Abstract
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder afflicting >500,000 patients in the United States alone. This age-related progressive disorder is typified by invariant loss of dopaminergic substantia nigra neurons (DAN), dystrophic neurites, the presence of α-synuclein (SYN) positive intracytoplasmic inclusions (Lewy bodies) in the remaining DAN, and activated microglia. As such, microglial activation and resultant increase in proinflammatory molecules have moved to the forefront of PD research as a potential pathobiologic mechanism of disease. Herein, we present data demonstrating early microglial activation in mice that over-express wild-type SYN, the release of SYN from a SYN overexpressing MN9D cell line, and dose-dependent SYN-mediated activation of primary microglial cultures with consequent increases in proinflammatory molecules. Furthermore, we provide evidence that the CD36 scavenger receptor and downstream kinases are involved in SYN-mediated microglial activation. Together, our data suggest an early role for SYN and inflammation in PD pathogenesis.
Keywords: microglia, CD36, Parkinson’s disease, synuclein, proinflammatory cytokines, MN9D
1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease with approximately 50,000 new cases per year in the United States. This age-related slowly progressive degenerative disease is characterized by the invariant loss of nigrostriatal dopamine neurons (DAN) and terminals (Duvoisin, 1992). The mechanism of neuron loss is obscure but thought to result from both genetic and environmental factors (Maguire-Zeiss and Federoff, 2003). Among familial cases, those kindreds harboring mutations in the gene α-synuclein (SYN) appear highly interesting owing to the presynaptic nature of the protein and its propensity to oligomerize. A second histopathologic feature of PD is the presence of eosinophilic proteinaceous ubiquitin- and α-synuclein-positive intracellular inclusions, Lewy bodies, within the surviving substantia nigra DAN (Baba et al., 1998; Irizarry et al., 1998). Another important feature of this and other neurodegenerative diseases is the accumulation of activated microglia (Croisier et al., 2005). Microglia are the resident immune cells in the brain and activation links inflammation and neurodegeneration in PD (Campbell, 2004; Hirsch et al., 2005; Kim and Joh, 2006; Liu and Hong, 2003; McGeer et al., 1988; McGeer and McGeer, 2004).
Microglia along with astrocytes comprise the innate immune response in the brain acting to surveil and mediate responses to foreign proteins, cell debris and various molecules (reviewed in (Kim and Joh, 2006)). Activation of microglia results in a change in cell morphology from a resting ramified shape to an amoeboid profile (reviewed in (Kreutzberg, 1996)). This change in morphology is accompanied by alterations in cell surface receptor expression, increased reactive oxygen species (ROS) production and release of chemokines and cytokines, such as TNFα and IL1β. While secreted soluble factors can be beneficial such as glia cell line-derived neurotrophic factor, the majority contribute to oxidative stress and are both proinflammatory and neurotoxic. Activated microglia can phagocytose foreign antigens, proliferate and recruit additional microglia to mediate the inflammatory response. In PD, it remains to be determined whether microglia actively participate in the initiation and progression of pathogenesis.
Among possible triggers of microglial activation in PD are damaged DANs, environmental toxins and/or extracellular SYN. We have previously postulated that SYN in concert with microglial activation, oxidative stress and protein aggregation converge upon a common PD pathogenic pathway (Maguire-Zeiss and Federoff, 2003). Wild-type SYN overexpression as well as point mutations within the human α-synuclein gene result in familial PD (Abbas et al., 1999; Kitada et al., 1998; Kruger et al., 1998; Polymeropoulos, 2000; Polymeropoulos et al., 1996; Polymeropoulos et al., 1997; Singleton et al., 2003; Sveinbjornsdottir et al., 2000). In some animal and cell culture models, wild-type and mutant SYN overexpression and/or neurotoxicant exposure lead to increased oxidative stress, SYN misfolding/aggregation and neuronal dysfunction (Ancolio et al., 2000; Beal, 2001; Dong et al., 2002; Hashimoto et al., 2003; Kahle et al., 2000; Lee et al., 2002; Manning-Bog et al., 2001; Manning-Bog et al., 2002; Masliah et al., 2000; Matsuoka et al., 2001; Przedborski et al., 2001a; Rathke-Hartlieb et al., 2001; Richfield et al., 2002; Song et al., 2004; Tabrizi et al., 2000; Thiruchelvam et al., 2004; van der Putten et al., 2000; Vila et al., 2000; Zhang et al., 2005). For example, 1,2,3,6-methyl-phenyl-tetrahydropyridine (MPTP) treatment of mice and monkeys has become a common method to achieve dopaminergic neuronal loss and produce a “model” of PD (Ali et al., 1993; Bankiewicz et al., 1990; Kowall et al., 2000; Langston et al., 1984a; Langston et al., 1984b; Miller et al., 2004; Przedborski et al., 2001b; Schmidt and Ferger, 2001; Seniuk et al., 1990; Tatton and Kish, 1997; Varastet et al., 1994). The linkage between toxicant injury and SYN aggregation was established by the finding that MPTP stimulates production, oxidative modification and aggregation of SYN (Dong et al., 2002; Gomez-Santos et al., 2002; Rathke-Hartlieb et al., 2001). MPTP-treatment has also been linked to glial activation, where microglial activation was followed by astrocytosis in both the substantia nigra and striatum (Kohutnicka et al., 1998; Thomas et al., 2004). Together these studies support that PD, despite different initiating mechanisms, follows a convergent pathogenic pathway involving SYN, microglial activation, oxidative stress and protein aggregation.
The mechanism by which SYN promotes neurodegeneration and whether microglia are contributory is unknown. SYN could activate microglia through a direct interaction or indirectly through release of neuronal-derived soluble factors. SYN-mediated microglial activation has been recently demonstrated in rat primary cultures, human microglia cultures and a monocytic cell line, THP-1, following treatment with exogenous SYN (Klegeris et al., 2006; Zhang et al., 2005). In vivo, microglial activation via direct interaction with SYN would require release of SYN from DAN and subsequent phagocytosis or receptor-mediated activation. In support of a direct effect, SYN release from cells via endoplasmic reticulum/Golgi-independent exocytosis and in response to neural activity has recently been demonstrated (Fortin et al., 2005; Lee et al., 2005) leaving extracellular SYN available for interaction with microglia. One known mechanism of microglial activation requires the interaction of “foreign” proteins with microglial membrane receptors. Multiple membrane receptors have been implicated in microglial activation and intracellular signal transduction pathways (Block et al., 2007). In particular, the class B scavenger receptor, CD36, has been shown to mediate the microglial proinflammatory response to another neurodegenerative protein, amyloid β (Coraci et al., 2002; El Khoury et al., 2003; Husemann et al., 2001; Kunjathoor et al., 2004; Medeiros et al., 2004; Moore et al., 2002; Verdier and Penke, 2004). Since structural studies demonstrate that the amyloid fibrils formed from amyloid β and SYN have similar characteristics we investigated whether SYN-induced microglial activation proceeds through CD36 (Serpell et al., 2000). Herein we present data demonstrating early microglial activation in an animal model of wild-type SYN overexpression, release of SYN from a SYN overexpressing dopaminergic-like cell line and dose-dependent SYN-mediated direct activation of primary microglia cultures with consequent increases in proinflammatory molecules. Furthermore we provide evidence that the CD36 scavenger receptor and downstream kinases are involved in SYN-mediated microglial activation. Together, our data suggests an early role for SYN and inflammation in PD pathogenesis.
2. Methods
2.1. Animals
The transgenic mouse model overexpressing human wild-type SYN under control of the 9-kb rat tyrosine hydroxylase promoter were previously developed in our laboratory (Richfield et al., 2002). We have since homozygoused the SYN locus for this model (SYNWT+/+). Age-matched male C57BL/6 mice were used as non-transgenic controls (NTG) in all experiments. CD36−/− mice were a gift of Drs. Andrew D. Luster and Kathryn Moore (El Khoury et al., 2003). All animal housing and procedures were performed in compliance with guidelines established by the University Committee of Animal Resources at the University of Rochester.
