Abstract
Study Objective:
To examine the effects of sleep on fear conditioning, extinction, extinction recall, and generalization of extinction recall in healthy humans.
Design:
During the Conditioning phase, a mild, 0.5-sec shock followed conditioned stimuli (CS+s), which consisted of 2 differently colored lamps. A third lamp color was interspersed but never reinforced (CS-). Immediately after Conditioning, one CS+ was extinguished (CS+E) by presentation without shocks (Extinction phase). The other CS+ went unextinguished (CS+U). Twelve hours later, following continuous normal daytime waking (Wake group, N = 27) or an equal interval containing a normal night's sleep (Sleep group, N = 26), conditioned responses (CRs) to all CSs were measured (Extinction Recall phase). It was hypothesized that the Sleep versus Wake group would show greater extinction recall and/or generalization of extinction recall from the CS+E to the CS+U.
Setting:
Academic medical center.
Subjects:
Paid normal volunteers.
Measurements and Results:
Square-root transformed skin conductance response (SCR) measured conditioned responding. During Extinction Recall, the Group (Wake or Sleep) × CS+ Type (CS+E or CS+U) interaction was significant (P = 0.04). SCRs to the CS+E did not differ between groups, whereas SCRs to the CS+U were significantly smaller in the Sleep group. Additionally, SCRs were significantly larger to the CS+U than CS+E in the Wake but not the Sleep group.
Conclusions:
After sleep, extinction memory generalized from an extinguished conditioned stimulus to a similarly conditioned but unextinguished stimulus. Clinically, adequate sleep may promote generalization of extinction memory from specific stimuli treated during exposure therapy to similar stimuli later encountered in vivo.
Citation:
Pace-Schott EF; Milad MR; Orr SP; Rauch SL; Stickgold R; Pitman RK. Sleep promotes generalization of extinction of conditioned fear. SLEEP 2009;32(1):19-26.
Keywords: Fear conditioning, extinction, generalization, human, emotional memory
IT IS OFTEN HYPOTHESIZED THAT SLEEP PLAYS AN EMOTIONAL REGULATORY ROLE.1–3 HOWEVER, THIS PUTATIVE NORMALIZING FUNCTION OF SLEEP HAS received remarkably little empirical study. Over the past decade, many studies have shown that sleep plays an important role in memory consolidation within multiple memory systems4 including that of emotional memory.5–8 The purpose of the current study was to examine the effects of sleep on the consolidation and generalization of extinction memory, which are of particular relevance to the pathophysiology and treatment of anxiety disorders.
Fear Conditioning and Extinction
Fear conditioning occurs when an emotionally neutral stimulus comes to be associated with an inherently aversive experience, thereby acquiring the capability of evoking a fearful response on its own. The aversive experience itself is termed an unconditioned stimulus (US). The initially neutral stimulus, which becomes associated with the aversive experience, is termed a conditioned stimulus (CS), and the subsequent response to it a conditioned response (CR). Extinction (reduction) of the CR occurs when the CS is repeatedly presented without the US. Current scientific opinion holds that extinction does not erase conditioned fear; rather it creates a new memory that coexists and competes with the fearful memory when the CS is again encountered.9–12 Evidence that the original CS/US association remains in memory includes the phenomena of spontaneous recovery, renewal, and reinstatement of conditioned fear.9–12
Animal Studies of Sleep, Fear Conditioning, and Extinction Memory
To date, there have been no reported studies in humans of the effect of sleep on recall of fear conditioning or extinction memory. However, sleep dependency for both phenomena has been reported in animal models. In rats, sleep deprivation preceding13 or following14 conditioning impairs consolidation of contextual (hippocampus-dependent) but not cued (hippocampus-independent) memory for conditioned fear. Interestingly, rapid eye movement (REM) sleep deprivation has been shown to impair extinction of cued but not contextual fear conditioning. REM deprivation following fear conditioning impaired later extinction learning,15 and REM sleep deprivation following extinction training impaired later extinction recall.16 For example, Fu et al.16 found that extinction decreased the percent of time the animal spent freezing after a conditioned fear cue from 75% to 49%. Twenty-four hours later, rats that had slept normally following extinction froze for 50% of cue duration, whereas those that were REM deprived immediately following extinction learning froze for 80% of cue duration, indicating a total failure to retain extinction learning. However, such loss of extinction memory did not occur when REM deprivation was delayed for 6 hours following extinction training.
There exist “REM windows” for memory consolidation during which REM deprivation can impair subsequent recall.17,18 It seems likely that the immediately post-extinction REM deprivation performed by Fu et al.16 overlapped with a REM window crucial to consolidating the memory of extinction learning. Critical periods of post-learning sleep may facilitate key synaptic, second messenger, gene transcriptional, and protein synthetic processes required for memory consolidation.19 Notably, experimental stressors, as well as cues associated with past stressors, can reduce subsequent REM sleep20 and thereby, in theory, interfere with memory consolidation.
Generalization of Extinction Memory
Research in humans has investigated conditioning to a CS+, followed by extinction of the same CS+, followed by retention testing to a generalization stimulus (GS+) that resembled the original CS+. Results indicated that extinction to the original CS+ successfully generalized such that it reduced responding to the GS+ during retention testing.21,22 However, the potential effect of sleep on generalization of extinction memory has not yet been examined.
