Abstract
Doxorubicin (DOX)-induced cardiotoxicity is thought to be mediated by the generation of superoxide anion radicals (superoxide) from redox cycling of DOX in cardiomyocyte mitochondria. Reduction of superoxide generates H2O2, which diffuses throughout the cell and potentially contributes to oxidant-mediated cardiac injury. The mitochondrial and cytosolic glutathione peroxidase 1 (Gpx1) primarily functions to eradicate H2O2. In this study, we hypothesize that Gpx1 plays a pivotal role in the clearance of H2O2 generated by DOX. To test this hypothesis, we compared DOX-induced cardiac dysfunction, mitochondrial injury, protein nitration, and apoptosis in Gpx1-deficient and wild type mouse hearts. The Gpx1-deficient hearts showed increased susceptibility to DOX-induced acute functional derangements than wild type hearts, including impaired contractility and diastolic properties, decreased coronary flow rate, and reduced heart rate. In addition, DOX treatment impaired the mitochondrial function of Gpx1-deficient hearts. Specifically, Gpx1-deficient hearts treated with DOX demonstrated an increased rate of NAD-linked state 4 respiration and a decline in the P/O ratio relative to wild type hearts, suggesting that DOX uncouples the electron transfer chain and oxidative phosphorylation in Gpx1-deficient hearts. Finally, apoptosis and protein nitration were significantly increased in Gpx1-deficient mouse hearts compared to wild type hearts. These studies suggest that Gpx1 plays significant roles in protecting DOX-induced mitochondrial impairment and cardiac dysfunction in the acute phase.
Keywords: doxorubicin, glutathione peroxidase deficiency, mitochondrial function, cardiac function, apoptosis, protein nitration
1. Introduction
Doxorubicin (DOX) is an anthracycline antibiotic that is widely used as a chemotherapeutic agent. However, the administration of DOX is known to induce numerous cardiotoxic effects, including transient arrhythmias, nonspecific electrocardiographic abnormalities, pericarditis, and acute heart failure (1,2). DOX can also cause congestive heart failure months or years after treatment. The mechanism of DOX-induced cardiac injury has been actively investigated, and several hypotheses have been suggested to explain the acute and chronic cardiotoxicity of DOX. These include the formation of free radicals, inhibition of enzymes and proteins, changes in cardiac muscle gene expression, alterations of mitochondrial membrane function, sensitization of Ca2+ release from sarcoplasmic reticulum channels, mitochondrial DNA damage and dysfunction (3), and induction of apoptosis (4, 5).
The free radical hypothesis of DOX-induced cardiotoxicity has gained significant support (6, 7). DOX is known to generate free radicals either by redox cycling between a semi-quinone form and a quinone form or through the formation of a DOX-Fe3+ complex (8). Molecular oxygen is reduced to superoxide anion (O2−), which is converted to other forms of reactive oxygen species such as hydrogen peroxide (H2O2) and hydroxyl radical (OH·). It is hypothesized that free radicals induce damage to the heart, an organ that is known to have a relatively low level of antioxidant enzymes such as superoxide dismutase (SOD) and catalase (9). In support of this hypothesis, DOX-induced cardiotoxicity can be inhibited by the overexpression of antioxidant enzymes MnSOD and catalase (10, 11).
MnSOD, which is located in mitochondria, specifically reduces superoxide to H2O2. In turn, H2O2 is decomposed by the enzymes catalase, glutathione peroxidase, and peroxiredoxin in mammalian cells (12, 13). Catalase is located in the peroxisomes of cells (14), so diffusion of H2O2 from other subcellular sites is necessary. The major isoform of glutathione peroxidase, glutathione peroxidase 1 (Gpx1), is present in both mitochondria and the cytosol (15). The location and low Km of Gpx1 for H2O2 may facilitate its ability to detoxify the H2O2 that is generated in the mitochondria of heart following DOX treatment (16, 17). It has been shown that an early and persistent decrease in Gpx1 after DOX treatment may contribute to DOX-induced cardiotoxicity (18, 19). Treatment with probucol increases cardiac Gpx1 activity, decreases lipid peroxidation, and improves mortality (18). All of these lines of evidence suggest that there Gpx1 may play a role in minimizing the cardiotoxicity of DOX.
If Gpx1 indeed does minimize DOX-induced cardiotoxicity, then it follows that Gpx1 deficiency would render hearts more susceptible to DOX-induced cardiotoxicity. To explore this hypothesis, cardiac performance was measured in a DOX-treated isolated mouse heart preparation as well as DOX-treated mouse hearts in vivo. In addition, this study measured the mitochondrial function, amount of apoptosis, and level of protein nitration in the hearts of DOX-treated Gpx1-dificient mice and their wild type littermates.
2. Materials and Methods
2.1. Materials
DOX HCl (2 mg/ml) was purchased from Bedford Laboratories (Bedford, OH). An ApopTag In Situ Oligo Ligation kit was obtained from Chemicon International (Temecula, CA). All other reagents were of the highest grades commercially available.
2.2. Animal studies
Gpx1 knockout mice (Gpx1−/−) were generated as described previously (20). Gpx1 is the predominant form of the Gpx isozymes, and little to no Gpx activity was detected in tissues of homozygous knockout mice. In the heart, Gpx activity in knockout mice is less than 10% of the total Gpx activity in wild type mice (20). Mice were maintained in AAALAC-accredited, climate-controlled facilities. Wild type Gpx1+/+ and Gpx1−/− mice (4–5 month old, in a C57BL/6 and 129SV mixed genetic background) were randomly assigned to four groups: Gpx1+/+, Gpx1+/+ + DOX, Gpx1−/− mice, or Gpx1−/− mice + DOX. A single dose of DOX (22.5 mg/kg, i.p.) or an equivalent volume of saline was injected. These animals were free of pathogens during the entire course of experiments. All the animal protocols were approved by the East Tennessee State University Animal Committee.
2.3. Isolation of cardiac mitochondria and measurement of mitochondrial respiratory and phosphorylating activities
Mouse cardiac mitochondria were isolated, and their respiratory and phosphorylating acivities measured according to the methods described previously (21). Respiratory control index (RCI) is defined as the ratio of the state 3 respiratory rate to the state 4 respiratory rate. ADP/O ratio (P/O ratio) was determined by dividing the amount (nmoles) of ADP added by the amount (natoms=nanoatoms) of oxygen consumed during state 3 respiration (22).
2.4. In vitro cardiac function measurement
In vitro mouse cardiac function was analyzed in a modified Langendorff perfusion model as previously described (23). Briefly, age- and gender-matched Gpx1+/+ and Gpx1−/− mice were injected with anticoagulant sodium heparin (500 units/kg, i.p.) 20 min prior to anesthetization with sodium pentobarbital (120 mg/kg, i.p.). Following anesthetization, mouse hearts were rapidly removed, cannulated, and perfused retrogradely at 37°C with a Krebs-Henseleit bicarbonate buffer. After 30 min perfusion to stabilize the cardiac function, mouse hearts were perfused with the buffer in the presence or absence of DOX for another 60 min. The left ventricular pressure trace was continuously recorded through a pressure transducer connected to a computerized data acquisition system (DATAQ Instruments, Inc., Akron, OH). The coronary effluent was collected and measured every 15 min. Cardiac function parameters, including heart rate (HR), left ventricular develop pressure (LVDP), and maximum development of pressure over time (±dP/dt), were analyzed from the recorded data with a software.
