Abstract
Although human and bovine γδ T cells were shown to express MHC class II antigen and function as APCs, attempts to determine if mouse γδ T cells have similar functions remained unsuccessful. We now show that γδ T cells derived from immunized mice also can be induced to express MHC class II and co-stimulatory molecules after activation in vitro, and are capable of antigen presentation. Using highly purified γδ T cells, we found that, unlike human γδ T cells, the expression of MHC class II molecules by mouse γδ T cells is limited to newly activated cells. Highest levels of MHC class II expression were seen on activated γδ T cells that had lost most surface-expressed γδ TCR while exhibiting increased levels of intracellular γδ TCR. In the absence of further stimulation, MHC class II expression gradually declined with the γδ T cells regaining their surface TCR. We also show that cytokine-activated γδ T cells can also express MHC class II antigen and exercise antigen-presenting activity.
Keywords: γδ T cell, Antigen presentation, MHC class II, Interleukin-17
1. Introduction
Mammalian hosts employ two interconnected systems − innate and adaptive immunity − to protect themselves from infection while minimizing immunopathology (Medzhitov and Janeway, 1997b; Peng et al., 2005; Trinchieri, 1995). The innate immune system senses infection through pattern-recognition receptors, which recognize conserved molecular features of pathogens that are unique to microbial life forms (Peng et al., 2005). According to current concepts, an effective immune response requires coordinated interaction between innate and adaptive immunity (Peng et al., 2005; Trinchieri, 1995). It is believed that the prompt innate immune response not only fills the gap of immunologic defense before full-effective adaptive responses become available, but also regulates the intensity and the pattern of adaptive response (Rajan et al., 2000; Tsuchiya et al., 2003; Kadowaki et al., 2000; Fernandez et al., 1999).
Early studies have shown that γδ T cells are an important cellular element invading inflamed organs (Ponomarev and Dittel, 2005; Skelsey et al., 2001) during the early phase of viral infection (Carding et al., 1990; Mukasa et al., 1997) and the inflammation involved in autoimmune diseases of CNS (encephalomyelitis) (Selmaj et al., 1991; Shimonkevitz et al., 1993) and intestines (colitis) (Fukushima et al., 1991; Tsuchiya et al., 2003). Studies have also shown that γδ T cells can either promote (Spahn et al., 1999; Shimonkevitz et al., 1993; Freedman et al., 1991; Hohlfeld et al., 1991) or inhibit (Tsuchiya et al., 2003) the development of autoimmune disease. It remains undetermined whether pathogenic γδ T cells are a distinct subset and differ from the γδ T cell subsets that regulate the immune response or whether the functional diversity is associated with the activation status of the γδ T cells.
Previous studies have shown that both human (Brandes et al., 2005) and bovine (Collins et al., 1998) γδ T cells are able to express MHC class II molecules and act as antigen-presenting cells. So far, attempts to demonstrate that mouse γδ T cells have similar functions were unsuccessful. However, we now show that the general observation that γδ T cells are capable of expressing MHC class II molecules and of presenting antigen to T cells expressing the αβ TCR can be extended to mice as well. The use of highly purified γδ T cells allowed us to demonstrate that high levels of MHC class II antigens and co-stimulatory molecules are expressed on recently activated mouse γδ Tcells when these cells downregulate their surface TCR, thus transiently loosing their phenotypical identity. Our results suggest, that the failure of demonstrating an ability of mouse γδ T cells to express MHC class II and to present antigen in the previous studies was probably due to the circumstance that human γδ T cells retain their surface TCR while expressing MHC class II molecules, whereas mouse γδ T cells express MHC class II molecules at high levels only shortly after having been activated, when their surface-expressed TCR is downregulated.
2. Methods
2.1. Animals and reagents
Female C57BL/6 (B6) mice (12- to 14-weeks-old) were purchased from Jackson Laboratory (Bar Harbor, ME), and were housed and maintained in the animal facilities of the University of Louisville. Recombinant mouse cytokines, including IL-1β, IL-2, IL-6, IL-7, IL-21, IL-23, and TGF-β, were purchased from R & D. (Minneapolis, MN). FITC-conjugated anti-αβ TCR, anti-γδ TCR (GL3) (Goodman and Lefrancois, 1989), anti-mouse MHC class II antibodies, anti-mouse CD40, and anti-mouse CD80, anti-mouse CD69 and anti-mouse CD62L antibodies were purchased from Biolegend (San Diego, CA). Beads-conjugated anti-FITC antibodies were purchased from Miltenyi Biotec (Aubum, CA, USA). IRBP1-20 (GPTHLFQPSLVLDMAKVLLD) is an uveitogenic peptide (Avichezer et al., 2000) and MOG35-55 (MEVG-WYRSPFSRVVHLYRNGK) is an encephalitogenic peptide in H-2b (C57BL/6) mice (Sun et al., 2003). Incomplete (IFA) or complete Freund's adjuvant (CFA) was obtained from Sigma (St. Louis, MO). Mouse TLR1-9 agonist kit was obtained from Invivogen (cat#: TLRL-kit 1m, San Diego, California), which includes TLR1/2 agonist Pam3CSK4, TLR2 agonist HKLM (Heat-killed Listeria monocytogenes), TLR3 agonist Poly(I:C), TLR4 agonist LPS-EK (E. coli K12), TLR5 agonist ST-FLA (Flagellin form S. typhimurium), TLR6/2 agonist FSL1 (a synthetic lipoprotein representing N-terminal part of the 44-kD lipoprotein LP44 of Mycoplasma salivarium), TLR7 agonist ssRNA40, and TLR8 agonist ODN1826.