2.2. Bacterial expression and purification of α-synuclein
The bacterial expression vector pRK172 containing wild-type human α-synuclein (SYN) cDNA was a kind gift of Dr. Giasson (Giasson et al., 1999). SYN was expressed in Escherichia coli BL21 (DE3) and purified as previously described (Maguire-Zeiss et al., 2006). SYN was stored in TE buffer (10 mM Tris–HCl, pH 7.5, and 1 mM EDTA) containing 20 mM sodium chloride (NaCl) at 4°C. The contamination of lipopolysaccharide (LPS) in the purified SYN was assessed using E-TOXATE test kits (Sigma, Saint Louis, MI). The detection limit of the kit was 0.06 Endotoxin Units (EU)/mL (10 EU=1 ng).
2.3. Cell Culture
2.3.1. MN9Dwtsyn cells
Human wildtype α-synuclein (syn) cDNA ((Giasson et al., 1999); a kind gift from Dr. Giasson) was subcloned into a tetracycline responsive autoregulated bi-directional expression vector ((Strathdee et al., 1999); pBig2i; a kind gift of Dr. Strathdee). The immortalized hybrid dopaminerigic cell line, MN9D, was a kind gift of Dr. Heller (Heller et al., 1996). MN9D cells were stably transfected with the pBig2isynIRESeGFP or pBig2ieGFP control construct and positive clones selected following incubation with hygromycin B (400 µg/mL media; Sigma; MN9DSYN; MN9DGFP). MN9D cells were maintained in Dulbecco’s modified Eagle’s medium (Sigma, D5648) containing 10% fetal bovine serum (FBS) and hygromycin B (200 µg/mL). SYN and GFP expression were induced with doxycycline (2.0 µg/mL media) for 48 hours. Cell lysates and conditioned media were analyzed for SYN and glucose-6-phosphate dehydrogenase by Western blot analysis as described.
2.3.2. Primary microglial cells
Cerebral cortices of neonatal mice (1-day old; C57BL/6 or CD36−/−) were stripped of meninges and minced in hepes balanced salt solution (HBSS; Mediatech Inc., Herndon, VA). Cells were dissociated in minimum essential media (MEM; Invitrogen, Frederick, MD) containing Earle’s salts, L-glutamine, 0.01% pyruvate, 0.6% glucose, 4% fetal bovine serum and 6% horse serum (complete medium), centrifuged, resuspended and plated into flasks containing 10 mL complete medium at a density of one brain per T75 flask. Cultures were grown at 37°C under 5% CO2. After 1 day, the flasks were tapped gently to remove cell debris, media removed and replaced with fresh complete media. Cultures were grown as above for approximately 12 days at which time the microglia were harvested by tapping the flasks and collecting the microglia-enriched containing medium. Microglia were pelleted by centrifugation (1000 rpm, 5 min), resuspended in MEM medium containing 0.01% pyruvate, 0.6% glucose, and 5% fetal bovine serum and enumerated. Primary microglial cells were plated in 24-well plates on cover glass (Carolina Biological Supply Company, Burlington, NC) at a density of 4 × 104 cells per well. Cells were incubated for 24 hours followed by the treatment with 10, 50 or 250 nM SYN. LPS (0.007 ng) and buffer treatment served as negative activation controls. All treatments were in triplicate. Following SYN treatment, cells were incubated for 24 hours under culture conditions and analyzed by immunocytochemistry (ICC).
2.4. Determination of TNFa levels
TNFα secretion into microglial-conditioned media following SYN treatment was measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
2.5. Fluorometric assay of ROS production
Primary microglia were plated in 24-well plates at the density of 1 × 105 cells per well for 24 hours followed by treatment with 250 nM SYN or buffer (control) for various times. Cells were washed once with HBSS and incubated with 2’, 7’-dichlorofluorescein diacetate (DCF-DA, Invitrogen, Carlsbad, CA) in HBSS at a concentration of 7.5 µg/mL for 1 hour at 37°C in the dark. DCF-DA-treated cells were washed with HBSS, and incubated for an additional 40 minutes in HBSS. DCF fluorescence intensities were measured using a Bio-Rad FluoromakTM Plate Reader (excitation at 485 nm and emission at 538 nm; Gain 20; Hercules, CA).
2.6. Immunological analysis
2.6.1. Antibodies
Antibodies were used at the following dilutions: rabbit anti-phospho-ERK (1:1000; Cell Signaling Technology, Danvers, MA), rabbit anti-ERK (1:1000; Cell Signaling Technology, Danvers, MA), rabbit anti-β-actin (1:20000; Sigma, Saint Louis, MI), mouse anti-SYN (1:1000; BD Bioscience, San Jose, CA), anti-glucose-6-phosphate dehydrogenase (1:2000; Abcam, Cambridge, MA), rabbit anti-ionized calcium-binding adaptor molecule 1 (Iba1; 1:5000 & 1:750; Wako, Richmond, VA), ECL anti-mouse or rabbit IgG horseradish peroxidase linked whole antibody (from sheep or donkey; 1:2000; GE Healthcare, Little Chalfront Buckinghamshire, UK).
2.6.2. Immunohistochemistry
2.6.2.1. Animals
One-month old SYNWT+/+ and NTG mice (n=4/genotype) were sacrificed, perfused with 4% paraformaldehyde (PFA), brains removed and post-fixed in 4% PFA overnight at 4°C. Brains were transferred to 20% sucrose overnight and stored in 30% sucrose at 4°C until sectioning. 30 µm brain sections were cut on a sliding microtome and stored in cryoprotectant (300 g/L sucrose and 30% ethylene glycol) at −20°C until immunohistochemical analyses (IHC). For IHC, sections were washed three times in 0.1M phosphate buffer solution (PBS; 80 g/L NaCl, 2 g/L KCl, 11.5 g/L Na2HPO4H2O, 2 g/L KH2PO4) for 10 minutes each followed by overnight incubation to remove the cryoprotectant. Sections were treated with 3% H2O2 in PBS for 20 minutes at room temperature (RT; 22°C) followed by permeablization in PBS containing 0.1% triton X-100 (v/v; PBS/0.1% TX-100) for 5 minutes. Sections were incubated in blocking solution containing PBS/10% filtered goat serum (v/v) for 1 hour at RT followed by incubation with rabbit anti-ionized calcium-binding adaptor molecule 1 (Iba1) antibody (1:5000; Wako, Richmond, VA) for 2 days at 4°C. Following 3 rinses in PBS/0.1% TX-100/0.1% goat serum for 10 minutes each at RT, sections were incubated in PBS/0.1%TX-100/1% goat serum containing 2° antibody (biotinylated goat anti-rabbit immunoglobulin; 1:1000; Vector Laboratories, Burlingame, CA) for 2 hours at RT followed by washes and colorimetric development (DAB; Vector Laboratories, Burlingame, CA). Immunostained sections were mounted on slides and sealed with cytoseal (Richard-Allan Scientific, Kalamazoo, MI). For image analysis, Iba1-positive cells were visualized using an Olympus AX-70 microscope equipped with a motorized stage (Olympus, Melville, NY) and the MCID 6.0 Elite Imaging Software (GE healthcare, Piscataway, NJ). Sections were tiled under 4X magnification. Four equal sections of substantia nigra (SN) from each mouse were analyzed in a blinded fashion. Eighty percent of the defined region of interest was assessed under 40X magnification. Two groups of morphologically distinct Iba1+ cells, resting (small cell bodies and thin processes) and activated (large cell bodies and thick processes) were counted.
