Summary
Signaling via the large protocadherin Fat (Ft), regulated in part by its binding partner Dachsous (Ds) and the Golgi-resident kinase Four-jointed (Fj), is required for a variety of developmental functions in Drosophila. Ft and, to a lesser extent, Ds, suppress overgrowth of the imaginal discs from which appendages develop and regulate the Hippo pathway [1-5] (reviewed in [6]). Ft, Ds and Fj are also required for normal planar cell polarity (PCP) in the wing, abdomen and eye, and for the normal patterning of appendages, including the spacing of crossveins in the wing and the segmentation of the leg tarsus (reviewed in [7-9]). Ft signaling was recently shown to be negatively regulated by the atypical myosin Dachs [10, 11]. We identify here an additional negative regulator of Ft signaling in growth control, PCP and appendage patterning, the Approximated (App) protein. We show that Approximated encodes a member of the DHHC family, responsible for the palmitoylation of selected cytoplasmic proteins, and provide evidence that App acts by controlling the normal subcellular localization and activity of Dachs.
Results and Discussion
approximated is required for patterning and normal PCP
Crossvein spacing and tarsal leg segmentation are extremely sensitive to changes in Ft activity; they are disrupted in weak Ft pathway mutants that have no obvious growth or PCP defects. Similar disruption occurs in app1 homozygotes: the distance between the anterior crossvein (ACV) and posterior crossvein (PCV) is reduced (Figure 1B) and one or more tarsal leg joints are lost or reduced (Figure S1B). app1 hemizygote wings also have weak PCP defects (Figure 1H) [12]. We used EMS to generate additional mutations that failed to complement app1. Of these, appe6 was semi-lethal in homozygotes and hemizygotes, and escaper adults had more extensive wing PCP defects, both proximally and in a distal region between the third and fourth longitudinal veins (Figures 1C, 1D and 1J). They also had abdominal PCP defects (Figure 1L): in the anterior compartment most hairs point in the normal, posterior direction, but polarity was disturbed around anterior-posterior (A/P) boundary (a6 and p3 in the nomenclature of [13]) and extensively reversed in the posterior compartment (p3). appe6 appears amorphic, as the defects were not noticeably stronger in hemizygotes.
Figure 1. app mutant phenotypes.
Bars in (A-F) compare the distance between the ACV and PCV in wild type (blue) and mutant (red) adult wings. Arrows in (A) and (D) show normal (blue) and abnormal (red) hair polarity. (A) Wild type. (B) app1 homozygote. (C) appe6 homozygote. (D) appe6 / Df(3L)ED4475. (E) Dorsal expression of UAS-app-RNAi with ap-gal4. (F) Rescue of appe6 with act-gal4 UAS-app-RA. (G-J) Close-ups of hair polarity in wing just proximal to ACV in (G) wild type, (H) app1 / Df(3L)BK9, (I) ap-gal4 UAS-app-RNAi, (J) appe6/Df(3L)ED4475. (K,L) Hair polarity in abdomens. Dark pigmentation marks the anterior. (K) Wild type. (L) appe6/Df(3L)ED4475. (M) Anti-Fmi staining (red, white) in pupal wing containing homozygous appe6 clones, marked by absence of GFP (green). Fmi is normally concentrated on the proximal (left) and distal (right) faces of cells; arrow shows cell face with abnormally polarized Fmi in an appe6 mutant cell.
The development of PCP in the pupal wing is accompanied by the polarized redistribution of the “core” planar polarity proteins to the proximal, distal, or proximal and distal faces of single wing cells [8]. PCP mutants can be separated depending on their effects on this polarization. Mutations in the core PCP proteins reduce the levels and block the polarization of the other core PCP proteins, while changes in ft, ds or fj, expression can reorient core PCP protein polarization along inappropriate axes [14-16]. We found that the levels of the core PCP protein Flamingo (Fmi, also known as Starry night) [17, 18] were not reduced in appe6 mutant clones (over 30 examined), and that Fmi polarization was in some cases reoriented (Figure 1M). This further supports App’s involvement in Ft signaling.