2.2. Enrichment of γδ T cells from IRBP-immunized C57BL/6 mice
Purified γδ T cells were prepared from the spleen and draining lymph nodes of immunized B6 mice using FITC-conjugated anti-mouse γδTCR. C57BL/6 (B6) mice were immunized subcutaneously with 200 μl of emulsion containing 200 μg of IRBP1-20 in CFA, distributed over six spots at the tail base and on the flank. At day 13 post-immunization (p.i.), T cells were isolated from lymph node cells and spleen cells by passage through a nylon wool column, then 1×107 cells in 2 ml of RPMI medium in a 6-well plate (Costar) were stimulated for 48 h with 10 μg/ml of IRBP1-20 in the presence of 1×107 irradiated syngeneic spleen cells (APCs) in the presence of IL-23 (10 ng/ml), then activated T cell blasts were separated by Ficoll gradient centrifugation and cultured for another 72 h in the same medium used for stimulation minus the peptide.
γδ T cells were purified from the in vivo IRBP1-20-primed splenic Tcells 5 days after the latter cells were stimulated in vitro with immunizing antigen. To separate γδ Tcells, the Tcells were first stained with FITC-conjugated anti-γδ TCR antibodies, followed by anti-FITC-microBeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) (Peng et al., 2006). The lymph node and spleen cells were first incubated for 30 min at 4 °C with FITC-conjugated anti-mouse γδ TCR, then for 15 min at 4 °C with anti-FITC Microbeads. The cells were then separated into bound and non-bound fractions on an autoMACSTM separator column (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), and washed with 15 ml of medium according to the manufacturer's protocol. The flow-through fractions containing γδ T cells or αβ T-enriched cells were collected. The purity of the isolated cell fraction was determined by flow cytometric analysis using FITC-conjugated anti-TCR antibodies and PE-conjugated Abs directed against γδ or αβ T cells (BD Bioscience, La Jolla, CA). Data collection and analysis were performed on a FACScalibur flow cytometer using CellQuest software (95% purified for γδ T cells). For further purification of γδ T cells, the residual αβ+ T cells were depleted using PE-conjugated anti-αβTCR antibody with anti-PE microbeads.
2.3. Immunofluorescence flow cytometry
Aliquots of 2×105 cells were double-stained with combinations of FITC- or PE-conjugated monoclonal antibodies against mouse αβTCR, pan-γδ TCR (GL3). Data collection and analysis were performed on a FACScaliber flow cytometer using CellQuest software.
2.4. Intracellular cytokine flow cytometry
Unfractionated or purified γδ T cells or αβ T IRBP1-20-specific T cells were stimulated in vitro with 50 ng/ml of PMA, 1 μg/ml of ionomycin, and 1 μg/ml of brefeldin A (Sigma, St. Louis, MO) for 4 h, then were washed, fixed, permeabilized overnight with Cytofix/Cytoperm buffer (eBioscience, San Diego, CA), and intracellularly stained with antibodies against mouse αβ-and γδ TCR and analyzed on a FACScalibur flow cytometer.
2.5. IRBP- and MOG-specific T cells
IRBP- (Shao et al., 2005) and MOG-specific (Sun et al., 2001) T cells were prepared as we previously described. Briefly, T cells from IRBP1-20- or MOG35-55-immunized B6 mice were isolated 13 days post-immunization (p.i.) from lymph node cells or spleen cells by passage through a nylon wool column, then 1×107 cells in 2 ml of RPMI medium in a 6-well plate (Costar) were stimulated with 20 μg/ml of IRBP1-20 in the presence of 1×107 irradiated syngeneic spleen cells as antigen-presenting cells (APCs). After 2 days, the activated lymphoblasts were isolated by gradient centrifugation on Lymphoprep (Robbins Scientific, Mountain View, CA) and cultured in RPMI 1640 medium supplemented with 15% IL-2-containing medium.
2.6. Proliferation assay
IRBP1-20- or MOG35-55-specific T cells were seeded at 4×105 cells/well in 96-well plates, then cultured at 37 °C for 48 h in a total volume of 200 μl medium with graded concentrations of related peptides in the presence of irradiated (2000 Rad) syngeneic spleen APCs (2 × 105), and [3H] thymidine incorporation during the last 8 h was assessed using a microplate scintillation counter (Packard). The proliferative response was expressed as the mean cpm±standard deviation (SD) of triplicate determinations.
