Abstract
We hypothesized that interferon-alpha (IFN-α) would enhance the apoptotic activity of bortezomib on melanoma cells. Combined treatment with bortezomib and IFN-α induced synergistic apoptosis in melanoma and other solid tumor cell lines. Apoptosis was associated with processing of procaspases-3, -7, -8, -9, and with cleavage of Bid and PARP. Bortezomib plus IFN-α was effective at inducing apoptosis in melanoma cells that over-expressed Bcl-2 or Mcl-1, suggesting that this treatment combination can overcome mitochondrial pathways of cell survival and resistance to apoptosis. The pro-apoptotic effects of this treatment combination were abrogated by a caspase-8 inhibitor, led to increased association of Fas and FADD prior to the onset of cell death, and were significantly reduced in cells transfected with a dominant-negative FADD construct or siRNA targeting Fas. These data suggest that bortezomib and IFN-α act through the extrinsic pathway of apoptosis via FADD-induced caspase-8 activation to initiate cell death. Finally, bortezomib and IFN-α displayed statistically significant anti-tumor activity as compared to either agent alone in both the B16 murine model of melanoma and in athymic mice bearing human A375 xenografts. These data support the future clinical development of bortezomib and IFN-α for malignant melanoma.
Keywords: interferon-alpha, bortezomib, velcade, proteasome inhibitor, melanoma, apoptosis
INTRODUCTION
Bortezomib is a novel anti-tumor compound that specifically and reversibly inhibits the 26S proteasome. The proteasome plays a critical role in the ordered, temporal degradation of transcription factors, cyclins and cyclin dependent kinase (CDK) inhibitors required for cell cycle progression in malignant and normal cells (1). Bortezomib has in vitro activity against a variety of tumor cell types (2–5). As a single agent, bortezomib has an acceptable toxicity profile and has demonstrated activity in patients with advanced multiple myeloma, non-Hodgkin's lymphoma, mantle cell lymphoma and non-small cell lung cancer (6–9). Bortezomib has also shown some clinical activity in other solid tumors, including advanced renal cell carcinoma (10, 11). Single agent bortezomib was tested in a phase II study of malignant melanoma, however, no responses were achieved when the drug was administered at 1.5 mg/m2 i.v. twice weekly for two weeks out of every three (12). Based on these studies, current research efforts in solid tumors are now focused on the use of bortezomib in combination with other pro-apoptotic agents (13–16).
Recombinant interferon-alpha (IFN-α) has been used in the treatment of malignant melanoma and renal cell carcinoma (RCC) and mediates the regression of metastatic disease in about 10-15% of patients (17, 18). Studies investigating the pro-apoptotic effects of IFN-α in tumor cell lines indicate that this cytokine can activate both the intrinsic and extrinsic pathways of apoptosis (19–24). Of note, IFN-α has been shown to increase the expression of cell cycle regulatory proteins (e.g. p21) in malignant cells, and proteins involved in the death receptor cascade (Fas, TRAIL), thereby sensitizing cells to apoptotic stimuli (25, 26). These observations suggested that IFN-α therapy might enhance the pro-apoptotic effects of proteasome inhibition in the setting of melanoma.
In the present study, we have demonstrated that treatment of melanoma cells with bortezomib and IFN-α synergistically induced apoptotic cell death via FADD-dependent activation of caspase-8. Importantly, this treatment combination could effectively induce apoptosis in cells that over-expressed Bcl-2 or Mcl-1, two relevant pathways of cellular survival and resistance to apoptosis in melanoma cells.
MATERIALS AND METHODS
Cell Lines
The B16F1 (murine), HT144 and A375 (human) melanoma, Kasumi-3 lymphoma and COLO-205 colorectal carcinoma cell lines were purchased from American Type Cell Culture Collection (ATCC, Manassas, VA). The 1259 MEL, 18105 MEL and MEL 39 human melanoma cell lines were obtained from Dr. Soldano Ferrone (Roswell Park Cancer Institute, Buffalo, NY). The JB/MS murine melanoma cell line was obtained from Dr. Vincent Hearing (National Cancer Institute, Bethesda, MD). Renal cell carcinoma cell lines RC-45 and RC-54 were obtained from Dr. Charles Tannenbaum (Cleveland Clinic Foundation, Cleveland, OH).
Reagents
Murine (mu) IFN-α was purchased from Access Biochemical (San Diego, CA). Recombinant human (hu) IFN-α was obtained from Schering-Plough, Inc. (Nutley, NJ). Recombinant human IFN-β was purchased from R & D Systems, Inc. (Minneapolis, MN). Bortezomib (Velcade®, PS-341) was obtained from Millennium Pharmaceuticals Inc, (Cambridge, MA). The irreversible proteasome inhibitor, MG-132 was purchased from Calbiochem, Inc. (San Diego, CA). The pan-caspase inhibitor, Z-VAD-FMK, caspase-8 inhibitor, Z-IETD-FMK, caspase-9 inhibitor, Z-LEHD-FMK, and negative control compound, Z-FA-FMK were purchased from R & D Systems, Inc. The pcDNA3-Bcl-2 vector was provided by Dr. A. Letai (Dana-Farber Cancer Institute, Boston, MA). The pCR3.1-Mcl-1 vector was a gift from Dr. H. Rabinowich (University of Pittsburgh Cancer Institute, Pittsburgh, PA). The FADD-dominant negative (FADD-DN) vector that expresses a truncated form of the FADD protein was provided by Dr. A. Taghiev (University of Iowa) (27). Fas-specific siRNA and negative control siRNA constructs were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Analysis of Apoptosis Via Annexin V/Propidium Iodide (PI) Staining
Phosphatidyl serine exposure was assessed in tumor cells by flow cytometry using APC-Annexin V and propidium iodide (PI; BD Pharmingen, San Diego, CA) as previously described (28). Each analysis was performed utilizing at least 10,000 events.
Immunoblot Analysis
Immunoblots were prepared as previously described and probed with antibodies specific for FADD, Fas, FasL, Bax (Santa Cruz Biotechnology, Santa Cruz, CA), human Bid (a BH3-only member of the Bcl-2 family), Bcl-2, Mcl-1, Bcl-xL, caspase-3, -7, -8, cleaved caspase-7, poly (ADP-ribose) polymerase (PARP) (Cell Signaling Technology), caspase-9 (Upstate Cell Signaling Solutions, San Francisco, CA), Noxa (Calbiochem, San Diego, CA) or β-actin (Sigma) (29). Following incubation with the appropriate horseradishperoxidase-conjugated secondary Ab, immune complexes were detected using the ECL Plus detection kit (Amersham Biosciences, Ayesbury, UK) and analyzed by quantitative densitometry using Optimas 6.51 image analysis software (Media Cybernetics, Carlsbad, CA).