2.5. Analysis of in vivo cardiac function
A single dose of DOX (22.5 mg/kg, i.p.) or an equivalent volume of saline was injected. Five days after DOX or saline administration, mice were injected with heparin (500 units/kg, i.p.) and anesthetized with 2% isoflurane. Each mouse was intubated with a 22-gauge soft catheter and ventilated with a rodent ventilator (Columbus Instruments International Corp., Columbus, OH) at a tidal volume of 0.3–0.5 ml and a respiratory rate of 120 breaths/min. After left thoracotomy, the pericardium was dissected to expose the heart. A microtip pressure–volume catheter (SPR-839; Millar Instruments, Houston, TX) was inserted through a 25-gauge apical stab into the left ventricle to measure steady state cardiac function. To change the cardiac preload, the inferior vena cava was occluded for 1 second. At the completion of the study, 10 µl of hypertonic saline (15%) was injected into the right atrium to calibrate Vp, the parallel volume. The signals were continuously recorded at a sampling rate of 1000 s−1 using an ARIA pressure-volume conductance system (Millar Instruments) coupled to a Powerlab/4SP A/D converter (AD Instruments, Mountain View, CA). All pressure–volume loop data were analyzed with a cardiac pressure–volume analysis program (PVAN3.4; Millar Instruments). At the end of function analysis, animals were anesthetized with tribromoethanol (270 mg/kg, i.p.). Hearts were removed and perfused for 2 min as Langendorff preparations to remove the remaining blood. Portions of the mid-ventricle were fixed in 4% buffered formaldehyde for 24 h, dehydrated, and embedded for immunological and apoptosis studies.
2.6. ISOL analysis
A 2-mm section of mid-ventricles was sliced, fixed in 4% buffered formaldehyde for 24 h, dehydrated, and embedded. Myocardial sections (5 µm) were mounted on siliconized slides and dried at 37°C overnight. In situ staining of DNA strand breaks in the serial section of each specimen was detected by the ApopTag ISOL (In Situ Oligo Ligation) kit using oligo A as previously described (24). The percentage of ISOL-positive myocytes was determined by counting 10 random fields per section under an Olympus BX40 microscope.
2.7. Immunohistochemistry
Immunostaining of nitrotyrosine in mouse hearts was performed using monoclonal anti-nitrotyrosine antibody at a dilution of 1:200 (Cayman, Ann Arbor, MI) at 4°C overnight. Antigen-antibody complexes were detected by the Super-sensitive alkaline phosphatase kit (BioGenex; San Ramon, CA) using fast red as a chromogen. Hematoxylin was used as a counterstain. Images were captured using an SPOT camera and transferred into research-based digital image analysis software (Image Pro Plus; Media Cybernetics, Silver Spring, MD). The extent of immunoreactivity in the LV was determined in the tissues by applying intensity thresholding analysis (25). The sum density of total LV image pixels in the 80-to-155 range was used as a semi-quantitative measure of relative immunoreactivity.
2.8. Statistical Analysis
All values were expressed as mean ± SEM. One-way ANOVA was used followed by Tukey’s multiple comparison tests if there were significant differences between groups. Significance of differences between two groups was established by Student’s t-test. Significance was indicated if P<0.05.
3. Results
Mouse hearts deficient in Gpx1 are more susceptible to DOX-induced cardiac dysfunction
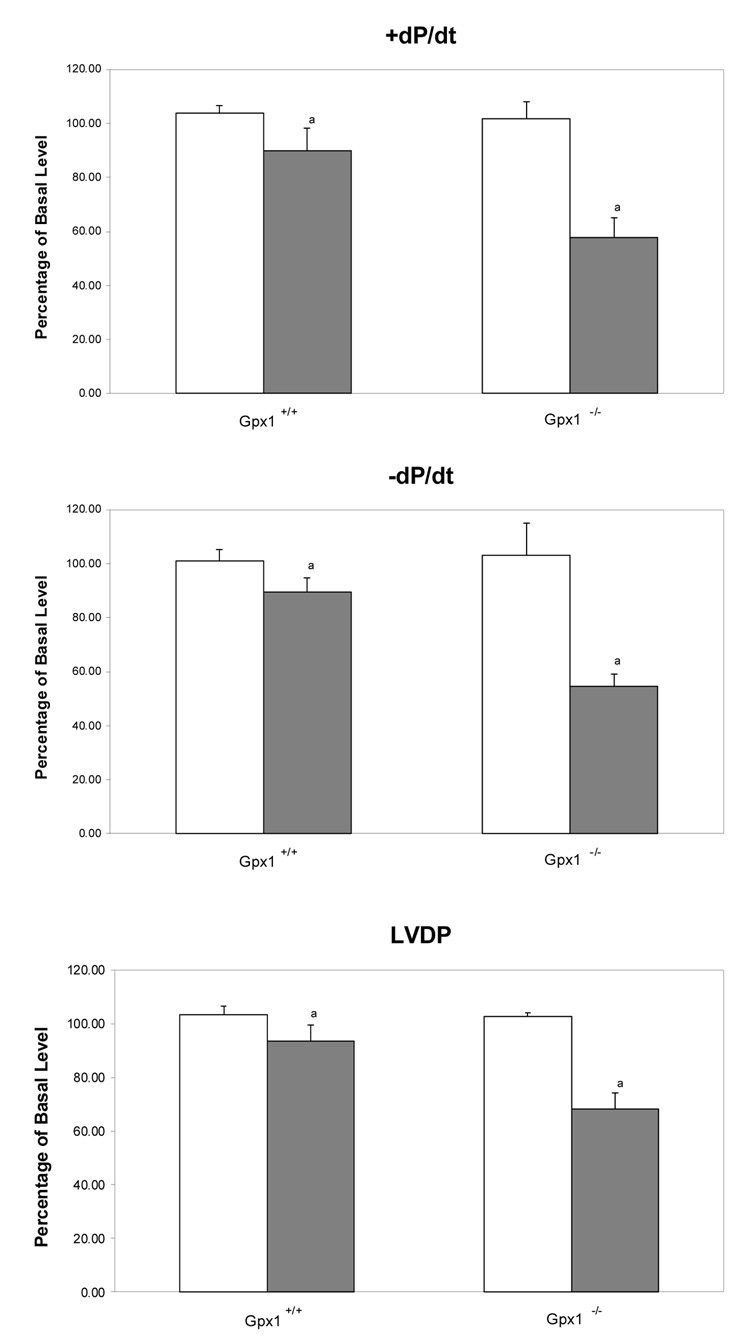
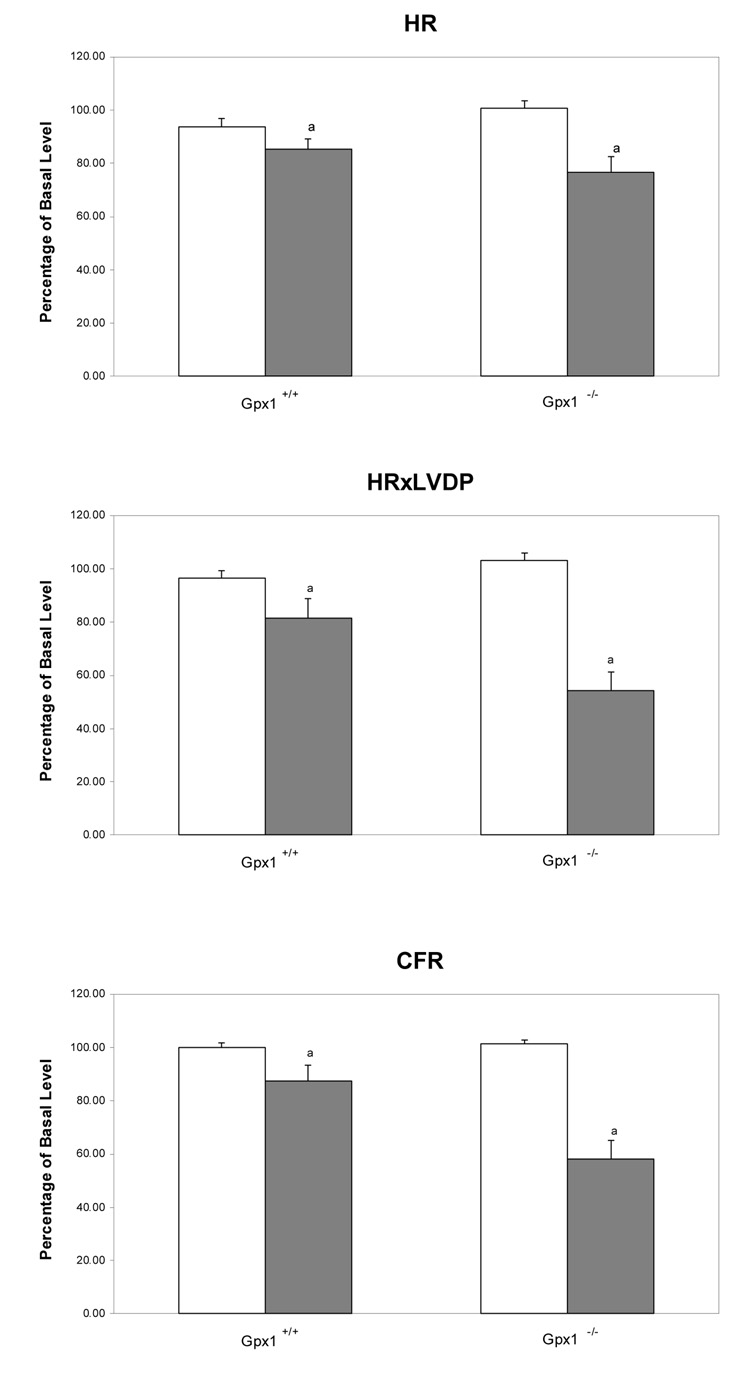
