Abstract
To investigate chemokine expression networks in chronic hepatitis C virus (HCV) infection, we used microarray analysis to determine chemokine expression in human infection and in chimpanzees experimentally infected with HCV. The CXCR3 chemokine family was highly expressed in both human and chimpanzee infection. CXCL10 was the only CXCR3 chemokine elevated in the serum, suggesting that it may neutralize any CXCR3 chemokine gradient established between the periphery and liver by CXCL11 and CXCL9. Thus, CXCR3 chemokines may not be responsible for recruitment of T lymphocytes but may play a role in positioning these cells within the liver. The importance of the CXCR3 chemokines, in particular CXCL11, was highlighted by replicating HCV (JFH-1) to selectively upregulate its expression in response to gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α). This selective upregulation was confirmed at the transcriptional level by using the CXCL11 promoter driving the luciferase reporter gene. This synergistic increase in expression was not a result of HCV protein expression but the nonspecific innate response to double-stranded RNA (dsRNA), as both in vitro-transcribed HCV RNA and the dsRNA analogue poly(I:C) increased CXCL11 expression and promoter activity. Furthermore, we show that CXCL11 is an IRF3 (interferon regulatory factor 3) response gene whose expression is selectively enhanced by IFN-γ and TNF-α. In conclusion, the CXCR3 chemokines are the most significantly expressed chemokines in chronic hepatitis C and most likely play a role in positioning T cells in the liver. Furthermore, HCV can selectively increase CXCL11 expression in response to IFN-γ and TNF-α stimulation that may play a role in the pathogenesis of HCV-related liver disease.
Hepatitis C virus (HCV) is the leading cause of chronic hepatitis and liver disease-related morbidity worldwide, with a significant proportion of infected individuals developing cirrhosis, hepatic failure, and/or hepatocellular carcinoma (8, 38). Chronic hepatitis C (CHC) is characterized by the presence of an inflammatory infiltrate having various degrees of severity in both portal tracts and hepatic parenchyma and resulting in piecemeal necrosis. The underlying mechanism(s) driving disease progression is not well understood; however, there is increasing evidence that a direct immune response to HCV-infected hepatocytes plays a role in the pathogenic process. HCV-specific T lymphocytes are the predominant cell population infiltrating the liver and are present at frequencies 10- to 30-fold higher than those in the peripheral circulation (11). There is also increasing evidence to suggest that extension of the inflammatory process from portal tracts into periportal and lobular regions could be fundamental in the development of progressive liver injury (6, 7). The factors that regulate the recruitment of T lymphocytes and other components of the inflammatory response to the HCV-infected liver are not well characterized; however, it has been hypothesized that chemokines and other cytokines play a major role.
In livers chronically infected with HCV, expression levels of CXCL10 and CXCL9 (both members of the CXCR3 chemokine family) are elevated, and recent studies from our laboratory have demonstrated that CXCL11 (also a CXCR3 chemokine) is also upregulated (2, 10, 12, 13, 39). In CHC, these chemokines are expressed by hepatocytes (10, 13), which, taken together with the observation that the majority of intrahepatic T lymphocytes express CXCR3 (10), suggests that the CXCR3 chemokine network may play a pivotal role in driving T lymphocytes to the liver and subsequent liver disease.
The CXCR3 ligands are predominantly type II interferon (IFN-γ) regulated, with a synergistic increase in expression occurring in the presence of tumor necrosis factor alpha (TNF-α) (13, 42). Considering that levels of IFN-γ and TNF-α are increased in HCV-infected liver and serum (28, 30), one might expect chemokines such as CXCL10, CXCL9, and CXCL11 to be upregulated. However, other chemokines, such as CXCL16, also known to be induced synergistically by IFN-γ and TNF-α (1) and to play a role in hepatitis (14), have not yet been demonstrated to be upregulated in the HCV-infected liver. This suggests that there are other mechanisms involved in the upregulation of the CXCR3 chemokines in the HCV-infected liver, one of which may be the virus itself. HCV and other Flaviviridae members have previously been shown to regulate various chemokine promoters. For instance, the interleukin-8 (IL-8) promoter has been reported to be modulated by Dengue virus NS5a protein (26) and HCV NS4a and 4b proteins (16), while the RANTES and MCP-1 promoters are transcriptionally activated by the HCV core (36, 40) and NS5a (40). In contrast, little is known regarding viral modulation of CXCR3 chemokine expression.
Detection of RNA viruses by the host occurs primarily through two types of double-stranded RNA (dsRNA) cytoplasmic pattern recognition receptors, retinoic acid-inducible gene I (RIG-I) and the pathogen-associated molecular pattern receptor known as Toll-like receptor 3 (TLR-3) (reviewed in reference 33). This pathway has been implicated in many RNA virus infections, and its importance in the host antiviral response is reinforced by the fact that many viruses have evolved mechanisms for evading this response, including HCV (41). dsRNA sensed by RIG-I or TLR-3 activates the latent transcription factors IRF3 (interferon regulatory factor 3) (through the adaptor molecule IPS-1 for RIG-I) and NF-κB (45). IRF3 and NF-κB translocate to the nucleus, where they bind to their cognate promoter elements and, together with other transcription factors, induce gene expression. Activated IRF3 binds to an interferon-stimulated response element (ISRE) present upstream of many interferon-stimulated genes (4, 15). IRF3 regulates the expression of a number of chemokine genes. For example, IRF3 induces expression of the chemokine RANTES by binding to an ISRE in the RANTES promoter, while dsRNA-induced expression of IL-8 is dependent on IRF3 activation and an ISRE within the IL-8 promoter element (23, 43). This suggests functional links between the innate antiviral response and inflammatory networks that would significantly impact the host response to viral infection.
There are significant gaps in our knowledge regarding factors responsible for recruitment of T lymphocytes into the HCV-infected liver. Using liver samples from human CHC patients and chimpanzees experimentally infected with HCV, we show that the CXCR3 chemokines are the most dominantly expressed chemokine family and that HCV replication in vitro can modulate expression of these chemokines through a dsRNA mechanism. Specifically, we show that CXCL11 expression is mediated by activation of the RIG-I pathway and is enhanced through stimulation with IFN-γ and TNF-α. Understanding T-lymphocyte trafficking and regulation of expression of chemokines involved in CHC has implications for understanding pathogenesis, vaccine design, and development of novel therapeutic strategies.
MATERIALS AND METHODS
Tissue, RNA isolation, and microarray analysis.
Liver biopsy samples were collected from nondiseased and HCV-infected patients and ethics approval was obtained as previously described (13). Nondiseased liver samples were obtained from sites distant from hepatic metastasis and deemed free of infection via histological analysis. All patients included in the study were negative for hepatitis B surface-antigen, and had no other causes of liver disease (Table 1). Total cellular RNA was isolated from liver biopsy samples and nondiseased liver samples by using an RNAqueous RNA extraction kit (Ambion, CA). Microarray analysis was performed as previously described (12), within the UTMB Molecular Genomics Core Facility, utilizing Affymetrix U133 plus 2.0 array gene chips. The gene expression profiles for HCV-infected liver biopsy samples were compared to those for pooled nondiseased liver samples as a baseline (n = 4), and transcripts demonstrating at least a 2.5-fold increase over those for pooled nondiseased liver samples were considered significantly elevated. Microarray analysis of liver samples from experimentally infected chimpanzees was performed as previously described (5).