Proliferation Assays
The effects of bortezomib and IFN-α on cell proliferation were tested in murine melanoma cell lines (B16F1, JB/MS), human melanoma cell lines (HT144, MEL 39), and human RCC cell lines (RC-45, RC-54). Cell proliferation was measured as optical density (O.D.) at 570 nm using the MTT Cell Proliferation Assay Kit according to manufacturer's instructions (ATCC, Manassas, VA). All assays were performed in triplicate as previously described (19).
Murine Tumor Models and Treatments
An i.p. tumor model of malignant melanoma was employed to determine whether treatment with the combination of bortezomib and IFN-α was superior in anti-tumor activity to either agent alone (19). Immune-competent, female C57BL/6 mice (n = 11 mice/group; 6-8 weeks of age; Taconic Farms, Inc., Germantown, NY) were injected i.p. on day 0 with 106 B16F1 murine melanoma cells. Beginning the next day mice received PBS, bortezomib (1.0 mg/kg twice weekly i.p.), muIFN-α (2×104 U/d i.p.), or IFN-α and bortezomib combined and monitored for survival. Human xenograft studies were conducted similar to other published reports investigating in vivo activity of bortezomib in athymic mice (5, 16). Briefly, female Balb/cnu/nu (athymic) mice (Taconic Farms, Inc.) were injected subcutaneously (s.c.) in the right flank with 2 × 106 human A375 melanoma cells (Day 0). Once tumors were palpable, mice were randomized to one of four treatment groups: [a] PBS, [b] IFN-α2b (2 × 104 U/d i.p.), [c] bortezomib (1.0 mg/kg twice weekly, s.c. peritumoral), [d] IFN-α and bortezomib combined. Bi-dimensional tumor measurements were obtained twice weekly using microcalipers. The weight of all animals receiving bortezomib or IFN-α and bortezomib combined was monitored for dosing and to assess toxicity throughout the study. Histologic analysis of apoptosis in formalin-fixed paraffin-embedded (FFPE) tumor xenografts was conducted using terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) as recommended by the manufacturer (Chemicon, Inc.). CD31 immunostaining was also used to evaluate angiogenesis in FFPE tumor xenografts. All samples were analyzed in a blinded fashion by an experienced pathologist (Dr. Ray Chaudhury), and the number of cells positive for each marker of interested were quantitated per 10 high-power fields.
Statistical Analysis
Analysis of variance (ANOVA) was used to determine if the two drugs (bortezomib and IFN-α) reduced cell proliferation in a synergistic manner. Regression diagnostics (i.e., residual plots and descriptive statistics) were used to check model assumptions. Median effect analysis was used to analyze the interaction between bortezomib and IFN-α as described by Chou and Talalay (30). The combination index (CI) evaluates dose information for each drug alone, and in combination, at a specified effect level to identify whether two drugs act to induce apoptosis in a synergistic manner (CI<1), an additive manner (CI=1) or an antagonistic fashion (CI>1). Calcusyn software from Biosoft (Cambridge, UK) was used for this multiple drug effect analysis. The analyses for synergistic apoptosis were conducted using data obtained from Annexin V/PI assays (% apoptotic cells), and were performed under the assumption that these drugs had independent modes of action and were therefore mutually non-exclusive. Median survival time in murine experiments was estimated by the Kaplan-Meier method and comparisons overall and between groups were made using the log-rank test. The Holm procedure is a modified Bonferroni type procedure which maintains the experiment-wise error rate and was used to make adjustments for multiple comparisons between dose groups. For human xenograft studies, tumor volume data were log transformed prior to statistical analyses to stabilize the variance of the measures and improve normality. Tumor volume data between the four groups on Day 25 were compared via ANOVA with a Tukey-Kramer adjustment for multiple comparisons. A mixed effects model was used to analyze the repeated volume measurements while adjusting for the correlation within mice. All analyses were performed in SAS (version 9.1, SAS Institute, Cary, NC). Adjusted p-values were considered significant at the 0.05 level and all tests were two-sided.
RESULTS
Treatment of Melanoma and RCC Cell Lines with Bortezomib and IFN-α Leads to Enhanced Apoptotic Cell Death
The ability of both bortezomib and IFN-α to induce tumor cell apoptosis when administered as single agents led us to examine the effects of combined therapy (3, 20). Human and murine melanoma cells were treated for 48 hours with PBS, bortezomib, IFN-α or both agents combined. Bortezomib in combination with IFN-α led to significantly increased levels of apoptosis as measured by Annexin V/PI staining compared to either agent alone in human melanoma (HT144, A375, 1259 MEL, 18105 MEL, MEL 39), renal cell carcinoma (RC-45, RC-54), colorectal carcinoma (COLO-205), lymphoma (Kasumi-3), and in multiple murine melanoma cell lines (JB/MS, B16F1; Figure 1A; Supplementary Figure S1A and data not shown; p<0.05). An irreversible proteasome inhibitor (MG-132) also induced synergistic apoptosis when combined with IFN-α (not shown). Time course experiments conducted in the B16F1 cell line revealed that apoptosis began at approximately 24 hours and reached maximal levels between 48 and 72 hours (Supplementary Figure S1B and data not shown). The order of treatment did not appear to be critical as cells treated with IFN-α separately followed by bortezomib separately or conversely, bortezomib followed by IFN-α displayed comparable cell death (Supplementary Figure S1C). Consistent with our previous observations, cells treated simultaneously with both agents displayed the greatest level of apoptosis. Bortezomib plus IFN-α induced apoptosis in melanoma cell lines with both functional p53 (A375, 18105 MEL, HT144) and in cell lines with a p53-mutant phenotype (SK-MEL-2) and did not induce apoptosis in normal peripheral blood mononuclear cells (data not shown). Treatment of human (HT144, MEL 39) and murine (B16F1, JB/MS) melanoma cell lines with bortezomib plus IFN-α also led to a significant reduction in cell proliferation as compared to either agent alone (Figure 1B; ANOVA interaction p<0.001). Of note, enhanced apoptosis of human melanoma cells was observed following treatment with bortezomib and IFN-β but not in response to bortezomib and IFN-γ (Supplementary Figure S2A-C).
Fig. 1. Bortezomib and IFN-α induce apoptosis in melanoma and renal cell carcinoma cell lines.
