Abstract
Although CD4+ T cells can have an important role in mediating lethal graft-versus-host disease (GVHD) directed to multiple minor histocompatibility antigens (miHA) following bone marrow transplantation (BMT), their precise characterization and effector function remains elusive. In this regard, T cell receptor (TCR) Vβ spectratype analysis has been a powerful tool for identifying donor CD4+ T cell populations expanding to host miHA following BMT in the major histocompatibility complex (MHC)-matched C57BL/6 (B6)->C.B10-H2b (BALB.B) model of lethal GVHD. Removal of all of the Vβ+ T cell families containing these responding cells from the donor inoculum has proven to be an effective means of preventing the development of GVHD. Previous studies have also found that of the eleven miHA-responding B6 CD4+Vβ+ T cell families, transplantation of Vβ2+ and Vβ11+ T cells, together, into lethally irradiated BALB.B mice appeared primarily responsible for the severity of resultant GVHD. Further focusing on these critical CD4 responses, in this study, we demonstrate that B6 CD4+Vβ11+ T cells, alone, can induce lethal GVHD in BALB.B recipients. In addition, immunohistochemical staining of host lingual and intestinal epithelial tissues supported the capacity of Vβ11+ T cells to infiltrate typical GVHD-associated target areas. To further characterize the specific CD4+Vβ11+ T cells involved in this anti-miHA response, TCR Vα spectratype analysis was performed and indicated that six Vα chains were utilized by this reactive population. These results provide further evidence that a restricted repertoire of T cell specificities, presumably recognizing a correspondingly low number of miHA, is sufficient for the induction of severe GVHD.
Keywords: T cell receptor, V-alpha chain, graft-versus-host disease, repertoire, minor histocompatibility antigens
INTRODUCTION
The wide diversity of the T cell repertoire is achieved by both the rearrangement of gene segments composing each TCRα and β chain, as well as by the combining of these two chains to form the final heterodimer structure. During a T cell response, the repertoire can become significantly skewed as a result of the significant clonal and/or oligoclonal expansion of antigen-specific T cells [1-3]. This same repertoire skewing can also be observed in the context of alloreactive responses, as in the case of the development of graft-versus-host disease (GVHD) following allogeneic blood and marrow transplantation (BMT) [4-7].
GVHD is mediated by T cells from the donor hematopoietic stem cell inoculum and is often characterized by immunopathological injury to the skin, intestinal tract, and liver, leading to morbidity and mortality [8]. Although it is well accepted that removal of donor T cells from the graft can significantly decrease the incidence and severity of GVHD, the lack of a functioning T cell repertoire in the recipient results in an increase in leukemic relapse, engraftment failure, and opportunistic infections [9]. An alternative approach is to identify and remove only those specific T cells primarily involved in the alloreactive anti-host response associated with GVHD, and thus preserving a large part of the T cell repertoire to provide the beneficial T cell-associated effects. In this regard, we have previously used TCRVβ CDR3-size spectratype analysis to characterize the skewing of the T cell repertoire in response to minor histocompatibility antigens (miHA) in the B6->BALB.B H2b-matched strain combination [10-12]. Utilizing the information obtained from this analysis to manipulate the donor inoculum by depleting the alloreactive Vβ families, we found improved survival rates for the transplantation recipients [5, 10-12]. Similarly, in the haploidentical B6->(B6xDBA2)F1 strain combination, spectratype analysis was also used to identify leukemia-reactive Vβ+ T cells, which when transplanted into lethally irradiated tumor-challenged mice could mediate an effective graft-versus-leukemia (GVL) response without GVHD development [4, 5, 10, 13, 14].
An appreciation for the role of the TCRα chain with regard to antigen recognition is evolving. Several studies using single α or β chain transgenic mice indicated that most responding T cells could use various β chains, but only one α chain [15-18]. These murine studies, indicating a contributing role of the TCRα chain in antigen recognition, were further supported by analysis of lesions in autoimmune and GVHD-associated patients [6, 17, 19, 20]. In addition, crystal structure analysis of a TCR interacting with a peptide-MHC II complex revealed that the α chain actually made more peptide contacts than the β chain [21]. Other studies showed a requirement for conservation within both α and β chains when mutations within the J regions could eliminate T cell responses to peptide [22]. Taken together, these results suggested that there may be a more significant role for the TCRα chain in antigen recognition than was originally thought. Consequently, we hypothesized that analysis of a specific CD4+ T cell response against miHA during the development of GVHD would reveal preferential Vα chain usage.