2.7. ELISA
IL-17 and IFN-γ were measured using commercially available ELISA kits (R&D Systems, Minneapolis, MN).
2.8. Statistical analysis
The data are expressed as the mean±SD of the results for at least three separate experiments.
3. Results
3.1. Preparation of highly purified γδ T cells from IRBP-immunized C57BL/6 mice
Total splenic T cells were enriched from naïve mice and from mice immunized with an uveitogenic peptide (IRBP1-20) for induction of EAU, by passing through nylon wool. As shown in Fig. 1A, the relative frequency of γδ T cells increased approximately 5-fold in the immunized mice. Subsequently, when the enriched splenic T cells of IRBP1-20-immunized B6 mice were cultured in medium supplemented with various cytokines for 3–5 days, the γδ T cells expanded more prominently in those cultures supplemented with cytokines IL-1, IL-7, and IL-23, but not with the other cytokines tested (Fig. 1B). The in vitro expanded γδ T cells could be purified using magnetic bead-sorting after staining with an FITC-labeled antibody specific for mouse pan-δ TCR segment (GL3) followed by anti-FITC antibodies (see Methods). As shown in Fig. 1C, γδ T cells enriched by this one-step procedure were approximately 95% pure. They were further enriched to 99% purity in a second step of depleting contaminating αβ T cells.
Fig. 1.
Preparation of purified γδ T cells. A). B6 mice induced for EAU expressed increased numbers of γδ T cells. T cells of naïve and IRBP-immunized B6 mice were enriched by passing through nylon wool and were stained with antibodies specific for mouse αβ TCR and γδ TCR. In addition, T cells from immunized mice were reassessed from expression of αβ- and γδ TCR after an in vitro stimulation with immunizing peptide in the presence of for 48 h. B) IL-1, IL-7 and IL-23 have greater effect promoting γδ T cell expansion in vitro. The numbers of γδ T cells in unfractionated splenic T cells from immunized B6 mice were assessed after 3 days of in vitro culture in medium containing the indicated inflammatory cytokines (10 ng/ml final concentration). The results showed that IL-1, IL-7, or IL23 stimulated γδ T cell expansion and had a synergistic effect when added together, whereas other cytokines had no effect. The results shown are the mean cpm values and are representative of those for 5 separate experiments, each involving pooled T cells from 8–10 IRBP1-20-immunized B6 mice; the SD was always less than 15%. C) Enrichment of γδ T cells using magnetic beads. One-step positive selection using GL3 antibody results in a cell population containing up to 95% γδ T cells (left panel). Further depletion of αβ T cells by removing T cells expressing the αβ TCR results in 99% γδ T cells (right panel).
3.2. Mouse γδ T cells expressed MHC class II antigen and down-regulated their surface-expressed TCR after in vitro activation
To determine whether mouse γδ T cells could express MHC class II antigen and function as APCs, highly purified (>98% purity) γδ T cells were exposed to a mitogenic dose of anti-CD3 plus anti-CD28 antibodies (10 μg/ml) in the absence of exogenous APC on 24-well plates. As shown in Fig. 2A, 72 h after exposure to anti-CD3, a majority of the cells expressed downregulated TCR (middle panel) and they partially regained the γδ TCR 6 days after CD3 stimulation (lower panel). Prior to in vitro stimulation, most, if not all, of the cells expressed γδ TCR, and approximately 40% of the cells expressed CD80.
Fig. 2.
Mouse γδ T cells show downregulation of TCR and upregulation of MHC class II antigens and co-stimulatory (CD40) molecules after in vitro stimulation with anti-CD3 and anti-CD28 antibodies. A). γδ T cells were enriched from IRBP-specific T cells derived from IRBP1-20-immunized B6 mice at day 13 post-immunization using magnetic separation, then were exposed to anti-CD3 and anti-CD28 antibodies pre-coated on 24-well plates, in the absence of exogenous APC. After 3 or 6 days of stimulation, the cells were assessed for surface TCR and co-stimulatory molecule expression. B) Demonstration of intracellular γδ TCR on activated γδ T cells that lost surface TCR. Resting γδ T cells express surface TCR, activated γδ T cells express TCR by intracellular staining but not surface staining. Results shown are from one representative experiment which was repeated 4 times.
The fact, that over 98% of the responder cells in culture are purified γδ T cells, and a majority of the cultured cells regained TCR 6 days after CD3 stimulation suggested the interpretation that the MHC class II-expressing cells shortly after in vitro activation were indeed γδ T cells rather than expanded cells of unknown origin. This was confirmed by intracellular staining for the γδ TCR of the newly activated γδ T cells. As shown in Fig. 2B, activated γδ T cells do not express detectable amount of surface TCR, which however, can be detected in the same T cells (lower panel) when we use intracellular staining (after fixation). Since these cells do not express surface TCR and the TCR can only be detected only after fixation, we conclude that the expression of TCR by the activated γδ T cell is limited in the intracellular compartment.