TABLE 1.
Liver biopsy samples
| Method, sample type, and sample no. | Inflammation scorea
|
Donor age (yr) | Genotype | Donor sexb | |
|---|---|---|---|---|---|
| Lobular | Portal | ||||
| Affymetrix | |||||
| HCV-infected liver | |||||
| 19G | 2 | 2 | 23 | 3a | M |
| 31G | 3 | 2 | 53 | 1b | M |
| 35G | 2 | 2 | 36 | 3a | F |
| 5G | 2 | 2 | 30 | 3a | M |
| 39 | 3 | 3 | 41 | 3a | M |
| 37G | 1 | 0 | 46 | 3a | F |
| 28 | 1 | 2 | 50 | 1b | M |
| 10 | 2 | 2 | 49 | 3a | M |
| NHLc | |||||
| C1 | 0 | 0 | 46 | M | |
| C2 | 0 | 0 | 56 | M | |
| C3 | 0 | 0 | 39 | M | |
| C4 | 0 | 0 | 53 | F | |
| Real-time PCRd | |||||
| 1 | 2 | 3 | 37 | 3 | M |
| 2 | 1 | 2 | 42 | 3 | M |
| 4 | 0 | 0 | 45 | 3 | M |
| 5 | 0 | 1 | 52 | 1 | M |
| 6 | 2 | 1 | 27 | 1 | M |
| 8 | 1 | 1 | 21 | 3 | F |
| 10 | 2 | 2 | 47 | 3 | M |
| 11 | 3 | 1 | 32 | 1 | M |
| 12 | 3 | 3 | 63 | 1 | F |
| 13 | 3 | 3 | 75 | 1 | M |
| 14 | 2 | 1 | 53 | 3 | M |
| 15 | 3 | 4 | 53 | 2 | M |
| 22 | 1 | 2 | 40 | 2 | F |
| 24 | 2 | 3 | 51 | 1 | F |
| 26 | 2 | 3 | 43 | 3 | F |
| 27 | 1 | 2 | 38 | 1 | M |
| 28 | 2 | 3 | 66 | 1 | F |
| 34 | 2 | 2 | 24 | 1 | M |
| 36 | 1 | 2 | 39 | 1 | M |
Scheuer classification.
M, male; F, female.
NHL, nondiseased human liver.
Performed on HCV-infected liver biopsy samples.
Liver histology.
Histological assessment of liver biopsy samples was performed by an experienced liver pathologist (A. Ruszkiewicz). Grading of each section was performed in a blind manner and in accordance with the Scheuer index (37).
RNA extraction, cDNA synthesis, and PCR analysis.
Real-time PCR analysis was utilized to quantitate mRNA corresponding to CXCL11, CXCL10, and CXCL9. Total cellular RNA was isolated from liver biopsy material or in vitro cell monolayers by using Trizol (Invitrogen, CA), and first-strand cDNA was synthesized and real-time PCR performed as described previously (13). The primers for cDNA detection were as follows: for CXCL11, 5′-CAAGGCTTCCCCATGTTCA-3′ and 5′-CCCAGGGCGTATGCAAAGA-3′; for CXCL10, 5′-TCCACGTGTTGAGATCATTGC-3′ and 5′-TCTTGATGGCCTTCGATTCTG-3′; for CXCL9, 5′-GAGTGCAAGGAACCCCAGTAGT-3′ and 5′-GGTGGATAGTCCCTTGGTTGGT-3′; and for RPLPO, control gene primers 5′-AGATGCAGCAGATCCGCAT-3′ and 5′-GGATGGCCTTGCGCA-3′.
Analysis of serum chemokine levels.
Sera were collected from 16 treatment-naïve CHC patients (average age = 45.4 ± 8.2) attending the Royal Adelaide Hospital liver clinic and from 16 healthy controls (average age = 39.4 ± 11.3) sourced from healthy blood donors attending the Australian Red Cross donation facility in Adelaide. Serum samples were processed within 2 h of collection and stored at −80°C until required. Samples were diluted appropriately in 0.1% bovine serum albumin and analyzed for CXCR3 chemokine expression. Enzyme-linked immunosorbent assay (ELISA) analysis was performed using R&D Systems antibody pairs, following the manufacturer's instructions, as previously described (13).
Cell lines.
Human hepatoma Huh-7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL, Grand Island, NY) supplemented with 10% fetal bovine serum, l-glutamine, penicillin, and streptomycin in a humidified 37°C/5% CO2 incubator.
JFH-1 infection of Huh-7 cells.
Huh-7 cells were seeded at 1.6 × 106 cells in a T75-cm3 flask for 24 h before being infected with 2 × 104 focus-forming units/ml of JFH-1 virus (provided by T. Wakita, National Institute of Infectious Diseases, Tokyo, Japan) (48) in a 2-ml volume of supplemented DMEM for 6 h. Following initial infection, the medium volume was increased to 10 ml and the cells were left for 48 or 72 h before being seeded for the luciferase assay or mRNA quantification experiments, respectively. Upon completion of the experiment, JFH-1-infected Huh-7 cells were analyzed to ensure HCV protein expression by using indirect immunofluorescence microscopy essentially as described previously (21), with the exception that cells were incubated at a 1/300 dilution of mixed sera taken from patients chronically infected with HCV and a fluorescein isothiocyanate-conjugated rabbit anti-human immunoglobulin G secondary antibody (Chemicon, Temecula, CA). Cells were visualized using an Olympus Provis AX70 microscope, and all experiments were performed at least in triplicate. All cells were at least 80% JFH-1 infected at the completion of the experiments, as determined by cell enumeration (data not shown).
CXCR3 chemokine promoter analysis.
DNA fragments comprising 934 and 1,013 bp and located upstream of the CXCL11 and CXCL9 ATG start sites, respectively, were cloned into the luciferase reporter vector pGL3 (Promega, WI). This was performed using directional cloning and the following primer pairs: for CXCL11, 5′-GGCTTCACCCCTCAACGAGTGG-3′ (forward) and 5′-GTTTGTTTTTTGCTGTTGCTGCTG-3′ (reverse), tagged with the NheI and XhoI restriction sites; and for CXCL9, 5′-TGATTATGGCCATTCTTGCAGG-3′ (forward) and 5′-AGTGATAGAATGGAGTTCCAAG-3′ (reverse), tagged with the XhoI and HindIII restriction sites. The CXCL10 promoter construct was a kind gift from R. M. Ransohoff (Lerner Research Institute, Cleveland, OH) (25).