To investigate the breadth of TCRα chain diversity, we again utilized the B6-> BALB.B strain combination and focused upon one of the two CD4+Vβ+ T cell populations that were previously observed to be directly involved in the severity of the disease [4]. Spectratype analysis had indicated that B6 CD4+ Vβ2+ and Vβ11+ T cells were skewed in the anti-host response, and the transplantation of an enriched population of both of these cells into lethally irradiated BALB.B recipients resulted in the development of lethal GVHD, whereas the remaining Vβ2-Vβ11- T cells did not [12]. Thus, in the current study, we sought to demonstrate the capacity of B6 CD4+Vβ11+ T cells, on their own, to induce GVHD, and to examine the complexity of this response by TCR Vα spectratype analysis. The results indicated that B6 CD4+ Vβ11+ T cells could be found infiltrating both lingual and intestinal sites of early immunopathological injury associated with GVHD. Furthermore, these reactive T cells utilized only six Vα chains, further demonstrating that an allogeneic response of limited scope can be of profound consequence in the development of GVHD.
MATERIALS AND METHODS
Mice
C57BL/6By (B6; H2b) and C.B10/LiMcdJ (BALB.B; H2b) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). In addition, BALB.B mice were provided from our own breeding colony. Male mice, 6-12 wk of age, were always used as donors while both female and male mice, 8-12 wk of age, were used as recipients. Mice were housed in a pathogen-free environment, housed in autoclaved microisolator cages, and were provided with autoclaved food and water ad libitum. Use of animals were approved by the Institutional Animal Care and Use Committees of both Thomas Jefferson University and Hackensack University Medical Center.
Isolation of CD4+ T Cells and GVHD Induction
BALB.B splenocytes (2×107) were injected intraperitoneally (i.p.) into B6 donor mice 17-21 days prior to GVHD induction. Spleens and lymph nodes (LN) were collected from the presensitized B6 mice, resuspended in Gey's balanced salt solution, containing 0.7% NH4Cl, to eliminate erythrocytes from the suspension, and washed with phosphate buffered saline (PBS; BioWhittaker, Walkerville, MI) containing 0.1% bovine serum albumin (BSA; Sigma, St. Louis, MO). B cells were then depleted from the spleen and LN cells by a panning procedure with plate-bound goat anti-mouse IgG (1:200 dilution; Cappel-Organon, Teknika, West Chester, PA; 1 hour, 4°C). Adherent cells were washed and resuspended in PBS containing 0.1% BSA (PBS/BSA) along with rat IgM anti-CD8 monoclonal antibody (mAb; 1:100 dilution of supernatants from hybridoma cells grown in CelLine flasks; BD Pharmingen; clone 3.168 [23]) and guinea pig complement (1:6 dilution; Rockland, Boyertown, PA; 45 minutes, 37°C) to deplete CD8+ T cells. Bone marrow cells were obtained from primed B6 mice by flushing femurs with PBS/BSA, and T cell-depleted bone marrow cells (ATBM) were prepared by incubation with anti Thy-1 mAb (J1j [24]; 1:100 dilution of supernatant) and complement for 45 minutes, 37°C.
For induction of GVHD, 2×106 ATBM cells along with 1.7-2.0×107 enriched CD4+ T cells were intravenously (i.v.) injected (0.25 ml PBS) into lethally irradiated (9.5 Gy, single dose or 13 Gy, split dose) BALB.B recipients, using a Mark I model 68A cesium source (J.L. Shephard, San Fernando, CA). Some Vβ11 transfer experiments were also performed at Hackensack University Medical Center, using a Gammacell 40 exactor (MDS Nordion, Ottawa, Ontario) with an exposure of 8.5 Gy. For Vβ11 transfer experiments, presensitized B6 CD4+ T cells were enriched, as described above, and positively-selected Vβ11+ cells (96-99% purity) and the remaining Vβ11- cells were sorted by magnetic cell separation using the VarioMACS system (Miltenyi Biotech, Auburn, CA). The number of Vβ11+ cells were injected at its % equivalent of the total unseparated CD4+ population. These cell populations along with ATBM (2×106) were injected i.v. into lethally irradiated (8.5-10 Gy) BALB.B recipients.
CDNA Isolation/Spectratype Analysis
Ten days following transplantation of 2×107 BALB.B-presensitized B6 CD4+ T cells (without ATBM cells) into irradiated (9.5 Gy) BALB.B mice, spleen and LN cells were harvested, enriched for CD4+ T cells, and Vβ11+ cells magnetically selected (97-99% purity, determined by flow cytometric analysis), as described above. Ultraspec (Biotecx Laboratories, Houston, TX) was added to the selected cells and RNA extracted according to manufacturer's instructions, and as previously described [10, 25]. RNA was precipitated in isopropanol, the pellet dissolved in DEPC water, heated for 10 minutes at 56°C, then combined with RNasin (40 U/μl), Moloney murine leukemia virus (M-MLV) reverse transcriptase buffer, oligo (dT)s (20 mmol/L), dNTPs (20 mmol/L), and M-MLV reverse transcriptase (300 U/uL) for 1.5 hours, 37°C. Reagents for cDNA synthesis were from Promega (Madison, WI), while the oligo (dT)s were synthesized in the Kimmel Cancer Center Oligo Synthesis Facility. RNA integrity was verified with β-actin primers.