In vitro cardiac function was analyzed in a mouse heart perfusion model to determine the role of Gpx1 in protecting hearts against DOX-induced cardiotoxicity. Our study found that DOX caused significantly more cardiac dysfunction in Gpx1-deficient (Gpx1−/−) mouse hearts than in wild type or Gpx−/− mice. However, as shown in Table 1, there was no difference in the basal levels of hemodynamic indices of cardiac function between the wild type mice and Gpx1−/− mice; however, perfusion with 5 µM DOX for 60 min resulted in both inotropic and chronotropic dysfunction. As shown in Fig. 1, hearts from Gpx1−/− mice showed a 44% suppression of +dP/dt (maximum rate of pressure development during systole), a 47% suppression of −dP/dt (maximum rate of pressure development during diastole), and a 34% suppression of LVDP (left ventricular developing pressure) following exposure to DOX for 60 min, compared to a 14% suppression of +dP/dt, a 12% suppression of −dP/dt, and a 10% reduction of LVDP from Gpx1+/+ mice. The chronotropic index of cardiac function HR decreased 9% in Gpx1+/+ hearts and 34% in Gpx1−/− mouse hearts following exposure to DOX for 60 min (P< 0.05 compared to that in the Gpx1+/+ mice). With HR×LVDP, which measures both inotropic and chronotropic changes, homozygous Gpx1−/−mouse hearts displayed a 49% reduction vs. a 14% reduction in Gpx1+/+ mice.
Table 1.
Basal levels of cardiac parameters of perfused mouse hearts
| Gpx1+/+ | Gpx1+/++DOX | Gpx1−/− | Gpx1−/−+DOX | |
|---|---|---|---|---|
| HR (beats/min) | 278±10 | 278±17 | 270±10 | 287±14 |
| LVDP (mmHg) | 75.3±4.5 | 82.4±2.2 | 80.4±1.5 | 76.8±2.3 |
| +dP/dt (mmHg/sec) | 2882±271 | 2580±100 | 2527±208 | 2617±99 |
| −dP/dt (mmHg/sec) | 1665±176 | 1612±72 | 1644±136 | 1773±93 |
| HR×LVDP | 22943±1377 | 23612±903 | 21769±1418 | 23471±587 |
| CFR (ml/min) | 1.38±0.10 | 1.28±0.05 | 1.29±0.07 | 1.27±0.09 |
Values represent the cardiac functional parameters before exposure to buffer only or buffer containing DOX and are expressed as mean ± SEM of six mouse hearts. Measurements of the maximum rates of pressure development (± dP/dt), left ventricular developed pressures (LVDP), heart rates (HR), and coronary flow rate (CFR) were made after 30 min of preliminary perfusion. There is no significant difference among all groups.
Fig. 1.
Effect of DOX on the cardiac function and coronary flow rate of hearts from Gpx1−/− and wild type mice. Mouse hearts were perfused in vitro with Krebs-Henseleit buffer as described in Materials and Methods. Cardiac function parameters are expressed as the recovery percentage after exposure to buffer or DOX compared to the basal levels measured in the first 30-min perfusion. Open and filled bars represent mouse hearts perfused with Krebs-Henseleit buffer only (as control) and Krebs-Henseleit buffer containing 5 µM DOX, respectively. Values are mean ± SEM of six hearts. Empty bars, hearts perfused with buffer only; filled bars, hearts perfused with buffer containing DOX. a, P < 0.01 vs. the same measurement of mouse hearts perfused with the buffer, or the Gpx1+/+ mouse hearts perfused with DOX.
The above data indicate that DOX induces significantly greater cardiac dysfunction in Gpx1−/− mice. In addition, the coronary flow rate during DOX perfusion was reduced from 43% in Gpx1−/− to 13% in the Gpx1+/+ mice, suggesting that the decrease of cardiac perfusion is either due to vascular constriction or endothelial damage following exposure to DOX. Overall, the in vitro cardiac function study suggests that knockout of the Gpx1 gene leads to significantly compromised cardiac systolic and diastolic function following exposure to DOX.