2.6.2.2. Cell Culture
Following treatment, cells were washed with 1X PBS for 5 minutes, fixed in 4% PFA at room temperature for 20 minutes, permeablized in 1X PBS containing 0.1% triton X-100 for 5 minutes and blocked for 1 hour with 1X PBS containing 10% goat serum. Cells were incubated overnight at 4°C with rabbit anti-Iba1 antibody (1:750; Wako, Richmond, VA) in blocking buffer, followed by a one hour incubation with a fluorescent 2° antibody (goat anti-rabbit IgG 594; 1:500; Invitrogen, Carlsbad, CA). Unbound 2° antibody was removed by washing with 1X PBS containing 0.1% triton X-100. Nuclei were stained with DAPI (1:5000) in 1X PBS for 5 min. Following two washes with 1X PBS the cover glasses were mounted to slides using Mowiol solution (333 g/L glycerol, 133 g/L Mowiol, 0.133 M Tris-HCl, pH 8.5). For enumeration of total and activated microglia, six random 20X ICC images per sample were taken using an Olympus AX70 microscope (Olympus, Melville, NY). Two distinct groups of cells were counted based on cell area and morphology (circularity or elongation) using ImageJ software (NIH, Bethesda, MD). Cells were considered inactivated if the cell area was less than 1 µm2 (27 × 27 pixels) and/or had thin and elongated morphology. All others were counted as activated.
2.6.3. Western blot analysis
Primary microglial cells were plated in 6-well plates at a density of 1 × 106 cells per well, incubated for 24 hours prior to treatment with 250 nM SYN or buffer only for 3, 5, or 10 minutes. Cells were washed in ice-cold PBS five times and lysed in RIPA buffer (50 mM Tris-HCl, pH 7.4; 1% NP-40; 0.25% sodium deoxycholate; 150 mM NaCl) containing protease inhibitors (1 mM EDTA; 1 mM phenylmethylsulphonyl fluoride (PMSF); 1 µg/mL aprotinin; 1 µg/mL leupeptin; 1 µg/mL pepstatin) and phosphatase inhibitors (1 mM Na3VO4; 1 mM NaF). Cell lysates were sonicated 3 times with 5-second bursts followed by gentle rotation for 1 hour at 4°C. Lysates were cleared by centrifugation at 13,000 rpm for 5 minutes. Thirty micrograms of protein was subjected to denaturing polyacrylamide gel electrophoresis ((Laemmli, 1970); 10% PAG-SDS) and electrophoretically transferred to PVDF membrane (PerkinElmer, Wellesley, MA). Membranes were blocked for 1 hour in TBST (20 mM Tris-HCl, 150 mM NaCl, 0.1% Tween) containing 5% non-fat dry milk (NFDM), incubated with 1° antibody in TBST containing 5% NFDM overnight with gentle shaking at 4°C, followed by 4 washes in TBST, and incubation with the appropriate HRP-conjugated 2° antibody. After washing for 3 × 5 minutes in TBST and 1 × 5 minutes in TBS, antibody:antigen complexes were visualized using a Western Lightning Chemiluminescence Reagent kit (PerkinElmer, Wellesley, MA). Subsequently, blots were stripped and re-probed with different 1° antibodies. β-actin served as the loading control.
2.7. Quantitative Real-time RT-PCR
2.7.1. Animals
RNA was isolated from microdissected substantia nigra (SN) and striatum (STR) of 1-month old SYNWT+/+ and NTG mice using TRIZOL (Invitrogen, Carlsbad, CA) (n = 8/genotype). RNA was treated with RQ1 DNase (Promega, Madixon, WI) followed by phenol:chloroform extraction and ethanol/lithium chloride precipitation. RNA integrity was determined using a bioanalyzer (Functional Genomic Center, University of Rochester, Rochester, NY). One microgram of total RNA was reverse transcribed using a High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). The quality of cDNA was assessed by β-actin PCR. Ten microliters of total cDNA from each sample was used for PCR on Taqman Low Density Array Micro Fluidic Cards which were preloaded with probes and primers for 15 targets and one endogenous control 18S rRNA. The assay for each target was performed in triplicate. Real-time PCR reactions were conducted using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). The results were analyzed using the relative quantification ΔΔCT method, normalizing the 1-month old SYNWT+/+ samples to age-matched NTG.
2.7.2. Cells
Primary microglial cells were plated in 12-well plates at a density of 2.6 × 105 per well for 24 hours followed by incubation with 250 nM SYN or buffer for various times. Each time point was performed in triplicate. Total RNA was extracted from primary microglial cells using Trizol reagent according to the manufacturer’s specifications (Invitrogen, Carlsbad, CA). Reverse transcription was conducted using a High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). cDNA samples were analyzed by the 7300 real time PCR system using quantitative real-time PCR Taqman Gene Expression Assays with specific primers and probes for the following targets: TNFα (Mm00443258_m1), COX2 (Mm00478374_m1), IL1 β (Mm00434228_m1), IL6 (Mm00446190_m1), IL10 (Mm00439616_m1), NOS2 (Mm00440485_m1), NOX2 (Mm00432775_m1) (Applied Biosystems, Foster City, CA). The quantification of target transcripts was based on a calibration curve. The calibration curve was made covering a concentration range of 2.4-1204 µg/mL of cDNA samples prepared from spleens of LPS-intraperitioneal (IP) injected mice.
3. Results
3.1. Microglial activation in young SYNWT+/+ mice
Whether activated microglia participate in the initiation and/or progression of PD remains unknown. In an effort to establish microglia activation as an early pathologic process in a model of PD, we utilized transgenic mice that overexpress human wild-type α-synuclein driven by a rat tyrosine hydroxylase (TH) promoter (SYNWT+/+). We have previously reported that heterozygous SYNwt+/− mice (hwα-SYN-5; (Richfield et al., 2002)) display increased dopamine transporter density, dystrophic neurites and greater decreases in locomotor activity following MPTP treatment. In this study the transgene locus is homozygosed (SYNWT+/+).
One-month old SYNWT+/+ and C57BL/6 (NTG) mice were examined for differences in microglial number and activation state. We enumerated Iba1+ microglia in the substantia nigra (SN) and striatum (STR) using immunohistochemistry followed by computer-assisted cell unbiased counting. The results demonstrate an increased number of activated microglia in the substantia nigra of 1-month old SYNWT+/+ compared to age-matched NTG mice (p=0.0001; Fig. 1A). Differences in striatal microglia counts did not reach significance (activated microglia in NTG = 258+64 and SYNWT+/+ = 485±166; p=0.2). In both the SN and STR the total number of microglia was unchanged suggesting a switch in the state of activation of resident microglia. The increased numbers of activated microglia in SYNWT+/+ mice suggests an association between microglial activation and SYN overexpression.
Fig. 1. Early expression of SYN activates microglia.
Iba1+ cells were enumerated from one-month old SYNWT+/+ and C57BL/6 (NTG) mice (n=4/genotype) following immunohistochemistry for the microglia marker, ionized calcium-binding adaptor molecule 1 (Iba1). Representative images of resting and activated microglia are shown (inset; 60X magnification; Olympus AX70, Melville, NY). Here, we demonstrate a statistically significant increase in activated SN microglia (A; *, p=0.0001). RNA from the SN and STR of one-month old SYNWT+/+ and NTG was subjected to qRT-PCR to determine changes in Iba1 and TNFα expression levels. There is a significant increase in Iba1 expression in the SN while TNFα expression is increased in both the SN and STR (B; #, p=0.03; *, p<0.05).
To identify molecules indicative of microglial activation within the nigrostriatal system, total RNA was extracted from the SN and STR and analyzed using quantitative real-time PCR (qRT-PCR). We demonstrate an increase in the expression of the microglial cell markers Iba 1 (SN; p=0.03) and F4/80 (STR; p=0.02, data not shown) in SYNWT+/+ compared to age-match NTG mice. Furthermore, we observe an elevation in TNFα expression in SYNWT+/+ SN and STR (p<0.05; Fig. 1B). The up-regulation of this proinflammatory molecule in one-month old SYNWT+/+ mice supports a role for inflammation and microglial activation in this model of SYN overexpression.