PCR Amplification of cDNA for Vα CDR3-Size Spectratyping
A fluorescent-labeled Cα constant primer (PE-Applied Biosystems, Foster City, CA) was used to amplify the 3' end of cDNA while primers specific for each of the Vα families were used to amplify the 5' end of the cDNA. Constant primers and primers for Vα families 1-13 were previously published [26]. The NCBI nucleotide database was used to search for sequences used to generate primers for Vα 13-22. A BLAST search of these primers revealed that they did not overlap with other non TCRα sequences. Primers for Vα 13-22 used were: Vα13 TGAGGCCGAGTTTAGGAAGA; Vα14 GAGTCCTCAG-TCCCTGGTTG; Vα15 AACGATTCTCCCTGCACATC; Vα16 CTGTAGTGCAGAG-CCCTTCC; Vα17 TTCCATCGGACTCATCATCA; Vα18 AGAAGCGCAGTGGA-AGACTC; Vα19 TGCCTCATACCTCTGTGCTG; Vα20 TTCTCACTGCACATCAC-AGC; Vα22 GGAAAGGGTCTCCACTTTG
PCR was performed using the reagents and methods described previously for TCR Vβ CDR3-size spectratype analysis [5, 10]. Briefly, cDNA was added to a mixture of MgCl2 (25 mmol/L), dNTPs (800μM), 10X Taq Polymerase Buffer B (Promega), Vα sense primer (20 μmol/L), Cαa antisense primer (12 μmol/L), and Taq polymerase (1 U/μl), in a volume of 50 μl. After 35 cycles of PCR, semi-nested PCR was performed as described above; using a fifth of the first round product, the product's respective Vα sense primer (20 μmol/L), and the fluorescently-labeled Cαβ antisense primer (12 μmol/L). After another 35 cycles of amplification, products were run on a sequencing gel.
Quantitation of CDR3-Size Usage
Vα spectratype analyses were performed three separate times for both control B6 and experimental BALB.B CD4+Vβ11+ T cells to obtain statistical significance and reproducibility of results. After PCR products were run on a sequencing gel, they were analyzed, as previously described [5], by the Genotyper Genescan software program (PE-Applied Biosystems), which depicts each PCR product as a histogram. Histogram peaks were labeled 1-6, with 1 assigned to the smallest and 6 to the largest sized peak. The average area under each experimental peak for each Vα family was compared to the average area under the corresponding peak in the B6 control mice. The experimental peak was considered skewed when the mean area under the peak exceeded the mean plus three times the standard deviation of the same B6 control histogram peak.
Histologic Staining and Immunohistochemistry
Techniques for immunohistochemistry of frozen sections were previously described [27, 28]. Briefly, murine tongue and distal ileum were removed, embedded in O.C.T. compound (Miles Laboratories, Elkhart, IN), and snap-frozen in liquid nitrogen. Sections (5 um) were cut on a cryostat, air dried, and then acetone-fixed at -20°C. Sections were blocked with PBS containing 5% BSA and 0.1% Tween-20 (PBS-BSA/Tween), then incubated for 1 hour with primary mAb, either FITC-murine-anti-Vβ5 or rat-anti-Vβ11 (both at 1:50 in PBS-BSA/Tween; Pharmingen). Secondary antibodies were biotinylated goat-anti-FITC and biotinylated goat anti-rat IgG, respectively (1:200 in PBS-BSA/Tween; Vector Labs, Burlingame, CA), and were incubated for 30 minutes, 20°C. A tertiary antibody of horse radish peroxidase (HRP)-conjugated anti-goat Ig was then added and developed with the NovaRed substrate kit (SK-4800; Vector Labs). Slides were counterstained with Mayer's hematoxylin solution for contrast.