To further validate our in vitro study findings, we investigated cardiac function in DOX-treated Gpx1−/− mice and their wild type littermates with a sophisticated in vivo functional study. As summarized in Table 2, there was no difference between the initial body weight and cardiac function of Gpx1+/+ mice and Gpx1−/− mice. DOX-treated mice showed numerous changes after 5 days of treatment. First, DOX treatment caused weight loss in both Gpx1−/− mice and Gpx1+/+ mice, but the weight loss was significantly greater in Gpx1−/− mice. Second, DOX treatment led to profound suppression of systolic cardiac function in both Gpx1−/− mice Gpx1+/+ mice, but the suppression was significantly higher in Gpx1−/− mice. Indeed, ejection fraction was significantly lower in Gpx1−/− mice compared to Gpx1+/+ mice (41.8 ± 1.71% vs. 51.2 ± 1.01%, P<0.05), which was the result of a greater suppression of end-systolic ventricular pressure (60.5 ± 2.68 mmHg vs. 67.1 ± 1.69 mmHg, P<0.05) and a greater elevation of end-systolic volume (9.19 ± 0.18 µl vs. 7.83 ± 0.26 µl, P<0.05). The pressure development rate of the left ventricle (+dP/dt) was significantly reduced in Gpx1−/− mice compared to the Gpx1+/+ group (4406 ± 331 mmHg/s vs. 5,566 ± 111 mmHg/s, P<0.05), which in return accounted for the greater suppression in preload recruitable stroke work (PRSW) in Gpx1−/− mice (44.5 ± 3.26 vs. 62.2 ± 2.96 mm Hg).
Table 2.
In vivo cardiac function of Gpx1+/+ and Gpx1−/− mice treated with or without DOX
| Parameter | Units | Gpx1+/++ Saline |
Gpx1−/− + Saline |
Gpx1+/++DOX | Gpx1−/− +DOX |
|---|---|---|---|---|---|
| Weight | g | 30.4±0.64a | 28.9±1.71a | 24.0±1.08b | 23.2±0.48 |
| HR | beats/min | 528±2a | 526±12a | 471±5a,b | 388±24 |
| CI | µl/g | 222±6a | 226±16a | 163±8a,b | 106±9 |
| SI | µl/g | 0.42±0.01a | 0.43±0.02a | 0.35±0.02a,b | 0.28±0.02 |
| EF | % | 71.4±0.56a | 70.02±0.67a | 51.2±1.01a,b | 41.8±1.71 |
| PRSW | mmHg | 91.1±2.92a | 87.2±4.51a | 62.2±2.96a,b | 44.5±3.26 |
| Systolic Function | |||||
| Pes | mmHg | 93.1±0.74a | 92.9±0.29a | 67.1±1.69a,b | 60.5±2.68 |
| Ves | µl | 5.10±0.13a | 5.03±0.07a | 7.83±0.26a,b | 9.19±0.18 |
| dP/dtmax | mmHg/s | 8093±226a | 8132±379a | 5566±111a,b | 4406±331 |
| dP/dtmax-Ved | mmHg/µl | 507±27a | 533±42a | 332±49b | 263±19 |
| Diastolic Function | |||||
| Ped | mmHg | 3.39±0.38a | 3.17±0.45a | 6.26±0.20b | 6.13±0.76 |
| Ved | µl | 17.82±0.15a | 17.26±0.23a | 16.02±0.25a,b | 14.87±0.50 |
| dP/dtmin | mmHg/s | 6932±165a | 7028±590a | 5599±142a,b | 3504±336 |
| τ (Weiss) | ms | 6.74±0.11a | 5.95±0.25a | 7.96±0.26a,b | 10.26±0.64 |
| β(beta) | mmHg/µl | 0.47±0.04 | 0.34±0.04 | 0.34±0.05 | 0.29±0.08 |
| Vascular Function | |||||
| Ea | mmHg/µl | 7.14±0.09a | 7.66±0.16a | 7.76±0.34a | 9.15±0.13 |
Gpx1+/+ or Gpx1−/− mice were injected with DOX (22.5 mg/kg, i.p.) or saline. Five days later, in vivo cardiac function was measured by the Millar conductance catheter system as described in Materials and Methods. Values are mean ± SEM of 6 mice.
P<0.05, Gpx1−/− mice + DOX vs. others
P<0.05, Gpx1+/+mice vs. Gpx1+/+mice + DOX.
HR, heart rate; Ved, end-diastolic volume; Ves, end-systolic volume; SI, stroke index (SI = SV/body wt); EF, ejection fraction; CI, cardiac index; PRSW, preload recruitable stroke work; Pes, end-systolic pressure; Ped, End-diastolic pressure; dP/dt max (or min), the maximal rate of pressure increasing (or decreasing); dP/dtmax-Ved, slope describing isovolumic contraction; τWeiss, the mono-exponential time constant of relaxation; β, slope of end-diastolic pressure-volume relationship; Ea, arterial elastance.
Finally, Gpx1−/− mice showed significantly worse diastolic function following DOX treatment than Gpx1+/+ mice. The end-diastolic volume was more reduced in Gpx1−/− mice compared to Gpx1+/+ mice (14.87 ± 0.50 µl vs. 16.02 ± 0.25 µl, P<0.05). LV diastolic dysfunction was also more impaired in Gpx1−/− mice, as evidenced by the suppressed maximal rate of LV pressure decreasing (5,599 ± 142 mmHg/s vs. 3504 ± 336 mmHg/s, P<0.05) and elevated time constant of isovolumic relaxation (7.96 ± 0.26 ms vs. 10.26 ± 0.64 ms). It is worthwhile to mention that although the slope of end-diastolic pressure-volume relationship (β) is lower in both Gpx1−/− and Gpx1+/+ mice following DOX treatment, we did not find a significant difference between the DOX-treated Gpx1−/− and Gpx1+/+ and saline-treated controls. DOX had a significant effect on peripheral vascular resistance (Ea) in Gpx1−/− mice, but not in Gpx1+/+ mice.
In summary, DOX treatment produced a profound impairment on cardiac performance in both Gpx1−/− and Gpx1+/+ mice, but the impairments were more pronounced in Gpx1−/− mice.
Mouse hearts deficient in Gpx1 are more susceptible to DOX-induced mitochondrial dysfunction
Mitochondria are the primary subcellular organelle in which DOX-induced generation of ROS is observed. Therefore, mitochondria are a direct target of oxidative stress. We measured the efficiency of mitochondrial respiration in mouse hearts one day after DOX administration to elucidate the biochemical abnormalities that occur in cardiac mitochondria in response to DOX treatment. We utilized NAD-linked substrates (glutamate and malate) in the measurement of mitochondrial respiration. DOX treatment had no effect on state 3 respiration in cardiac mitochondria of either wild type or Gpx1−/− (Fig. 2A). However, DOX caused a 64% increase in the rate of state 4 respiration in cardiac mitochondria of Gpx1−/− mice as compared to saline-treated counterparts. However, this was not observed in cardiac mitochondria of wild type mice (Fig. 2B).
Fig. 2.