3.2. SYN activates microglia and promotes TNFα release
We next pursued studies that would elucidate a mechanism underlying microglia activation in SYNWT+/+. Given recent demonstration of SYN in human CSF and blood plasma (Borghi et al., 2000; El-Agnaf et al., 2003) as well as in the conditioned media of SYN expressing cells (Lee et al., 2005; Sung et al., 2005) we analyzed dopaminergic MN9D cells engineered to overexpress SYN and green fluorescent protein (GFP) under the regulation of doxycycline (MN9DSYN; Fig. 2). Using an autoregulated bi-directional tetracycline-responsive expression vector designed by Strathdee et al., we were able to coordinately regulate the expression of human wild-type SYN and GFP with doxycycline in MN9D cells (Strathdee et al., 1999). These inducible cell lines are capable of tightly regulated expression of human wild-type SYN and GFP. The parental MN9D cells or MN9D cells with regulated GFP alone (MN9DGFP) do not express SYN (Fig. 2) (Heller et al., 2000; Heller et al., 1996). MN9DSYN and MN9DGFP were grown in the absence and presence of doxycycline (DOX). Following treatment, conditioned media was collected (M) and lysates prepared from the adherent cells (L). Samples were subjected to SDS-PAG electrophoresis followed by western blot analysis for SYN. As expected, SYN is highly expressed in cell lysates from DOX-treated MN9DSYN cells (intracellular SYN) while untreated cells have low SYN levels. The SYN from untreated MN9DSYN cells is likely due to promoter leakage and not endogenous SYN since MN9DGFP lysates do not have detectable levels of this protein (Fig. 2). In contrast, only the conditioned media from MN9DSYN cells is replete with SYN (Fig. 2; M). Blots were also probed against the ubiquitous cellular protein, glucose-6-phosphate dehydrogenase (G6PD), to control for non-specific protein release. G6PD is absent from all conditioned media and as expected present in all cell lysates. Together, these results indicate that SYN is released from MN9DSYN cells. This mechanism may also make SYN available to activate resident microglia in vivo.
Fig. 2. SYN is released from MN9DSYN cells.
MN9D cells overexpressing SYN and/or GFP under the regulation of tetracycline were treated with or without the tetracycline analog, doxcycline (DOX), for 48 hours. Conditioned media (M) was collected and cell lysates (L) prepared. Fifty microliters of conditioned media and either 1 µg (SYN blot) or 20 µg (G6PD blot) of cell lysate were analyzed by Western blot analysis for SYN and glucose-6-phosphate dehydrogenase (G6PD). Here we demonstrate the presence of SYN but not endogenous G6PD in conditioned media from MN9DSYN DOX-treated cells (MN9DSYN; M+DOX).
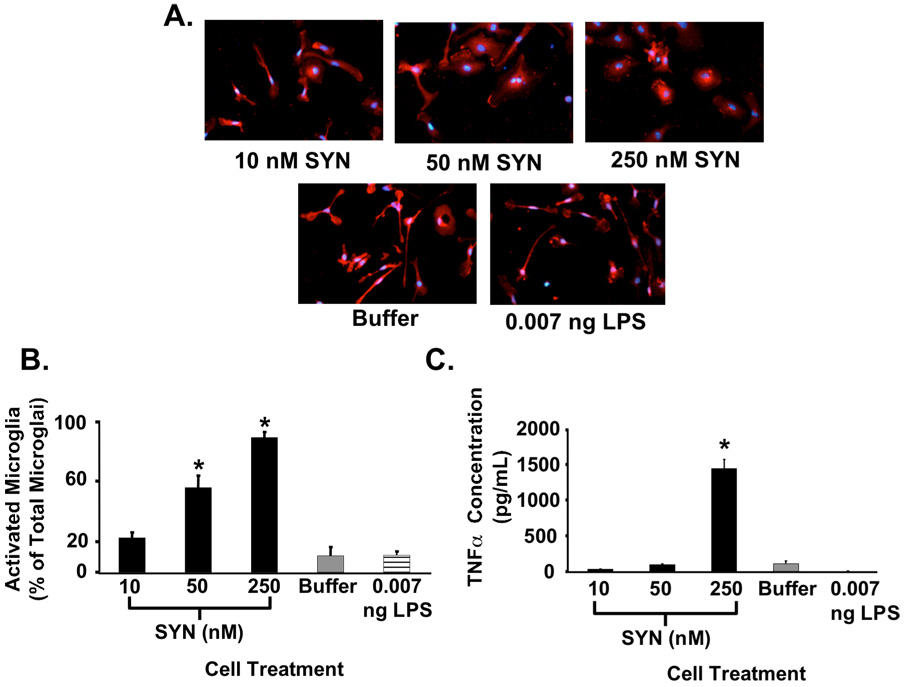
To examine the effect of SYN on microglial activation, we treated NTG mouse primary microglial-enriched cultures with various amounts of SYN. Prior to treatment with recombinant SYN (250 mM) the amount of contaminating lipopolysaccaride (LPS) that co-purified with SYN was determined. Parallel cultures of microglia were then treated with this amount of LPS (0.007 ng) as a control. Treated microglia responded to SYN in a dose-dependent manner, with a distinct change in cell size and morphology (Fig. 3A). We scored the Iba1+ microglia as activated when the cell body was amoeboid and less elongated (Fig. 3A, compare 250 mM SYN with buffer or LPS). As shown in Figure 3B, there was a significant SYN-mediated dose-dependent increase in activated microglia. However, there was no significant increase in microglial activation in control cultures (buffer or 0.007ng LPS treated). These data illustrate SYN specific activation of microglia.
Fig. 3. Extracellular SYN activates cultured microglia.
Primary microglia-enriched cultures were prepared from the cerebral cortices of neonatal mice (1-day old C57Bl/6). Cells were incubated with buffer, SYN (10, 50, or 250 nM) or 0.007 ng LPS for 24 hours (n=3/condition). Activated Iba1+ microglia were enumerated following Iba1 immunocytochemistry (A, B; 40X magnification; red=Iba1; blue=DAPI). Two distinct groups of cells were counted based on cell area and morphology (circularity or elongation) using ImageJ software (NIH, Bethesda, MD). Cells were considered inactivated if the cell area was less than 1 µm2 (27 × 27 pixels) or displayed thin and elongated morphology. All other Iba1+ cells were considered “morphologically” activated. Here, we demonstrate a concentration-dependent increase in activated microglia following SYN treatment (*, p<0.05). Likewise, conditioned media from SYN-treated microglia demonstrate an increase in TNFα expression and release (C). Buffer only and 0.007 ng LPS served as negative controls (*; p<0.05).
Since our SYNWT+/+ mice exhibit increased TNFα expression at an early age and activated microglia demonstrate enhanced expression and release of proinflammatory cytokines and chemokines, we determined whether SYN promoted microglial secretion of TNFα. We observed a dose-dependent increase in TNFα secretion following SYN treatment (Fig. 3C). Again, neither buffer nor LPS contamination contributed to the TNFα secretion in the SYN-treated microglial cultures. These data demonstrate that SYN is capable of direct induction of TNFα from microglia and suggest that in SYNWT+/+ mice a similar mechanism may be contributory.