Hypotheses
The current study tested the effects of normal sleep on extinction memory and its generalization in healthy young adults using the protocol of Milad et al.23 Fear conditioning was established to 2 different conditioned stimuli (CS+s) using a mild electric shock as the US and skin conductance response (SCR) as a measure of the CR. Immediately following conditioning, one CS+ was extinguished (CS+E) whereas the other went unextinguished (CS+U). Conditioning and extinction were performed either in the morning or evening. Conditioned responses to both types of CS+ were then tested following 12 hours of a normal day's continuous waking (Wake group) or an equal duration containing a normal night's sleep (Sleep group). There were 2 hypotheses as to group differences when extinction was recalled. The first was that a period of sleep would enable more extinction recall than an equal period of waking, as manifested by lower SCRs to the CS+E in the Sleep versus Wake group. The second hypothesis was that sleep would enable generalization of extinction from a previously extinguished to a previously unextinguished stimulus, as manifested by a smaller difference in SCRs to the CS+U compared to the CS+E in the Sleep versus Wake group.
METHODS
Subjects
Fifty-nine paid volunteers (30 female), mean age = 23 years (SD = 4, range = 18–35) were recruited by advertisements on internet web sites and responded to a posted telephone number. Anonymity during screening was assured by instructing potential subjects not to give their name when leaving a call-back number or during the telephone screening until they were determined eligible to participate and invited to do so. Of 59 subjects recruited, data from 53 were analyzed (see “Response Scores” below).
Exclusion criteria included current neurological or medical conditions, any history of seizures, significant head trauma, DSM IV Axis I mental disorder, sleep disorder, use of psychiatric medication, average sleep per night < 6 or > 10 hours, inability or unwillingness to keep a regular sleep schedule, smoking, excessive caffeine or alcohol consumption, and current use of any sleep-altering drugs. A 23-item telephone screening questionnaire specifically addressed each exclusion criterion. Excessive caffeine consumption was defined as self-report of > 5 cups or glasses per day. Excess alcohol consumption was defined as self-report of > 12 drinks per week or of problems with alcohol or drug abuse.
Before beginning the study, subjects were again screened using an extended 104-question Habitual Sleep Questionnaire24 that contained queries addressing: (i) general sleep habits, hygiene and circadian preference; (ii) substance use (including recreational drugs and prescribed and over-the-counter medications) and associated sleep problems; (iii) each primary sleep disorder including subtypes of insomnia;25 (iv) medical conditions, especially those affecting sleep (e.g., fibromyalgia, pain syndromes); (v) DSM-IV Axis I disorders;26 and (vi) current psychosocial stressors. Based upon the results, one initially accepted subject was excluded for a recent history of psychiatric medication. Another subject was excluded for color-blindness. (These 2 are not included in above totals.)
Excessive daytime sleepiness was assessed using the Epworth Sleepiness Scale27 (ESS), and sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI).28
For female subjects, 70% (all except the first 9 recruited who were approximately equally distributed between the 2 groups) were studied during the early follicular phase (as determined from self-report) because work from this laboratory suggests that extinction memory is influenced by menstrual cycle phase.29 All procedures were approved by the Partners (Massachusetts General Hospital) Human Research Committee, and all subjects gave written informed consent after the procedures had been fully explained.
PROCEDURE
Pre-Study Week
During the week prior to the experiment, subjects were asked to maintain a regular sleep schedule consisting of ≥ 7 hours in bed each night with bedtime no later than 02:00. Subjects also filled out a nightly sleep diary.30 During this week, subjects were asked to refrain entirely from alcohol, recreational drugs and daytime napping. In addition, on the study days, subjects were asked not to use caffeine and not to nap.
Protocol
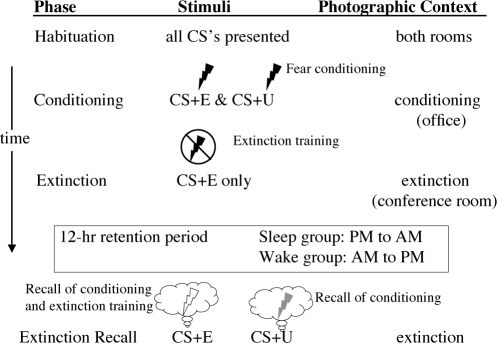
The experimental protocol consisted of 4 experimental phases: Habituation, Conditioning, Extinction, and Extinction Recall (Figure 1).23,31 Because they were not informative, data from the Habituation phase are not presented in the current report. The Habituation, Conditioning, and Extinction phases occurred sequentially either between 08:30 and 10:30 (Wake group, N = 29) or 19:30 and 21:30. (Sleep group, N = 30). The Extinction Recall phase occurred at 21:30 following 12 hours of continuous waking (Wake group) or at 08:30 following a 12-hour period containing a normal night's sleep (Sleep group).
Figure 1.