Identifying app
We mapped app to a portion of 69A2-A4 containing seven known or predicted genes (Figure S2A). app1, appe1, appe3 and appe6 all contained mutations in the 5′ coding exons of one of these, the CG5620 Flybase gene prediction (Figure 2B). We constructed a UAS-driven RNAi transgene corresponding to the 5′ end of CG5620 and expressed it in developing dorsal wings using ap-gal4; this produced app-like wing and PCP defects on the dorsal surface (Figures 1E and 1I)
Figure 2. App structure and localization and its effects of Ft and Ds.
(A) ClustalW lineup of region of App containing the DHHC-CRD and common to App-PA, -PB and -PC (predicted) with human ZDHHC9 and yeast ERF2. Lines overlie the transmembrane (TM) domains (black) and the DHHC-CRD (red). Identical (black) and similar (gray) amino acids are shaded. (B) App mutations and predicted topology of App-PA. Green shows the portion of the App common region, shared by App-PA, -PB and -PC (predicted), that was used to generate anti-App-common. Blue shows the App-PA-specific C-terminus, used to generate anti-App-PA. The C-termini of the App-PA, -PB and -PC isoforms diverge at the transition from green to blue. (C, C’) Loss of anti-App-PA staining from homozygous appe6 clone, marked by absence of GFP, in wing imaginal disc. (D) Anti-App-PA at cell cortex in embryo. (E, F) Apical anti-App-PA staining in wing disc. These confocal sections are through a fold in the wing disc such that cells are oriented apical up and basal down in the image. (E) App-PA (green, white in E’) is apical to DE-cadherin (red). (F) App-PA (green, white in F’) overlaps Ds (red, white in F”). (G, H) Normal anti-Ft staining (red in G ,white in G’) and anti-Ds staining (red in H, white in H’) in homozygous appe6 clones in wing disc, marked by absence of GFP (green). (I) Phalloidin staining showing hair polarity (red, white) in pupal wing (33 hr after puparium formation) containing appe6 clone marked by absence of GFP (green). Region shown is L4 around the PCV. Hairs in the (green) wild type cells point distally (right), while in the clone many hairs show abnormal posterior and proximal orientation, as in app homozygote wings (compare with Figures 1A and 1D). (J). Normal hair polarity near anterior-posterior compartment boundary in wing expressing UAS-app-RA in the posterior with en-gal4.
However, the 3′ end of the CG5620 coding prediction is in error (Figure S2B), as our 3′RACE products (and a recent Drosophila Genome Project EST [19]) use instead the 3′ exon of the adjacent CG17144 prediction. We call this transcript app-RA and show below that the corresponding App-PA protein is produced in flies. Another EST predicts a shorter transcript we call app-RB. We did not find transcripts covering the entire final coding exon of CG5620 in embryonic or larval cDNA libraries or by RACE. However, the full CG5620 prediction is conserved in Drosophila pseudoobscura suggesting that it might be utilized; we term this app-RC (predicted).
We rescued the wing and leg defects of app homozygotes by expressing UAS-app-RA, UAS-app-RB or UAS-app-RC with either act-gal4 or en-gal4 (act-gal4 UAS-app-RA in Figures 1F and S1F). Overexpression of higher levels of UAS-app with strong drivers such as ap-gal4 or tub-gal4 also disrupted PCP in the proximal wing and abdomen (see Figure 4K).
Figure 4. Interactions between App and Dachs.