Results shown in Fig. 2A demonstrated that over 50% of the total cells examined 24 h after in vitro stimulation with anti-CD3 antibody expressed MHC class II molecules. FACS analysis after staining for co-stimulatory molecules further showed that activated γδ T cells also expressed increased levels of CD80 and CD40 molecules.
3.3. The class II antigen-expressing γδ T cells are effective APCs
To determine if the activated γδ T cells can function as APC, we stimulated highly purified γδ T cells with a combination of anti-CD3 and anti-CD28 antibodies for 48 h. The activated Tcell blasts were separated from the dead cells by Ficoll gradient centrifugation, FACS analyzed, and then treated with Mitomycin C (100 μg/ml, 37 °C, 60 min). Graded numbers of thus treated γδ T cells were then mixed with antigen-specific responder αβ T cells in the presence of specific antigens. Fig. 3 shows a comparison of the Tcell-stimulatory effect of activated γδ T cells and professional APC (irradiated spleen cells). In this experiment, 96-well plates were pre-seeded with 1×105/well MMC-treated γδ Tcells with IRBP1-20 (10 μg/ml). For each well, 5×104 responder T cells were added. As shown, the γδ T cells are potent APCs in the stimulation of IRBP-specific T cell activation.
Fig. 3.

Antigen-presenting function of activated γδ T cells. γδ T cells were cultured in 24-well plate with or without an exposure to anti-CD3 and anti-CD28 antibodies for 24 h. They were inactivated by mitomycin C treatment, and seeded to 96-well plate (1×105/well). Then, 5×104 IRBP-specific and MOG-specific T cells were added to each culture. Proliferation was measured by adding 3H-thymidine. The proliferative response is expressed as the mean cpm±SD for triplicate wells. The results shown are the mean cpm values and are representative of those for 4 separate experiments; the SD was always less than 15%.
To further determine whether γδ T cells isolated from mice with EAU can interact with disease-related or irrelevant autoreactive T cells, we have also tested the AP function of γδ T cells on the activation of T cells specific for a non-related encephalitogenic peptide (MOG35-55). As shown in Fig. 3, the γδ T cells isolated from mice with induced EAU were capable of presenting antigen for MOG-specific T cells as well, and they were almost as effective as splenic APC in stimulating the syngeneic antigen-specific T cells.
3.4. Activation of γδ Tcells by TLR ligands and proinflammatory cytokines
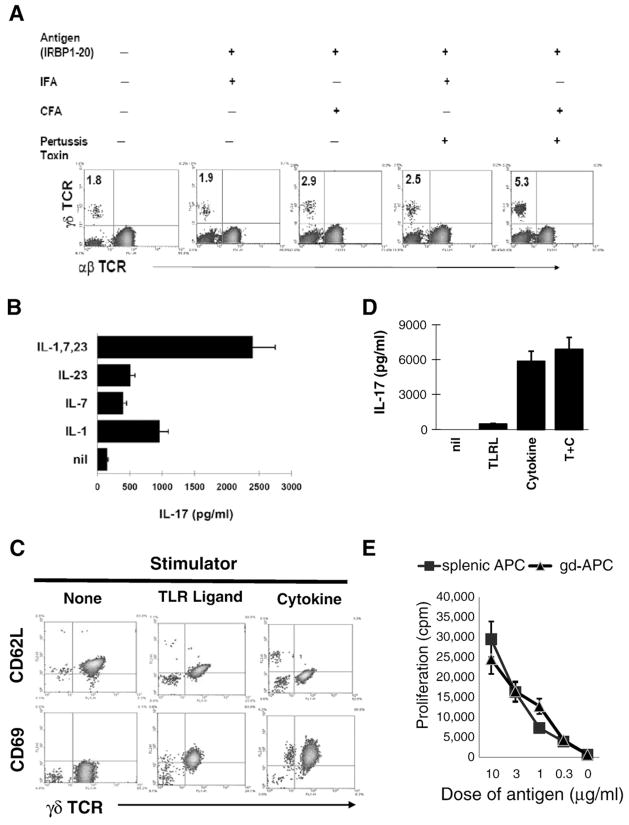
To determine whether immunizing antigen or microbial protein in CFA and/or pertussis toxin (PTX) caused γδ T cell activation in immunized mice, groups of B6 mice were immunized with antigen in IFA or CFA with or without PTX. The results in Fig. 4A show that antigen plus CFA or antigen plus IFA and PTX induced an increase in γδ T cells; and, a combination of antigen in CFA plus PTX induced a greater number of γδ T cells. Since mice immunized with antigen in IFA alone did not show the increase in the number of γδ T cells when compared to untreated mice, we conclude that the uveitogen (IRBP1-20) itself does not stimulate γδ T cell activation and expansion in vivo; rather, γδ T cell activation in such cases, is driven by exposure to either microbial antigen in the CFA and PTX or by proinflammatory cytokines induced in these mice. To test the latter hypothesis, purified γδ T cells were exposed to the cytokines (IL-1, IL-7, and IL-23) that had been found to have a stimulatory effect on γδ T cells (Fig. 2). As demonstrated in Fig. 4B, exposure to IL-1, IL-7 and IL-23 induced γδ T cells to produce IL-17, and combination of the three cytokine had a synergistic stimulatory effect on the γδ T cells. Fig. 4C showed that both the combined cytokines and pooled ligands for TLR 2, 3, 4, and 9 at final concentration of 10 ng/ml, induced increased expression of CD69 and decreased expression of CD62L. However, as shown in Fig. 4D, only the combined cytokines or the cytokines plus the TLR ligands but not the TLR ligands alone, induced IL-17 production in the γδ T cells.