3.3. SYN-mediated microglial activation induces a pro-inflammatory molecular cascade
Previous work shows robust microglial activation is accompanied by an increase in the production of various cytokines, including IL1β and IL6 (McGeer et al., 2005) as well as inflammation-related enzymes, such as cyclooxygenase 2 (COX2) and inducible nitric oxide synthase (iNOS/NOS2) (Knott et al., 2000). To determine whether these molecules are involved in SYN-mediated microglial activation, we examined the temporal pattern of microglia-mediated inflammatory mRNAs elaborated in response to SYN. Microglia were incubated with 250 nM SYN for 0.25, 0.75, 2, 4, 8 or 24 hours. Total RNA isolated from treated microglia at each time point was subjected to qRT-PCR and TNFα, IL1β, IL6, COX2, NOX2 and iNOS transcript levels determined (Fig. 4A). TNFα mRNA expression displayed a steep increase over time, peaked at 4 hours followed by a sharp decline by 8 hours (Fig. 4A). However, TNFα protein expression was delayed with a peak at 8 hours and a minor decrease by 24 hours (data not shown). COX2, IL1β and IL6, exhibited a less robust temporal increase and decline in expression compared with TNFα. In contrast, the temporal induction of NOX2 was delayed and peaked at 8 hours, while iNOS expression was delayed in onset but continued to increase through 24 hours. These data indicate that SYN triggers microglial-derived proinflammatory events including up-regulation of cytokines, enzymes and subsequently mediators of reactive oxygen species generation. Moreover, the temporal pattern of cellular ROS production increase in the SYN-treated microglia cultures mirrors the increase in iNOS expression (Fig. 4B).
Fig. 4. SYN induces expression of proinflammatory molecules.
Primary microglia-enriched cultures were treated with 250 nM SYN or buffer only for 0.25, 0.75, 2, 4, 8, 24 hours. RNA extracted from treated cells was subjected to qRT-PCR to evaluate the temporal expression of TNFα, IL1β, IL6, COX2, NOX2 and iNOS (A). Cellular ROS was determined using 2’, 7’-dichlorofluorescein diacetate (B; *, p<0.05; DCF). Here we demonstrate SYN- and time-dependent changes in proinflammatory molecule expression and cellular ROS production consistent with increased microglial activation.
3.4. A role for CD36 in SYN-mediated microglial activation
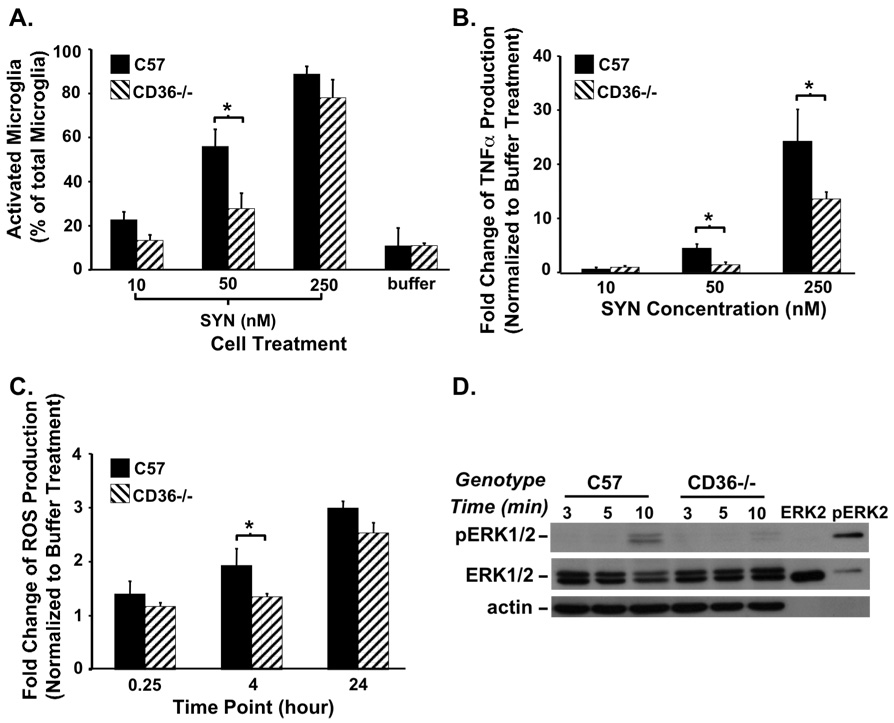
Multiple membrane receptors have been implicated in microglial activation and intracellular signal transduction pathways including the class B scavenger receptor, CD36 (Block et al., 2007). Since CD36 mediates the amyloid β activation of microglia and amyloid β and SYN form similar fibrillar structures, we examined CD36 as a mediator of SYN-mediated microglial activation. To elucidate a role for CD36 in the process, primary microglial cultures were prepared from CD36-deficient (CD36−/−) and C57BL/6 (C57; wild type) mice, treated with increasing amounts of recombinant human SYN and subjected to Iba1+ immunohistochemistry to enumerate activated microglia (Fig. 5). We find that while CD36−/− and C57 microglial cultures exhibit a dose-dependent response to SYN, CD36−/− cultures have an attenuated response (Fig. 5A). Similarly, SYN-mediated microglial activation increases TNFα secretion but is significantly blunted in CD36−/− cultures (Fig. 5B). In addition, less cellular ROS was produced by CD36−/− SYN-treated microglia, in comparison with C57 SYN treated cells (Fig. 5C). Lastly, we investigated the downstream signaling pathway elicited in response to CD36 receptor ligation. Since the ERK pathway is activated by CD36 interaction with amyloid β we examined the effect of SYN-treatment on ERK phosphorylation in wild type (C57) and CD36−/− microglia lysates (Ho et al., 2005). Cultured microglia were treated with 250 nM SYN for 3, 5 or 10 minutes, cell lysates prepared and subjected to Western blot analysis. Total ERK protein was present in both C57 and CD36−/− lysates at all time points (Fig. 5D; ERK1/2) while phosphorylated ERK was present only after 10 minutes of SYN treatment of wild type (C57) microglia (pERK). The pERK signal density from SYN-treated CD36−/− microglia cultures is markedly diminished relative to wild type cultures. These observations support a role for this scavenger receptor in SYN-mediated microglial activation.
Fig. 5. Attenuation of SYN-mediated microglial activation in CD36−/− cultures.
Primary microglia-enriched cultures were prepared from the cerebral cortices of neonatal wild type C57BL/6 and CD36-null mice (C57 and CD36−/−). Cells were incubated with SYN (10, 50, or 250 nM) or buffer alone for 24 hours followed by immunocytochemistry for Iba1. Here we demonstrate a reduction in activated microglia from CD36−/− cultures (A; *, p<0.05). The amount of secreted TNFα protein from microglia conditioned media was also reduced in the CD36 null cultures (B; *, p<0.05). ROS production was determined following incubation of microglia-enriched cultures with 250 nM SYN for 0.25, 4 or 24 hours. At 4 hours there was a significant decrease in ROS in the CD36−/− cultures (C; *, p<0.05). To evaluate the effect of SYN and CD36 status on phospho-ERK1/2 (pERK1/2) production; 1 × 106 primary microglia-enriched cells were plated, incubated with 250 nM SYN for 3, 5, or 10 minutes and cell lysates prepared. Cell lysates (30 µg) as well as 10 µl of purified non-phospho-ERK2 and phospho-ERK2 were analyzed by Western blot analysis using anti-phospho-ERK1/2, ERK1/2 and β-actin antibodies (D). Overall, these data demonstrate a reduced effect of SYN on CD36 knock-out cultures compared to C57 suggesting this scavenger receptor is in part responsible for SYN-dependent microglial activation.