Promoter studies were performed via transfection of constructs into naïve and JFH-1-infected Huh-7 cells. Cells (7 × 104) were transfected with 400 ng of a reporter construct either alone or in combination with (i) 400 ng of HCV protein-expressing plasmid (pCDNA3.1 CP7, NS3, and NS4a; pCDNA6 NS3/4a and 3-5B; and pCMV NS5a-Flag and NS5b-Flag, all kind donations from Kui Li, University of Texas Medical Branch) (9, 17, 20); (ii) RIG-I-wt, RIG-N, IRF3-wt, or IRF3-5D (all kind donations from Stephen Polyak, University of Washington, Seattle, WA) (22, 45), using Fugene 6 reagent (Roche, Indianapolis, IN); or (iii) in vitro-transcribed JFH-1 or 5′ untranlated region (5′UTR) RNA (JFH-1) transfection 24 h after reporter construct transfection as described below. Ten nanograms of PhRL-TK, a Renilla-expressing vector, was also cotransfected to normalize transfection efficiency. Cultures were incubated for 24 h before treatment with 1,000 U/ml IFN-γ either alone or in combination with 40 ng/ml TNF-α (Peprotech, Rehovot, Israel) for a further 24 h. Luciferase activity was measured using the luciferase assay system (Promega) and a Turner TD 20/20 luminometer. All experiments were performed at least in triplicate.
Treatment of cells with dsRNA, interferon, and TNF-α.
Huh-7 cells were seeded at 2 ×105 in a six-well plate without antibiotics and allowed to settle for 24 h before RNA transfection with either 2 μg of T7 RNA polymerase-derived JFH-1 RNA, HCV 5′UTR (ApaI-digested JFH-1), or poly(I)-poly(C) RNA (Sigma, MO), using DMRIE C (Invitrogen). Transfection reagent was removed at 4 h and replaced with either complete DMEM or IFN-γ (1,000 units/ml) in combination with TNF-α (40 ng/ml) for 4 h. RNA was then harvested and real-time PCR performed as described previously (12). Experiments involving poly(I)-poly(C) RNA stimulation of the CXCR3 promoters were performed as described above 24 h after transfection of the CXCR3 luciferase promoter constructs into Huh-7 cells. All experiments were performed at least in triplicate.
Statistical analysis.
The nonparametric Spearman correlation was used for all correlations. Student t tests and the Wilcoxon rank sum method were utilized to analyze the distributions of two normally distributed and nonnormally distributed independent data sets. All statistical analysis was performed using SPSS 10.
RESULTS
The CXCR3 ligands are highly expressed in CHC.
We have previously reported elevated expression of the CXCR3 ligand CXCL11 in CHC (12). To gain further insight into possible global chemokine networks at play in CHC, we further interrogated our array data to identify differential expression levels of chemokine transcripts in livers of both human CHC patients and chimpanzees experimentally infected with HCV. Analysis of liver samples from eight humans chronically infected with HCV revealed seven chemokine transcripts elevated in at least three out of eight patients (Table 2). Most striking were the CXCR3 ligands CXCL10, CXCL11, and CXCL9, which were upregulated in all eight patients, with average mRNA increases of 62-, 21-, and 19-fold, respectively, compared with levels for nondiseased liver samples. The CCR5 ligand RANTES was also upregulated in six out of eight patients (average increase of 7.6-fold) and, along with the three CXCR3 ligands, has previously been reported to be elevated in CHC (10, 12, 13, 18, 39). Other chemokines found to be upregulated in fewer patients were GCP-2, Mip-3β, and SCM-1β.
TABLE 2.
Chronic HCV levels
| Host and ligand | Receptor | No. of subjectsa with upregulation | Range (avg) of fold increase |
|---|---|---|---|
| Human | |||
| IP-10 (CXCL10) | CXCR3 | 8 | 42-97 (62.0) |
| I-TAC (CXCL11) | CXCR3 | 8 | 39-147 (21.4) |
| Mig (CXCL9) | CXCR3 | 8 | 2.5-30 (19.2) |
| GCP-2 (CXCL6) | CXCR1/2 | 3 | 30-45 (37.3) |
| RANTES (CCL5) | CCR5 | 6 | 2.5-21 (7.6) |
| MIP-3β (CCL19) | CCR7 | 3 | 26-39 (34.0) |
| SCM-1beta (XCL2) | XCR1 | 5 | 3.5-5 (4.1) |
| Chimpanzee | |||
| IP-10 (CXCL10) | CXCR3 | 18 | 2-57 (24.3) |
| I-TAC (CXCL11) | CXCR3 | 18 | 3.5-97 (28.0) |
| Mig (CXCL9) | CXCR3 | 2 | NDb |
| RANTES (CCL5) | CCR5 | -c | - |
Humans (n = 8) or chimpanzees (n = 18).
ND, not determined.
Decreasing trend.
We next investigated whether a similar profile of chemokine expression occurs in chimpanzees chronically infected with HCV. Analysis of Affymetrix GeneChip data from 18 chimpanzees chronically infected with HCV revealed that CXCL10 and CXCL11 were the most significantly elevated chemokines (24.3- and 28-fold, respectively); however, in contrast to what was found for humans, CXCL9 and RANTES were not elevated. Together with the human data, this suggests that the CXCR3 chemokines play a significant role in recruitment of T lymphocytes into the liver and that, while similarities exist, there are also differences between the livers of chimpanzees and humans chronically infected with HCV.
Real-time PCR was performed on 19 separate liver biopsy samples from CHC patients to analyze the relative distributions of the CXCR3 ligands in individual patients (Fig. 1). All patients had increased mRNA levels of all three chemokines, with the average increases above the levels for pooled nondiseased human liver samples being 17.54-, 31.95-, and 15.23-fold for CXCL11, CXCL10, and CXCL9, respectively (Fig. 1B). However, the upregulation of these three chemokines is not uniform in all patients; this is further supported by analysis of the correlation of these individual data sets, where upregulation of CXCL11 correlates with that of CXCL10 and CXCL9 (P < 0.001 and P = 0.01, respectively) (Fig. 1B) whereas no correlation can be seen when increases of CXCL9 and CXCL10 for different patients are compared. These data are suggestive of some form of variable stimulation of these transcripts within the livers of CHC patients, perhaps in addition to that by endogenous IFN-γ and TNF-α.
FIG. 1.
CXCR3 ligand mRNA is increased in liver biopsy samples from CHC patients. (A) Real-time PCR analysis of liver biopsy samples taken from CHC patients was able to demonstrate an increase in all CXCR3 ligands for all patients compared to the level for pooled nondiseased human liver (NHL) samples. (B) Average increases in CXCR3 ligand mRNA for all patients plotted demonstrated significant distribution differences (P = 0.017), with means of 17.54-, 31.95-, and 15.23-fold above those seen for NHL samples for CXCL11, CXCL10, and CXCL9, respectively. ANOVA, analysis of variance.
CXCR3 ligand expression correlates with liver inflammation.