RESULTS
GVHD Potential of CD4+Vβ11+T Cells in BALB.B Recipients
In a previous study of the GVHD-associated B6 anti-BALB.B CD4+ TCR Vβ repertoire it was found that although Vβ skewing was indicative of alloreactive expansion, it did not always correlate with the potential for those particular Vβ+ cells to mediate severe GVHD [24]. In this case, the combined transfer of detectably skewed B6 CD4+Vβ10+ and Vβ12+ T cells into irradiated BALB.B recipients did not result in severe GVHD, whereas combined transfer of Vβ2+ and Vβ11+ T cells was a very potent mediator of disease. In order to more precisely examine the repertoire diversity of the TCR Vα chain during the development of GVHD, we sought to first determine the extent of B6 CD4+Vβ11+ T cell involvement during this response when transferred alone into BALB.B recipients. BALB.B-presensitized B6 CD4+Vβ11+ T cells were enriched (Figure 1A) by magnetic cell sorting using anti-Vβ11 mAb, as previously described [4] and transferred (5.6×105 cells/mouse; i.v.) into lethally irradiated BALB.B recipients along with 2×106 ATBM cells. Control mice received 2×106 ATBM cells, either alone or along with 2×107 unfractionated B6 CD4+ T cells. The results indicated that both unfractionated CD4+ and CD4+Vβ11+ T cells, alone, were able to mediate lethal GVHD, while the recipients receiving no T cells exhibited 100% survival (p≤0.001; Figure 1B). Analysis of recipient body weight also indicated equivalent significant weight loss in the two T cell groups throughout the course of the 80-day experiment (Figure 1C).
Fig 1.
B6 CD4+Vβ11+ T Cells Infiltrate BALB.B Target Organs During GVHD
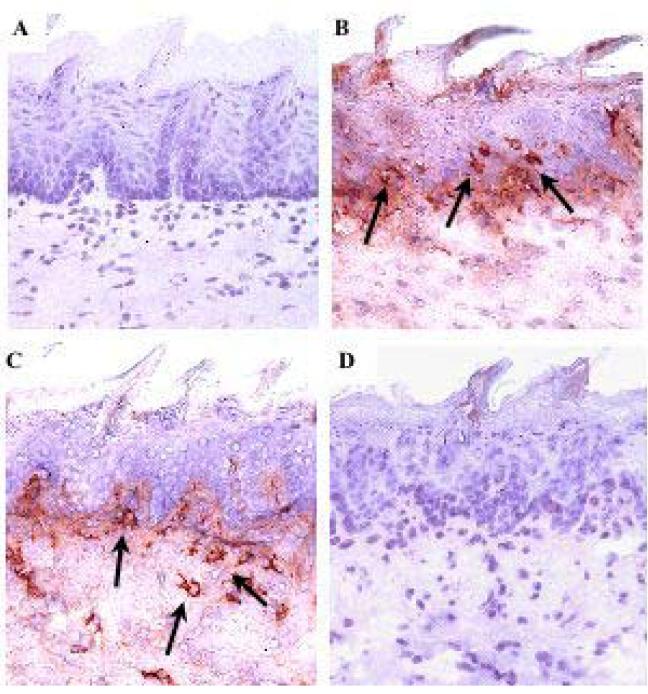
The skin and intestinal tract are two major target organs affected during the immunopathological development of GVHD. In human skin, alloreactive T cell infiltration is concentrated around the epidermal rete ridges located at the interface of the dermis and epidermal layers [29]. Although murine skin does not exhibit rete ridge structures, the counterpart to human skin can be found in the rete-like prominences (RLP) of the dorsal tongue squamous epithelium, and this site is also targeted by T cells mediating GVHD [29, 30]. We have previously used in situ analysis of lingual epidermal injury to confirm the presence of several spectratype-skewed B6 Vβ families in GVHD development in BALB.B mice, mediated by CD4+ T cells at day 30 post-transplant [5]. Here, we investigated whether CD4+Vβ11+ T cells could infiltrate murine RLP and crypts of the small intestine in the absence of CD4+ T cells from other Vβ family members. Immunohistochemical analysis of lingual epithelium demonstrated that transfer of either unfractionated B6 CD4+ or CD4+Vβ11+ T cells into lethally irradiated BALB.B recipients resulted in lymphocyte infiltration within the superficial submucosa and epithelium of both dorsal lingual tissue (Figures 2B and C, respectively) and intestinal crypts 9 days following transplant (Figures 3B and C, respectively), as compared to recipients receiving either ATBM (Figures 2A and 3A) or Vβ11-depleted CD4+ T cells (Figures 2D and 3D). Peak pathology was observed on day 15 in both the lingual epithelium and the small intestine, as reflected by the number of apoptotic cells within these given tissues (Figure 4). The total number of T cells, including theVβ11+ T cell population, seemed to be declining by day 15 in the tongue, although it was still increasing in the intestinal crypts by that time point. The number of apoptotic cells in a syngeneic B6->B6 control group (lethally irradiated B6 mice transplanted with B6 ATBM and 2.0×107 enriched CD4+ T cells) was less than 0.1/mm for lingual and less than 0.1/crypt for intestinal tissues, and the number of non-reactive (not skewed in the spectratype analysis)Vβ5+ T cells infiltrating those same tissues was negligible at both time points (data not shown). These data supported the capacity of B6 CD4+Vβ11+ T cells to infiltrate at least two major BALB.B GVHD-associated target tissues in the absence of other Vβ families, consistent with their ability to mediate lethal disease.