(A-C) Wings and wing hair PCP in d1 (A), dGC13 / Df(2L)ED623 (B) and dGC13 / Df(2L)ED623 ; appe6(C). (D,E). Abdominal PCP defects in dGC13 /Df (2L)ED623 (D) and dGC13 / Df(2L)ED623; appe6(E). (F,G). Reduction of apical cell cortex Dachs::V5 (red, white) in homozygous appe6 clones marked by absence of GFP (green). (F) shows focus on apical level in wing disc, while (G) shows a confocal section through a fold in disc, such that cells in the image are oriented apical up and basal down. (H,I) Dachs::V5 levels (red, white) in wing disc without (H) and with (I) coexpression of App-PA (green). Expression is limited to the right (posterior) using en-gal4. (J-L) PCP in abdomens overexpressing Dachs::V5 (J), App-PA (K) and both (L). (M) appe6 PCP defect between distal L4 and L5 (M) rescued on dorsal surface by dorsal expression of Dachs::V5 with ap-gal4 (M’).
App is a DHHC protein
app encodes a member of the DHHC protein family, responsible for adding palmitates to cytoplasmic proteins [20, 21]. Eukaryotes contain multiple members of the DHHC family, with 8 predicted in yeast, 23 in mammals [22] and 20 in Drosophila. The region common to all predicted App isoforms contains four predicted transmembrane domains, with a DHHC cysteine-rich domain (DHHC-CRD) located between the second and third transmembrane domains (Figures 2A and 2B). It is likely that the DHHC-CRD is located on the cytoplasmic side of the membrane as in the yeast DHHC protein AKR1 [23].
Alignments using the region common to the App isoforms indicate that App is in the same sub-family as the human ZDHHCs 9, 14 and 18 [22] and is the Drosophila protein most similar to yeast ERF2 [24] (Figures 2A, S2C and S3). The similarity between App and these proteins drops off at the C-terminal end of the common region (Figure S2C). The divergent C-termini of App-PA and App-PC (predicted) have no significant similarity to each other or to other proteins in the database outside the Drosophilids, except for a short region at the C-terminus of App-PA that is similar to predicted App homologs from the insects Tribolium and Apis. App-PB has a much shorter C-terminus. Since any of the putative app transcripts rescued the app leg and wing phenotypes (see above), the different C-termini are dispensable for these phenotypes.
app1 contains a missense mutation N-terminal to the DHHC-CRD domain and an aberration that introduces a frameshift predicted to truncate the C-terminal end of App-PA (Figure 2B). appe3 contains a missense mutation changing a conserved cysteine in the DHHC-CRD. Since the DHHC-CRD is required for palmitoyltransferase activity [25], this supports a role for palmitoylation in Ft signaling. appe6 and appe1 contain nonsense mutations predicted to truncate the protein prior to or towards the end of the DHHC-CRD, respectively, and therefore appe6 is likely null for App function.
App is concentrated at the apical cell cortex but does not affect the levels or distribution of Ft or Ds
We generated two antisera, one specific for App-PA and one to the common region (Figure 2B). Both antisera uniformly stained embryos, imaginal discs and pupal wings, and staining was lost from mitotic homozygous appe6 clones, confirming the expression of the App-PA isoform (anti-App-PA in Figures 2C-2F and S4; similar results with anti-App-common). There is no obvious asymmetric distribution of the App protein along the proximo-distal or anterior-posterior axes of imaginal discs or pupal wings. However, staining was especially strong in the apical cell cortex (Figures 2D and S4), and this concentration did not extend more basally to the adherens junction marker DE-cadherin (Figure 2E) or the septate junction marker Discs large 1 (data not shown). This is similar to the distribution of Ft and Ds [15] and there is overlap between the regions where App, Ds and Ft are concentrated (Figure 2F). This result is surprising as human ZDHHC9, 14 and 18 and yeast ERF2 are concentrated in the Golgi or ER; only a few, less similar, ZDHHCs have been detected at the plasma membrane [24, 26-28]. We did not observe any significant overlap between App and Golgi or ER markers in wing discs (data not shown). While App must traffic through the ER and Golgi, these results suggest that App is active in the plasma membrane, in or near the apical region where Ft and Ds are concentrated.