In the present study, CXCL11, CXCL10, and CXCL9 mRNA levels were found to correlate significantly with the Scheuer classification lobular inflammation scores for each biopsy sample (P = 0.001, P = 0.014, and P = 0.002, respectively) (Fig. 2). However, a significant correlation between chemokine mRNA increase and portal inflammation was demonstrated only for CXCL11 (P = 0.033). No correlations were observed between chemokine mRNA levels and total Scheuer scores or fibrosis scores (data not shown). Collectively, these results suggest that the CXCR3 ligands may play a role in immune-mediated pathogenesis of hepatitis C, with CXCL11 potentially having the more dominant role.
FIG. 2.
CXCR3 ligand mRNA expression correlates with liver inflammation in CHC. Blinded histological analysis of HCV liver biopsy sections revealed that Scheuer scores for lobular inflammation significantly correlated with the increases in CXCL11, CXCL10, and CXCL9 mRNA levels compared to the levels for pooled nondiseased human liver (NHL) samples. A significant correlation between mRNA increase and portal inflammation score was seen only for CXCL11. NS, not significant.
CXCR3 chemokine expression in sera from CHC patients versus healthy controls.
Sera taken from CHC patients (n = 16) or healthy controls (n = 16) were analyzed using ELISA for CXCL11, CXCL10, and CXCL9. Figure 3 demonstrates that only CXCL10 levels were significantly increased in the sera of HCV patients compared with levels for healthy controls (P = 0.0037). The mean CXCL11, CXCL10, and CXCL9 protein concentrations in sera from CHC patients were 368, 2,218, and 633 pg/ml, respectively, and those in sera from healthy controls were 261, 1,405, and 693 pg/ml, respectively. These results suggest that, unlike for CXCL10, a significant chemokine gradient exists for CXCL11 and CXCL9 between the liver (increased levels) (Fig. 1) and the peripheral circulation (no increase) (Fig. 3).
FIG. 3.
CXCL11 and CXCL9 serum levels are not increased in CHC. ELISA for CXCR3 ligand levels in sera taken from CHC patients demonstrated mean values of 368, 2,218, and 633 pg/ml for CXCL11, CXCL10, and CXCL9, respectively, and those in sera from healthy controls were 261, 1,405, and 693 pg/ml, respectively, indicating significant increases in serum CXCL10 for CHC patients compared to levels for healthy controls (P = 0.007).
HCV replication regulates CXCR3 expression in response to IFN-γ and TNF-α.
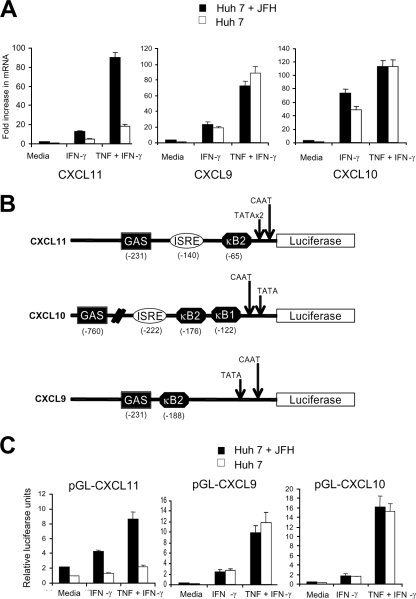
We have previously shown that HCV replication can increase CXCL11 expression following stimulation of HCV genomic replicon cells with IFN-γ and TNF-α (13). To extend this observation, we next investigated the role of HCV replication on CXCL9 and CXCL10 expression following IFN-γ and TNF-α stimulation by using the HCV JFH-1 cell culture system. Preliminary experiments showed that the sensitivity of our ELISA was not sufficient to detect CXCL11 in the Huh-7 line used for JFH-1 experiments, precluding ELISA analysis. Hence, mRNA expression of CXCR3 ligands was used for JFH-1 experiments. JFH-1 infection of Huh-7 cells demonstrated a significant (P < 0.01) increase in CXCL11 mRNA under IFN-γ and IFN-γ/TNF-α stimulation; however, no significant differences were observed for CXCL9 or CXCL10 mRNA (Fig. 4A). IFN-γ is known to inhibit HCV replication, and these experiments were performed at 16 h poststimulation, at which point HCV replication is still active, as determined via immunofluorescence staining (results not shown). These results suggest that HCV replication can selectively increase expression of CXCL11 but not CXCL9 or CXCL10 in response to IFN-γ and TNF-α.
FIG. 4.
Replicating HCV modulates CXCL11 expression. (A) Real-time PCR analysis of Huh-7 cells with or without JFH-1 infection 16 h following treatment with medium alone, 1,000 U/ml IFN-γ, or 1,000 u/ml IFN-γ plus 40 ng/ml TNF-α. (B) Schematic diagram of the CXCR3 promoters. (C) Luciferase assays were performed on Huh-7 cells with or without JFH-1 replication and transfected with luciferase reporter constructs outlined in panel B in the presence of no stimulation or stimulation with 1,000 U/ml IFN-γ with or without 40 mg/ml TNF-α.
HCV replication activates CXCR3 ligand transcription via a dsRNA mechanism.
To examine the effect of HCV replication on CXCR3 ligand transcription, the CXCL11, CXCL9, and CXCL10 promoters were cloned upstream of the luciferase reporter gene (Fig. 4B). JFH-1-infected Huh-7 cells were transfected with CXCR3 chemokine promoter-luciferase constructs and then stimulated with either IFN-γ alone or IFN-γ and TNF-α for 24 h. Cell lines replicating HCV JFH-1 (Fig. 4C) showed significant increases (six- to sevenfold) in luciferase output in the presence of IFN-γ and TNF-α for the CXCL11 promoter-luciferase reporter plasmid (P < 0.01), compared with control levels. No selective increase was noted following stimulation with IFN-γ/TNF-α for the CXCL9 or CXCL10 promoter constructs (Fig. 4C).
To determine if HCV protein expression was responsible for the selective transcriptional activation of the CXCL11 promoter, reporter assays were repeated in the presence of individual HCV proteins or the complete complement of nonstructural proteins by using published expression constructs (HCV protein expression was confirmed in an immunofluorescence assay using anti-HCV patient sera or specific anti-HCV antibodies where appropriate; results not shown). As shown in Fig. 5, expression of HCV proteins did not modulate CXCR3 ligand promoter activity in either untreated or stimulated (IFN-γ or TNF-α) Huh-7 cells. Collectively, these results demonstrate that HCV replication is able to selectively increase transcription of CXCL11 but not CXCL9 or CXCL10. However, this activation does not appear to be due to the actions of any single HCV protein.
FIG. 5.
Individual HCV protein expression does not modulate the CXCR3 ligand promoters. Huh 7 cells were transiently transfected with luciferase reporter constructs driven by either the CXCL11, the CXCL10, or the CXCL9 promoter and an expression plasmid encoding HCV NS3, 3/4a, 4a, 4b, 5a, 5b, C-p7, NS3-5b, or enhanced green fluorescent protein (E-gfp) as a control. Cells were treated for 24 h with either medium alone or 1,000 U/ml IFN-γ plus 40 ng/ml TNF-α before luciferase readings were taken.