(A) The pro-apoptotic effects of IFN-α (104 U/mL), bortezomib (10 nM), or both agents combined were evaluated in human and murine melanoma cell lines (A375 and B16F1) respectively and in human RCC cell lines (RC-54) at 48 hours post-treatment. (B) Human and murine melanoma cell lines were cultured with bortezomib (B; 10 nM), IFN-α (104 U/ml) or both agents combined for 48 hours. Cells treated with PBS served as negative controls in each assay. Cell proliferation was measured as optical density (O.D.) at 570 nm using an MTT cell proliferation assay. Error bars denote the standard deviations of triplicate wells, and graphs are representative of data obtained from at least 3 individual experiments. (C) Synergistic induction of apoptosis in HT144 melanoma cells by bortezomib plus IFN-α. HT144 cells were treated for 48 hours with various doses of bortezomib alone (0.625 - 80 nM), IFN-α alone (0.625 × 103 − 80 × 103 U/mL) or in combination and examined for apoptosis via Annexin V/PI staining. This data was used to calculate the median effect plots. The fraction of cells undergoing apoptosis, or ‘effect’ is presented on the y-axis. The median effect data presented were derived from n = 3 individual experiments and have been averaged. ♦ = Bortezomib + IFN-α; ▲ = Bortezomib; ■ = IFN-α. (D) Combination indices (CI) are presented for HT144 melanoma cells following treatment with bortezomib and IFN-α combined at four doses that demonstrated synergy (5 - 80 nM bortezomib + 5 × 103 − 80 × 103 IFN-α). Data shown were derived from n = 3 individual experiments, where each data point represents an individual experiment. The combination index plots generated present CI values with their respective fraction affected levels for the four dose level combinations of IFN-α and bortezomib.
Apoptosis Induced by Bortezomib and IFN-α is Synergistic
Using the median effect method we next assessed if bortezomib and IFN-α induced synergistic apoptosis (31). HT144 melanoma cells were treated for 48 hours with various doses of bortezomib, IFN-α, or both agents combined (5 dose combinations) and then analyzed for apoptosis via Annexin V-PI staining. Dose-effect plots indicated that increasing the dosage of IFN-α had little effect on the induction of apoptosis, while bortezomib treatment resulted in dose-dependent increases in apoptosis (Figure 1C). At each paired dose, the pro-apoptotic effect was further enhanced following combined treatment with bortezomib plus IFN-α (Figure 1C). Dose combinations of 5 nM bortezomib and 5 × 103 U/mL IFN-α or greater resulted in combination indices consistently below 1.0, suggesting a dose-dependent, synergistic effect on apoptosis of HT144 melanoma cells (Figure 1C-D).
Combined Treatment With Bortezomib and IFN-α Induces Processing of Effector Caspases and poly (ADP-ribose) polymerase (PARP)
Enhanced processing of the major effector caspases (caspase-3, and -7) to their active forms and cleavage of poly (ADP-ribose) polymerase (PARP; a target of activated effector caspases) was observed at 48 hours following treatment of the HT144 human melanoma cell line with bortezomib plus IFN-α (Figure 2A). This pattern of caspase activation and cleavage of PARP was reproducible in multiple human melanoma cell lines (1259 MEL, A375, HT144, 18105 MEL, MEL 39; Supplementary Figure S3 and data not shown). Enhanced cleavage of caspase-3 in the human HT144 and murine B16F1 melanoma cell line following dual treatment was confirmed by flow cytometry (Figure 2B and data not shown).
Fig. 2. Treatment with bortezomib and IFN-α results in cleavage of caspases-3, -7, and PARP.
(A) The HT144 human melanoma cell line was treated for 48 hours with PBS (P), IFN-α (α; 104 U/mL), bortezomib (B; 5 nM or 10 nM) or both agents combined (C) and evaluated by immunoblot analysis for caspase-3, caspase-7, cleaved caspase-7, and cleaved PARP (arrows). Membranes were probed with an anti-β-actin Ab as a loading control. Similar results were obtained in multiple human melanoma cell lines (A375, 18105 MEL, 1259 MEL). (B) Levels of cleaved caspase-3 were measured by flow cytometry with a FITC-conjugated rabbit anti-active caspase-3 mAb (Asp175; Cell Signaling Technology) in the HT144 melanoma cell line 48 hours following treatment. Shaded histograms represent staining with cleaved caspase-3 Ab. Voltage was set based on appropriate isotype control Abs (M1). The percentage of cells positive for cleaved caspase-3 is given in each histogram. (C) Inhibition of caspase activation reverses the pro-apoptotic effects of bortezomib and IFN-α treatment. HT144 cells were treated with PBS, IFN-α (104 U/mL), bortezomib (10 nM) or both agents combined in the presence of the pancaspase inhibitor Z-VAD-FMK or a negative control compound (Z-FA-FMK) at a 50 μM concentration for 48 hours.
Bortezomib and IFN-α induce caspase-dependent apoptosis
We next sought to determine the dependence of cell death on caspase activation. HT144 human melanoma cells were treated with PBS, IFN-α, bortezomib or both agents combined in the presence of either the pan-caspase inhibitor Z-VAD-FMK (50 μM) or Z-F-A-FMK (negative control). The pan-caspase inhibitor Z-VAD-FMK significantly inhibited apoptotic cell death at the 48 hour time point in response to bortezomib plus IFN-α (mean % of Annexin V-positive cells = 10.08% ± 3.37; n = 3 experiments, (p = 0.04) as compared to similarly treated cells cultured in the presence of the negative control compound (mean % of Annexin V-positive cells = 35.84% ± 13.85; Figure 2C and Supplementary Figure S4). These data suggest that the synergistic death induced by bortezomib and IFN-α is dependent on caspase activation.