The experimental protocol involved a sequential fear conditioning, extinction and extinction recall protocol completed in 2 sessions separated by 12 hours. Habituation, Conditioning and Extinction phases took place at the first session occurring in the morning for the Wake group and the evening for the Sleep group. Following 12 hours that contained a normal night's sleep in the Sleep group and continuous waking in the Wake group, subjects completed the Extinction Recall phase. During the Conditioning phase, fear conditioning was established to 2 separate CS+ stimuli by following their presentation with a mild electric-shock US. Conditioning to one CS+ (the CS+E) was subsequently extinguished during the Extinction phase while the other remained unextinguished (the CS+U). CS+ stimuli were presented within photographic images of two different rooms (contexts) displayed on a computer screen. One room was the Conditioning phase context and the other the Extinction phase context. At the Extinction Recall phase that followed the 12-hour delay, both types of CS+ stimuli were presented within the Extinction phase's context.
Prior to the experiment, subjects selected their own US level. Increasing intensities of a 0.5-sec mild electric shock (from 0.2 to 4.0 milliamperes across up to 8 increments) were administered using a Coulbourn Transcutaneous Aversive Finger Stimulator (Coulbourn Instruments, Allentown, PA) until the subject chose a level that was “highly annoying but not painful”.32 Shock increments were then stopped, and subjects were informed that the level they had selected would be used throughout the subsequent experiment.
During all phases except Habituation, there were 32 CS trials (presentations). These consisted of digital photographs of 3 differently colored lamps (blue, red, or yellow) displayed on a computer screen within the image of 2 different photographic environments (contexts), viz., an office or a conference room. The office served as the “conditioning context” in which the CS+s were usually followed by the US. The conference room served as the “extinction context” in which CSs occurred without USs. In all phases, the different CSs were presented in pseudo-random order.
During the Habituation phase, subjects viewed each possible combination of the 3 CS colors and 2 contexts for a total of 6 presentations. Subjects were told they would not be shocked during this initial phase. After Habituation and before beginning Conditioning, subjects were told that, henceforth, shocks could occur at any time during the experiment. In actuality, however, subjects received shocks only during the Conditioning phase. Expectation of shocks throughout the experiment allowed subjects to learn that the CS+E was not followed by the shock specifically in the extinction context. Their extinction memory could thereby be later aided by this context during the Extinction Recall phase. Maintaining ambiguity as to the context in which the shock did or did not occur thus allowed subjects, on their own, to slowly form this extinction memory and its specific linkage to the extinction context.
During the Conditioning phase, 16 CS+s (8 each of 2 different colors) were presented in the conditioning context. The 0.5-sec US immediately followed the offset of 10 of 16 CS+ presentations (5 of the 8 of each CS+ color). Such partial reinforcement schedules have been shown to be more effective than 100% reinforcement schedules for maintaining fear conditioning and preventing too-rapid extinction of SCR responses.33 Sixteen randomly interspersed presentations of the third lamp color were never paired with the US (CS-). In classical conditioning, use of a CS- is useful in order to demonstrate that conditioning, as opposed to sensitization, to the CS+s, has occurred. In the current study, responses to CS-s confirmed that differential conditioning had indeed occurred.
During the Extinction phase, one CS+ color unaccompanied by the US (termed the CS+E) appeared 16 times in the extinction context, along with 16 interspersed CS- presentations. The other CS+ color remained unextinguished (termed the CS+U, see Figure 1). During the Extinction Recall phase 12 hours later, 8 CS+Es and 8 CS+Us were presented again in the extinction context with no USs, with 16 CS-s again interspersed.
After Milad et al.23 during the Conditioning and Extinction Recall phases, all 8 CS+Es were presented in a block (with 8 interspersed CS−s), as were all 8 CS+Us (with 8 interspersed CS−s). Milad et al. found that such blocked presentations improve conditioning to the respective CS+s. The order in which the blocked CS+Es and CS+Us were presented (i.e., whether the block of 8 CS+Es or the block of 8 CS+Us appeared first) was counterbalanced across phases and subjects. The colors assigned to be CS+E, CS+U and CS− (red, blue, or yellow) were also counterbalanced across subjects. In all phases, each trial lasted a total of 9 sec, with the context picture appearing initially alone for 3 sec, and then one of the CS+s or the CS− appeared within this context picture for an additional 6 sec. The inter-trial interval (ITI), measured from CS offset to next context onset, averaged 15 sec and varied pseudo-randomly between 12 and 18 sec.
Physiological Measurements
Two 9-mm (sensor diameter) Sensor Medics Ag/AgCl radiotranslucent electrodes (BioPac Systems Inc., Goleta, CA) were filled with isotonic paste and attached to the hypothenar surface of the nondominant hand separated by 14 mm. Skin conductance level (SCL) was measured in microSiemens (μS) using a Coulbourn Isolated Skin Conductance Coupler (S71-23) within a Coulbourn Modular Instruments System (Allentown, PA). Digitization of analog signals was performed by a Coulbourn Lablinc Analog-to-Digital Converter (V19-16), and digital values were sampled at 5 Hz and stored in a personal computer.
Response Scores
A SCR score was calculated for each trial by subtracting the mean SCL during the last 2 sec of the context-alone presentation from the peak SCL that occurred during the 6-sec CS presentation. Subtraction of peak SCL to the CS from the SCL to context alone allowed accurate measurement of the SCR that occurred specifically to the CS, un-confounded by any response to the context alone. All SCRs were square-root transformed. If the untransformed SCR value was negative, the negative sign was retained after calculating the square root of the SCR's absolute value.32 A total of 6 subjects (5 females, 3 in Sleep group) were excluded from the analyses because they failed to show evidence of a CR, i.e., no more than one SCR to a CS+ during the Conditioning phase (excluding the first CS+ presentation, which occurs before any US) exceeded 0.05 μS (untransformed, see Reference34). With these exclusions, the final sample comprised 53 subjects, with 26 in the Sleep group (11 females) and 27 in the wake group (14 females). For simplicity, the 2 CS+s are termed “CS+E” and “CS+U” for the Conditioning, as well as the Extinction Recall phase even though their differentiation did not occur until the Extinction phase.