Fig 2.
Fig 3.
Fig 4.

B6 CD4+Vβ11+ T Cells Combine With All TCR Vα Chains
Given the likelihood that the TCR utilizes both of its α and β chains during donor T cell recognition of recipient miHA, we next investigated the scope of TCRα chain usage by the B6 CD4+Vβ11+ T cells associated with the GVHD response. Certain TCR Vα chains have been shown to have a preference for recognition of either MHC class I or II molecules [31]. Similarly, certain TCR Vα chains have also been reported to exhibit a preference for combining with certain TCR Vβ chains, as well [32]. In order to determine whether there was any preexisting TCRVα-bias for combining with Vβ11, we enriched B6 lymphocytes for CD4+Vβ11+ T cells by magnetic sorting and used similar methodology as for the TCRVβ chain spectratyping to amplify each of the 21 TCRVα families from this population. The resulting histograms for each of the Vβ11-associated TCRVα families were complex and unskewed, exhibiting a Gaussian distribution of CDR3-size (Figure 5), demonstrating that there was no inherent bias for a particular rearranged Vα chain to combine with the Vβ11 chain.
Fig 5.
Limited TCR Vα Chain Involvement During the GVHD Response
In order to determine whether there would be restricted TCRα chain usage by B6 CD4+Vβ11+ T cells in response to stimulation by miHA during the development of GVHD, we first transplanted 2×107 BALB.B-presensitized unfractionated B6 CD4+ T cells i.v. into lethally irradiated BALB.B mice. Ten days later, spleens and LN were harvested from BALB.B recipients and CD4+Vβ11+ T cells were positively-selected by magnetic cell separation, (98% purity). TCRVα spectratype analysis was performed, and the CDR3-size distribution for 20 resolvable Vα families was examined, in comparison to control spectratypes from normal B6 CD4+Vβ11+ T cells. The results, summarized in Figure 6, indicated significant skewing (p<0.05) in 6 of the 20 tested Vα families. Vα9, 13, 14, and 18 exhibited skewing in one peak, while Vα6 and 22 each had skewing in two peaks. These data indicated that a limited number of TCRVα chains combine with the population of B6 CD4+Vβ11+ T cells that are responding to BALB.B miHA in the context of a GVHD response.
Fig 6.
DISCUSSION
We initially observed that purified CD4+ T cells were able to mediate severe lethal GVHD in the B6->BALB.B strain combination, and that this response could be due to a limited number of reactive specificities [4, 33]. Subsequent studies, utilizing TCRVβ spectratype analysis to examine the scope of this response, through biased CDR3-size skewing, suggested that the Vβ2 and 11 families might have a dominant role in potentiating disease [5, 11, 12]. This notion was supported by the observation that Vβ11+ T cells exhibited a two-fold increase in infiltration of the lingual epithelium of BALB.B recipients transplanted with B6 CD4+ T cells. In addition, Vβ2 and 11 were both found to be consistently skewed in the spleen at days 7, 11, 15, 33, and 40 post-transplant, throughout the course of the GVHD response [11]. The important role of these cells was further confirmed by experiments in which B6 CD4+ Vβ2+ and Vβ11+ enriched T cells were co-transplanted into lethally irradiated BALB.B recipients and they elicited severe GVHD. Furthermore, exclusion of these two Vβ families from the donor CD4+ T cell inoculum resulted in significantly reduced GVHD and increased survival [12].
It was not clear however, whether either one of these two Vβ families could mediate GVHD when transferred individually into recipients. It could be argued that infiltration of the one CD4+Vβ+ T cell type into a particular tissue could be required to cause a threshold amount of tissue destruction, generating cryptic epitopes that would then be recognized by the other Vβ+ T cell, resulting in significant target tissue damage [34]. Alternatively, biased usage of a Vβ chain found in the peripheral lymphoid system, indicative of a response to miHA, does not always correlate with the ability of that same Vβ family member to mediate immunopathology in all target tissues [35]. This may be because of the inability of those T cells to gain access to particular tissue sites due to either the absence of specific adhesion molecules or the lack of tissue-specific miHA expression [36-39]. The current study extended our earlier results by demonstrating that transplanted B6 CD4+Vβ11+ T cells, alone, could cause GVHD-associated weight loss and mortality. In addition, immunohistochemical analysis demonstrated that these cells can infiltrate both lingual and intestinal epithelium in BALB.B recipients, suggesting appropriate expression of the targeted miHA in both target tissues.