Gpx1-deficient mouse hearts are more susceptible to DOX-induced declines in NAD-linked electron transfer and energy coupling capacities in mitochondria. (A) DOX treatment had no effect on NAD-linked state 3 respiration in cardiac mitochondria of either wild type or Gpx1 knockout mice. (B) However, the same treatment caused an increase in the rate of state 4 respiration in cardiac mitochondria of Gpx1 knockout mice, but not wild type mice. a, p < 0.001 vs. saline-treated Gpx1 knockout mice and saline- or DOX-treated wild type mice. The efficiency of NAD-linked oxidative phosphorylation in cardiac mitochondria, as determined by RCI (C) and P/O ratio (D), was more severely affected in by DOX treatment in Gpx1 knockout mice than in wild type mice. In (C), b indicates p < 0.001 vs. saline-treated wild type mice. In (D), c indicates p < 0.01 vs. saline-treated wild type mice. In (C) and (D), a indicates p < 0.001 vs. saline-treated Gpx1 knockout and saline- or DOX-treated wild type mice. Values are mean ± SEM for all figures. N ≥ 4 mice for each experiment.
RCI is derived from the ratio of the rate of state 3 respiration to the rate of state 4 respiration. Thus, a change in the state 4 respiratory rate would lead to an altered RCI. RCI of cardiac mitochondria from Gpx1−/− mice treated with DOX was 56% of the RCI of saline-treated mice (Fig. 2C). Although the rates of state 3 and state 4 respiration in cardiac mitochondria of wild type mice were statistically unaffected by DOX treatment, a relatively modest decrease (26%) of RCI was observed in these samples.
The decrease in RCI of Gpx1−/− mitochondria in response to DOX treatment was greater than wild type mitochondria (p<0.001). DOX treatment also decreased the P/O ratio in wild type or Gpx1−/− mice. The P/O ratio of the latter samples was lower than the former (p < 0.001) (Fig. 2D).
The effect of DOX on mitochondrial respiration with FAD-linked substrate (succinate) was not as dramatic as the effect with NAD-linked substrates. DOX treatment did not alter the rates of state 3 and state 4 respiration and RCI in wild type and Gpx1−/− mice (Fig. 3A–C). The P/O ratio of Gpx1−/− cardiac mitochondria, but not wild type mitochondria, was decreased by 20%, as compared to mitochondria isolated from saline-treated counterparts (Fig. 3D).
Fig. 3.
Effect of Gpx1 deficiency on FAD-linked electron transfer and energy coupling capacities in cardiac mitochondria of wild type and Gpx1 knockout mice in response to DOX treatment. Gpx1 deficiency had no effect on FAD-linked state 3 respiration (A), state 4 respiration (B), and RCI (C) of cardiac mitochondria from either wild type or Gpx1 knockout mice. The P/O ratio of cardiac mitochondria from Gpx1 knockout mice, but not wild type mice, decreased after DOX treatment (D). In (D), a indicates p < 0.001 vs. saline-treated Gpx1 knockout and saline- or DOX-treated wild type mice. Values are mean ± SEM for all figures. N ≥ 4 mice for each experiment.
These studies demonstrate that DOX treatment impairs mitochondrial respiration in hearts of wild type and Gpx1−/− mice. However, the extent of functional decline in cardiac mitochondria of Gpx1 knockout mice is significantly increased.
Gpx1-deficient hearts undergo enhanced apoptosis
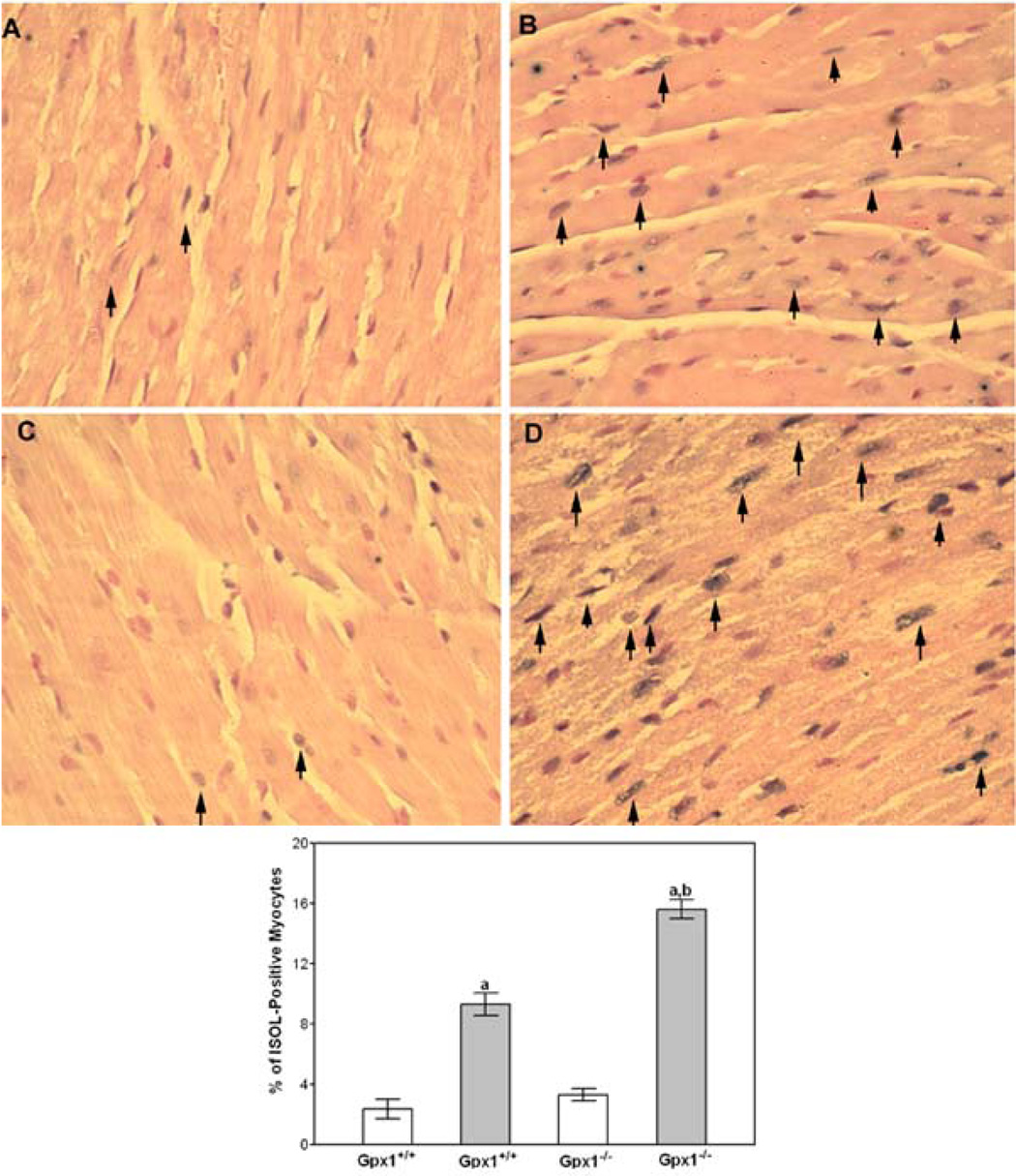
Apoptosis is observed in cultured cardiomyocytes and hearts of DOX-treated animals (4, 5). The ISOL method, which is more specific for double-stranded DNA breaks during apoptosis, was used to identify apoptotic myocytes in DOX-treated mouse hearts. The results showed that 9 ± 0.8% of cardiomyocytes in DOX-treated Gpx1+/+ mice were ISOL-positive (Fig. 4). In contrast, there were 15 ± 0.64% ISOL-positive cardiomyocytes in DOX-treated mouse Gpx1−/− hearts.