Bortezomib and IFN-α can overcome elevated expression of pro-survival proteins and promote apoptosis in melanoma cells
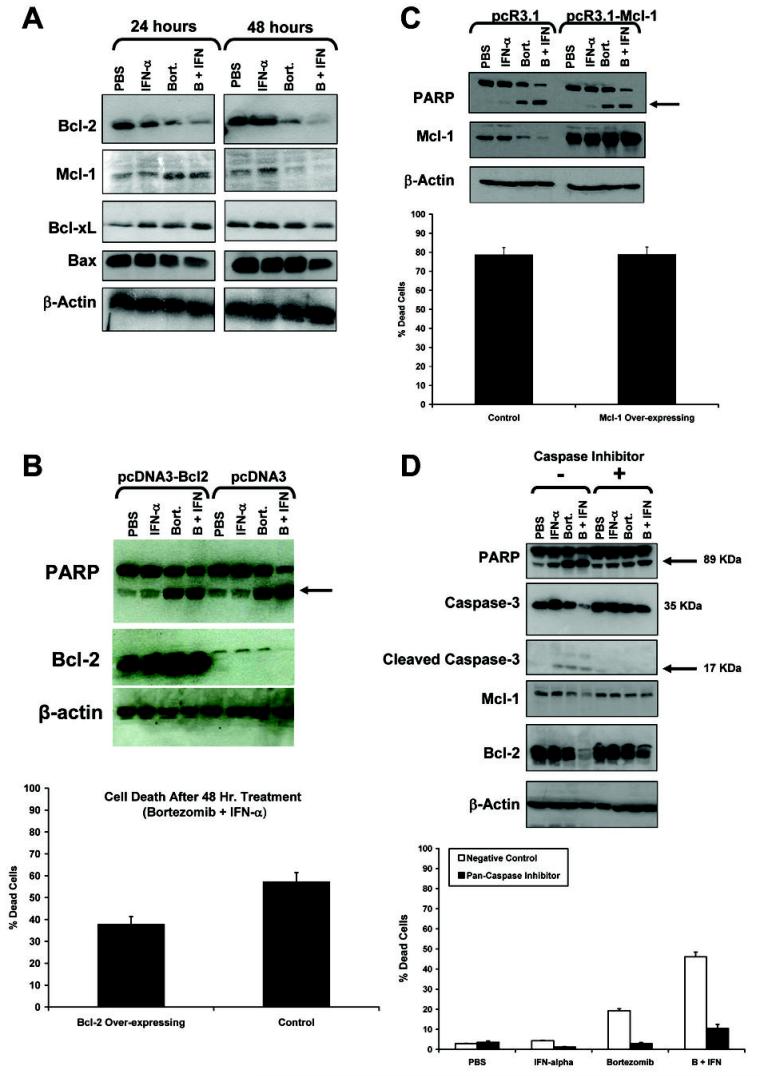
The Bcl-2 family of mitochondrial proteins inhibit apoptosis by stabilizing the mitochondrial membrane (32) and down-regulation of these proteins has been shown to contribute to the induction of caspase-mediated apoptosis in response to bortezomib (33). Mcl-1 promotes cell survival through its antagonistic interactions with the pro-apoptotic protein Bax, while Bcl-2 and Bcl-xL have been shown to directly antagonize Bak (34). The Bcl-2 and Mcl-1 proteins are often over-expressed in melanoma and have been shown to affect the susceptibility of melanoma cells to apoptotic stimuli in other systems (35–37). Decreased levels of Bcl-2 and Mcl-1 were also observed following treatment of melanoma cell lines with bortezomib and IFN-α (Figure 3A). In contrast, Bcl-xL and Bax expression was not affected by treatment with bortezomib or bortezomib plus IFN-α (Figure 3A). The ability of this treatment combination to stimulate processing of effector caspases and down-regulate levels of pro-survival Bcl-2 and Mcl-1 proteins initially suggested that bortezomib and IFN-α might promote the intrinsic pathway of apoptosis by modulation of protein targets within the mitochondria. To further test the importance of the intrinsic pathway in mediating apoptosis, and to determine whether this treatment combination could induce apoptosis in the presence of high Bcl-2 levels, Bcl-2 was over-expressed in the human A375 melanoma cell line. Twenty-four hours following transient transfection with either the pcDNA3-Bcl-2 vector or pcDNA3 control vector, cells were treated for an additional 48 hours with PBS, bortezomib, IFN-α or both agents combined. Cells transfected with the unmodified pcDNA3 vector displayed 60% cell death and a characteristic decrease in Bcl-2 protein expression 48 hours following treatment with bortezomib plus IFN-α (Figure 3B). However, melanoma cells transfected with the pcDNA3- Bcl-2 construct remained sensitive to the pro-apoptotic effects of bortezomib plus IFN-α, as indicated by the cleavage of PARP (arrow; lanes 4 and 8). Also, cell death as measured by trypan blue exclusion, was only slightly reduced at 48 hours, despite the presence of high levels of Bcl-2 at all time points in all four conditions.
Fig. 3. Bortezomib and IFN-α induce apoptosis in melanoma cells that over-express Bcl-2 and Mcl-1.
(A) Combined treatment with bortezomib (10 nM) plus IFN-α (104 U/mL) results in decreased levels of Bcl-2 and Mcl-1 and no change in the expression of Bcl-xL and Bax. A375 melanoma cells were treated with PBS (P), IFN-α (α; 104 U/mL), bortezomib (B; 20nM) or both agents combined (C) 24 hours following transient transfection with the (B) pcDNA3-Bcl-2 construct, (C) pcR3.1-Mcl-1 construct or empty vector (negative control). Lysates were evaluated for Bcl-2 expression and cleavage of PARP via immunoblot analysis. Viability of cells used to make lysates in each experiment was evaluated using trypan blue staining. Transfection efficiency in all experiments was typically > 90% as determined by transfection of A375 cells with a green fluorescent protein (GFP)-expression vector in parallel. All blots shown are representative of n = 3 experiments with similar results. Error bars are derived from n = 3 separate experiments in the A375 melanoma cell line. Comparable data were obtained using cells stably transfected with either construct following selection of transfected clones for 7 days with G418 (data not shown). (D) Decreased levels of Bcl-2 and Mcl-1 are the result of caspase-activation. A375 cells were treated PBS (P), IFN-α (α; 104 U/mL), bortezomib (B; 10nM) or both agents combined (C) in the presence of the pan-caspase inhibitor Z-VAD-FMK or a negative control compound (Z-FA-FMK) at a 50 μM concentration for 48 hours. Cell lysates were analyzed by immunoblot analysis for caspase-3 cleavage, and for Mcl-1 and Bcl-2 expression. Membranes were also probed with an anti-β-actin Ab to control for loading. Arrows denote cleaved forms of caspase-3 or PARP. Viability of cells used to make lysates for immunoblot analysis in each experiment was evaluated using trypan blue staining.
Experiments using the pCR3.1-Mcl-1 vector indicated that melanoma cells over-expressing Mcl-1 also remained sensitive to the pro-apoptotic effects of bortezomib and IFN-α as compared to cells transfected with the negative control vector (Figure 3C). Interestingly, Bcl-2 and Mcl-1 levels did not decrease when cells were treated with bortezomib and IFN-α in the presence of a pan-caspase inhibitor (Figure 3D). These results indicated that the observed reductions in levels of Bcl-2 and Mcl-1 were likely due to the cleavage of these proteins by activated caspases and were not due to a direct modulation of Bcl-2 or Mcl-1 by bortezomib and IFN-α. Taken together, these data suggest that bortezomib and IFN-α may be an effective means of promoting cell death in cells that have developed resistance to apoptosis due to elevated expression of pro-survival proteins.
Bortezomib and IFN-α induce apoptosis via FADD-mediated caspase-8 activation
Our previous data suggested that bortezomib and IFN-α may induce apoptosis of melanoma cells through a mechanism that is independent of the mitochondrial, intrinsic pathway of apoptosis. We therefore investigated the role of the extrinsic pathway of apoptosis in mediating bortezomib and IFN-α-induced cell death. While caspase-9 is activated via the mitochondrial pathway of apoptosis, the extrinsic pathway of apoptosis involves processing of pro-caspase-8 and subsequent cleavage of Bid (a pro-apoptotic BH3-only protein) in response to the binding of death receptors by their cognate ligands (e.g. TRAIL, TNF-α, FasL) (38). Immunoblot analysis revealed that treatment of human melanoma cell lines with bortezomib plus IFN-α led to enhanced processing of initiator caspase-8, caspase-9 and reduced levels of native Bid, a proapoptotic BH3-only protein (Figure 4A). The caspase-9 inhibitor Z-LEHD-FMK did not significantly inhibit apoptosis of melanoma cells in response to bortezomib and IFN-α (data not shown) however, the caspase-8 inhibitor Z-IETD-FMK significantly inhibited cell death at the 48 hour time point (mean % of Annexin V-positive cells = 20%± 5.4; n = 3 experiments) when compared to cells cultured in the presence of a negative control inhibitor (mean % of Annexin V-positive cells = 71% ± 4.2; p=.004) (Figure 4B). These data suggest that caspase-8 is activated first in response to treatment with bortezomib and IFN-α and that caspase-9 is processed in a secondary fashion by activated caspase-8. Interestingly, no detectable levels of TNF-α, TRAIL or FasL protein were evident in cell culture supernatants from melanoma cell lines following treatment with bortezomib and IFN-α (data not shown). While FasL and Fas proteins were detectable in whole cell lysates from untreated melanoma cell lines, treatment with bortezomib and IFN-α did not alter the expression level of these proteins in any cell line tested (data not shown).