Since antigen recognition may involve both the α and β TCR chains, we sought to investigate the extent to which B6 CD4+Vβ11-associated Vα was restricted in the response to miHA. Using CDR3-size spectratype analysis, we found that six TCRVα families (Vα 6, 9, 13, 14, 18, and 22) were skewed within the Vβ11+ T cell response obtained during the development of GVHD in BALB.B recipients. This biased TCRVα usage suggested recognition of a limited number of targeted BALB.B miHA during this Vβ11+ response. It is also interesting to note that spectratype histograms for the Vα16 and Vα19 families were not able to be resolved in the anti-miHA B6 CD4+Vβ11+ T cell population. The failure to generate these PCR products is not likely due to the inability of these particular Vα's to combine with the Vβ11+ protein, since both Vα16 and Vα19 histograms were successfully generated for normal CD4+Vβ11+ T cells. Rather, it is possible that the Vα16 and Vα19 families may somehow be deleted in vivo from the responding B6 CD4+Vβ11+ T cell population.
Previous data from our laboratory supports the hypothesis that a limited number of BALB.B miHA are being targeted during the B6 CD4+ T cell response, as evidenced by inter-recombinant inbred strain GVHD analysis and Vβ spectratype analysis of the responding B6 T cells [5, 11, 32]. Here, we have extended the characterization of the miHA-reactive B6 CD4+Vβ11+ T cells through analysis of the TCRVα chain and confirm that there is restricted usage by these T cells and thereby implying that there may only be a single, or at most a few, miHA responsible for eliciting this severe immunopathological response. The relevance of this finding is that, ultimately, if we can identify these important miHA, it might allow us to focus on similar types of antigens in the clinical setting in order to avoid severe GVHD development. Further focus on the combinational diversity of TCRVα with TCRVβ, along with varying TCR expression patterns within sites of tissue damage, will contribute to our understanding of GVHD directed against miHA.
ACKNOWLEDGMENTS
We are grateful for the expert technical assistance of Diana Whitaker-Menezes and Qian Zhan for histological preparation and immunohistochemical staining. We also appreciate the discussion of the work with Michael Appel. This research was supported by U.S. Public Health Service Research Grants HL-98895 and HL-55593 from the National Heart, Lung, and Blood Institute and CA-40358 from the National Cancer Institute.
REFERENCES
- 1.Nikolich-Zugich J, Slifka MK. Messaoudi I: The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4(2):123–32. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 2.Argaet VP, Schmidt CW, Burrows SR, et al. Dominant selection of an invariant T cell antigen receptor in response to persistent infection by Epstein-Barr virus. J Exp Med. 1994;180(6):2335–40. doi: 10.1084/jem.180.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsumoto Y, Yoon WK, Jee Y, et al. Complementarity-determining region 3 spectratyping analysis of the TCR repertoire in multiple sclerosis. J Immunol. 2003;170(9):4846–53. doi: 10.4049/jimmunol.170.9.4846. [DOI] [PubMed] [Google Scholar]
- 4.Friedman TM, Statton D, Jones SC, Berger MA, Murphy GF, Korngold R. Vbeta spectratype analysis reveals heterogeneity of CD4+ T-cell responses to minor histocompatibility antigens involved in graft-versus-host disease: correlations with epithelial tissue infiltrate. Biol Blood Marrow Transplant. 2001;7(1):2–13. doi: 10.1053/bbmt.2001.v7.pm11215694. [DOI] [PubMed] [Google Scholar]
- 5.Friedman TM, Statton D, Jones SC, Berger MA, Murphy GF, Korngold R. Vbeta spectratype analysis reveals heterogeneity of CD4+ T-cell responses to minor histocompatibility antigens involved in graft-versus-host disease: correlations with epithelial tissue infiltrate. Biol Blood Marrow Transplant. 2001;7(1):2–13. doi: 10.1053/bbmt.2001.v7.pm11215694. [DOI] [PubMed] [Google Scholar]
- 6.Hirokawa M, Matsutani T, Saitoh H, et al. Distinct TCRAV and TCRBV repertoire and CDR3 sequence of T lymphocytes clonally expanded in blood and GVHD lesions after human allogeneic bone marrow transplantation. Bone Marrow Transplant. 2002;30(12):915–23. doi: 10.1038/sj.bmt.1703730. [DOI] [PubMed] [Google Scholar]