To investigate if the selective increase in CXCL11 transcription noted above was a result of dsRNA signaling, we examined the ability of dsRNA to act synergistically with IFN-γ and TNF-α to increase CXCR3 ligand promoter activity and de novo mRNA expression. First, we transfected Huh-7 cells with the CXCR3 ligand promoter reporter constructs, followed by transfection with dsRNA in the form of poly(I:C) or in vitro-transcribed HCV RNA representing either the complete HCV genome or the 5′UTR. Transfection of the synthetic dsRNA analogue poly(I:C) resulted in a small increase in CXCR3 ligand promoter activity (Fig. 6A). However, in the presence of IFN-γ/TNF-α and poly(I:C) stimulation, there was a synergistic increase in CXCL11 promoter activity compared to the level for IFN-γ/TNF-α stimulation alone. This was not observed for CXCL9 or CXCL10 promoter activity (Fig. 6A). A selective synergistic increase in promoter activity was also seen for CXCL11 (but not CXCL9 or CXCL10) following transfection of either the HCV 5′UTR or the complete HCV genome, each of which has the capacity to result in significant regions of dsRNA (Fig. 6B) (41).
FIG. 6.
dsRNA is able to act synergistically with IFN-γ/TNF-α to increase CXCL11 mRNA expression. Huh-7 cells were transiently transfected for 24 h with various CXCR3 ligand promoter constructs before being transfected with poly(I:C) (A) or JFH-1 full-length or 5′UTR RNA (B) for 4 hours and then stimulated with medium alone or 1,000 U/ml IFN-γ in combination with 40 ng/ml TNF-α for 16 h before luciferase analysis. (C) Huh 7 cells were transfected with JFH-1 RNA or poly(I:C), allowed to settle for 4 hours, and then stimulated with medium alone or 1,000 U/ml IFN-γ alone or in combination with 40 ng/ml TNF-α for 4 h before analysis via real-time PCR for CXCL11, CXCL10, and CXCL9 mRNA.
We next examined the ability of in vitro-transcribed HCV JFH-1 RNA to stimulate de novo mRNA production of CXCR3 ligands and the effect of IFN-γ and TNF-α stimulation. Transfection of Huh-7 cells with JFH-1 RNA followed by IFN-γ/TNF-α stimulation for 8 h resulted in mRNA increases of 525-, 306-, and 63-fold for CXCL11, CXCL9, and CXCL10, respectively, compared to the levels for untreated cells (Fig. 6C). The increase for CXCL11 mRNA was significant (P < 0.01), but those for CXCL9 and CXCL10 mRNA were not, as treatment of Huh-7 cells with IFN-γ/TNF-α alone demonstrated mRNA increases of 302-, 284-, and 61-fold for CXCL11, CXCL9, and CXCL10, respectively, compared to the levels for untreated cells. Furthermore, similar results were seen following stimulation with poly(I:C), IFN-γ, and TNF-α (Fig. 6C). Thus, this selective response of CXCL11 expression seems not to be specific for HCV RNA but may be part of a more generic cellular response to dsRNA.
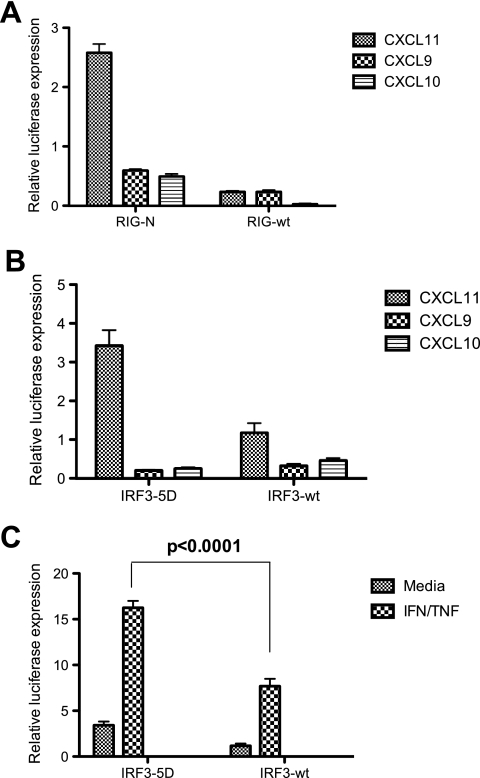
dsRNA sensor proteins selectively increase CXCL11 expression following IFN and TNF stimulation.
Our previous experiments established that the selective activation of CXCL11 transcription to IFN-γ and TNF-α was mediated by a dsRNA response. To characterize this effect in more detail, we investigated the dsRNA RIG-I signaling pathway that is central for triggering the host response to HCV infection. Activation of this pathway results in IRF3 and NF-κB activation following either viral infection or dsRNA stimulation (9, 27, 41, 45). Using transfection of a constitutively active RIG-I molecule (RIG-N) together with the CXCR3 ligand promoters resulted in a significant increase (threefold) for CXCL11 transcription, but not for CXCL9 or CXCL10 transcription, that was independent of dsRNA stimulation (Fig. 7A). As predicted, transfection of RIG-wt did not stimulate promoter activity. It is surprising that the CXCL10 promoter was not activated, considering that it contains an ISRE and suggests that other factors are required in addition to IRF3 for activation of CXCL10 transcription. As predicted, the CXCL9 promoter was not responsive to RIG-N, presumably due to the absence of an ISRE within the CXCL9 transcriptional promoter (Fig. 4B).
FIG. 7.
CXCL11 is an IRF3 responsive gene. Huh-7 cells were transiently transfected with various CXCR3 ligand promoter constructs in combination with RIG-N or RIG-wt (A) or IRF3-5D or IRF3-wt (B) before luciferase analysis at 24 h posttransfection. (C) The CXCL11 promoter was cotransfected with the IRF3-5D or IRF3-wt plasmids and left for 24 h before being treated for 8 h with either medium alone or 1,000 U/ml IFN-γ and 40 ng/ml TNF-α before luciferase analysis.
RIG-I signals to IRF3 and is essential for regulation of the transcription of a number of other chemokines, such as RANTES (23) and IL-8 (43). Furthermore, IRF3 activation is fundamental to establishing the host response to HCV and is a target of the HCV NS3/4a protease, which blocks innate sensing of dsRNA (9). Therefore, we next investigated the effects of IRF3 expression on CXCR3 ligand transcription by using IRF3-wt and a constitutively active form of IRF3 (IRF3-5D) that has previously been described (22). Under basal conditions, IRF3-5D resulted in a significant increase (3.5-fold; P < 0.01) in CXCL11 transcription while there was no increase with IRF3-wt, as expected (Fig. 7B). In concordance with the RIG-I data presented above and the lack of an ISRE within the CXCL9 promoter, IRF3-5D did not stimulate CXCL9 or CXCL10 promoter activity (Fig. 7B). Interestingly, in the presence of IRF3-5D and stimulation with IFN-γ and TNF-α, a selective increase in CXCL11 promoter activity compared to the level for transfection with IRF3-wt was seen (17-fold versus 7-fold; P < 0.0001) (Fig. 7C). Collectively, our data suggest that HCV infection can selectively activate CXCL11 transcription synergistically in the presence of IFN-γ and TNF-α via the RIG-I/IRF3 pathway.