Fig. 4.
γδ T cell-stimulatory effect of cytokine and TLR ligands. Expansion of γδ T cell in immunized mice is driven by molecules in CFA (mycobacteria) and pertussis toxin, but not by the uveitogenic antigen (IRBP1-20). B6 mice were immunized with the uveitogenic peptide (IRBP1-20) in IFA or CFA with or without PTX treatment. Mice treated with peptide and IFA did not have an appreciable increase in the number of γδ T cells, whereas mice treated with either or both CFA and PTX showed increased numbers of γδ T cell. The results shown are representative of those for 3 separate experiments. B). IL-17 production by γδ T cells. γδ T cells were purified from IRBP1-20 immunized B6 mice. 3×105/well cells in 96-well plate were exposed to the indicated cytokines for 48 h, and IL-17 levels in the cell supernatants were assessed by ELISA. C) Purified γδ T cells expressed higher levels of CD69 and lower levels of CD62L after exposure to IL-1, IL-7, and IL-23 or/and ligands for TLR 2,3,4 and 9 at final concentration of 10 ng/ml. Expression of CD69 and CD62L by purified γδ T cells before and after an exposure to cytokines and TLR ligands was determined. D). Antigen-presenting activity of γδ T cell activated by cytokines and TLR ligands. Purified γδ T cells were exposed to cytokines or TLR ligands for 24 h. They were killed by mitomycin C treatment, and seeded to 96-well plate (1×105/well). Then, 5×104 IRBP-specific T cells were added to each culture in the presence or absence of antigen. IFN-γ levels in the stimulated cell supernatants were assessed 48 h after the co-culture by ELISA (E).
Finally, we have also tested whether the cytokine-activated γδ T cells gained antigen-presenting activity. To do so, purified γδ T cells were exposed to a combination of cytokines for 24 h. After a treatment with mitomycin C, they were tested for AP function by inducing IFN-γ production of IRBP-specific Tcell. Fig. 4E shows that after an exposure to cytokines, γδ T cells gained an ability of stimulating IRBP-specific T cells to produce IFN-γ.
4. Discussion
Innate immunity plays an essential role in host defense by eliciting rapid inflammatory reactions and orchestrating adaptive immune responses to microbial infection (Mak and Ferrick, 1998; Medzhitov and Janeway, 1997a; Born et al., 1999). Like cells of the macrophage/dendritic lineage and NK/NKT cells, T cells expressing the γδ TCR are critical components of the innate immune system. Previous studies have shown that the γδ T cells play a major role linking innate and adaptive immunity (Mak and Ferrick, 1998). Studies have shown that γδ T cells are the early infiltrating cells of inflammation caused by infection (Ladel et al., 1995; Mukasa et al., 1997; Andrew and Carding, 2005; Carding and Egan, 2000) and autoimmune diseases (Fukushima et al., 1991; Hohlfeld et al., 1991; Mukasa et al., 1997; Peterman et al., 1993; Spahn et al., 1999). The severity of myelin oligodendrocyte glycoprotein peptide 33–35-induced EAE in mice with a disrupted TCR δ chain gene was significantly decreased (Spahn et al., 1999). In addition, γδ T cells have both effector and immunoregulatory functions (Born et al., 2006; Haas et al., 1993; Mak and Ferrick, 1998). Depletion of γδ T cells from LNC resulted in diminished severity of EAE in recipient mice, both clinically and histopathologically (Odyniec et al., 2004; Tagawa et al., 2004).
Unlike T cells expressing the αβ TCR, γδ T cells represent a minor subset of human peripheral T cells (1%–10%). These cells have limited diversity of TCR, and they recognize antigen in MHC-restricted or unrestricted fashion (Born et al., 2006; Haas et al., 1993; Mak and Ferrick, 1998). The patho-physiologic function of γδ T cells remains elusive, though evidence has been accumulated indicating that γδ T cells play a role in the “first line of defense” against a broad spectrum of invasive microorganisms such as mycobacteria (D'Souza et al., 1997; Janis et al., 1989; Kabelitz et al., 1990; Lockhart et al., 2006; O'Brien et al., 1989). In early inflammation, γδ T cells are the major infiltrating cells of the inflamed organs involved in viral infection, and in autoimmunity of unknown etiology.