Statistical Analyses
Group comparisons of demographic, caffeine and alcohol use, sleepiness and sleep quality were performed with unpaired t- and Mann-Whitney U-tests. For all psychophysiological analyses, square-root transformed SCR served as the dependent variable. Three-factor repeated-measures analyses of variance (ANOVAs) were performed separately for the Conditioning and Extinction Recall phases. CS+ Type (CS+E, CS+U) and Trials (nested within CS+ Type) were treated as within-subjects factors; Group (Sleep, Wake) was treated as a between-subjects factor. For the Conditioning phase, this 3-factor analysis was used to check for baseline differences between Group or CS+ Type. For the Extinction Recall phase, the hypothesis that extinction would generalize from the CS+E to the CS+U to a greater extent in the Sleep group was tested using the Group × CS+ Type interaction. Additionally, for the Extinction Recall phase, a 2-factor (Trials, Group) (ANOVA) was conducted on SCRs to CS+E trials only. This latter analysis allowed testing the hypothesis that a period of sleep would enable more extinction recall than an equal period of waking using the main effect of Group. For the Extinction phase analyses, the CS+ Type factor was dropped because no CS+Us were presented during this phase. Because twice as many CS+s of one type were presented in the Extinction phase relative to the other phases, SCRs were averaged over successive 2-trial blocks.
RESULTS
Group Characteristics
There was no significant group difference between ages of subjects (Sleep: mean 22.9, SD 4.3; Wake mean 23.6, SD 4.1), sleepiness (ESS: Sleep: mean 5.92, SD 3.12; Wake: mean 5.19, SD 2.95) or sleep quality (PSQI: Sleep: mean 3.23, SD 2.05; Wake: mean 3.04, SD 2.14). Similarly, no group differences were seen for self-reported caffeine (Sleep: mean 1.08, SD 1.01; Wake: mean 1.01, SD 0.90) or alcohol (Sleep: mean 2.48, SD 2.71; Wake: mean 2.72, SD 2.84) use.
Conditioning and Extinction Phases
During the Conditioning phase, there was a significant Trials main effect (F(6,306) = 12.5, P = 0.0001) and no significant Group main effect. As would be expected, because the CS+E and CS+U were not differentiated in the Conditioning phase, there was no significant CS+ Type main effect or Trials × CS+ Type, CS+ Type × Group, or Trials × CS+ Type × Group interactions. During the Extinction phase, there was a significant Trials main effect (F(7,357) = 9.0, P = 0.0001), but no significant Group main effect or Trials × Group interaction.
Extinction Recall Phase
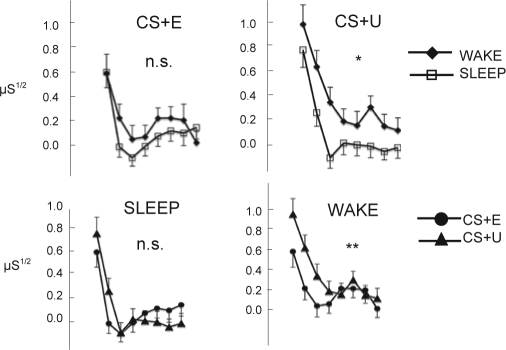
During the Extinction Recall phase, the Group main effect for responses to the CS+E alone was not significant (F(1,51) = 1.3, P = 0.26). There was a significant Trials effect (F(7,357) = 8.0, P < 0.0001), but no significant Group × Trials interaction. Therefore the hypothesis that sleep would enable more extinction retention than waking was not supported. However, collapsed across trials, the group effect size was 0.31 (Cohen's d; Sleep < Wake), and the 95% upper confidence limit was 0.24 μS1/2. This effect size is not inconsequential, and the failure to detect this group difference may be due to limited statistical power.
There was, however, a significant Group × CS+ Type interaction (F(1,51) = 4.27, P = 0.04). Because the Trials × CS+ Type × Group interaction was not significant, the Group × CS+ Type interaction was decomposed by means of separate analyses within each Group (lower 2 graphs in Figure 2) and each CS+ Type (upper 2 graphs). Decomposing by Group revealed no significant main effect for CS+ Type in the Sleep group, whereas the CS+ Type main effect was highly significant in the Wake group (F(1,26) = 8.33, P = 0.008), which demonstrated larger SCRs to the CS+U compared to the CS+E. Decomposing by CS+ type, the Group main effect was not significant for the CS+E (as detailed above), whereas for the CS+U, Wake subjects showed significantly larger SCRs compared to Sleep subjects (F(1,51) = 6.66, P = 0.013). Therefore, the hypothesis that sleep would enable generalization of extinction from a previously extinguished to a previously unextingushed stimulus was supported.
Figure 2.