However, App does not detectably regulate Ft and Ds levels or their binding. The binding between Ft and Ds stabilizes both proteins at the cell surface in wing discs [14-16], but appe6 clones did not affect Ft or Ds levels or distribution (Figures 2G-2H). Creating artificial boundaries of ft or ds expression also strongly polarizes wing PCP [14-16], as do boundaries of the Fj kinase that phosphorylates Ft and Ds and modulates their levels [14, 15, 29-31]. If App affected Ft-Ds levels or binding we would also expect App boundaries to affect PCP. However, small to moderately sized appe6 clones did not affect PCP, while large clones only affected PCP in the regions of the wing where defects were observed in appe6 homozygotes (Figure S5). There was no tendency to reorient hairs at clone boundaries (Figures 2I and S5), and only rarely did regions with altered PCP affected PCP in adjacent wild type cells; these may be due to altered cell interactions mediated by the core polarity proteins. Sharp boundaries of UAS-app-RA misexpression (e.g. driven with the posterior driver en-gal4) also had no effect on PCP (Figure 2J). Anti-App staining was not altered in wing discs by ft or ds clones (data not shown). Thus, despite their co-localization, there is no evidence that App physically interacts for Ft or Ds.
app mutants partially rescue the viability, growth and PCP defects of ft mutants
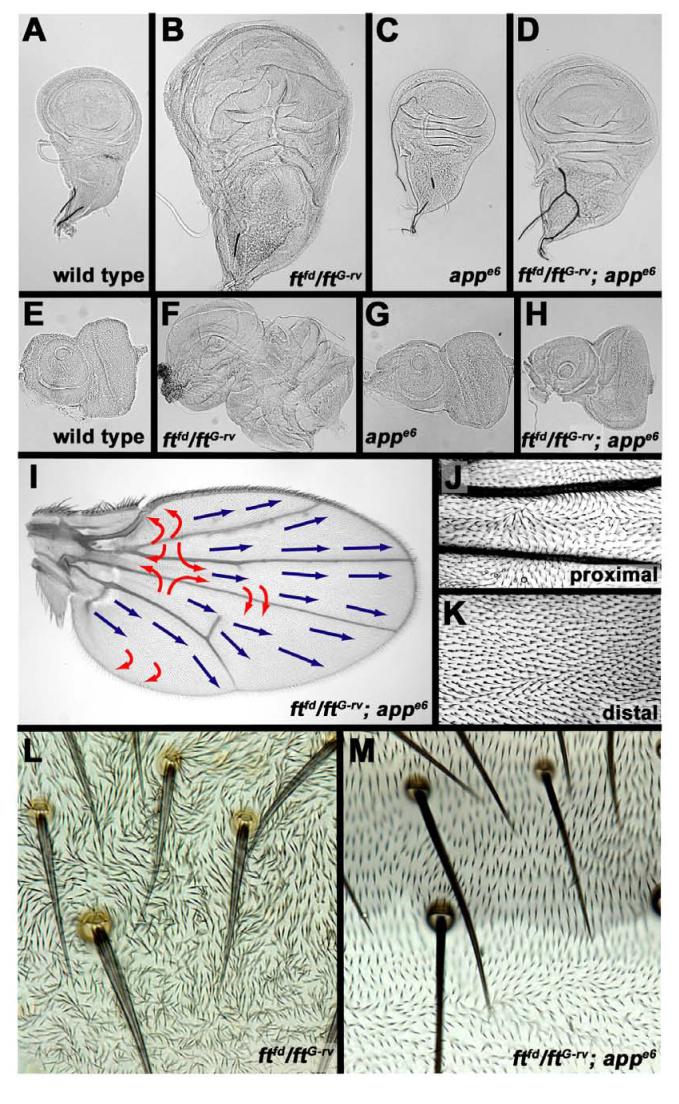
PCP defects, reduced crossvein spacing and lost tarsal leg joints can be caused by either gains or losses in Ft signaling [15, 16, 30, 32], so we examined the phenotypes of app ft double mutants. ftfd and ftG-rv are likely null alleles predicted to truncate Ft N-terminal to its transmembrane region [33]. ftfd homozygotes and ftfd/ftG-rv die during late pupal stages with strongly overgrown imaginal discs (compare Figures 3A and 3E with Figures 3B and 3F) and disc-derived tissues; late pupal abdomens are not overgrown, but have strong PCP defects (Figure 3L). These phenotypes were partially rescued in ftfd ; appe6 and ftfd/ftG-rv ; appe6 flies: overgrowth and extra folding of imaginal discs was suppressed (Figures 3D and 3H) and lethality and abdominal PCP defects were reduced (Figure 3M). PCP was still defective in proximal portions of the wing but was normal in the distal wing (Figures 3I-3K), in contrast to the distal defects in viable ft18 wings [33]. Thus, App acts genetically downstream of and in opposition to Ft in both growth control and PCP.