Fig. 4.
Effect of DOX on apoptosis in hearts from Gpx1−/− mice. ISOL procedure was performed in paraffin-embedded myocardial sections as described in Materials and Methods. Representative staining pattern of hearts from Gpx1+/+ (A), DOX + Gpx1+/+ (B), Gpx1−/− (C), and DOX + Gpx1−/− were shown. Arrows indicate ISOL-positive myocytes. Immunolabeled nuclei of myocytes were determined by random counting of 10 fields per section. Each bar represents mean ± SEM of six hearts. a=P<0.05, DOX vs. respective control mice, b=P<0.05, DOX + Gpx1−/− mice vs. DOX + Gpx1+/+ mice.
Mouse hearts deficient in Gpx1 display increased protein nitration
Protein nitration was investigated by the immunostaining of 3-nitrotyrosine (3-NT) in heart sections of DOX-treated Gpx1+/+ and Gpx1−/− mice and controls. Diffused cytoplasmic staining was observed throughout the heart sections. The immunostaining density of 3-NT was analyzed using a digital image system. The background of 3-NT staining was negligible in both Gpx1+/+ and Gpx1−/− control mouse hearts (data not shown). DOX caused elevated immunostaining of 3-NT in mouse hearts; and the density was three-fold higher in Gpx1−/−hearts relative to Gpx1+/+ hearts (Fig. 5B, 5C). Little staining was observed in the absence of primary nitrotyrosine antibody (Fig. 5A)
Fig. 5.
Immunohistochemical staining of mouse hearts with anti-nitrotyrosine antibody. Sections from DOX + Gpx1+/+ (panel B) or DOX + Gpx1−/− mouse hearts (panel C) were stained with anti-tyrosine antibody. Staining pattern of DOX + Gpx1−/− heart in the absence of specific antibody was shown in Panel A. Semi-quantitative analysis was performed using Image Pro Plus. Each bar represents mean ± SEM of 4 hearts. a=P<0.05, Gpx1−/− mice + DOX vs. Gpx1+/+ mice + DOX.
4. Discussion
Free radicals mediate DOX-induced cardiotoxicity. However, a minimal set of studies have investigated the role of the antioxidant enzyme Gpx1 in DOX-induced cardiotoxicity. We hypothesized that if Gpx1 plays a critical role in preventing DOX-induced cardiotoxicity, then mouse hearts overexpressing this enzyme will exhibit a resistant phenotype. Conversely, we hypothesized that mouse hearts deficient in this enzyme will exhibit a susceptible phenotype. We previously demonstrated that overexpression of Gpx1 in the heart attenuated cardiac dysfunction and improved mitochondrial complex respiration activity (21). Our current study demonstrates that Gpx1 deficiency dramatically exacerbates DOX-induced in vivo cardiac dysfunction. We also show that DOX-induced impairment in mitochondrial respiratory activity was more profound in mice lacking Gpx1. It may be possible that impaired mitochondrial activity leads to increased H2O2, increased protein nitration, and ultimately cardiomyocyte apoptosis.
Our in vitro studies demonstrated that Gpx1 partially reversed the DOX-induced deficits, including impaired contractility and diastolic function, decreased coronary flow rate, and reduced HR (Fig. 1). The toxic effects of DOX have been observed on perfused rat hearts in other studies (26, 27). Although the mechanism responsible for these alterations of cardiac function is uncertain, it has been previously shown that OH· formation in DOX-perfused rat hearts is involved in the toxic response (7). Free radicals are known to have a detrimental effect on endogenous vascular regulators, such as nitric oxide (NO), a strong vascular dilator. Coronary vascular constriction or endothelial damage could play a role as well. Pelikan et al. has found that DOX elevates coronary resistance in DOX-perfused rat heart (27), which may explain why the coronary flow decreased in DOX-perfused Gpx1−/− mouse hearts but not in wild type mouse hearts in our study. It is likely that the cardioprotective effects of Gpx1 can be primarily attributed to its antioxidant activity. However, it should be noted that heart rate was significantly decreased following exposure to DOX in our in vitro cardiac function study, which was done in the absence of pacing. Reduction of heart rate likely has a negative impact on cardiac function and contractility due to underperfusion of cardiac muscle. The exact mechanism of DOX cardiotoxicity would ideally be explored in an isolated and paced working heart, although to date, this has never been technically achieved in mouse hearts.
Our in vivo cardiac function study shows that DOX significantly decreased CO, EF, and PRSW, indicating heart failure in these mice. DOX treatment induced both systolic and diastolic dysfunction, which was magnified in Gpx1−/− mice. DOX–induced decrease in dP/dtmax-Ved suggests an effect on the calcium cycling proteins during isovolumic contraction, while DOX–induced decrease in τ suggests an effect on SERCA2a and/or phospholamban during isovolumic relaxation. An increase in Ea suggests that arterial vascular resistance is affected by DOX in Gpx1−/− mice. There was no change in β in DOX-treated mice, suggesting that there is no effect on the cardiac extracellular matrix during the passive filling phase.
Mitochondria are the major intracellular ATP generation sites; their swelling and destruction in human and animal hearts following exposure to DOX are well documented in many studies (28, 29). To further explore the possible role of Gpx1 in mitochondrial damage, we assessed mitochondrial respiration function from DOX- or saline-treated mouse hearts. Our studies showed that DOX treatment significantly compromises the efficiency of mitochondrial respiration in hearts of both wild type and Gpx1 knockout mice. However, the functional decline of cardiac mitochondria in knockout mice was significantly more severe, suggesting that Gpx1 mediates mitochondrial protection subsequent to DOX-induced damage.
The effect of DOX on cardiac mitochondrial respiration has been previously reported in a number of reports. The size of the effect varies according to differences in the dosage of DOX treatment, the genetic background of the mice, and the species (mice vs. rats) used in the studies (30–34). In our study, DOX at 22.5 mg/kg had no effect on NAD- or FAD-linked state 3 respiration in mitochondria of either wild type or Gpx1 knockout mice, suggesting that DOX does not affect the rate of ATP synthesis. Nonetheless, DOX treatment caused a more profound uncoupling of oxidative phosphorylation from the electron transport chain in NAD-linked respiration in mitochondria of Gpx1-deficient hearts compared to those of normal hearts, as suggested by the decreased P/O ratios of these samples. The uncoupling was also observed in FAD-linked respiration in mitochondria of Gpx1-deficient hearts but not wild type hearts. Finally, it should be noted that the rate of NAD-linked state 4 respiration was significantly increased upon DOX administration in cardiac mitochondria of Gpx1 knockout mice, but not wild type mice. This increased state 4 respiratory rate suggests that mitochondria cannot maintain a proper chemiosmotic gradient, possibly because DOX treatment significantly damages the mitochondrial inner membrane. On the other hand, FAD-linked state 4 respiration was not affected in Gpx1-deficient cardiac mitochondria, indicating that the DOX-induced increase in the rate of state 4 respiration is substrate-specific. The mechanisms by which substrates affect state 4 respiration remain to be determined.