Average square-root transformed SCRs during each trial of the Extinction Recall phase illustrating the significant Group × CS+ Type interaction in a 3-factor, repeated-measures ANOVA. Upper 2 graphs: interaction decomposed by CS+ Type. Lower 2 graphs: interaction decomposed by Group. Error bars represent standard error. μS: microSiemens; n.s. P > 0.1; *P < 0.05; **P < 0.01.
Additionally, there was a significant CS+ Type × Trials interaction (F(7,357) = 4.00, P < 0.001), indicating that SCRs to CS+E trials decreased more rapidly, compared to CS+U trials, regardless of Group.
DISCUSSION
Because responding to the extinguished CS+ during extinction recall was not significantly smaller in the Sleep than in the Wake group, the results do not provide support for the hypothesis that sleep enhances extinction memory. However, the magnitude of the 95% upper confidence limit indicates that failure to find such a group difference could have been due to type II error.
What the results do support is the suggestion that sleep promotes generalization of extinction memory from an extinguished CS+ to a similarly conditioned, but unextinguished, CS+. Following fear conditioning and extinction training, when the 12-hour delay before testing of extinction recall contained a normal day's continuous waking, larger SCRs to unextinguished vs. extinguished CS+s were observed, as would have been expected. In contrast, responses to the 2 CS+ types were comparable when this delay contained a night's sleep. Comparably small post-delay SCRs to the extinguished CS+ in the 2 groups, but significantly larger responses in the Wake group to the unextinguished CS+, indicate that this group difference resulted from diminished responding to the unextinguished CS+ in the Sleep group rather than loss of extinction memory for the extinguished CS+.
Related Experimental Observations
The observed generalization of extinction memory following sleep bears a similarity to recent observations from experiments in healthy humans that compared normal sleep with normal waking, the results of which suggest that sleep mediates changes in the relationships of memory traces to one another. For example, sleep has been shown to: promote false recall of words with the same gist as previously learned words,35 enhance remote nonverbal, transitive inference reasoning,36 augment preferential visual recall of emotional foreground versus neutral background37 and promote explicit38 and implicit39 discovery of rules embedded in experimental tasks.
The current findings also resemble the elicitation of extinction memory by stimuli that are perceptually similar, but not identical, to the CS+.40 For example, Vervliet et al.22 showed that extinction of a conditioned response to an original CS+ extended to novel stimuli that were physically similar to it. The current results suggest that, with intervening sleep, an even more powerful generalization of extinction can occur since the “generalization stimulus” (CS+U) had itself actually been associated with the US (i.e., fear conditioned).
In a recent rat study, generalization of extinction memory was enhanced pharmacologically using the N-methyl-D-aspartate (NMDA) receptor partial agonist D-cycloserine (DCS). Ledgerwood et al.41 conditioned rats to both light and tone CS+s using the same loud-noise US. The CR to the light CS+ was extinguished, after which half of the rats received DCS injection and the other half saline. DCS not only improved extinction memory (less freezing) for the extinguished light CS+, but it also decreased freezing to the unextinguished tone CS+, suggesting that extinction memory had generalized across perceptual modalities. The current data suggest that, like DCS, sleep can enhance generalization of extinction memory from an extinguished CS+ to a similarly conditioned, but unextinguished, CS+. This raises the interesting possibility that sleep-mediated generalization of extinction memory may involve NMDA-receptor processes. Notably, in rodents, hippocampal long-term potentiation, an NMDA-receptor dependent process, can be blocked by sleep restriction42 as well as by REM deprivation.43
Sleep and the Brain Bases of Extinction Memory
Data from animal studies have produced a detailed model of the brain bases of extinction learning, retention and expression that implicates circuitry linking the amygdala, ventromedial prefrontal cortex, and hippocampus.9–12 Neuroimaging studies of extinction recall have shown involvement of homologous structures in humans.23,33,44,45 Notably, activity in these same regions markedly increases in the transition from NREM to REM sleep.46–48 Dysfunction in the first 2 of these structures, subsumed under an “anterior paralimbic REM activation area” by Nofzinger et al.,47 has been implicated in anxiety disorders.49 A recent fMRI study found greater amygdala activation and less amygdala-medial prefrontal (mPFC) functional connectivity in response to emotional stimuli following sleep deprivation.50 Therefore, sleep loss might specifically impair retention and expression of extinction memory via interference with mPFC-amygdala circuitry.
Possible Clinical Significance of Sleep Effects on Extinction Memory Generalization
It has long been recognized that generalization of extinction memory following exposure therapy for anxiety disorders is necessary for successful treatment since, outside the treatment setting, the patient encounters stimuli that elicit conditioned fear but differ in varying degrees from the specific stimulus extinguished in therapy.21,22,51 For example fearful responding may re-emerge when the patient encounters exemplars of a feared category of objects (e.g., spiders) that differ from the specific exemplar (e.g., species of spider) for which fear was extinguished in therapy.21,51 Such phenomena involve failure of extinction memory to generalize from the treatment setting to diverse stimuli evoking conditioned fears in the real world. Clinical strategies to maximize such generalization include presenting a variety of exemplars in a class of feared objects,51 presenting feared objects in multiple contexts,52 and in vivo exposure sessions.53 Since sleep disruption is extremely common in anxiety disorders,54 the current results suggest 2 additional strategies for maximizing the generalization of extinction memory following exposure therapy: first, promoting good sleep quality and second, scheduling sessions late in the day.