4. Discussion
The main goal of this work was to evaluate early changes in the nigrostriatal system resulting from the expression of human wild-type synuclein (SYN). SYN appears to be an important molecular contributor in Parkinson’s disease (PD) pathogenesis because it is localized to the vulnerable dopaminergic neurons, overexpression or mutations within the SYN gene are linked to familial disease, neurotoxicant exposure alters SYN conformation and the protein is found in the hallmark pathologic feature, the Lewy body (Ancolio et al., 2000; Dong et al., 2002; Kitada et al., 2000; Kruger et al., 1998; Maroteaux et al., 1988; Mezey et al., 1998; Polymeropoulos, 2000; Polymeropoulos et al., 1997; Singleton et al., 2003; Spillantini et al., 1998). Although the mechanism of SYN-mediated pathogenesis has remained obscure this protein has been linked with microglial activation in primary glial cultures and human PD brains (Croisier et al., 2005; Kim and Joh, 2006; Klegeris et al., 2006; Zhang et al., 2005). Therefore, we began our studies by investigating the state of microglia in our transgenic mouse model of human wild-type SYN overexpression (SYNWT+/+). Previously, we demonstrated changes in dopamine transporter, dopamine metabolism and dystrophic neurites in heterozygous SYN animals (SYNWT+/−; (Richfield et al., 2002)). In an effort to raise the gene product’s level we homozygoused the transgene locus for human wild-type α-synuclein and utilized these animals for the studies presented here. Using Iba1 as a marker of microglial cells we enumerated total and activated microglia in the striatum and substantia nigra of SYNWT+/+ and C57BL/6 (non-transgenic, NTG) mice. Our findings of increased numbers of amoeboid Iba1+ cells as well as increased expression of TNFα in one-month old SYNWT+/+ mice compared with NTG mice points to microglial activation and inflammation as early initiators of PD pathogenesis. Furthermore, the microglial activation in our one-month old SYNWT+/+ mice precedes by almost 2 years the DAN death in this model suggesting that microglial activation is not a response to cell death in the localized microenvironment (Thiruchelvam et al., 2004).
Since there is no evidence of DAN demise and liberation of intracellular proteins at one-month of age in our transgenic mice we reasoned that the activation of microglia might result from specific release of SYN. Other studies provide evidence for neuronal activity-dependent release of SYN and exocytosis through an endoplasmic reticulum/Golgi-independent mechanism (Fortin et al., 2005; Lee et al., 2005). As we could not directly measure release from DAN in vivo we turned to the study of a dopaminergic cell line, MN9D, engineered to induce SYN expression in response to doxycycline. Our findings of SYN release together with our transgenic data support the hypothesis that SYN is released from neurons into the interstitial space where the protein would be available to stimulate microglia. We are aware that detection of released SYN in our transgenic animals would establish the mechanism of SYN-mediated microglial activation and this is currently under investigation.
To test whether SYN directly activates microglia we prepared primary microglia-enriched cultures from C57BL/6 mice and subjected them to exogenously applied SYN. Cultured microglia were activated in both a dose- and time-dependent manner following incubation with SYN demonstrating a direct effect of SYN. We then sought to determine the temporal progression of microglial-derived proinflammatory molecule changes during SYN-mediated activation. TNFα, IL1β, and IL6 are proinflammatory cytokines frequently produced during CNS inflammation and their increased expression in response to SYN was not unexpected (Block et al., 2007; Campbell, 2004; Kim and Joh, 2006; Kreutzberg, 1996; McGeer et al., 2005; McGeer and McGeer, 2004; Peyrin et al., 1999). TNFα demonstrated the most dramatic increase and the most precipitous decrease following SYN-mediated activation while IL1β and IL6 maintained expression throughout the treatment paradigm. In our model, SYN treatment also led to increased cytokine expression with concomitant up-regulation of COX2, NOX2, iNOS, and microglial-derived reactive oxygen species setting in motion a self-propagating vicious cycle that we hypothesize would engender microglia-mediated neuronal cell death.
Once we established that SYN activates microglia through direct contact we investigated whether the known microglial-localized scavenger receptor, CD36, might be involved in SYN’s actions. CD36 was examined since this pattern recognition receptor was previously shown to mediate the action of amyloid β (Aβ) which also forms neurotoxic protein conformers (Bamberger et al., 2003; Coraci et al., 2002; El Khoury et al., 2003; Husemann et al., 2002; Medeiros et al., 2004; Moore et al., 2002). We prepared microglia-enriched cultures from CD36 knockout mice and demonstrated a decrease in the percent of activated microglia as well as a dampening of the proinflammatory response to SYN relative to C57BL/6 microglia. Moreover in CD36 null microglia, phosphorylation of ERK2, a downstream kinase activated by CD36 ligation, was diminished in response to SYN. Diminution of ERK2 phosphorylation would be expected to dampen ROS production and indeed, we observed an attenuation of ROS production in the CD36 knockout cultures. However, it is likely that SYN is acting through more than one mechanism since the CD36 knockout cultures retained a percentage of activated microglia, as well as TNFα and ROS levels that exceeded buffer treatment.
Taken together these data support an early role for SYN in initiating inflammation and contributing to nigrostriatal pathology. The unexpected finding of a robust change in microglia activation in our transgenic model at such an early age well in advance of other pathological findings (ie. loss of TH+ neurons and changes in DA neurochemistry and behavior) suggest that SYN is a early pathogenic mediator. We propose that the direct interaction of SYN with microglia triggers proinflammatory cytokine expression and reactive oxygen species that commences a self-propagating cycle of inflammation and which we speculate is a major cause of delayed dopaminergic neuronal death. This work implicates microglia as an important early mediator of PD pathogenesis and accordingly represents a productive target for early therapeutic interventions.
Acknowledgements
We thank Catherine Dunn, Susan Kang and Eric Yehling for expert technical assistance. This study was supported by DAMD17-03-1-0009 to HJF; KV was supported by the Interdepartmental Neuroscience Training Grant (T32 NS7489).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement for Authors
KM-Z, XS, RG, LP and KV do not have any actual or potential conflicts of interest.
HF discloses the following: “I am a founder and equity owner of AmpliVex and MedGenesis. I have received consulting honoraria from the High Q Foundation, Spinal Muscular Atrophy NINDS Advisory Board, and speakers’ honoraria from non-academic entities Amgen, GE Healthcare, Sirtris Pharmaceuticals, the Ernst Scherring Foundation and the Institute on the Study of Aging. I have received research support from the NINDS, NIA, DoD, Johnson and Johnson and Abbott Laboratories.”