DISCUSSION
In view of the known association between T-lymphocyte infiltration and negative prognosis in CHC, it is important to investigate the mechanisms governing recruitment of these cells to livers chronically infected with HCV. However, to date there is a paucity of information regarding the factors responsible for recruitment of T lymphocytes and other immune cells to the liver in CHC. Nevertheless, it is hypothesized that the CXCR3 ligands CXCL10, CXCL9, and CXCL11 play a central role in the chemotaxis of activated T lymphocytes to this organ (2, 10, 12, 13, 28, 39). The results herein demonstrate for the first time that the CXCR3 ligand family, while dominantly expressed in CHC, is differentially expressed in the liver and peripheral blood samples of these patients. Interestingly, we were able to confirm that the upregulation of these three chemokines correlated with lobular inflammation in CHC but that only CXCL11 transcript upregulation correlated with portal inflammation seen in these patients. This observation was explored further in an in vitro setting, where we demonstrated for the first time that replicating HCV is able to selectively modulate the expression of these chemokines.
Apart from the significant increase in expression of the CXCR3 ligands, other chemokine transcripts, including RANTES, CXCL6, Mip-3β, and SCM-1β, were also upregulated, although to a lesser degree and in a smaller number of patients. RANTES, also a T-lymphocyte chemoattractant, has been shown in a number of reports to be upregulated in CHC (19, 31). CXCL6 has recently been demonstrated to be upregulated in advancing HCV fibrosis (3) and is an active chemoattractant for neutrophils (44). To the best of our knowledge, Mip-3β and SCM-1 have not previously been described to be upregulated in CHC. Both chemokines promote the chemotaxis of T cells, with Mip-3β also being actively involved in the migration of naïve T lymphocytes and mature dendritic cells (34, 46).
Previous data suggest that CXCR3 chemokine expression is driven primarily within the HCV-infected liver by IFN-γ and TNF-α (28-30). However, it is also plausible that other factors, such as viral replication, may play a role, as has been demonstrated for other chemokines, including IL-8 (16, 26). In the present study, replicating HCV enhanced the expression of CXCL11 but not that of CXCL10 or CXCL9 in response to IFN-γ and TNF-α at the level of transcription. This transcriptional activation was not a result of any individual HCV protein but was part of the innate cellular response to dsRNA, as both in vitro-transcribed HCV RNA and synthetic dsRNA [poly(I:C)] selectively increased CXCL11 expression. Expression of other chemokines, such as IL-8, has been shown to be induced by dsRNA (43). This could point to a common mechanism of induction of expression of certain chemokines in response to viral infection, probably via one or more pattern recognition receptors. The CXCR3 ligand promoters all have relatively similar structures (Fig. 4B), and it is therefore difficult to reconcile the selective increase in CXCL11 expression over that of CXCL10 and CXCL9. Activation of the cellular innate dsRNA response by RIG-I signaling is central for triggering the host response to HCV infection. IRF3 signals downstream of RIG-I activation, and given that IRF3 activation is essential for the transcriptional activation of the chemokines RANTES and IL-8, we investigated if the CXCR3 ligand chemokines were also regulated by IRF3 and if this was the basis for selective CXCL11 activation by IFN-γ and TNF-α. We found that CXCL11 was a primary IRF3 response gene, in contrast to CXCL9 and CXCL10. This is not surprising, as the CXCL9 transcriptional promoter does not contain an ISRE. However, the presence of an ISRE within the CXCL10 promoter and the lack of IRF3 activation suggest that other transcription factors in addition to IRF3 are required for maximal CXCL10 transcription in response to dsRNA. Interestingly, we further showed that the selective increase in CXCL11 transcription noted in the presence HCV replication and IFN-γ and TNF-α stimulation was attributable to activation of the dsRNA response via IRF3.
Given that the HCV NS3/4a protease blocks RIG-I-dependent signaling of IRF3 by its targeted proteolysis of IPS-1 (27), it is difficult to conceive what role this selective expression of CXCL11 may play in the pathogenesis of HCV-related liver disease. However, the host response to HCV infection may be transiently activated prior to control by NS3/4a, allowing for expression of CXCL11 by direct activation of IRF3, which is further enhanced by IFN-γ and TNF-α, which are both expressed within the HCV-infected liver. Our data indicate that the dsRNA-activated RIG-I pathway can drive the expression of CXCL11, thus connecting innate defense signaling to the inflammatory response to viral infection, which is further enhanced by stimulation with IFN-γ and TNF-α.
The attraction of T lymphocytes to a site of infection requires a chemokine gradient between the infected site and the peripheral circulation. In CHC, a difference in expression of CXCR3 ligands between the chronically infected liver and the peripheral blood would therefore be expected. Indeed, CXCL9 and CXCL11 show significant differences in expression between the liver and peripheral circulation in CHC patients; however, in contrast, CXCL10 is expressed at high levels in the serum and liver. It is therefore unlikely that the CXCR3 chemokines have a role to play in extravasation of T lymphocytes into the liver, as the high concentrations of CXCL10 in the blood are likely to neutralize the gradient, as has been shown previously (24). It is more likely that these chemokines play a role in the movement of T lymphocytes within the liver lobule and that another chemokine-receptor pair is responsible for initial T-lymphocyte extravasation. A candidate pair for this might be CCR5/RANTES; RANTES is known to be present at low concentrations in the periphery circulation of CHC patients (35) and was demonstrated to be upregulated in the livers of CHC patients in this study and others (47). Moreover, CCR5 is known to be expressed on activated T cells (2). Interestingly, RANTES was not increased in the chronic chimpanzee infection and may reflect the lower inflammatory disease noted in these animals.
The findings of this study raise the question as to why HCV would increase CXCL11 levels within the liver, considering that the prime function of the inflammatory infiltrate is removal of HCV-infected hepatocytes. Our data suggest that an increase in expression of CXCR3 ligands is a normal host response to the presence of the virus. However, another possible explanation is that increasing CXCL11 levels within the HCV-infected liver may be advantageous to the virus in this regard. It has previously been demonstrated that CXCL11 is a natural antagonist for CCR5, unlike the other CXCR3 ligands (32). Given that the majority of the T lymphocytes in the livers of CHC patients are CCR5/CXCR3 positive (2), it is possible that CCR5 is involved in extravasation of T lymphocytes into the livers of CHC patients and that the ability of HCV replication to increase CXCL11 levels may be a viral defense mechanism for neutralizing the CCR5 receptor and decreasing T-lymphocyte recruitment into the liver lobule. This in turn may promote chronic inflammation in the HCV-infected liver, contributing to chronicity.