Figure 3. Rescue of ft mutant phenotypes by loss of app.
(A-H) Comparison of overgrowth in wing (A-D) and eye (E-H) imaginal discs. (A,E) wild type. (B,F) ftfd /ftG-rv. (C,G) appe6 homozygotes. (D,H) ftfd /ftG-rv ; appe6. (I) PCP in wing of ftfd /ftG-rv ; appe6. (J,K) polarity in ftfd /ftG-rv ; appe6 wing, limited to regions proximal to ACV (J) and distally between L3 and L4 (K). (L) Hair polarity in pharate ftfd /ftG-rv abdomen. (M) Partially rescued hair polarity in adult ftfd /ftG-rv ; appe6 abdomen.
App affects the levels and distribution of Dachs
The effects of app mutations on Ft signaling are strikingly similar to those caused by reducing the function of the atypical myosin Dachs [11]. Like app mutations, dachs (d) mutations partially suppress the overgrowth and PCP defects caused by loss of Ft [10, 11]. The adult phenotypes are also similar, although the defects in d null mutants are stronger than those of appe6. Null dGC13 hemizygotes and hypomorphic d1 homozygotes reduce tarsal leg segmentation (Figures S1H and S1I) and the distance between the ACV and PCV and cause mild wing PCP defects quite similar to those observed in null app mutants (compare Figures 4A and 4B to Figures 1B-1D; details in Figures S6 and S7). Like appe6 clones, dGC13 clones had PCP defects when in regions of the wing where defects occur in d homozygotes, and Fmi polarization was reoriented in d1 pupal wings (Figure S5E). d mutants also had abdominal PCP defects similar to those in app mutants: polarity was almost normal in the anterior compartment, but abnormal near A/P compartment boundary and reversed in the posterior compartment (Figure 4D).
We therefore examined the effects of App on the levels and distribution of a V5-tagged Dachs protein. Dachs::V5 normally accumulates at higher levels in the apical cell cortex in wing imaginal discs [11], overlapping the region of high anti-App staining (Figures S4A, S10A and S10B). Apical Dachs accumulation was greatly reduced, although not completely eliminated, in appe6 clones (Figures 4F-4G”). We did not detect changes in the levels of basolateral or cytoplasmic Dachs::V5. While App affects Dachs accumulation at the apical cell cortex, anti-App staining was normal in d mutant clones (data not shown).
Co-overexpression of App and Dachs::V5 greatly increased the accumulation of Dachs at the cell cortex compared with the expression of Dachs::V5 alone (compare Figures 4H to 4I). Co-expression of App also increased the efficacy of Dachs in growth and PCP. Even though overexpression of App-RA did not obviously increase growth, co-expression of App-RA and Dachs caused greater overgrowth than did the expression of Dachs alone (Figure S8). Co-overexpression of Dachs and App caused more extreme PCP defects in the wing and abdomen than did the overexpression of App alone, while overexpression of Dachs::V5 alone did not affect PCP (compare Figures 4J-4L; more examples in Figure S9).