In our study, we showed increased protein nitration in Gpx1-deficient mice, suggesting that DOX-induced ROS exert their deleterious effects partly by increasing protein nitration. Previous studies have shown that DOX treatment of the heart raises nitric oxide levels by increasing iNOS expression (35). NO and superoxide anion react to generate peroxynitrite, a potent oxidant that partially mediates DOX-induced cardiotoxicity by causing nitrosative stress on proteins, lipids, and nucleic acids (36). Mitochondria are particularly sensitive targets for peroxynitrite-induced toxicity, which may lead to their impaired function resulting in further production of superoxide and H202. Pacher et al. established a pivotal role of peroxynitrite in DOX-induced cardiac dysfunction by demonstrating that FP-15, a peroxynitrite decomposition catalyst, protected against protein nitration and cardiac dysfunction (37). Furthermore, both H202 and peroxynitrite can induce DNA damage and lead to the overactivation of the nuclear enzyme PARP-1, which may also contribute to DOX-induced cardiotoxicity (38).
DOX-induced reactive oxygen species and reactive nitrogen species also activate matrix metalloproteinases, such as MMP-2 and MMP-9 (39,40). These matrix degrading enzymes have been associated with cardiac failure. Whether DOX treatment leads to higher MMP-2 or MMP-9 levels in Gpx1−/− mouse hearts warrants further investigation.
Several groups have reported that cardiomyocyte apoptosis is involved in cardiac injury induced by anthracycline antibiotics (4, 5, 41–43). Peroxynitrite has been found to cause apoptosis in cardiomyocytes (44). As shown in Fig. 4, Gpx1 hearts displayed a significant increase in ISOL-positive apoptosis. These results support a recent study that showed that oxidative stress is involved in DOX-induced apoptosis and that this stress can be inhibited by Gpx1.
In summary, our study demonstrates that Gpx1 deficiency renders hearts more susceptible to DOX-induced cardiotoxicity. This cardiotoxic effect involves mitochondrial dysfunction, an increase in apoptosis, and an increase in protein nitration. These findings suggest a therapeutic role for agents such as probucol (18) and melatonin (45, 46), both of which increase Gpx1 activity in the heart. This possibility warrants further study.
Acknowledgements
We are grateful for Dr. Douglas F. Larson, University of Arizona, for his technical advice on the LV loop analysis. This study was supported by NIH grant HL087271, a grant from the Department of Veterans Affairs Merit Review and a grant-in-aid from the American Heart Association Southeast Affiliate to B.H.L.C.; and by American Heart Association Midwest Affiliate grants 0455876Z and 0655631Z to Y.-S. H. The use of equipment in the Imagine and Cytometry Facility Core was supported by a Center grant P30 ES06639.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Billingham ME, Mason JW, Bristow MR, Daniels JR. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat. Rep. 1978;62:865–872. [PubMed] [Google Scholar]
- 2.Bristow MR, Thompson PD, Martin RP, Mason JW, Billingham ME, Harrison DC. Early anthracycline cardiotoxicity. Am. J. Med. 1978;65:823–832. doi: 10.1016/0002-9343(78)90802-1. [DOI] [PubMed] [Google Scholar]
- 3.Olson RD, Mushlin PS. Doxorubicin cardiotoxicity: analysis of prevailing hypotheses. FASEB J. 1990;4:3076–3086. [PubMed] [Google Scholar]
- 4.Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000;60:1789–1792. [PubMed] [Google Scholar]
- 5.Kumar D, Kirshenbaum L, Li T, Danelisen I, Singal P. Apoptosis in isolated adult cardiomyocytes exposed to adriamycin. Ann. N. Y. Acad. Sci. 1999;874:156–168. doi: 10.1111/j.1749-6632.1999.tb09233.x. [DOI] [PubMed] [Google Scholar]
- 6.Monti E, Prosperi E, Supino R, Bottiroli G. Free radical-dependent DNA lesions are involved in the delayed cardiotoxicity induced by adriamycin in the rat. Anticancer Res. 1995;15:193–197. [PubMed] [Google Scholar]
- 7.Rajagopalan S, Politi PM, Sinha BK, Myers CE. Adriamycin-induced free radical formation in the perfused rat heart: implications for cardiotoxicity. Cancer Res. 1988;48:4766–4769. [PubMed] [Google Scholar]
- 8.Davies KJ, Doroshow JH. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J. Biol. Chem. 1986;261:3060–3067. [PubMed] [Google Scholar]
- 9.Doroshow JH, Locker GY, Myers CE. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J. Clin. Invest. 1980;65:128–135. doi: 10.1172/JCI109642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang YJ, Chen Y, Epstein PN. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J. Biol. Chem. 1996;271:12610–12616. doi: 10.1074/jbc.271.21.12610. [DOI] [PubMed] [Google Scholar]
- 11.Yen HC, Oberley TD, Vichitbandha S, Ho Y-S, St Clair DK. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J. Clin. Invest. 1996;98:1253–1260. doi: 10.1172/JCI118909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 13.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 14.van der Bosch H, Schutgens RB, Wanders RJ, Tager JM. Biochemistry of peroxisomes. Annu. Rev. Biochem. 1992;61:157–197. doi: 10.1146/annurev.bi.61.070192.001105. [DOI] [PubMed] [Google Scholar]
- 15.Esworthy RS, Ho Y-S, Chu F-F. The Gpx1 gene encodes mitochondrial glutathione peroxidase in the mouse liver. Arch. Biochem. Biophys. 1997;340:59–63. doi: 10.1006/abbi.1997.9901. [DOI] [PubMed] [Google Scholar]
- 16.Flohé L, Loschen G, Gunzler WA, Eichele E, Glutathione peroxidase V. The kinetic mechanism. Hoppe Seylers Z. Physiol. Chem. 1972;353:987–999. doi: 10.1515/bchm2.1972.353.1.987. [DOI] [PubMed] [Google Scholar]
- 17.Makino N, Mochizuki Y, Bannai S, Sugita Y. Kinetic studies on the removal of extracellular hydrogen peroxide by cultured fibroblasts. J. Biol. Chem. 1994;269:1020–1025. [PubMed] [Google Scholar]
- 18.Li T, Singal PK. Adriamycin-induced early changes in myocardial antioxidant enzymes and their modulation by probucol. Circulation. 2000;102:2105–2110. doi: 10.1161/01.cir.102.17.2105. [DOI] [PubMed] [Google Scholar]