Such sleep-related interventions may be particularly relevant to treating individuals with posttraumatic stress disorder (PTSD). Individuals with PTSD have been shown to have an abnormal propensity toward fear conditioning,32 impaired extinction of conditioned fear responses32,55 as well as poorer recall of such extinction.56 Among the anxiety disorders, sleep disturbances are particularly common in PTSD.57 For example, a recent meta-analysis identified increased stage 1 NREM, decreased slow wave sleep (SWS), and increased rapid eye movement density in REM as consistent alterations of sleep in PTSD versus control groups.57 Moreover, sleep abnormalities following traumatic experiences, such as shortened duration of REM periods, may predict later development of PTSD.58 Notably, a recent rat study has shown amelioration of fear-conditioning-induced sleep disruption by extinction training.59
Generalization of therapeutic extinction learned in exposure therapy may be especially important in treating PTSD, in which patients may have developed fear conditioning to multiple stimuli in more than one modality that immediately preceded a traumatic event and have subsequently become signals of impending danger.60 Additional generalization and multiplication of fear responses in PTSD may also occur via second order fear conditioning.61 Aggressive treatment of the prominent sleep problems in PTSD patients may therefore augment the efficacy of psychotherapeutic interventions.
A specific role for REM sleep in emotion regulation is suggested by its enhancement of emotional memory,5 its disruption by experimental stressors20 and the fact that REM abnormalities are characteristic and possibly predictive of PTSD.57,58 Therefore, in any sleep interventions designed to enhance response to exposure therapy for PTSD, specifically protecting the integrity of REM sleep may prove important. For example, since the majority of aminergic antidepressants inhibit REM sleep,62 the choice of therapeutic agents used to treat mood disorders comorbid with PTSD might strive to minimize such effects.
Potential Confounding Factors and Limitations of the Current Study
The following findings militate against generally reduced reactivity to conditioned fearful stimuli following sleep as a confounding explanation of the current results. First, Milad et al.31 employed a no-extinction control group in a study with a single CS+. Unlike the experimental group in which extinction training immediately followed conditioning, no extinction training took place in controls prior to a 24-hour retention period that contained a normal night's sleep. Following this retention period, SCRs were significantly larger in the no-extinction group despite its having slept; actually they were comparable to SCRs during conditioning itself.
Using the current two-CS+ protocol, Milad et al.23 showed full retention of responses to the CS+U following a 24-hour delay that contained a night's sleep, again arguing against a general reduction of reactivity following sleep. Although those results appear to contradict the current observations of overnight extinction generalization to the CS+U, it is important to note that conditioning and extinction training in Milad et al.23 took place from 07:30–10:00, i.e., well before bedtime. Such subjects, therefore, spent the equivalent time awake before their normal evening bedtime as those in the Wake group of the current study. Studies in humans63,64 and animals16,17 have demonstrated delimited post-training windows of opportunity for the effects of sleep on memory. The generalization of extinction memory observed here may be contingent upon sleep's occurring within a delimited post-training interval.
A second potential confound of the present results involves the possibility of a circadian effect, whereby SCRs are lower in the morning than in the evening. Circadian patterns of SCL and SCRs to International Affective Picture System stimuli have been reported in which a morning minimum is followed by a mid-afternoon peak, which is then followed by a decline to evening levels that remain greater than morning levels.65 However, the fact that mean SCRs to CS+s during conditioning and extinction trials did not differ between the evening (Sleep group) and morning (Wake group) constitutes strong evidence that circadian effects on SCR are not responsible for the current findings.
Nonetheless, there exists evidence in rodents that acquisition of conditioning is facilitated by training at the peak versus trough of the circadian adrenocorticotropic hormone (ACTH) cycle.66 Therefore, circadian changes in brain levels of ACTH (as well as other neuroendocrine factors) may have influenced acquisition of conditioning and extinction learning in Sleep and Wake groups in a manner that influenced later extinction recall despite the observed lack of immediate circadian effects on conditioning and extinction. These findings suggest that an important control study in humans might compare not only conditioning and extinction learning, but also extinction recall between morning and evening testing without intervening sleep in either group (i.e., with a shorter extinction retention interval).
Conclusions
In healthy human subjects, concurrent fear conditioning to 2 different stimuli, followed by extinction of conditioned responding to one of the stimuli, led to generalization of extinction to the other stimulus when sleep, but not wake, followed extinction training. Failure of sleep-facilitated generalization of extinction memory offers a potential basis for the role of diminished sleep in the pathophysiology of anxiety disorders such as PTSD, and it suggests the importance of good sleep following cognitive behavior therapies.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Stickgold has received research support from and consulted for Takeda, Actelion, and Sepracor; is on the advisory board of, has received research support from, and has consulted for Merck; has received research support from Jazz Pharmaceuticals; and has consulted for Epix Pharmaceuticals. Dr. Rauch has received research support Medtronics, Cyberonics, and Cephalon; has consulted for Novartis, Neurogen, Sepracor, and Primedia; and attended an advisory board meeting for Medtronics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Work was performed at Massachusetts General Hospital, Charlestown, MA. EPS is supported by NIDA grant R01DA11744 to Robert T. Malison. We thank Heike Croteau for laboratory assistance and Elaine O'Rourke for administrative assistance.