It is likely that much or all of the app mutant phenotype is mediated by the reduction of effective Dachs at apical cell cortex. The effects of app and d mutants are not additive: double mutants for null app and d alleles resembled the stronger d null phenotype, as expected if App works by controlling Dachs activity (Figures 4B-4E). Since App affects Dachs post-transcriptionally it is unlikely that overexpressed Dachs would fully rescue the app null. Nonetheless, overexpression of UAS-d with ap-gal4 or en-gal4 rescued the wing PCP defects normally found in the distal wing of appe6 mutants (Figures 4M and 4M’), and partly rescued the crossvein spacing and leg joint defects of appe6 (Figures S1G and S6). That Dachs retains some activity in the absence of App is consistent with the low but significant levels of Dachs that remain at the apical cell cortex in appe6 clones (Figures 4F-4G). Different DHHC proteins can palmitoylate the same target (e.g. [34]), so other Drosophila DHHC proteins may be supplying residual activity in the absence of App.
Conclusions
The App palmitoyltransferase acts in opposition to the Ft pathway, largely or wholly by controlling the apical cell cortex localization and the activity of the atypical myosin Dachs. This localization is likely required for full Dachs activity. For growth control, this localization would place Dachs near not only Ft but also the Hippo pathway member Warts. Warts is concentrated near the cell cortex, with an apical bias that overlaps the region of strong App and Dachs accumulation (Figures S10C and S10D). Dachs binds Warts and may thereby regulate the Hippo pathway, accounting for its effects on Ft-dependent growth control [4].
As shown here and elsewhere [11], Dachs also modulates the effects of Ft signaling on PCP. It is not clear if this modulation is also mediated through changes in Warts activity. Warts is thought to act by changing the activity of the transcription factor Yorkie [6] which would not directly confer polarity. Moreover, Dachs was reported to accumulate preferentially on the distal faces of some wing disc cells [11], suggesting that Dachs is involved in cell polarization independent on any effect on transcription, likely via as-yet unknown binding partners.
It remains possible that Dachs is palmitoylated by App. However, there is no precedent for palmitoylation of a myosin, nor does Dachs score highly using an algorithm that detects palmitoylation sites [35]. Nor have we detected Dachs palmitoylation using the acylbiotin-exchange technique [36]. The effect of App may thus be less direct, palmitoylating a binding partner or regulator of Dachs. While a myosin, portions of Dachs are unique and lack known protein interaction motifs [11, 37]. Warts is the only proven binding partner for Dachs, but appe6 clones did not affect the levels or cell cortex localization of Myc-tagged Warts in wing discs (Figure S10E).
Given that the human and yeast DHHC proteins that App most resembles palmitoylate many targets [24, 25, 34], the adult phenotypes of app mutants are surprisingly specific to the Ft pathway. One known target of ERF2 and ZDHHC9 is Ras, whose activity relies on membrane localization through both farnesylation and palmitoylation. Intriguingly, the Ras and MAPK pathways interact with the Ft pathway in growth control [38]. However, reducing Ras activity causes loss of wing veins, a phenotype not observed in app mutations, and does not cause the PCP and appendage patterning defects of app and d mutants (e.g. [38-40]). Moreover, reducing Ras activity via expression of a dominant negative EGF receptor did not affect the levels of Dachs::V5 in wing discs (Figure S11). The different subcellular distributions of App, to the cell cortex, and ZDHHC9 and ERF2, to endomembranes, suggests they have different roles and targets, despite their strong similarity at the amino-acid level.