T-cell trafficking in the HCV-infected liver is a complicated event, perhaps involving multiple chemokine networks. We have demonstrated that the CXCR3 family members CXCL11, CXCL9, and CXCL10 are significantly increased in the livers of CHC patients and chimpanzees experimentally infected with HCV and are likely to play a role in T-lymphocyte traffic within the liver lobule. A better understanding of the roles these chemokines play in driving T-lymphocyte accumulation in the HCV-infected liver will further aid in the development of therapeutic intervention strategies for inhibiting or reducing the excessive inflammation observed in CHC.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia.
We acknowledge T. Wakita from the Department of Virology, National Institute of Infectious Diseases, Tokyo, Japan, for the JFH-1 HCV clone.
Footnotes
Published ahead of print on 5 November 2008.
REFERENCES
- 1.Abel, S., C. Hundhausen, R. Mentlein, A. Schulte, T. A. Berkhout, N. Broadway, D. Hartmann, R. Sedlacek, S. Dietrich, B. Muetze, B. Schuster, K. J. Kallen, P. Saftig, S. Rose-John, and A. Ludwig. 2004. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J. Immunol. 1726362-6372. [DOI] [PubMed] [Google Scholar]
- 2.Apolinario, A., P. L. Majano, E. Alvarez-Perez, A. Saez, C. Lozano, J. Vargas, and C. Garcia-Monzon. 2002. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am. J. Gastroenterol. 972861-2870. [DOI] [PubMed] [Google Scholar]
- 3.Asselah, T., I. Bieche, I. Laurendeau, V. Paradis, D. Vidaud, C. Degott, M. Martinot, P. Bedossa, D. Valla, M. Vidaud, and P. Marcellin. 2005. Liver gene expression signature of mild fibrosis in patients with chronic hepatitis C. Gastroenterology 1292064-2075. [DOI] [PubMed] [Google Scholar]
- 4.Barnes, B., B. Lubyova, and P. M. Pitha. 2002. On the role of IRF in host defense. J. Interferon Cytokine Res. 2259-71. [DOI] [PubMed] [Google Scholar]
- 5.Bigger, C. B., B. Guerra, K. M. Brasky, G. Hubbard, M. R. Beard, B. A. Luxon, S. M. Lemon, and R. E. Lanford. 2004. Intrahepatic gene expression during chronic hepatitis C virus infection in chimpanzees. J. Virol. 7813779-13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunt, E. M. 2000. Grading and staging the histopathological lesions of chronic hepatitis: the Knodell histology activity index and beyond. Hepatology 31241-246. [DOI] [PubMed] [Google Scholar]
- 7.Di Bisceglie, A. M., Z. D. Goodman, K. G. Ishak, J. H. Hoofnagle, J. J. Melpolder, and H. J. Alter. 1991. Long-term clinical and histopathological follow-up of chronic posttransfusion hepatitis. Hepatology 14969-974. [DOI] [PubMed] [Google Scholar]
- 8.Di Bisceglie, A. M., L. H. Simpson, M. T. Lotze, and J. H. Hoofnagle. 1994. Development of hepatocellular carcinoma among patients with chronic liver disease due to hepatitis C viral infection. J. Clin. Gastroenterol. 19222-226. [DOI] [PubMed] [Google Scholar]
- 9.Foy, E., K. Li, C. Wang, R. Sumpter, Jr., M. Ikeda, S. M. Lemon, and M. Gale, Jr. 2003. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 3001145-1148. [DOI] [PubMed] [Google Scholar]
- 10.Harvey, C. E., J. J. Post, P. Palladinetti, A. J. Freeman, R. A. Ffrench, R. K. Kumar, G. Marinos, and A. R. Lloyd. 2003. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J. Leukoc. Biol. 74360-369. [DOI] [PubMed] [Google Scholar]
- 11.He, X. S., B. Rehermann, F. X. Lopez-Labrador, J. Boisvert, R. Cheung, J. Mumm, H. Wedemeyer, M. Berenguer, T. L. Wright, M. M. Davis, and H. B. Greenberg. 1999. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc. Natl. Acad. Sci. USA 965692-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helbig, K. J., D. T. Lau, L. Semendric, H. A. Harley, and M. R. Beard. 2005. Analysis of ISG expression in chronic hepatitis C identifies viperin as a potential antiviral effector. Hepatology 42702-710. [DOI] [PubMed] [Google Scholar]
- 13.Helbig, K. J., A. Ruszkiewicz, L. Semendric, H. A. Harley, S. R. McColl, and M. R. Beard. 2004. Expression of the CXCR3 ligand I-TAC by hepatocytes in chronic hepatitis C and its correlation with hepatic inflammation. Hepatology 391220-1229. [DOI] [PubMed] [Google Scholar]
- 14.Heydtmann, M., P. F. Lalor, J. A. Eksteen, S. G. Hubscher, M. Briskin, and D. H. Adams. 2005. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J. Immunol. 1741055-1062. [DOI] [PubMed] [Google Scholar]
- 15.Hiscott, J. 2007. Convergence of the NF-kappaB and IRF pathways in the regulation of the innate antiviral response. Cytokine Growth Factor Rev. 18483-490. [DOI] [PubMed] [Google Scholar]
- 16.Kadoya, H., M. Nagano-Fujii, L. Deng, N. Nakazono, and H. Hotta. 2005. Nonstructural proteins 4A and 4B of hepatitis C virus transactivate the interleukin 8 promoter. Microbiol. Immunol. 49265-273. [DOI] [PubMed] [Google Scholar]
- 17.Konan, K. V., T. H. Giddings, Jr., M. Ikeda, K. Li, S. M. Lemon, and K. Kirkegaard. 2003. Nonstructural protein precursor NS4A/B from hepatitis C virus alters function and ultrastructure of host secretory apparatus. J. Virol. 777843-7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusano, F., Y. Tanaka, F. Marumo, and C. Sato. 2000. Expression of C-C chemokines is associated with portal and periportal inflammation in the liver of patients with chronic hepatitis C. Lab. Investig. 80415-422. [DOI] [PubMed] [Google Scholar]
- 19.Leroy, V., I. Vigan, J. F. Mosnier, T. Dufeu-Duchesne, M. Pernollet, J. P. Zarski, P. N. Marche, and E. Jouvin-Marche. 2003. Phenotypic and functional characterization of intrahepatic T lymphocytes during chronic hepatitis C. Hepatology 38829-841. [DOI] [PubMed] [Google Scholar]
- 20.Li, K., E. Foy, J. C. Ferreon, M. Nakamura, A. C. Ferreon, M. Ikeda, S. C. Ray, M. Gale, Jr., and S. M. Lemon. 2005. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc. Natl. Acad. Sci. USA 1022992-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, K., T. Prow, S. M. Lemon, and M. R. Beard. 2002. Cellular response to conditional expression of hepatitis C virus core protein in Huh7 cultured human hepatoma cells. Hepatology 351237-1246. [DOI] [PubMed] [Google Scholar]