REFERENCES
- 1.Cartwright R, Luten A, Young M, Mercer P, Bears M. Role of REM sleep and dream affect in overnight mood regulation: a study of normal volunteers. Psychiatry Res. 1998;81:1–8. doi: 10.1016/s0165-1781(98)00089-4. [DOI] [PubMed] [Google Scholar]
- 2.Levin R, Nielsen TA. Disturbed dreaming, posttraumatic stress disorder, and affect distress: a review and neurocognitive model. Psychol Bull. 2007;133:482–528. doi: 10.1037/0033-2909.133.3.482. [DOI] [PubMed] [Google Scholar]
- 3.Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses. Sleep Med Rev. 2008;12:185–95. doi: 10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–66. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 5.Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8:112–9. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner U, Hallschmid M, Rasch B, Born J. Brief sleep after learning keeps emotional memories alive for years. Biol Psychiatry. 2006;60:788–90. doi: 10.1016/j.biopsych.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 7.Hu P, Stylos-Allan M, Walker MP. Sleep facilitates consolidation of emotional declarative memory. Psychol Sci. 2006;17:891–8. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- 8.Holland P, Lewis PA. Emotional memory: selective enhancement by sleep. Curr Biol. 2007;17:R179–81. doi: 10.1016/j.cub.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 9.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–58. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- 11.Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–84. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- 12.Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–60. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Ruskin DN, Liu C, Dunn KE, Bazan NG, LaHoste GJ. Sleep deprivation impairs hippocampus-mediated contextual learning but not amygdala-mediated cued learning in rats. Eur J Neurosci. 2004;19:3121–4. doi: 10.1111/j.0953-816X.2004.03426.x. [DOI] [PubMed] [Google Scholar]
- 14.Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–76. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silvestri AJ. REM sleep deprivation affects extinction of cued but not contextual fear conditioning. Physiol Behav. 2005;84:343–9. doi: 10.1016/j.physbeh.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Fu J, Li P, Ouyang X, et al. Rapid eye movement sleep deprivation selectively impairs recall of fear extinction in hippocampus-independent tasks in rats. Neuroscience. 2007;144:1186–92. doi: 10.1016/j.neuroscience.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 17.Smith C. Sleep states and memory processes. Behav Brain Res. 1995;69:137–45. doi: 10.1016/0166-4328(95)00024-n. [DOI] [PubMed] [Google Scholar]
- 18.Smith C. Sleep states and memory processes in humans: procedural versus declarative memory systems. Sleep Med Rev. 2001;5:491–506. doi: 10.1053/smrv.2001.0164. [DOI] [PubMed] [Google Scholar]
- 19.Graves L, Pack A, Abel T. Sleep and memory: a molecular perspective. Trends Neurosci. 2001;24:237–43. doi: 10.1016/s0166-2236(00)01744-6. [DOI] [PubMed] [Google Scholar]
- 20.Pawlyk AC, Morrison AR, Ross RJ, Brennan FX. Stress-induced changes in sleep in rodents: models and mechanisms. Neurosci Biobehav Rev. 2008;32:99–117. doi: 10.1016/j.neubiorev.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vervliet B, Vansteenwegen D, Baeyens F, Hermans D, Eelen P. Return of fear in a human differential conditioning paradigm caused by a stimulus change after extinction. Behav Res Ther. 2005;43:357–71. doi: 10.1016/j.brat.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Vervliet B, Vansteenwegen D, Eelen P. Generalization of extinguished skin conductance responding in human fear conditioning. Learn Mem. 2004;11:555–8. doi: 10.1101/lm.77404. [DOI] [PubMed] [Google Scholar]
- 23.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: Evidence for occult insomnia. Drug Alcohol Depend. 2006;82:238–49. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 25.International classification of sleep disorders: Diagnostic and coding manual. Diagnostic Classification Steering Committee, Thorpy MJ, Chairman. Rochester, Minnesota: American Sleep Disorders Association; 1990. [Google Scholar]
- 26.Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) New York: New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- 27.Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994;17:703–10. doi: 10.1093/sleep/17.8.703. [DOI] [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.Milad MR, Goldstein JM, Orr SP, et al. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behav. Neurosci. 2006;120(6):1196–203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- 30.Pace-Schott EF, Stickgold R, Muzur A, et al. Sleep quality deteriorates over a binge--abstinence cycle in chronic smoked cocaine users. Psychopharmacology (Berl) 2005;179:873–83. doi: 10.1007/s00213-004-2088-z. [DOI] [PubMed] [Google Scholar]
- 31.Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42:456–64. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 32.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–8. [PubMed] [Google Scholar]
- 33.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 34.Orr SP, Lasko NB, Shalev AY, Pitman RK. Physiologic responses to loud tones in Vietnam veterans with posttraumatic stress disorder. J Abnorm Psychol. 1995;104:75–82. doi: 10.1037//0021-843x.104.1.75. [DOI] [PubMed] [Google Scholar]
- 35.Payne JD, Propper R, Walker MP, Stickgold R. Sleep increases false recall of semantically related words in the Deese-Roedinger-McDermott memory task. Sleep. 2006;29(Suppl):A373. [Google Scholar]