- 7.Margolis DA, Casper JT, Segura AD, et al. Infiltrating T cells during liver graft-versus-host disease show a restricted T-cell repertoire. Biol Blood Marrow Transplant. 2000;6(4):408–15. doi: 10.1016/s1083-8791(00)70017-6. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara JL, Levy R, Chao NJ. Pathophysiologic mechanisms of acute graft-vs.-host disease. Biol Blood Marrow Transplant. 1999;5(6):347–56. doi: 10.1016/s1083-8791(99)70011-x. [DOI] [PubMed] [Google Scholar]
- 9.Kernan NA, Collins NH, Juliano L, Cartagena T, Dupont B, O'Reilly RJ. Clonable T lymphocytes in T cell-depleted bone marrow transplants correlate with development of graft-v-host disease. Blood. 1986;68(3):770–3. [PubMed] [Google Scholar]
- 10.Friedman TM, Gilbert M, Briggs C, Korngold R. Repertoire analysis of CD8+ T cell responses to minor histocompatibility antigens involved in graft-versus-host disease. J Immunol. 1998;161(1):41–8. [PubMed] [Google Scholar]
- 11.Friedman TM, Jones SC, Statton D, Murphy GF, Korngold R. Evolution of responding CD4+ and CD8+ T-cell repertoires during the development of graft-versus-host disease directed to minor histocompatibility antigens. Biol Blood Marrow Transplant. 2004;10(4):224–35. doi: 10.1016/j.bbmt.2003.12.303. [DOI] [PubMed] [Google Scholar]
- 12.Jones SC, Friedman TM, Murphy GF, Korngold R. Specific donor Vbeta-associated CD4 T-cell responses correlate with severe acute graft-versus-host disease directed to multiple minor histocompatibility antigens. Biol Blood Marrow Transplant. 2004;10(2):91–105. doi: 10.1016/j.bbmt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Patterson AE, Korngold R. Cross-protective murine graft-versus-leukemia responses to phenotypically distinct myeloid leukemia lines. Biol Blood Marrow Transplant. 2000;6(5A):537–47. doi: 10.1016/s1083-8791(00)70063-2. [DOI] [PubMed] [Google Scholar]
- 14.Patterson AE, Korngold R. Infusion of select leukemia-reactive TCR Vbeta+ T cells provides graft-versus-leukemia responses with minimization of graft-versus-host disease following murine hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7(4):187–96. doi: 10.1053/bbmt.2001.v7.pm11349805. [DOI] [PubMed] [Google Scholar]
- 15.Brandle D, Burki K, Wallace VA, et al. Involvement of both T cell receptor V alpha and V beta variable region domains and alpha chain junctional region in viral antigen recognition. Eur J Immunol. 1991;21(9):2195–202. doi: 10.1002/eji.1830210930. [DOI] [PubMed] [Google Scholar]
- 16.Engel I, Hedrick SM. Site-directed mutations in the VDJ junctional region of a T cell receptor beta chain cause changes in antigenic peptide recognition. Cell. 1988;54(4):473–84. doi: 10.1016/0092-8674(88)90068-2. [DOI] [PubMed] [Google Scholar]
- 17.Mantovani S, Palermo B, Garbelli S, et al. Dominant TCR-alpha requirements for a self antigen recognition in humans. J Immunol. 2002;169(11):6253–60. doi: 10.4049/jimmunol.169.11.6253. [DOI] [PubMed] [Google Scholar]
- 18.Yokosuka T, Takase K, Suzuki M, et al. Predominant role of T cell receptor (TCR)-alpha chain in forming preimmune TCR repertoire revealed by clonal TCR reconstitution system. J Exp Med. 2002;195(8):991–1001. doi: 10.1084/jem.20010809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsutani T, Yoshioka T, Tsuruta Y, et al. Restricted usage of T-cell receptor alpha-chain variable region (TCRAV) and T-cell receptor beta-chain variable region (TCRBV) repertoires after human allogeneic haematopoietic transplantation. Br J Haematol. 2000;109(4):759–69. doi: 10.1046/j.1365-2141.2000.02080.x. [DOI] [PubMed] [Google Scholar]
- 20.Oksenberg JR, Stuart S, Begovich AB, et al. Limited heterogeneity of rearranged T-cell receptor V alpha transcripts in brains of multiple sclerosis patients. Nature. 1991;353(6339):94. doi: 10.1038/353094a0. [DOI] [PubMed] [Google Scholar]
- 21.Reinherz EL, Tan K, Tang L, et al. The crystal structure of a T cell receptor in complex with peptide and MHC class II. Science. 1999;286(5446):1913–21. doi: 10.1126/science.286.5446.1913. [DOI] [PubMed] [Google Scholar]
- 22.Turner SJ, Cose SC, Carbone FR. TCR alpha-chain usage can determine antigen-selected TCR beta-chain repertoire diversity. J Immunol. 1996;157(11):4979–85. [PubMed] [Google Scholar]