Supplementary Material
Acknowledgements
This work was supported by a National Institutes of Health grant (NS028202) and a National Science Foundation grant (IBN0416586).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Willecke M, Hamaratoglu F, Kango-Singh M, Udan R, Chen CL, Tao C, Zhang X, Halder G. The Fat cadherin acts through the Hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16:2090–2100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Silva E, Tsatskis Y, Gardano L, Tapon N, McNeill H. The tumor-suppressor gene fat controls tissue growth upstream of Expanded in the Hippo signaling pathway. Curr Biol. 2006;16:2081–2089. doi: 10.1016/j.cub.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Bennett FC, Harvey KF. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr Biol. 2006;16:2101–2110. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 4.Cho E, Feng Y, Rauskolb C, Maitra S, Fehon R, Irvine KD. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 5.Feng Y, Irvine KD. Fat and Expanded act in parallel to regulate growth through Warts. Proc Natl Acad Sci USA. 2007;104:20362–20367. doi: 10.1073/pnas.0706722105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saucedo LJ, Edgar BA. Filling out the Hippo pathway. Nat Rev Mol Cell Biol. 2007;8:613–621. doi: 10.1038/nrm2221. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat Rev Genet. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strutt H, Strutt D. Long-range coordination of planar polarity in Drosophila. Bioessays. 2005;27:1218–1227. doi: 10.1002/bies.20318. [DOI] [PubMed] [Google Scholar]
- 9.Tree DR, Ma D, Axelrod JD. A three-tiered mechanism for regulation of planar cell polarity. Semin Cell Dev Biol. 2002;13:217–224. doi: 10.1016/s1084-9521(02)00042-3. [DOI] [PubMed] [Google Scholar]
- 10.Cho E, Irvine KD. Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development. 2004;131:4489–4500. doi: 10.1242/dev.01315. [DOI] [PubMed] [Google Scholar]
- 11.Mao Y, Rauskolb C, Cho E, Hu WL, Hayter H, Minihan G, Katz FN, Irvine KD. Dachs: an unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development. 2006;133:2539–2551. doi: 10.1242/dev.02427. [DOI] [PubMed] [Google Scholar]
- 12.Lindsley DL, Zimm GG. The Genetics of Drosophila melanogaster. Academic Press; New York: 1992. [Google Scholar]
- 13.Struhl G, Barbash DA, Lawrence PA. Hedgehog organises the pattern and polarity of epidermal cells in the Drosophila abdomen. Development. 1997;124:2143–2154. doi: 10.1242/dev.124.11.2143. [DOI] [PubMed] [Google Scholar]
- 14.Strutt H, Strutt D. Nonautonomous planar polarity patterning in Drosophila: dishevelled-independent functions of frizzled. Dev Cell. 2002;3:851–863. doi: 10.1016/s1534-5807(02)00363-5. [DOI] [PubMed] [Google Scholar]
- 15.Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- 16.Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–3794. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- 17.Chae J, Kim MJ, Goo JH, Collier S, Gubb D, Charlton J, Adler PN, Park WJ. The Drosophila tissue polarity gene starry night encodes a member of the protocadherin family. Development. 1999;126:5421–5429. doi: 10.1242/dev.126.23.5421. [DOI] [PubMed] [Google Scholar]
- 18.Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, Takeichi M, Uemura T. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- 19.Stapleton M, Carlson J, Brokstein P, Yu C, Champe M, George R, Guarin H, Kronmiller B, Pacleb J, Park S, et al. A Drosophila full-length cDNA resource Genome Biol 20023, RESEARCH0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 21.Nadolski MJ, Linder ME. Protein lipidation. Febs J. 2007;274:5202–5210. doi: 10.1111/j.1742-4658.2007.06056.x. [DOI] [PubMed] [Google Scholar]
- 22.Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 palmitoylating enzymes. Neuron. 2004;44:987–996. doi: 10.1016/j.neuron.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Politis EG, Roth AF, Davis NG. Transmembrane topology of the protein palmitoyl transferase Akr1. J Biol Chem. 2005;280:10156–10163. doi: 10.1074/jbc.M411946200. [DOI] [PubMed] [Google Scholar]