- 36.Ellenbogen JM, Hu PT, Payne JD, Titone D, Walker MP. Human relational memory requires time and sleep. Proc Natl Acad Sci U S A. 2007;104:7723–8. doi: 10.1073/pnas.0700094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payne JD, Stickgold R, Swanberg K, Kensinger E. Sleep preferentially enhances memory for emotional components of scenes. Psychol Sci. 2008;19:781–8. doi: 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427:352–5. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- 39.Djonlagic I, Rosenfeld A, Stickgold R. Sleep-dependent aspects of category learning. Sleep. 2005;28(Suppl) [Google Scholar]
- 40.Vervliet B, Vansteenwegen D, Eelen P. Generalization gradients for acquisition and extinction in human contingency learning. Exp Psychol. 2006;53:132–42. doi: 10.1027/1618-3169.53.2.132. [DOI] [PubMed] [Google Scholar]
- 41.Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biol Psychiatry. 2005;57:841–7. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 42.Kopp C, Longordo F, Nicholson JR, Luthi A. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J Neurosci. 2006;26:12456–65. doi: 10.1523/JNEUROSCI.2702-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishikawa A, Kanayama Y, Matsumura H, Tsuchimochi H, Ishida Y, Nakamura S. Selective rapid eye movement sleep deprivation impairs the maintenance of long-term potentiation in the rat hippocampus. Eur J Neurosci. 2006;24:243–8. doi: 10.1111/j.1460-9568.2006.04874.x. [DOI] [PubMed] [Google Scholar]
- 44.Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A. 2005;102:10706–11. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–11. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maquet P, Ruby P, Maudoux A, et al. Human cognition during REM sleep and the activity profile within frontal and parietal cortices: a reappraisal of functional neuroimaging data. Prog Brain Res. 2005;150:219–27. doi: 10.1016/S0079-6123(05)50016-5. [DOI] [PubMed] [Google Scholar]
- 47.Nofzinger EA, Buysse DJ, Germain A, et al. Increased activation of anterior paralimbic and executive cortex from waking to rapid eye movement sleep in depression. Arch Gen Psychiatry. 2004;61:695–702. doi: 10.1001/archpsyc.61.7.695. [DOI] [PubMed] [Google Scholar]
- 48.Braun AR, Balkin TJ, Wesenten NJ, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120:1173–97. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- 49.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep--a prefrontal amygdala disconnect. Curr Biol. 2007;17:R877–8. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Rowe MK, Craske MG. Effects of varied-stimulus exposure training on fear reduction and return of fear. Behav Res Ther. 1998;36:719–34. doi: 10.1016/s0005-7967(97)10017-1. [DOI] [PubMed] [Google Scholar]
- 52.Vansteenwegen D, Vervliet B, Iberico C, Baeyens F, Van den Bergh O, Hermans D. The repeated confrontation with videotapes of spiders in multiple contexts attenuates renewal of fear in spider-anxious students. Behav Res Ther. 2007;45:1169–79. doi: 10.1016/j.brat.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 53.Foa E, Hembree E, Rothbaum B. Emotional processing of traumatic experiences. Therapist Guide: Oxford University Press; 2007. Prolonged exposure therapy for PTSD. [Google Scholar]
- 54.Mellman TA. Sleep and anxiety disorders. Psychiatr Clin North Am. 2006;29:1047–58. doi: 10.1016/j.psc.2006.08.005. abstract x. [DOI] [PubMed] [Google Scholar]
- 55.Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther. 2007;45:2019–33. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 56.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–20. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44:660–9. doi: 10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- 58.Mellman TA, Pigeon WR, Nowell PD, Nolan B. Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J Trauma Stress. 2007;20:893–901. doi: 10.1002/jts.20246. [DOI] [PubMed] [Google Scholar]
- 59.Wellman LL, Yang L, Tang X, Sanford LD. Contextual fear extinction ameliorates sleep disturbances found following fear conditioning in rats. Sleep. 2008;31:1035–42. [PMC free article] [PubMed] [Google Scholar]
- 60.Ehlers A, Hackmann A, Steil R, Clohessy S, Wenninger K, Winter H. The nature of intrusive memories after trauma: the warning signal hypothesis. Behav Res Ther. 2002;40:995–1002. doi: 10.1016/s0005-7967(01)00077-8. [DOI] [PubMed] [Google Scholar]
- 61.Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatry. 2007;164:1684–92. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- 62.Vogel GW, Buffenstein A, Minter K, Hennessey A. Drug effects on REM sleep and on endogenous depression. Neurosci Biobehav Rev. 1990;14:49–63. doi: 10.1016/s0149-7634(05)80159-9. [DOI] [PubMed] [Google Scholar]
- 63.Stickgold R, James L, Hobson JA. Visual discrimination learning requires sleep after training. Nat Neurosci. 2000;3:1237–8. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- 64.Gais S, Lucas B, Born J. Sleep after learning aids memory recall. Learn Mem. 2006;13:259–62. doi: 10.1101/lm.132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hot P, Leconte P, Sequeira H. Diurnal autonomic variations and emotional reactivity. Biol Psychol. 2005;69:261–70. doi: 10.1016/j.biopsycho.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Pagano RR, Lovely RH. Diurnal cycle and ACTH facilitation of shuttlebox avoidance. Physiol Behav. 1972;8:721–3. doi: 10.1016/0031-9384(72)90102-3. [DOI] [PubMed] [Google Scholar]