References
- Abbas N, Lucking CB, Ricard S, Durr A, Bonifati V, De Michele G, Bouley S, Vaughan JR, Gasser T, Marconi R, et al. A wide variety of mutations in the parkin gene are responsible for autosomal recessive parkinsonism in Europe. French Parkinson's Disease Genetics Study Group and the European Consortium on Genetic Susceptibility in Parkinson's Disease. Hum Mol Genet. 1999;8:567–574. doi: 10.1093/hmg/8.4.567. [DOI] [PubMed] [Google Scholar]
- Ali SF, David SN, Newport GD. Age-related susceptibility to MPTP-induced neurotoxicity in mice. Neurotoxicology. 1993;14:29–34. [PubMed] [Google Scholar]
- Ancolio K, Alves da Costa C, Ueda K, Checler F. Alpha-synuclein and the Parkinson's disease-related mutant Ala53Thr-alpha-synuclein do not undergo proteasomal degradation in HEK293 and neuronal cells. Neurosci Lett. 2000;285:79–82. doi: 10.1016/s0304-3940(00)01049-1. [DOI] [PubMed] [Google Scholar]
- Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankiewicz KS, Plunkett RJ, Jacobowitz DM, Porrino L, di Porzio U, London WT, Kopin IJ, Oldfield EH. The effect of fetal mesencephalon implants on primate MPTP-induced parkinsonism. Histochemical and behavioral studies. J Neurosurg. 1990;72:231–244. doi: 10.3171/jns.1990.72.2.0231. [DOI] [PubMed] [Google Scholar]
- Beal MF. Experimental models of Parkinson's disease. Nat Rev Neurosci. 2001;2:325–334. doi: 10.1038/35072550. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Borghi R, Marchese R, Negro A, Marinelli L, Forloni G, Zaccheo D, Abbruzzese G, Tabaton M. Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson's disease and normal subjects. Neurosci Lett. 2000;287:65–67. doi: 10.1016/s0304-3940(00)01153-8. [DOI] [PubMed] [Google Scholar]
- Campbell A. Inflammation, neurodegenerative diseases, and environmental exposures. Ann N Y Acad Sci. 2004;1035:117–132. doi: 10.1196/annals.1332.008. [DOI] [PubMed] [Google Scholar]
- Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella GK, Luster AD, Silverstein SC, El-Khoury JB. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am J Pathol. 2002;160:101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:14. doi: 10.1186/1742-2094-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Ferger B, Feldon J, Bueler H. Overexpression of Parkinson's disease-associated alpha-synucleinA53T by recombinant adeno-associated virus in mice does not increase the vulnerability of dopaminergic neurons to MPTP. J Neurobiol. 2002;53:1–10. doi: 10.1002/neu.10094. [DOI] [PubMed] [Google Scholar]
- Duvoisin RC. Overview of Parkinson's disease. Ann N Y Acad Sci. 1992;648:187–193. doi: 10.1111/j.1749-6632.1992.tb24537.x. [DOI] [PubMed] [Google Scholar]
- El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197:1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf OM, Salem SA, Paleologou KE, Cooper LJ, Fullwood NJ, Gibson MJ, Curran MD, Court JA, Mann DM, Ikeda S, et al. Alpha-synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. Faseb J. 2003;17:1945–1947. doi: 10.1096/fj.03-0098fje. [DOI] [PubMed] [Google Scholar]
- Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH. Neural activity controls the synaptic accumulation of alpha-synuclein. J Neurosci. 2005;25:10913–10921. doi: 10.1523/JNEUROSCI.2922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Uryu K, Trojanowski JQ, Lee VM. Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem. 1999;274:7619–7622. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- Gomez-Santos C, Ferrer I, Reiriz J, Vinals F, Barrachina M, Ambrosio S. MPP+ increases alpha-synuclein expression and ERK/MAP-kinase phosphorylation in human neuroblastoma SH-SY5Y cells. Brain Res. 2002;935:32–39. doi: 10.1016/s0006-8993(02)02422-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Masliah E. Transgenic models of alpha-synuclein pathology: past, present, and future. Ann N Y Acad Sci. 2003;991:171–188. [PubMed] [Google Scholar]
- Heller A, Freeney A, Hessefort S, Villereal M, Won L. Cellular dopamine is increased following exposure to a factor derived from immortalized striatal neurons in humans. Neurosci Lett. 2000;295:1–4. doi: 10.1016/s0304-3940(00)01589-5. [DOI] [PubMed] [Google Scholar]
- Heller A, Price S, Won L. Glial-derived neurotrophic factor (GDNF) induced morphological differentiation of an immortalized monoclonal hybrid dopaminergic cell line of mesencephalic neuronal origin. Brain Res. 1996;725:132–136. doi: 10.1016/0006-8993(96)00345-9. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S, Hartmann A. Neuroinflammatory processes in Parkinson's disease. Parkinsonism Relat Disord. 2005;11 Suppl 1:S9–S15. doi: 10.1016/j.parkreldis.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Ho GJ, Drego R, Hakimian E, Masliah E. Mechanisms of cell signaling and inflammation in Alzheimer's disease. Curr Drug Targets Inflamm Allergy. 2005;4:247–256. doi: 10.2174/1568010053586237. [DOI] [PubMed] [Google Scholar]
- Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC. Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia. 2002;40:195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- Husemann J, Loike JD, Kodama T, Silverstein SC. Scavenger receptor class B type I (SR-BI) mediates adhesion of neonatal murine microglia to fibrillar beta-amyloid. J Neuroimmunol. 2001;114:142–150. doi: 10.1016/s0165-5728(01)00239-9. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Growdon W, Gomez-Isla T, Newell K, George JM, Clayton DF, Hyman BT. Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson's disease and cortical Lewy body disease contain alpha-synuclein immunoreactivity. J Neuropathol Exp Neurol. 1998;57:334–337. doi: 10.1097/00005072-199804000-00005. [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, Okochi M, Leimer U, van Der Putten H, Probst A, et al. Subcellular localization of wild-type and Parkinson's disease- associated mutant alpha -synuclein in human and transgenic mouse brain. J Neurosci. 2000;20:6365–6373. doi: 10.1523/JNEUROSCI.20-17-06365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson's disease. Exp Mol Med. 2006;38:333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Matsumine H, Hattori N, Shimura H, Minoshima S, Shimizu N, Mizuno Y. Progress in the clinical and molecular genetics of familial parkinsonism. Neurogenetics. 2000;2:207–218. doi: 10.1007/s100489900083. [DOI] [PubMed] [Google Scholar]
- Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG, McGeer PL. alpha-Synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Knott C, Stern G, Wilkin GP. Inflammatory regulators in Parkinson's disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci. 2000;16:724–739. doi: 10.1006/mcne.2000.0914. [DOI] [PubMed] [Google Scholar]
- Kohutnicka M, Lewandowska E, Kurkowska-Jastrzebska I, Czlonkowski A, Czlonkowska A. Microglial and astrocytic involvement in a murine model of Parkinson's disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Immunopharmacology. 1998;39:167–180. doi: 10.1016/s0162-3109(98)00022-8. [DOI] [PubMed] [Google Scholar]
- Kowall NW, Hantraye P, Brouillet E, Beal MF, McKee AC, Ferrante RJ. MPTP induces alpha-synuclein aggregation in the substantia nigra of baboons. Neuroreport. 2000;11:211–213. doi: 10.1097/00001756-200001170-00041. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kunjathoor VV, Tseng AA, Medeiros LA, Khan T, Moore KJ. beta-Amyloid promotes accumulation of lipid peroxides by inhibiting CD36-mediated clearance of oxidized lipoproteins. J Neuroinflammation. 2004;1:23. doi: 10.1186/1742-2094-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langston JW, Irwin I, Langston EB, Forno LS. 1-Methyl-4-phenylpyridinium ion (MPP+): identification of a metabolite of MPTP, a toxin selective to the substantia nigra. Neurosci Lett. 1984a;48:87–92. doi: 10.1016/0304-3940(84)90293-3. [DOI] [PubMed] [Google Scholar]
- Langston JW, Langston EB, Irwin I. MPTP-induced parkinsonism in human and non-human primates--clinical and experimental aspects. Acta Neurol Scand Suppl. 1984b;100:49–54. [PubMed] [Google Scholar]
- Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL. Human alpha-synuclein-harboring familial Parkinson's disease-linked Ala- 53 --> Thr mutation causes neurodegenerative disease with alpha- synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- Maguire-Zeiss KA, Federoff HJ. Convergent pathobiologic model of Parkinson's disease. Ann N Y Acad Sci. 2003;991:152–166. doi: 10.1111/j.1749-6632.2003.tb07473.x. [DOI] [PubMed] [Google Scholar]
- Maguire-Zeiss KA, Wang CI, Yehling E, Sullivan MA, Short DW, Su X, Gouzer G, Henricksen LA, Wuertzer CA, Federoff HJ. Identification of human alpha-synuclein specific single chain antibodies. Biochem Biophys Res Commun. 2006;349:1198–1205. doi: 10.1016/j.bbrc.2006.08.127. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes upregulation and aggregation of alpha- synuclein in mice. J Biol Chem. 2001;13:13. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes up-regulation and aggregation of alpha- synuclein in mice: paraquat and alpha-synuclein. J Biol Chem. 2002;277:1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Vila M, Lincoln S, McCormack A, Picciano M, LaFrancois J, Yu X, Dickson D, Langston WJ, McGowan E, et al. Lack of nigral pathology in transgenic mice expressing human alpha- synuclein driven by the tyrosine hydroxylase promoter. Neurobiol Dis. 2001;8:535–539. doi: 10.1006/nbdi.2001.0392. [DOI] [PubMed] [Google Scholar]
- McGeer EG, Klegeris A, McGeer PL. Inflammation, the complement system and the diseases of aging. Neurobiol Aging. 2005;26 Suppl 1:94–97. doi: 10.1016/j.neurobiolaging.2005.08.008. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann N Y Acad Sci. 2004;1035:104–116. doi: 10.1196/annals.1332.007. [DOI] [PubMed] [Google Scholar]
- Medeiros LA, Khan T, El Khoury JB, Pham CL, Hatters DM, Howlett GJ, Lopez R, O'Brien KD, Moore KJ. Fibrillar amyloid protein present in atheroma activates CD36 signal transduction. J Biol Chem. 2004;279:10643–10648. doi: 10.1074/jbc.M311735200. [DOI] [PubMed] [Google Scholar]
- Mezey E, Dehejia AM, Harta G, Tresser N, Suchy SF, Nussbaum RL, Brownstein MJ, Polymeropoulos MH. Alpha synuclein is present in Lewy bodies in sporadic Parkinson's disease. Mol Psychiatry. 1998;3:493–499. doi: 10.1038/sj.mp.4000446. [DOI] [PubMed] [Google Scholar]
- Miller RM, Callahan LM, Casaceli C, Chen L, Kiser GL, Chui B, Kaysser-Kranich TM, Sendera TJ, Palaniappan C, Federoff HJ. Dysregulation of gene expression in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse substantia nigra. J Neurosci. 2004;24:7445–7454. doi: 10.1523/JNEUROSCI.4204-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KJ, El Khoury J, Medeiros LA, Terada K, Geula C, Luster AD, Freeman MW. A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J Biol Chem. 2002;277:47373–47379. doi: 10.1074/jbc.M208788200. [DOI] [PubMed] [Google Scholar]
- Peyrin JM, Lasmezas CI, Haik S, Tagliavini F, Salmona M, Williams A, Richie D, Deslys JP, Dormont D. Microglial cells respond to amyloidogenic PrP peptide by the production of inflammatory cytokines. Neuroreport. 1999;10:723–729. doi: 10.1097/00001756-199903170-00012. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH. Genetics of Parkinson's disease. Ann N Y Acad Sci. 2000;920:28–32. doi: 10.1111/j.1749-6632.2000.tb06901.x. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Higgins JJ, Golbe LI, Johnson WG, Ide SE, Di Iorio G, Sanges G, Stenroos ES, Pho LT, Schaffer AA, et al. Mapping of a gene for Parkinson's disease to chromosome 4q21-q23. Science. 1996;274:1197–1199. doi: 10.1126/science.274.5290.1197. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Chen Q, Vila M, Giasson BI, Djaldatti R, Vukosavic S, Souza JM, Jackson-Lewis VV, Lee VM, Ischiropoulos H. Oxidative post-translational modifications of alpha-synuclein in the 1- methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. J Neurochem. 2001a;76:637–640. doi: 10.1046/j.1471-4159.2001.00174.x. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem. 2001b;76:1265–1274. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- Rathke-Hartlieb S, Kahle PJ, Neumann M, Ozmen L, Haid S, Okochi M, Haass C, Schulz JB. Sensitivity to MPTP is not increased in Parkinson's disease-associated mutant alpha-synuclein transgenic mice. J Neurochem. 2001;77:1181–1184. doi: 10.1046/j.1471-4159.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Thiruchelvam MJ, Cory-Slechta DA, Wuertzer C, Gainetdinov RR, Caron MG, Di Monte DA, Federoff HJ. Behavioral and neurochemical effects of wild-type and mutated human alpha-synuclein in transgenic mice. Exp Neurol. 2002;175:35–48. doi: 10.1006/exnr.2002.7882. [DOI] [PubMed] [Google Scholar]
- Schmidt N, Ferger B. Neurochemical findings in the MPTP model of Parkinson's disease. J Neural Transm. 2001;108:1263–1282. doi: 10.1007/s007020100004. [DOI] [PubMed] [Google Scholar]
- Seniuk NA, Tatton WG, Greenwood CE. Dose-dependent destruction of the coeruleus-cortical and nigral-striatal projections by MPTP. Brain Res. 1990;527:7–20. doi: 10.1016/0006-8993(90)91055-l. [DOI] [PubMed] [Google Scholar]
- Serpell LC, Berriman J, Jakes R, Goedert M, Crowther RA. Fiber diffraction of synthetic alpha-synuclein filaments shows amyloid-like cross-beta conformation. Proc Natl Acad Sci U S A. 2000;97:4897–4902. doi: 10.1073/pnas.97.9.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Song DD, Shults CW, Sisk A, Rockenstein E, Masliah E. Enhanced substantia nigra mitochondrial pathology in human alpha-synuclein transgenic mice after treatment with MPTP. Exp Neurol. 2004;186:158–172. doi: 10.1016/S0014-4886(03)00342-X. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee CA, McLeod MR, Hall JR. Efficient control of tetracycline-responsive gene expression from an autoregulated bi-directional expression vector. Gene. 1999;229:21–29. doi: 10.1016/s0378-1119(99)00045-1. [DOI] [PubMed] [Google Scholar]
- Sung JY, Park SM, Lee CH, Um JW, Lee HJ, Kim J, Oh YJ, Lee ST, Paik SR, Chung KC. Proteolytic cleavage of extracellular secreted {alpha}-synuclein via matrix metalloproteinases. J Biol Chem. 2005;280:25216–25224. doi: 10.1074/jbc.M503341200. [DOI] [PubMed] [Google Scholar]
- Sveinbjornsdottir S, Hicks AA, Jonsson T, Petursson H, Gugmundsson G, Frigge ML, Kong A, Gulcher JR, Stefansson K. Familial aggregation of Parkinson's disease in Iceland. N Engl J Med. 2000;343:1765–1770. doi: 10.1056/NEJM200012143432404. [DOI] [PubMed] [Google Scholar]
- Tabrizi SJ, Orth M, Wilkinson JM, Taanman JW, Warner TT, Cooper JM, Schapira AH. Expression of mutant alpha-synuclein causes increased susceptibility to dopamine toxicity. Hum Mol Genet. 2000;9:2683–2689. doi: 10.1093/hmg/9.18.2683. [DOI] [PubMed] [Google Scholar]
- Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77:1037–1048. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam MJ, Powers JM, Cory-Slechta DA, Richfield EK. Risk factors for dopaminergic neuron loss in human alpha-synuclein transgenic mice. Eur J Neurosci. 2004;19:845–854. doi: 10.1111/j.0953-816x.2004.03139.x. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther. 2004;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- van der Putten H, Wiederhold KH, Probst A, Barbieri S, Mistl C, Danner S, Kauffmann S, Hofele K, Spooren WP, Ruegg MA, et al. Neuropathology in mice expressing human alpha-synuclein. J Neurosci. 2000;20:6021–6029. doi: 10.1523/JNEUROSCI.20-16-06021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varastet M, Riche D, Maziere M, Hantraye P. Chronic MPTP treatment reproduces in baboons the differential vulnerability of mesencephalic dopaminergic neurons observed in Parkinson's disease. Neuroscience. 1994;63:47–56. doi: 10.1016/0306-4522(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Verdier Y, Penke B. Binding sites of amyloid beta-peptide in cell plasma membrane and implications for Alzheimer's disease. Curr Protein Pept Sci. 2004;5:19–31. doi: 10.2174/1389203043486937. [DOI] [PubMed] [Google Scholar]
- Vila M, Vukosavic S, Jackson-Lewis V, Neystat M, Jakowec M, Przedborski S. Alpha-synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP. J Neurochem. 2000;74:721–729. doi: 10.1046/j.1471-4159.2000.740721.x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. Faseb J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]