bainton D. F. Changes in pH within the phagocytic vacuoles of human neutrophils and monocytes. Lab Invest. 1978 Sep;39(3):179–185. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Maloney P. C., Schneider H., Brandt B. L. Production and degradation of serogroup B Neisseria meningitidis polysaccharide. Infect Immun. 1972 Nov;6(5):657–658. doi: 10.1128/iai.6.5.657-661.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe W. R., Carling P. C., Bruins S., Greely A. The relation of K-antigen to virulence of Escherichia coli. J Infect Dis. 1975 Jan;131(1):6–10. doi: 10.1093/infdis/131.1.6. [DOI] [PubMed] [Google Scholar]
- McCabe W. R., Kaijser B., Olling S., Uwaydah M., Hanson L. A. Escherichia coli in bacteremia: K and O antigens and serum sensitivity of strains from adults and neonates. J Infect Dis. 1978 Jul;138(1):33–41. doi: 10.1093/infdis/138.1.33. [DOI] [PubMed] [Google Scholar]
- McCracken G. H., Jr, Sarff L. D., Glode M. P., Mize S. G., Schiffer M. S., Robbins J. B., Gotschlich E. C., Orskov I., Orskov F. Relation between Escherichia coli K1 capsular polysaccharide antigen and clinical outcome in neonatal meningitis. Lancet. 1974 Aug 3;2(7875):246–250. doi: 10.1016/s0140-6736(74)91413-5. [DOI] [PubMed] [Google Scholar]
- Merrill D., Hartley T. F., Claman H. N. Electroimmunodiffusion (EID): a simple, rapid method for quantitation of immunoglobulins in dilute biological fluids. J Lab Clin Med. 1967 Jan;69(1):151–159. [PubMed] [Google Scholar]
- Nicholson A. M., Glynn A. A. Investigation of the effect of K antigen in Escherichia coli urinary tract infections by use of a mouse model. Br J Exp Pathol. 1975 Dec;56(6):549–553. [PMC free article] [PubMed] [Google Scholar]
- Ohman L., Magnusson K. E., Stendahl O. Mannose-specific and hydrophobic interaction between Escherichia coli and polymorphonuclear leukocytes--influence of bacterial culture period. Acta Pathol Microbiol Immunol Scand B. 1985 Apr;93(2):125–131. doi: 10.1111/j.1699-0463.1985.tb02863.x. [DOI] [PubMed] [Google Scholar]
- Orskov F., Sharma V., Orskov I. Influence of growth temperature on the development of Escherichia coli polysaccharide K antigens. J Gen Microbiol. 1984 Oct;130(10):2681–2684. doi: 10.1099/00221287-130-10-2681. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3973–3986. [PubMed] [Google Scholar]
- SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. I. GENETICS. Nature. 1964 Mar 28;201:1298–1299. doi: 10.1038/2011298a0. [DOI] [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens P., Chu C. L., Young L. S. K-1 antigen content and the presence of an additional sialic acid-containing antigen among bacteremic K-1 Escherichia coli: correlation with susceptibility to opsonophagocytosis. Infect Immun. 1980 Sep;29(3):1055–1061. doi: 10.1128/iai.29.3.1055-1061.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W., Messner P., Parton R. Effect of the growth environment on cell-envelope components of Escherichia coli in relation to sensitivity to human serum. J Med Microbiol. 1981 Feb;14(1):9–19. doi: 10.1099/00222615-14-1-9. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]