- 22.Lin, R., P. Genin, Y. Mamane, and J. Hiscott. 2000. Selective DNA binding and association with the CREB binding protein coactivator contribute to differential activation of alpha/beta interferon genes by interferon regulatory factors 3 and 7. Mol. Cell. Biol. 206342-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, R., C. Heylbroeck, P. Genin, P. M. Pitha, and J. Hiscott. 1999. Essential role of interferon regulatory factor 3 in direct activation of RANTES chemokine transcription. Mol. Cell. Biol. 19959-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, Y. Q., R. T. Poon, J. Hughes, Q. Y. Li, W. C. Yu, and S. T. Fan. 2005. Desensitization of T lymphocyte function by CXCR3 ligands in human hepatocellular carcinoma. World J. Gastroenterol. 11164-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majumder, S., L. Z. Zhou, P. Chaturvedi, G. Babcock, S. Aras, and R. M. Ransohoff. 1998. Regulation of human IP-10 gene expression in astrocytoma cells by inflammatory cytokines. J. Neurosci. Res. 54169-180. [DOI] [PubMed] [Google Scholar]
- 26.Medin, C. L., K. A. Fitzgerald, and A. L. Rothman. 2005. Dengue virus nonstructural protein NS5 induces interleukin-8 transcription and secretion. J. Virol. 7911053-11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 4371167-1172. [DOI] [PubMed] [Google Scholar]
- 28.Napoli, J., G. A. Bishop, P. H. McGuinness, D. M. Painter, and G. W. McCaughan. 1996. Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology 24759-765. [DOI] [PubMed] [Google Scholar]
- 29.Nelson, D. R., R. P. Gonzalez-Peralta, K. Qian, Y. Xu, C. G. Marousis, G. L. Davis, and J. Y. Lau. 1997. Transforming growth factor-beta 1 in chronic hepatitis C. J. Viral Hepat. 429-35. [DOI] [PubMed] [Google Scholar]
- 30.Nelson, D. R., H. L. Lim, C. G. Marousis, J. W. Fang, G. L. Davis, L. Shen, M. S. Urdea, J. A. Kolberg, and J. Y. Lau. 1997. Activation of tumor necrosis factor-alpha system in chronic hepatitis C virus infection. Dig. Dis. Sci. 422487-2494. [DOI] [PubMed] [Google Scholar]
- 31.Nischalke, H. D., J. Nattermann, H. P. Fischer, T. Sauerbruch, U. Spengler, and F. L. Dumoulin. 2004. Semiquantitative analysis of intrahepatic CC-chemokine mRNas in chronic hepatitis C. Mediators Inflamm. 13357-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petkovic, V., C. Moghini, S. Paoletti, M. Uguccioni, and B. Gerber. 2004. I-TAC/CXCL11 is a natural antagonist for CCR5. J. Leukoc. Biol. 76701-708. [DOI] [PubMed] [Google Scholar]
- 33.Pichlmair, A., and C. Reis e Sousa. 2007. Innate recognition of viruses. Immunity 27370-383. [DOI] [PubMed] [Google Scholar]
- 34.Robbiani, D. F., R. A. Finch, D. Jager, W. A. Muller, A. C. Sartorelli, and G. J. Randolph. 2000. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell 103757-768. [DOI] [PubMed] [Google Scholar]
- 35.Roe, B., S. Coughlan, J. Hassan, A. Grogan, G. Farrell, S. Norris, C. Bergin, and W. W. Hall. 2007. Elevated serum levels of interferon-gamma-inducible protein-10 in patients coinfected with hepatitis C virus and HIV. J. Infect. Dis. 1961053-1057. [DOI] [PubMed] [Google Scholar]
- 36.Ruggieri, A., M. Franco, I. Gatto, A. Kumar, and M. Rapicetta. 2007. Modulation of RANTES expression by HCV core protein in liver derived cell lines. BMC Gastroenterol. 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheuer, P. J. 1991. Classification of chronic viral hepatitis: a need for reassessment. J. Hepatol. 13372-374. [DOI] [PubMed] [Google Scholar]
- 38.Seeff, L. B. 2002. Natural history of chronic hepatitis C. Hepatology 36S35-S46. [DOI] [PubMed] [Google Scholar]
- 39.Shields, P. L., C. M. Morland, M. Salmon, S. Qin, S. G. Hubscher, and D. H. Adams. 1999. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J. Immunol. 1636236-6243. [PubMed] [Google Scholar]
- 40.Soo, H. M., A. Garzino-Demo, W. Hong, Y. H. Tan, Y. J. Tan, P. Y. Goh, S. G. Lim, and S. P. Lim. 2002. Expression of a full-length hepatitis C virus cDNA up-regulates the expression of CC chemokines MCP-1 and RANTES. Virology 303253-277. [DOI] [PubMed] [Google Scholar]
- 41.Sumpter, R., Jr., Y. M. Loo, E. Foy, K. Li, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale, Jr. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 792689-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tensen, C. P., J. Flier, S. S. Rampersad, S. Sampat-Sardjoepersad, R. J. Scheper, D. M. Boorsma, and R. Willemze. 1999. Genomic organization, sequence and transcriptional regulation of the human CXCL 11(1) gene. Biochim. Biophys. Acta 1446167-172. [DOI] [PubMed] [Google Scholar]
- 43.Wagoner, J., M. Austin, J. Green, T. Imaizumi, A. Casola, A. Brasier, K. S. Khabar, T. Wakita, M. Gale, Jr., and S. J. Polyak. 2007. Regulation of CXCL-8 (interleukin-8) induction by double-stranded RNA signaling pathways during hepatitis C virus infection. J. Virol. 81309-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wuyts, A., N. Van Osselaer, A. Haelens, I. Samson, P. Herdewijn, A. Ben-Baruch, J. J. Oppenheim, P. Proost, and J. Van Damme. 1997. Characterization of synthetic human granulocyte chemotactic protein 2: usage of chemokine receptors CXCR1 and CXCR2 and in vivo inflammatory properties. Biochemistry 362716-2723. [DOI] [PubMed] [Google Scholar]
- 45.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5730-737. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida, T., T. Imai, S. Takagi, M. Nishimura, I. Ishikawa, T. Yaoi, and O. Yoshie. 1996. Structure and expression of two highly related genes encoding SCM-1/human lymphotactin. FEBS Lett. 39582-88. [DOI] [PubMed] [Google Scholar]
- 47.Zeremski, M., L. M. Petrovic, and A. H. Talal. 2007. The role of chemokines as inflammatory mediators in chronic hepatitis C virus infection. J. Viral Hepat. 14675-687. [DOI] [PubMed] [Google Scholar]
- 48.Zhong, J., P. Gastaminza, G. Cheng, S. Kapadia, T. Kato, D. R. Burton, S. F. Wieland, S. L. Uprichard, T. Wakita, and F. V. Chisari. 2005. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. USA 1029294-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]