Abstract
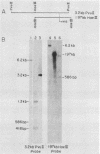
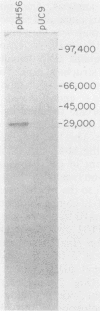
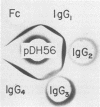
DNA containing the 5' end of the M-12 structural gene was used as a probe in colony hybridizations in an attempt to clone the M-76 gene from an M-type 76 group A streptococcal strain. A single positive colony was detected, and Southern hybridization analysis of plasmid DNA isolated from this colony indicated that the insert DNA had homology to the 5' end of the M-12 structural gene. Subclones were constructed to define the limits of the M-76 gene, and sonicates of these subclones were reacted with M-76-specific antiserum in immunodiffusion. A sonicate of one subclone, JM83(pDH56), reacted strongly with the M-76-specific antiserum but also reacted with preimmune rabbit serum. Protein expressed from this subclone bound immunoglobulin from horse and pig, as well as human myeloma immunoglobulin G (IgG) representing all four subclasses and purified human IgG Fc fragments. This indicated that JM83(pDH56) expressed a protein with characteristics previously attributed to the IgG Fc receptor protein from group A streptococci. Western blot analysis indicated that the cloned IgG Fc receptor protein had a molecular weight of approximately 29,000. Binding studies showed that the Fc receptor gene is expressed by the M-type 76 strain from which it was cloned and by an M- variant.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen P., Grubb A., Grubb R., Samuelsson G., Schalén C., Svensson M. L. Demonstration of the non-identity between the Fc receptor for human IgG from group A streptococci type 15 and M protein, peptidoglycan and the group specific carbohydrate. Acta Pathol Microbiol Scand C. 1979 Jun;87C(3):257–261. [PubMed] [Google Scholar]
- Christensen P., Johansson B. G., Kronvall G. Interaction of streptococci with the Fc fragment of IgG. Acta Pathol Microbiol Scand C. 1976 Apr;84(2):73–76. doi: 10.1111/j.1699-0463.1976.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Manjula B. N., Scott J. R. Streptococcal M6 protein expressed in Escherichia coli. Localization, purification, and comparison with streptococcal-derived M protein. J Exp Med. 1984 Apr 1;159(4):1083–1095. doi: 10.1084/jem.159.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. N. M proteins of group A streptococci. Bacteriol Rev. 1974 Mar;38(1):57–86. doi: 10.1128/br.38.1.57-86.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb A., Grubb R., Christensen P., Schalén C. Isolation and some properties of an IgG Fc-binding protein from group A streptococci type 15. Int Arch Allergy Appl Immunol. 1982;67(4):369–376. doi: 10.1159/000233049. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Wallis J. Colony hybridization. Methods Enzymol. 1979;68:379–389. doi: 10.1016/0076-6879(79)68027-8. [DOI] [PubMed] [Google Scholar]
- Guss B., Eliasson M., Olsson A., Uhlén M., Frej A. K., Jörnvall H., Flock J. I., Lindberg M. Structure of the IgG-binding regions of streptococcal protein G. EMBO J. 1986 Jul;5(7):1567–1575. doi: 10.1002/j.1460-2075.1986.tb04398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G. A surface component in group A, C, and G streptococci with non-immune reactivity for immunoglobulin G. J Immunol. 1973 Nov;111(5):1401–1406. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lancefield R. C. THE ANTIGENIC COMPLEX OF STREPTOCOCCUS HAEMOLYTICUS : I. DEMONSTRATION OF A TYPE-SPECIFIC SUBSTANCE IN EXTRACTS OF STREPTOCOCCUS HAEMOLYTICUS. J Exp Med. 1928 Jan 1;47(1):91–103. doi: 10.1084/jem.47.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre E. B., Kronvall G. Heterogeneity of nonimmune immunoglobulin Fc reactivity among gram-positive cocci: description of three major types of receptors for human immunoglobulin G. Infect Immun. 1977 Sep;17(3):475–482. doi: 10.1128/iai.17.3.475-482.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Reis K. J., Boyle M. D., Ayoub E. M. Identification of distinct Fc-receptor molecules on streptococci and staphylococci. J Clin Lab Immunol. 1984 Feb;13(2):75–80. [PubMed] [Google Scholar]
- Rýc M., Wagner M., Wagner B., Havlicek J. Electron microscopic study of the location of the Fc-reacting factor on group A streptococci. Microbios. 1982;34(135):7–15. [PubMed] [Google Scholar]
- Spanier J. G., Cleary P. P. A DNA substitution in the group A streptococcal bacteriophage SP24. Virology. 1983 Oct 30;130(2):514–522. doi: 10.1016/0042-6822(83)90104-6. [DOI] [PubMed] [Google Scholar]
- Spanier J. G., Cleary P. P. Bacteriophage control of antiphagocytic determinants in group A streptococci. J Exp Med. 1980 Nov 1;152(5):1393–1406. doi: 10.1084/jem.152.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanier J. G., Jones S. J., Cleary P. Small DNA deletions creating avirulence in Streptococcus pyogenes. Science. 1984 Aug 31;225(4665):935–938. doi: 10.1126/science.6089334. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yarnall M., Boyle M. D. Identification of a unique receptor on a group A streptococcus for the Fc region of human IgG3. J Immunol. 1986 Apr 1;136(7):2670–2673. [PubMed] [Google Scholar]