It was hypothesized that augmented recruitment and enhanced function of APCs is one of the key steps linking innate to adaptive immunity (Brandes et al., 2005; Collins et al., 1998; Moser and Brandes, 2006). Previous studies have shown that human (Brandes et al., 2005) and bovine (Collins et al., 1998 ) γδ T cells are capable of expressing MHC class II molecules, and act as APCs. Earlier attempts to verify that the mouse cell counterparts have similar functions were not successful. We observed that a number of surface markers of T cells are downregulated in newly activated mouse T cells (data not shown). This drove us to determine whether activated γδ T cells downregulate their surface TCR, and therefore might have escaped detection in certain studies. To do so, we prepared highly (>98%) purified γδ T cells. Remarkably, after stimulation with the antibody specific for mouse CD3, a majority of the γδ T cells expressed MHC class II antigens and increased levels of co-stimulatory molecules such as CD40 and CD80. When tested as APC, these cells greatly stimulated syngenic antigen-specific αβ T cells in the presence of relevant antigens. Our results demonstrate, for the first time, that mouse γδ T cells are capable to express MHC class II antigens and co-stimulatory molecules, and function as APCs. Our study additionally shows that γδ T cells expand rapidly if cultured in medium containing cytokines such as IL-1, IL-7, and IL-23. Given that these cytokines are produced by innate immune cells such as macrophage and dendritic cells, it is likely that exposure to viral and bacterial products, which induced production of these cytokines, subsequently leads to activation of γδ T cells, which are then enabled to activate the antigen-specific adaptive immune response.
It is interesting to note that an increased number of γδ T cells was recruited into lymphoid organs such as the spleen, after immunization with antigens and CFA, which contains microbial products of mycobacteria. In addition, when cultured in growth factor-containing medium, the γδ T cells expanded more vigorously to cytokines such as IL-1 and IL-23, both of which are predominantly the products of innate immune cells such as macrophages and dendritic cells. Given that γδ T cells are early infiltrating cells of the inflamed organ, it is likely that during early phases of infections, cells of the macrophage lineage produce cytokines such as IL-1 and IL-23, which, in turn, cause recruitment, expansion, and activation of γδ Tcells. Conceivably, activated γδ T cells producing inflammatory cytokines further promote the subsequent, adaptive immune responses. Given that APCs play a major role in the subsequent adaptive immune response, investigators believe that recruitment and maturation of dendritic cells are essential. However, since γδ T cells are the early inflammatory cells, it is likely that such cells may play important role in the early immune response as well, not only by acting as effecter and regulatory cells, by also as APCs.
The γδ T cells of this study were isolated from C57BL/6 mice immunized with an uveitogenic antigen IRBP1-20, rather than from naïve mice, due to that the study is part of our research project investigating the role γδ T cells in activation of IL-17+ uveitogenic T cells. Our studies demonstrated that γδ T cells play a major role in the activation of the IL-17+ uveitogenic T cells (data not shown and paper in preparation). We have observed that while the number of γδ T cells in naïve mouse is very low, it is greatly increased in the immunized mice and further expanded after in vitro culture. It is likely that the same cell isolated from naïve mice or from mice immunized with different antigens might express distinct structural and functional phenotypes. Our future study should determine whether specific immunization procedures promote the differentiation, expansion, and activation of the γδ T cell subset(s) with unique immunologic functions.
We have observed that the freshly isolated γδ T cells from immunized mice are partially activated in vivo and express elevated amount of CD69 molecules. Although the separation procedure using magnetic beads and antibodies specific for γδ TCR likely might cause the observed cell activation, we considered it unlikely, because similarly separated αβ T cells do not express such activation molecules (data not shown). Since mice immunized with antigen and IFA did not significantly promote the expansion of γδ T cells in vivo whereas mice immunized with antigen and CFA expressed elevated numbers of γδ Tcells, we considered the possibility that microbial protein in the CFA might play a role in promoting the γδ Tcell activation in vivo. To support such a hypothesis, we exposed γδ T cells to TLR ligands and cytokines in vitro. Our results showed that although individual TLR ligands only weekly stimulated the γδ T cell activation, pooled together, these molecules have a stronger effect stimulating γδ T cell activation. However, in vitro studies showed that a combination of IL-1, IL-7 and IL-23, but not other cytokines tested, has a greater effect on γδ T cell activation and expansion. Given that induction of autoimmune diseases by immunization of genetically disease-prone animals with antigen requires the CFA and/or PTX, it seems likely that the microbial antigens in such reagents trigger the innate cell elements such as γδ T cells. Subsequently induced cytokines then might further promote the activation of γδ T cells, and their potential to drive a stronger and faster development of the adaptive αβ T cell response.