- 24.Swarthout JT, Lobo S, Farh L, Croke MR, Greentree WK, Deschenes RJ, Linder ME. DHHC9 and GCP16 constitute a human protein fatty acyltransferase with specificity for H- and N-Ras. J Biol Chem. 2005;280:31141–31148. doi: 10.1074/jbc.M504113200. [DOI] [PubMed] [Google Scholar]
- 25.Lobo S, Greentree WK, Linder ME, Deschenes RJ. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J Biol Chem. 2002;277:41268–41273. doi: 10.1074/jbc.M206573200. [DOI] [PubMed] [Google Scholar]
- 26.Bartels DJ, Mitchell DA, Dong X, Deschenes RJ. Erf2, a novel gene product that affects the localization and palmitoylation of Ras2 in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:6775–6787. doi: 10.1128/mcb.19.10.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohno Y, Kihara A, Sano T, Igarashi Y. Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim Biophys Acta. 2006;1761:474–483. doi: 10.1016/j.bbalip.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Zhao L, Lobo S, Dong X, Ault AD, Deschenes RJ. Erf4p and Erf2p form an endoplasmic reticulum-associated complex involved in the plasma membrane localization of yeast Ras proteins. J Biol Chem. 2002;277:49352–49359. doi: 10.1074/jbc.M209760200. [DOI] [PubMed] [Google Scholar]
- 29.Zeidler MP, Perrimon N, Strutt DI. The four-jointed gene is required in the Drosophila eye for ommatidial polarity specification. Curr Biol. 1999;9:1363–1372. doi: 10.1016/s0960-9822(00)80081-0. [DOI] [PubMed] [Google Scholar]
- 30.Zeidler MP, Perrimon N, Strutt DI. Multiple roles for four-jointed in planar polarity and limb patterning. Dev Biol. 2000;228:181–196. doi: 10.1006/dbio.2000.9940. [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa HO, Takeuchi H, Haltiwanger RS, Irvine KD. Four-jointed Is a Golgi kinase that phosphorylates a subset of cadherin domains. Science. 2008;321:401–404. doi: 10.1126/science.1158159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adler PN, Charlton J, Liu J. Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development. 1998;125:959–968. doi: 10.1242/dev.125.5.959. [DOI] [PubMed] [Google Scholar]
- 33.Matakatsu H, Blair SS. Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development. 2006;133:2315–2324. doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]
- 34.Roth AF, Wan J, Bailey AO, Sun B, Kuchar JA, Green WN, Phinney BS, Yates JR, 3rd, Davis NG. Global analysis of protein palmitoylation in yeast. Cell. 2006;125:1003–1013. doi: 10.1016/j.cell.2006.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou F, Xue Y, Yao X, Xu Y. CSS-Palm: palmitoylation site prediction with a clustering and scoring strategy (CSS) Bioinformatics. 2006;22:894–896. doi: 10.1093/bioinformatics/btl013. [DOI] [PubMed] [Google Scholar]
- 36.Drisdel RC, Alexander JK, Sayeed A, Green WN. Assays of protein palmitoylation. Methods. 2006;40:127–134. doi: 10.1016/j.ymeth.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 37.Tzolovsky G, Millo H, Pathirana S, Wood T, Bownes M. Identification and phylogenetic analysis of Drosophila melanogaster myosins. Mol Biol Evol. 2002;19:1041–1052. doi: 10.1093/oxfordjournals.molbev.a004163. [DOI] [PubMed] [Google Scholar]
- 38.Garoia F, Grifoni D, Trotta V, Guerra D, Pezzoli MC, Cavicchi S. The tumor suppressor gene fat modulates the EGFR-mediated proliferation control in the imaginal tissues of Drosophila melanogaster. Mech Dev. 2005;122:175–187. doi: 10.1016/j.mod.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Galindo MI, Bishop SA, Greig S, Couso JP. Leg patterning driven by proximal-distal interactions and EGFR signaling. Science. 2002;297:256–259. doi: 10.1126/science.1072311. [DOI] [PubMed] [Google Scholar]
- 40.Martin-Blanco E, Roch F, Noll E, Baonza A, Duffy JB, Perrimon N. A temporal switch in DER signaling controls the specification and differentiation of veins and interveins in the Drosophila wing. Development. 1999;126:5739–5747. doi: 10.1242/dev.126.24.5739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.