Fig. 4. Bortezomib and IFN-α act via FADD-induced caspase-8 activation to initiate cell death.

(A) Cell lysates were evaluated by immunoblot analysis for processing of pro-caspase-8 (55 kDa) into its cleaved 43 kDa and 41 kDa fragments, pro-caspase-9 (47 kDa) into its cleaved 37 kDa and 35 kDa fragments, and for levels of native Bid protein (22 kDa) following a 48 hour treatment with PBS, IFN-α (104 U/mL), bortezomib (10nM) or both agents combined. (B) A375 cells were treated with PBS, IFN-α, bortezomib or both agents combined in the presence of the Z-IETD-FMK caspase-8 inhibitor or Z-FA-FMK negative control compound at a 50 μM concentration for 48 hours. (C) Fas and FADD associate prior to bortezomib and IFN-α induced apoptosis. Following a 16 hour treatment with PBS, IFN-α, bortezomib or both agents combined, cell lysates were immunoprecipitated with an anti-Fas antibody, and blots were probed with an anti-FADD antibody (or anti-Fas antibody to control for loading). Comparable data were obtained in 1259 MEL, 18105 MEL and A375 cell lines. (D) Bortezomib and IFN-α induced apoptosis is inhibited by a FADD-DN construct. Twenty-four hours following transient transfection with the pcDNA3-FADD-DN or pcDNA3 vectors, A375 melanoma cells were treated for an additional 48 hours with PBS, IFN-α, bortezomib or both agents combined. Lysates were evaluated for FADD expression (or its lower molecular weight, truncated form) and cleavage of PARP as a marker of apoptosis via immunoblot analysis. Viability of cells used to make lysates for immunoblot analysis in each experiment was evaluated using trypan blue staining.
Importantly, immunoprecipitation of cell lysates with an anti-Fas Ab followed by immunoblot analysis with an anti-FADD Ab revealed that there was a significant increase in the association between these two proteins prior to the onset of cell death following a 16 hour co-treatment with bortezomib and IFN-α (Figure 4C). The association of Fas and FADD and subsequent activation of caspase-8 and induction of apoptosis can occur via both FasL-dependent and FasL- independent mechanisms (39). Importantly, bortezomib and IFN-α were significantly less effective at inducing apoptosis in melanoma cells transfected with a dominant negative FADD construct that lacks the death effector domain, and therefore cannot bind pro-caspase-8 (FADDDN; Figure 4D). Similarly, siRNA-mediated reduction of cellular Fas inhibited cell death mediated via bortezomib and IFN-α (Supplementary Figure S5). We also observed a decrease in the basal expression of FADD in cells treated with bortezomib and IFN-α at the 48 hour time point. This event is likely due to non-specific cleavage of intracellular proteins by activated caspases as was observed for Bcl-2 and Mcl-1 (Figure 3D). Together these data suggest that bortezomib and IFN-α activate caspase-8 and induce apoptosis following the association of Fas and FADD.
Combined Administration of Bortezomib and IFN-α Prolongs Survival in a Murine Model of Malignant Melanoma
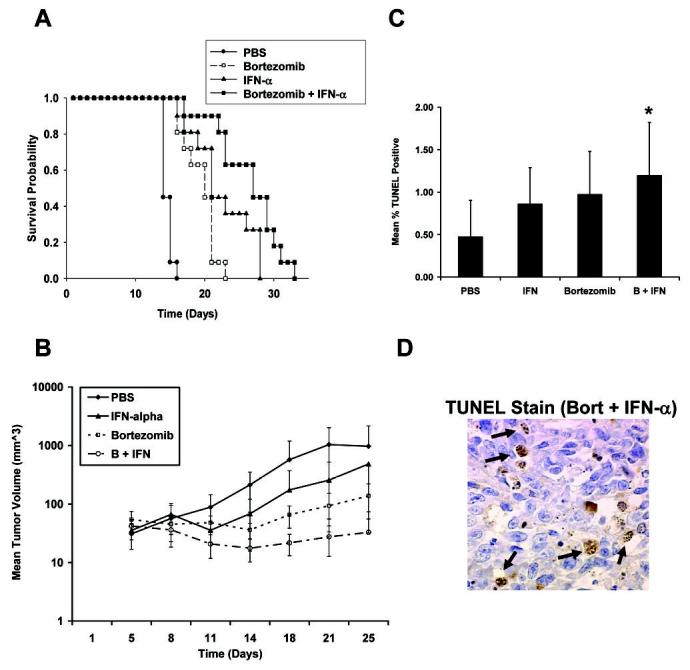
The effects of combined treatment with bortezomib and IFN-α were studied in an intraperitoneal (i.p.) murine model of B16 malignant melanoma in which immune competent mice routinely succumb to fatal disease burden after 12-14 days (19). Daily i.p. administration of IFN-α alone led to a significant improvement in survival of tumor bearing mice compared to mice treated with PBS alone [PBS median survival = 15 days (95% CI = 14-15); IFN-α median survival = 21 days (95% CI = 17-28+; p = 0.02)]. Mice receiving bortezomib as a single agent (twice weekly) also displayed a significant improvement in survival compared to PBS-treated mice [bortezomib median survival = 21 days (95% CI = 17-21; p < 0.01)]. However, the median survival of mice treated with bortezomib and IFN-α combined was significantly enhanced when compared to mice treated with PBS (p<0.001), IFN-α (p = 0.02) or bortezomib alone (p<0.001) [bortezomib plus IFN-α median survival 27 days (95% CI = 22-30)] (Figure 5A). No remarkable alterations in the weight or behavior of tumorbearing mice were evident when IFN-α was administered with bortezomib (Supplementary Figure S6).
Fig. 5.