Abbreviations
- EAU
experimental autoimmune uveitis
- IRBP
interphotoreceptor retinoid-binding protein
- APC
antigen-presenting cell
- MHC
major histocompatibility complex
Footnotes
Supported in part by NIH grants NEI-EY014366 and EY017373, Vision Research Infrastructure Development (R24 EY015636), and the Commonwealth of Kentucky Research Challenge Trust Fund. DS is a recipient of a senior investigator award from Research to Prevent Blindness.
References
- Andrew EM, Carding SR. Murine γδ T cells in infections: beneficial or deleterious? Microbes Infect. 2005;7:529–536. doi: 10.1016/j.micinf.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Avichezer D, Chan CC, Silver PB, Wiggert B, Caspi RR. Residues 1–20 of IRBP and whole IRBP elicit different uveitogenic and immunological responses in interferon gamma deficient mice. Exp Eye Res. 2000;71:111–118. doi: 10.1006/exer.2000.0860. [DOI] [PubMed] [Google Scholar]
- Born W, Cady C, Jones-Carson J, Mukasa A, Lahn M, O'Brien R. Immunoregulatory functions of γδ T cells. Adv Immunol. 1999;71:77–144. [PubMed] [Google Scholar]
- Born WK, Reardon CL, O'Brien RL. The function of γδ T cells in innate immunity. Curr Opin Immunol. 2006;18:31–38. doi: 10.1016/j.coi.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human γδ T cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- Carding SR, Egan PJ. The importance of γδ T cells in the resolution of pathogen-induced inflammatory immune responses. Immunol Rev. 2000;173:98–108. doi: 10.1034/j.1600-065x.2000.917302.x. [DOI] [PubMed] [Google Scholar]
- Carding SR, Allan W, Kyes S, Hayday A, Bottomly K, Doherty PC. Late dominance of the inflammatory process in murine influenza by γ/δ+ T cells. J Exp Med. 1990;172:1225–1231. doi: 10.1084/jem.172.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RA, Werling D, Duggan SE, Bland AP, Parsons KR, Howard CJ. γδ Tcells present antigen to CD4+ ab Tcells. J Leuko Biol. 1998;63:707–714. doi: 10.1002/jlb.63.6.707. [DOI] [PubMed] [Google Scholar]
- D'Souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, Orme IM. An anti-inflammatory role for γδ T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–1221. [PubMed] [Google Scholar]
- Fernandez NC, Lozier A, Flament C, Ricciardi-Castognoli P, Bellet D, Suter M, Perricaudet M, Tursz T, maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- Freedman MS, Ruijs TCG, Selin LK, Antel JP. Peripheral blood γδ T cells lyse fresh human brain-derived oligodendrocytes. Ann Neurol. 1991;30:794–800. doi: 10.1002/ana.410300608. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Masuda T, Ohtani H, Sasaki I, Funayama Y, Matsuno S, Nagura H. Immunohistochemical characterization, distribution, and ultrastructure of lymphocytes bearing T-cell receptor γδ in inflammatory bowel disease. Gastroenterology. 1991;101:670–678. doi: 10.1016/0016-5085(91)90524-o. [DOI] [PubMed] [Google Scholar]
- Goodman T, Lefrancois L. Intraepithelial lymphocytes. Anatomical site, not T cell receptor form, dictates phenotype and function. J Exp Med. 1989;170:1569–1581. doi: 10.1084/jem.170.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas W, Pereira P, Tonegawa S. γδ cells. Ann Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R, Engel AG, Ii K, Harper MC. Polymyositis mediated by T lymphocytes that express the γδ receptor. N Eng J Med. 1991;324:877–881. doi: 10.1056/NEJM199103283241303. [DOI] [PubMed] [Google Scholar]
- Janis EM, Kaufmann SHE, Schwartz RH, Pardoll DM. Activation of γδ T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989;244:713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- Kabelitz D, Bender A, Schondelmaier S, Schoel B, Kaufmann SHE. A large fraction of human peripheral blood γδ+ T cells is activated by Mycobacterium tuberculosis but not by its 65-kD heat shock protein. J Exp Med. 1990;171:667–679. doi: 10.1084/jem.171.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon a/b-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladel CH, Blum C, Dreher A, Reifenberg K, Kaufmann SH. Protective role of /γδ T cells and a/b T cells in tuberculosis. Eur J Immunol. 1995;25:2877–2881. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]
- Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- Mak TW, Ferrick DA. The γδ T-cell bridge — linking innate and acquired immunity. Nat Med. 1998;4:764–765. doi: 10.1038/nm0798-764. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997a;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997b;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Moser B, Brandes M. γδ T cells: an alternative type of professional APC. Trends Immunol. 2006;27:112–118. doi: 10.1016/j.it.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Mukasa A, Lahn M, Pflum EK, Born W, O'Brien RL. Evidence that the same γδ T cells respond during infection-induced and autoimmune inflammation. J Immunol. 1997;159:5787–5794. [PubMed] [Google Scholar]
- O'Brien RL, Happ MP, Dallas A, Palmer E, Kubo R, Born WK. Stimulation of a major subset of lymphocytes expressing T cell receptor γδ by an antigen derived from Mycobacterium tuberculosis. Cell. 1989;57:667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Odyniec A, Szczepanik M, Mycko MP, Stasiolek M, Raine CS, Selmaj KW. Gammadelta T cells enhance the expression of experimental autoimmune encephalomyelitis by promoting antigen presentation and IL-12 production. J Immunol. 2004;173:682–694. doi: 10.4049/jimmunol.173.1.682. [DOI] [PubMed] [Google Scholar]
- Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- Peng Y, Shao H, Ke Y, Zhang P, Xiang J, Kaplan HJ, Sun D. In vitro activation of CD8 interphotoreceptor retinoid-binding protein-specific T cells requires not only antigenic stimulation but also exogenous growth factors. J Immunol. 2006;176:5006–5014. doi: 10.4049/jimmunol.176.8.5006. [DOI] [PubMed] [Google Scholar]
- Peterman GM, Spencer C, Sperling AI, Bluestone JA. Role of γδ T cells in murine collagen-induced arthritis. J Immunol. 1993;151:6546–6558. [PubMed] [Google Scholar]
- Ponomarev ED, Dittel BN. γδ T cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand-dependent mechanism. J Immunol. 2005;174:4678–4687. doi: 10.4049/jimmunol.174.8.4678. [DOI] [PubMed] [Google Scholar]
- Rajan AJ, Asensio VC, Campbell IL, Brosnan CF. Experimental autoimmune encephalomyelitis on the SJL mouse: effect of γδ T cell depletion on chemokine and chemokine receptor expression in the central nervous system. J Immunol. 2000;164:2120–2130. doi: 10.4049/jimmunol.164.4.2120. [DOI] [PubMed] [Google Scholar]
- Selmaj K, Brosnan CF, Raine CS. Colocalization of lymphocytes bearing γδ T-cell receptor and heat shock protein hsp65+ oligodendrocytes in multiple sclerosis. Proc Natl Acad Sci USA. 1991;88:6452–6456. doi: 10.1073/pnas.88.15.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H, Peng Y, Liao T, Wang M, Song M, Kaplan HJ, Sun D. A shared epitope of the interphotoreceptor retinoid-binding protein (IRBP) recognized by the CD4+ and CD8+ autoreactive T cells. J Immunol. 2005;175:1851–1857. doi: 10.4049/jimmunol.175.3.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimonkevitz R, Colburn C, Burnham JA, Murray RS, Kotzin BL. Clonal expansions of activated gamma/γδ T cells in recent-onset multiple sclerosis. Proc Natl Acad Sci USA. 1993;90:923–927. doi: 10.1073/pnas.90.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelsey ME, Mellon J, Niederkorn JY. γδ Tcells are needed for ocular immune privilege and corneal graft survival. J Immunol. 2001;166:4327–4333. doi: 10.4049/jimmunol.166.7.4327. [DOI] [PubMed] [Google Scholar]
- Spahn TW, Issazadah S, Salvin AJ, Weiner HL. Decreased severity of myelin oligodendrocyte glycoprotein peptide 33–35-induced experimental autoimmune encephalomyelitis in mice with a disrupted TCR d chain gene. Eur J Immunol. 1999;29:4060–4071. doi: 10.1002/(SICI)1521-4141(199912)29:12<4060::AID-IMMU4060>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, Wekerle H, Raine CS. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 Mice. J Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- Sun D, Zhang Y, Wei B, Peiper SC, Shao H, Kaplan HJ. Encephalitogenic activity of truncated myelin oligodendrocyte glycoprotein (MOG) peptides and their recognition by CD8+ MOG-specific T cells on oligomeric MHC class I molecules. Int Immunol. 2003;15:261–268. doi: 10.1093/intimm/dxg023. [DOI] [PubMed] [Google Scholar]
- Tagawa T, Nishimura H, Yajima T, Hara H, Kishihara K, Matsuzaki G, Yoshino I, Maehara Y, Yoshikai Y. Vδ1+ γδ T cells producing CC chemokines may bridge a gap between neutrophils and macrophages in innate immunity during Escherichia coli infection in mice. J Immunol. 2004;173:5156–5164. doi: 10.4049/jimmunol.173.8.5156. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Ann Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T, Fukuda S, Hamada H, Nakamura A, Kohama Y, Ishikawa H, Tsujikawa K, Yamamoto H. Role of γδ T cells in the inflammatory response of experimental colitis mice. J Immunol. 2003;171:5507–5513. doi: 10.4049/jimmunol.171.10.5507. [DOI] [PubMed] [Google Scholar]