- 23.Sarmiento M, Glasebrook AL, Fitch FW. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980;125(6):2665–72. [PubMed] [Google Scholar]
- 24.Bruce J, Symington FW, McKearn TJ, Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981;127(6):2496–501. [PubMed] [Google Scholar]
- 25.Gorski J, Yassai M, Zhu X, et al. Circulating T cell repertoire complexity in normal individuals and bone marrow recipients analyzed by CDR3 size spectratyping. Correlation with immune status. J Immunol. 1994;152(10):5109–19. [PubMed] [Google Scholar]
- 26.Casanova JL, Romero P, Widmann C, Kourilsky P, Maryanski JL. T cell receptor genes in a series of class I major histocompatibility complex-restricted cytotoxic T lymphocyte clones specific for a Plasmodium berghei nonapeptide: implications for T cell allelic exclusion and antigen-specific repertoire. J Exp Med. 1991;174(6):1371–83. doi: 10.1084/jem.174.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitaker-Menezes D, Jones SC, Friedman TM, Korngold R, Murphy GF. An epithelial target site in experimental graft-versus-host disease and cytokine-mediated cytotoxicity is defined by cytokeratin 15 expression. Biol Blood Marrow Transplant. 2003;9(9):559–70. doi: 10.1016/s1083-8791(03)00288-x. [DOI] [PubMed] [Google Scholar]
- 28.Korngold R, Jameson BA, McDonnell JM, et al. Peptide analogs that inhibit IgE-Fc epsilon RI alpha interactions ameliorate the development of lethal graft-versus-host disease. Biol Blood Marrow Transplant. 1997;3(4):187–93. [PubMed] [Google Scholar]
- 29.Sale GE, Raff RF, Storb R. Stem cell regions in filiform papillae of tongue as targets of graft-versus-host disease. Transplantation. 1994;58(11):1273–5. [PubMed] [Google Scholar]
- 30.Gilliam AC, Whitaker-Menezes D, Korngold R, Murphy GF. Apoptosis is the predominant form of epithelial target cell injury in acute experimental graft-versus-host disease. J Invest Dermatol. 1996;107(3):377–83. doi: 10.1111/1523-1747.ep12363361. [DOI] [PubMed] [Google Scholar]
- 31.Sim BC, Lo D, Gascoigne NR. Preferential expression of TCR V alpha regions in CD4/CD8 subsets: class discrimination or co-receptor recognition? Immunol Today. 1998;19(6):276–82. doi: 10.1016/s0167-5699(98)01257-2. [DOI] [PubMed] [Google Scholar]
- 32.Uematsu Y. Preferential association of alpha and beta chains of the T cell antigen receptor. Eur J Immunol. 1992;22:603–606. doi: 10.1002/eji.1830220247. [DOI] [PubMed] [Google Scholar]
- 33.Korngold R, Leighton C, Mobraaten LE, Berger MA. Inter-strain graft-vs.-host disease T-cell responses to immunodominant minor histocompatibility antigens. Biol Blood Marrow Transplant. 1997;3(2):57–64. [PubMed] [Google Scholar]
- 34.el-Shami K, Tirosh B, Bar-Haim E, et al. MHC class I-restricted epitope spreading in the context of tumor rejection following vaccination with a single immunodominant CTL epitope. Eur J Immunol. 1999;29(10):3295–301. doi: 10.1002/(SICI)1521-4141(199910)29:10<3295::AID-IMMU3295>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 35.Kubo K, Yamanaka K, Kiyoi H, et al. Different T-cell receptor repertoires between lesions and peripheral blood in acute graft-versus-host disease after allogeneic bone marrow transplantation. Blood. 1996;87(7):3019–26. [PubMed] [Google Scholar]
- 36.Griem P, Wallny HJ, Falk K, et al. Uneven tissue distribution of minor histocompatibility proteins versus peptides is caused by MHC expression. Cell. 1991;65(4):633–40. doi: 10.1016/0092-8674(91)90095-g. [DOI] [PubMed] [Google Scholar]
- 37.Miconnet I, de la Selle V, Tucek C, Huchet R, Bonardelle D, Bruley-Rosset M. Tissue distribution and polymorphism of minor histocompatibility antigens involved in GVHR. Immunogenetics. 1994;39(3):178–86. doi: 10.1007/BF00241258. [DOI] [PubMed] [Google Scholar]
- 38.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105(11):4191–9. doi: 10.1182/blood-2004-12-4726. Epub 2005 Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiocchia G, Boissier MC, Fournier C. Therapy against murine collagen-induced arthritis with T cell receptor V beta-specific antibodies. Eur J Immunol. 1991;21(12):2899–905. doi: 10.1002/eji.1830211202. [DOI] [PubMed] [Google Scholar]