(A) Treatment with bortezomib plus IFN-α enhances survival in a murine model of malignant melanoma. B16F1 cells (106) were injected i.p. into C57BL/6 mice (n = 11 per treatment group). One day following tumor challenge, mice were treated intraperitoneally with either PBS (negative control), bortezomib alone (1 mg/kg, twice weekly), IFN-α alone (2 × 104 U/daily), or a combination of bortezomib and IFN-α. (B) Treatment with bortezomib plus IFN-α inhibits the growth of human melanoma xenografts in athymic mice. Human A375 cells (2 × 106) were injected s.c. into Balb/cnu/nu mice (n = 4-6 per group). Mice were treated with PBS, bortezomib, IFN-α or both agents combined. Data represents the mean tumor volume within each group (mm3) while the error bars represent the standard deviation between individual animals. (C) Mice were euthanized on Day 25 whereupon tumors were harvested and stained by TUNEL, and percentages of apoptotic cells were quantified manually. Columns, mean (n = 4-6 per group); bars, standard deviation. *, p = 0.038 versus PBS-treated controls. (D) A representative tumor section from mice treated with Bortezomib and IFN-α combined is shown with arrows depicting TUNEL+ cells used for quantitation (400X magnification).
A tumor xenograft model of human A375 melanoma in Balb/cnu/nu mice was also employed to evaluate the anti-tumor effects of this treatment combination in vivo. Administration of IFN-α2b or bortezomib as single agents led to a significant inhibition of tumor growth as compared to control mice treated with PBS (p < 0.05). Co-administration of bortezomib and IFN-α2b to A375-bearing animals also led to a statistically significant inhibition of tumor growth as compared to either agent alone (p = 0.047 vs. bortezomib; p = 0.0004 vs. IFN-α; Figure 5B). Histologic analysis revealed significantly greater apoptosis in tumors derived from mice treated with bortezomib and IFN compared with tumors from PBS-treated mice as determined by TUNEL staining (p = 0.038; ANOVA; Figure 5C-D). No significant differences in the percentage of TUNEL-positive cells were observed in tumors from combination treated mice as compared to mice treated with IFN-α or bortezomib as single agents (p's > 0.05). Prior studies have demonstrated that combined treatment with bortezomib and IFN-α2b led to inhibition of angiogenesis in athymic mice bearing human bladder cancer tumors (5). However, no difference in the microvessel density (MVD) of tumors as detected by CD31 immunostaining was observed in any treatment group of the present study (Supplementary Figure S7).
DISCUSSION
We have demonstrated that bortezomib and IFN-α induce synergistic apoptosis in human melanoma cell lines and that cell death was dependent upon caspase activation. The proapoptotic effects of this treatment combination were significantly reduced following inhibition of caspase-8 and in the presence of a dominant negative form of the FADD protein or Fas-specific siRNA. Combined treatment with bortezomib and IFN-α also led to a significant prolongation of survival in a murine model of melanoma, and significantly reduced tumor growth in a xenograft model of human melanoma in athymic mice as compared to either agent alone. The present study demonstrates that this treatment combination may have utility for the treatment of melanoma.
Our initial experiments showed that treatment of melanoma cell lines with bortezomib and IFN-α led to processing of pro-caspase-9 and decreased levels of Bcl-2 and Mcl-1. These data suggested that the mitochondrial pathway could be involved in promoting apoptosis in response to this treatment combination (40). Previous studies by Nencioni et al. have eloquently demonstrated that apoptosis induced by proteasome inhibition and TRAIL may involve both the cleavage of Bcl-2 to a fragment with putative pro-apoptotic activity and the elimination of anti-apoptotic Mcl-1 (33). However, Bcl-2 and Mcl-1 protein levels remained stable in the presence of a caspase inhibitor and over-expression of these pro-survival proteins or caspase-9 inhibition did not protect melanoma cells from the apoptotic program induced by bortezomib plus IFN-α. Therefore, reduced levels of these proteins in this panel of cell lines post-treatment were likely the result of non-specific cleavage by activated caspase proteins, rather than an initiating event of the apoptotic program. The activation of caspase-9 and decreased expression levels of Bcl-2 and Mcl-1 in this system likely serves to amplify the process of apoptosis once it has been initiated.
We subsequently hypothesized that this treatment combination might lead to increased production of death receptor ligands (i.e. TRAIL, TNF-α, FasL) by the melanoma cells. Previous reports have shown either bortezomib or IFN-α can activate the extrinsic pathway of apoptosis in this manner (41). In addition, Papageorgiou et al. have demonstrated that combined therapy with IFN-α and bortezomib enhances apoptosis of human bladder cancer cell lines by a TRAIL-associated mechanism (5). Although treatment with a caspase-8 inhibitor led to a significant decrease in cell death in the present study, the endogenous expression of TRAIL, TNF-α, or FasL was not observed in either culture supernatants or lysates from melanoma cells treated with bortezomib and IFN-α (data not shown). Furthermore, previous studies have shown that TRAIL expression is not induced in A375 melanoma cells following treatment with type I IFNs (42), but A375 cells remained sensitive to the pro-apoptotic effects of combined therapy with bortezomib and IFN-α.
Despite the absence of a defined death receptor ligand, we observed an increased association between Fas and FADD in multiple melanoma cell lines prior to the onset of cell death. Furthermore, transfection of A375 melanoma cells with a FADD-DN construct or Fas-specific siRNA significantly inhibited the pro-apoptotic effects of bortezomib and IFN-α. Although increased binding between Fas and FasL can activate caspase-8 and initiate the apoptotic process (43), other reports have suggested that processing of pro-caspase-8 can be a downstream consequence of Bid cleavage (44). However, the timing of Bid cleavage in the present model does not support this conclusion. Our data suggest that caspase-8 activation and subsequent apoptosis is initiated by Fas trimerization and its association with FADD independent of FasL (39). The precise molecular events leading to an association between Fas and FADD proteins in the present model are under investigation.
The anti-tumor properties of bortezomib have been attributed to inhibition of IκB degradation, which leads to inactivation of NF-κB, a pro-survival transcription factor that is constitutively activated in many melanoma cell lines (16, 45, 46). However, our preliminary data indicated that bortezomib did not decrease the levels of NF-BκDNA binding or transcriptional activity in melanoma cell lines as measured by gel shift assay or analysis of NF-κB reporter activity in cells transfected with the 3xκB-Luc NF-κB promoter luciferase construct (data not shown). In addition, other groups have recently shown that inhibition of NF-κB activity accounts for only a portion of the anti-tumor activity of bortezomib (47, 48). Previous studies by Qin et al. and Fernandez et al. have also described a role for the BH3-only protein Noxa in mediating the proapoptotic effects of proteasome inhibition in melanoma cell lines (49, 50). However, we did not detect an increase in Noxa transcript or protein in these particular melanoma cell lines following treatment with bortezomib plus IFN-α as compared to bortezomib alone (Supplementary Figure S8). These observations suggest that the pro-apoptotic effects of bortezomib may proceed by different mechanisms depending on the melanoma cell line under study and are augmented in a unique manner when co-administered with IFN-α.