- 19.Sazuka Y, Tanizawa H, Takino Y. Effect of adriamycin on the activities of superoxide dismutase, glutathione peroxidase and catalase in tissues of mice. Jpn. J. Cancer Res. 1989;80:89–94. doi: 10.1111/j.1349-7006.1989.tb02250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho Y-S, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, Funk CD. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J. Biol. Chem. 1997;272:16644–16651. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- 21.Xiong Y, Liu X, Lee C-P, Chua BHL, Ho Y-S. Attenuation of doxorubicin-induced contractile and mitochondrial dysfunction in mouse heart by cellular glutathione peroxidase. Free Radic. Biol Med. 2006;41:46–55. doi: 10.1016/j.freeradbiomed.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Lee CP, Sciamanna M, Peterson PL. Intact rat brain mitochondria from a single animal: Preparation and properties. Meth. Toxicol. 1993;2:41–49. [Google Scholar]
- 23.Liu X, Chen Z, Chua CC, Ma YS, Youngberg GA, Hamdy R, Chua BH. Melatonin as an effective protector against doxorubicin-induced cardiotoxicity. Am. J Physiol. Heart Circ. Physiol. 2002;283:H254–H263. doi: 10.1152/ajpheart.01023.2001. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Chua CC, Ho Y-S, Hamdy RC, Chua BH. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2313–H2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 25.Mihm MJ, Coyle CM, Jing L, Bauer JA. Vascular peroxynitrite formation during organic nitrate tolerance. J. Pharmacol. Exp. Ther. 1999;291:194–198. [PubMed] [Google Scholar]
- 26.Ganey PE, Carter LS, Mueller RA, Thurman RG. Doxorubicin toxicity in perfused rat heart. Decreased cell death at low oxygen tension. Cir. Res. 1991;68:1610–1613. doi: 10.1161/01.res.68.6.1610. [DOI] [PubMed] [Google Scholar]
- 27.Pelikan PC, Weisfeldt ML, Jacobus WE, Miceli MV, Bulkley BH, Gerstenblith G. Acute doxorubicin cardiotoxicity: functional, metabolic, and morphologic alterations in the isolated, perfused rat heart. J. Cardiovasc. Pharmacol. 1986;8:1058–1066. [PubMed] [Google Scholar]
- 28.Lambertenghi-Deliliers G, Zanon PL, Pozzoli EF, Bellini O. Myocardial injury induced by a single dose of adriamycin: an electron microscopic study. Tumori. 1976;62:517–528. doi: 10.1177/030089167606200506. [DOI] [PubMed] [Google Scholar]
- 29.Lefrak EA, Rosenheim JS, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302–314. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Childs AC, Phaneuf SL, Dirks AJ, Philips T, Leewenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. 2002;62:4592–4598. [PubMed] [Google Scholar]
- 31.Kawasaki S, Akiyama S, Kurokawa T, Kataoka M, Dohmitsu K, Kondoh K, Yamauchi M, Ito K, Watanabe T, Sugiyama S, Ozawa T, Matsuyama M, Takagi H. Polyoxyethylene-modified superoxide dismutase reduces side effects of adriamycin and mitomycin C. Jpn. J. Cancer Res. 1992;83:899–906. doi: 10.1111/j.1349-7006.1992.tb01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumura M, Nishioka K, Fuijii T, Yoshibayashi M, Nozaki K, Nakata Y, Temma S, Ueda T, Mikawa H. Age-related acute adriamycin cardiotoxicity in mice. J. Mol. Cell. Cardiol. 1994;26:899–905. doi: 10.1006/jmcc.1994.1107. [DOI] [PubMed] [Google Scholar]
- 33.Praet M, Pollakis G, Goormaghtigh E, Ruysschaert JM. Damages of the mitochondrial membrane in adriamycin treated mice. Cancer Lett. 1984;25:89–96. doi: 10.1016/s0304-3835(84)80030-0. [DOI] [PubMed] [Google Scholar]
- 34.Yen H-C, Oberley TD, Gairola CG, Szweda LI, St Clair DK. Manganese superoxide dismutase protects mitochondrial complex I against adriamycin-induced cardiomyopathy in transgenic mice. Arch. Biochem. Biophys. 1999;362:59–66. doi: 10.1006/abbi.1998.1011. [DOI] [PubMed] [Google Scholar]
- 35.Weinstein DM, Mihm MJ, Bauer JA. Cardiac peroxynitrite formation and left ventricular dysfunction following doxorubicin treatment in mice. J. Pharmacol. Exp. Ther. 2000;294:396–401. [PubMed] [Google Scholar]
- 36.Pacher P, Bechkman JS, Liaudet L. Nitric Oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virág L, Deb A, Szabó E, Ungvári Z, Wolin MS, Groves JT, Szabó C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Basic Sci. Rep. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- 38.Pacher P, Liaudet L, Bai P, Virag L, Mabley JG, Haskó G, Szabó C. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J. Pharmacol. Exp. Ther. 2002;300:862–867. doi: 10.1124/jpet.300.3.862. [DOI] [PubMed] [Google Scholar]
- 39.Bai P, Mabley JG, Liaudet L, Virag L, Szabó C, Pacher P. Matrix metalloproteinase activation is an early event in doxorubicin-induced cardiotoxicity. Oncol. Rep. 2004;11:505–508. [PubMed] [Google Scholar]
- 40.Kizaki K, Ito R, Okada M, Yoshioka K, Uchide T, Temma K, Mutoh K, Uechi M, Hara Y. Enhanced gene expression of myocardial matrix metalloproteinases 2 and 9 after acute treatment with doxorubicin in mice. Pharm. Res. 2006;53:341–346. doi: 10.1016/j.phrs.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Wang GW, Klein JB, Kang YJ. Metallothionein inhibits doxorubicin-induced mitochondrial cytochrome c release and caspase-3 activation in cardiomyocytes. J. Pharmacol. Exp. Ther. 2001;298:461–468. [PubMed] [Google Scholar]
- 42.Zhu W, Zou Y, Aikawa R, Harada K, Kudoh S, Uozumi H, Hayashi D, Gu Y, Yamazaki T, Nagai R, Yazaki Y, KomuroI I. MAPK superfamily plays an important role in daunomycin-induced apoptosis of cardiac myocytes. Circulation. 1999;100:2100–2107. doi: 10.1161/01.cir.100.20.2100. [DOI] [PubMed] [Google Scholar]
- 43.Mukhopadhyay P, Bátkai S, Rajesh M, Czifra N, Harvey-White J, Haskó G, Zsengeller Z, Gerard NP, Liaudet L, Kunos G, Pacher P. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J. Am. Coll. Cardiol. 2007;50:528–536. doi: 10.1016/j.jacc.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levrand S, Vannay-Bouchiche C, Pesse B, Pacher P, Fiehl F, Waeber B, Liaudet L. Peroxynitrite is a major trigger of cardiomyocyte apoptosis in vitro and in vivo. Free Rad. Biol. Med. 2006;41:886–895. doi: 10.1016/j.freeradbiomed.2006.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dziegiel P, Murawska-Cialowicz E, Jethon Z, Januszewska L, Podhorska-Okolow M, Surowiak P, Zawadzki M, Rabczynski J, Zabel M. Melatonin stimulates the activity of protective antioxidative enzymes in myocardial cells of rats in the course of doxorubicin intoxication. J Pineal Res. 2003;35:183–187. doi: 10.1034/j.1600-079x.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 46.Pablos MI, Agapito MT, Gutierrez R, Recio JM, Reiter RJ, Barlow-Walden L, Acuna-Castroviejo D, Menendez-Pelaez A. Melatonin stimulates the activity of the detoxifying enzyme glutathione peroxidase in several tissues of chicks. J Pineal Res. 1995;9:111–115. doi: 10.1111/j.1600-079x.1995.tb00178.x. [DOI] [PubMed] [Google Scholar]