Together these pre-clinical data suggest that combined treatment with bortezomib and IFN-α represents a novel treatment strategy for inducing a direct, pro-apoptotic effect on tumor cells. We are currently evaluating the safety and tolerability of this treatment combination in an investigator-initiated phase I clinical trial at our institution. Elucidation of the pathways of cell death induced by this treatment combination may help to identify key molecular targets that govern the viability of melanoma cells that can be further manipulated to provide an anti-tumor effect.
ACKNOWLEDGMENTS
We thank John Byrd, M.D. for critical review of this manuscript, and The OSU CCC Analytical Cytometry, Histology and Biostatistics Shared Resources.
Grant Support: The Harry J. Lloyd Charitable Trust, The Melanoma Research Foundation, The Valvano Foundation for Cancer Research Award (to G.B. Lesinski), National Institutes of Health (NIH) Grants CA84402, K24-CA93670 (to W.E. Carson), P30-CA16058, P01-CA95426 (to M.A. Caligiuri), The Valvano Foundation for Cancer Research Award (to W.E. Carson), and Millennium Pharmaceuticals, Inc., and Johnson & Johnson Pharmaceutical Research & Development, L.L.C.
Supplementary Material
REFERENCES
- 1.Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–22. [PubMed] [Google Scholar]
- 2.Adams J. Preclinical and clinical evaluation of proteasome inhibitor PS-341 for the treatment of cancer. Curr Opin Chem Biol. 2002;6:493–500. doi: 10.1016/s1367-5931(02)00343-5. [DOI] [PubMed] [Google Scholar]
- 3.Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–6. [PubMed] [Google Scholar]
- 4.Khan T, Stauffer JK, Williams R, et al. Proteasome inhibition to maximize the apoptotic potential of cytokine therapy for murine neuroblastoma tumors. J Immunol. 2006;176:6302–12. doi: 10.4049/jimmunol.176.10.6302. [DOI] [PubMed] [Google Scholar]
- 5.Papageorgiou A, Kamat A, Benedict WF, Dinney C, McConkey DJ. Combination therapy with IFN-alpha plus bortezomib induces apoptosis and inhibits angiogenesis in human bladder cancer cells. Mol Cancer Ther. 2006;5:3032–41. doi: 10.1158/1535-7163.MCT-05-0474. [DOI] [PubMed] [Google Scholar]
- 6.Fanucchi MP, Fossella FV, Belt R, et al. Randomized phase II study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:5025–33. doi: 10.1200/JCO.2006.06.1853. [DOI] [PubMed] [Google Scholar]
- 7.Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127:165–72. doi: 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa M, Yu WG, Umehara K, et al. Multiple roles of interferon-gamma in the mediation of interleukin 12-induced tumor regression. Cancer Res. 1998;58:2426–32. [PubMed] [Google Scholar]
- 9.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–98. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 10.Davis NB, Taber DA, Ansari RH, et al. Phase II trial of PS-341 in patients with renal cell cancer: a University of Chicago phase II consortium study. J Clin Oncol. 2004;22:115–9. doi: 10.1200/JCO.2004.07.165. [DOI] [PubMed] [Google Scholar]
- 11.Kondagunta GV, Drucker B, Schwartz L, et al. Phase II trial of bortezomib for patients with advanced renal cell carcinoma. J Clin Oncol. 2004;22:3720–5. doi: 10.1200/JCO.2004.10.155. [DOI] [PubMed] [Google Scholar]
- 12.Markovic SN, Geyer SM, Dawkins F, et al. A phase II study of bortezomib in the treatment of metastatic malignant melanoma. Cancer. 2005;103:2584–9. doi: 10.1002/cncr.21108. [DOI] [PubMed] [Google Scholar]
- 13.Nawrocki ST, Sweeney-Gotsch B, Takamori R, McConkey DJ. The proteasome inhibitor bortezomib enhances the activity of docetaxel in orthotopic human pancreatic tumor xenografts. Mol Cancer Ther. 2004;3:59–70. [PubMed] [Google Scholar]
- 14.Cusack JC, Liu R, Houston M, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001;61:3535–40. [PubMed] [Google Scholar]
- 15.Bold RJ, Virudachalam S, McConkey DJ. Chemosensitization of pancreatic cancer by inhibition of the 26S proteasome. J Surg Res. 2001;100:11–7. doi: 10.1006/jsre.2001.6194. [DOI] [PubMed] [Google Scholar]
- 16.Amiri KI, Horton LW, LaFleur BJ, Sosman JA, Richmond A. Augmenting chemosensitivity of malignant melanoma tumors via proteasome inhibition: implication for bortezomib (VELCADE, PS-341) as a therapeutic agent for malignant melanoma. Cancer Res. 2004;64:4912–8. doi: 10.1158/0008-5472.CAN-04-0673. [DOI] [PubMed] [Google Scholar]
- 17.Bukowski RM. Cytokine therapy for metastatic renal cell carcinoma. Semin Urol Oncol. 2001;19:148–54. [PubMed] [Google Scholar]
- 18.Lens MB, Dawes M. Interferon alfa therapy for malignant melanoma: a systematic review of randomized controlled trials. J Clin Oncol. 2002;20:1818–25. doi: 10.1200/JCO.2002.07.070. [DOI] [PubMed] [Google Scholar]
- 19.Lesinski GB, Anghelina M, Zimmerer J, et al. The anti-tumor effects of interferon-alpha are abrogated in a STAT1-deficient mouse. J Clin Invest. 2003;112:170–80. doi: 10.1172/JCI16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thyrell L, Erickson S, Zhivotovsky B, et al. Mechanisms of Interferon-alpha induced apoptosis in malignant cells. Oncogene. 2002;21:1251–62. doi: 10.1038/sj.onc.1205179. [DOI] [PubMed] [Google Scholar]
- 21.Kelly JD, Dai J, Eschwege P, et al. Downregulation of Bcl-2 sensitises interferon-resistant renal cancer cells to Fas. Br J Cancer. 2004;91:164–70. doi: 10.1038/sj.bjc.6601895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panaretakis T, Pokrovskaja K, Shoshan MC, Grander D. Interferon-alpha-induced apoptosis in U266 cells is associated with activation of the proapoptotic Bcl-2 family members Bak and Bax. Oncogene. 2003;22:4543–56. doi: 10.1038/sj.onc.1206503. [DOI] [PubMed] [Google Scholar]
- 23.Papageorgiou A, Lashinger L, Millikan R, et al. Role of tumor necrosis factor-related apoptosis-inducing ligand in interferon-induced apoptosis in human bladder cancer cells. Cancer Res. 2004;64:8973–9. doi: 10.1158/0008-5472.CAN-04-1909. [DOI] [PubMed] [Google Scholar]
- 24.Chawla-Sarkar M, Leaman DW, Jacobs BS, Borden EC. IFN-beta pretreatment sensitizes human melanoma cells to TRAIL/Apo2 ligand-induced apoptosis. J Immunol. 2002;169:847–55. doi: 10.4049/jimmunol.169.2.847. [DOI] [PubMed] [Google Scholar]
- 25.Bearzatto A, Orlandi L, De Marco C, Daidone MG, Zaffaroni N. Lack of p21waf1 and p27kip1 protein induction by interferon-alpha2a in human melanoma cell lines. Melanoma Res. 1999;9:457–63. doi: 10.1097/00008390-199910000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y, Wang S, Yue BG, Gobl A, Oberg K. Effects of interferon alpha on the expression of p21cip1/waf1 and cell cycle distribution in carcinoid tumors. Cancer Invest. 2002;20:348–56. doi: 10.1081/cnv-120001180. [DOI] [PubMed] [Google Scholar]
- 27.Guseva NV, Taghiyev AF, Rokhlin OW, Cohen MB. Contribution of death receptor and mitochondrial pathways to Fas-mediated apoptosis in the prostatic carcinoma cell line PC3. Prostate. 2002;51:231–40. doi: 10.1002/pros.10095. [DOI] [PubMed] [Google Scholar]
- 28.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 29.Kuzumaki T, Kobayashi T, Ishikawa K. Genistein induces p21(Cip1/WAF1) expression and blocks the G1 to S phase transition in mouse fibroblast and melanoma cells. Biochem Biophys Res Commun. 1998;251:291–5. doi: 10.1006/bbrc.1998.9462. [DOI] [PubMed] [Google Scholar]
- 30.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 31.Chou TC. Relationships between inhibition constants and fractional inhibitions in enzyme-catalyzed reactions with different numbers of reactants, different reaction mechanisms, and different types of mechanisms of inhibition. Mol Pharmacol. 1974;10:235–47. [PubMed] [Google Scholar]
- 32.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 33.Nencioni A, Wille L, Dal Bello G, et al. Cooperative cytotoxicity of proteasome inhibitors and tumor necrosis factor-related apoptosis-inducing ligand in chemoresistant Bcl-2-overexpressing cells. Clin Cancer Res. 2005;11:4259–65. doi: 10.1158/1078-0432.CCR-04-2496. [DOI] [PubMed] [Google Scholar]
- 34.Qin JZ, Xin H, Sitailo LA, Denning MF, Nickoloff BJ. Enhanced Killing of Melanoma Cells by Simultaneously Targeting Mcl-1 and NOXA. Cancer Res. 2006;66:9636–45. doi: 10.1158/0008-5472.CAN-06-0747. [DOI] [PubMed] [Google Scholar]
- 35.Bowen AR, Hanks AN, Allen SM, Alexander A, Diedrich MJ, Grossman D. Apoptosis regulators and responses in human melanocytic and keratinocytic cells. J Invest Dermatol. 2003;120:48–55. doi: 10.1046/j.1523-1747.2003.12010.x. [DOI] [PubMed] [Google Scholar]
- 36.Raisova M, Hossini AM, Eberle J, et al. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol. 2001;117:333–40. doi: 10.1046/j.0022-202x.2001.01409.x. [DOI] [PubMed] [Google Scholar]
- 37.Thallinger C, Wolschek MF, Wacheck V, et al. Mcl-1 antisense therapy chemosensitizes human melanoma in a SCID mouse xenotransplantation model. J Invest Dermatol. 2003;120:1081–6. doi: 10.1046/j.1523-1747.2003.12252.x. [DOI] [PubMed] [Google Scholar]
- 38.Chawla-Sarkar M, Lindner DJ, Liu YF, et al. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–49. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- 39.Shao RG, Cao CX, Nieves-Neira W, Dimanche-Boitrel MT, Solary E, Pommier Y. Activation of the Fas pathway independently of Fas ligand during apoptosis induced by camptothecin in p53 mutant human colon carcinoma cells. Oncogene. 2001;20:1852–9. doi: 10.1038/sj.onc.1204264. [DOI] [PubMed] [Google Scholar]
- 40.Nikrad M, Johnson T, Puthalalath H, Coultas L, Adams J, Kraft AS. The proteasome inhibitor bortezomib sensitizes cells to killing by death receptor ligand TRAIL via BH3- only proteins Bik and Bim. Mol Cancer Ther. 2005;4:443–9. doi: 10.1158/1535-7163.MCT-04-0260. [DOI] [PubMed] [Google Scholar]
- 41.Zhu H, Zhang L, Dong F, et al. Bik/NBK accumulation correlates with apoptosisinduction by bortezomib (PS-341, Velcade) and other proteasome inhibitors. Oncogene. 2005;24:4993–9. doi: 10.1038/sj.onc.1208683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chawla-Sarkar M, Leaman DW, Borden EC. Preferential induction of apoptosis by interferon (IFN)-beta compared with IFN-alpha2: correlation with TRAIL/Apo2L induction in melanoma cell lines. Clin Cancer Res. 2001;7:1821–31. [PubMed] [Google Scholar]
- 43.Curtin JF, Cotter TG. Live and let die: regulatory mechanisms in Fas-mediated apoptosis. Cell Signal. 2003;15:983–92. doi: 10.1016/s0898-6568(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 44.Broaddus VC, Dansen TB, Abayasiriwardana KS, et al. Bid mediates apoptotic synergy between tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and DNA damage. J Biol Chem. 2005;280:12486–93. doi: 10.1074/jbc.M408190200. [DOI] [PubMed] [Google Scholar]
- 45.An J, Sun Y, Fisher M, Rettig MB. Maximal apoptosis of renal cell carcinoma by the proteasome inhibitor bortezomib is nuclear factor-kappaB dependent. Mol Cancer Ther. 2004;3:727–36. [PubMed] [Google Scholar]
- 46.Nawrocki ST, Carew JS, Pino MS, et al. Aggresome disruption: a novel strategy to enhance bortezomib-induced apoptosis in pancreatic cancer cells. Cancer Res. 2006;66:3773–81. doi: 10.1158/0008-5472.CAN-05-2961. [DOI] [PubMed] [Google Scholar]
- 47.Hideshima T, Chauhan D, Richardson P, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277:16639–47. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 48.Zheng B, Georgakis GV, Li Y, et al. Induction of cell cycle arrest and apoptosis by the proteasome inhibitor PS-341 in Hodgkin disease cell lines is independent of inhibitor of nuclear factor-kappaB mutations or activation of the CD30, CD40, and RANK receptors. Clin Cancer Res. 2004;10:3207–15. doi: 10.1158/1078-0432.ccr-03-0494. [DOI] [PubMed] [Google Scholar]
- 49.Qin JZ, Ziffra J, Stennett L, et al. Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res. 2005;65:6282–93. doi: 10.1158/0008-5472.CAN-05-0676. [DOI] [PubMed] [Google Scholar]
- 50.Fernandez Y, Verhaegen M, Miller TP, et al. Differential regulation of noxa in normal melanocytes and melanoma cells by proteasome inhibition: therapeutic implications. Cancer Res. 2005;65:6294–304. doi: 10.1158/0008-5472.CAN-05-0686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.