Abstract
Endothelial nitric oxide synthase-derived NO and its derivative, peroxynitrite (ONOO-), suppresses oxygen consumption by nitration of mitochondrial proteins after reperfusion. However, very few nitrated proteins are identified to date. In this paper, ischemia/reperfusion (I/R) injury was induced in mouse heart by ligation and release of the left anterior descending coronary artery. Western blotting showed that tyrosine nitration was higher in I/R hearts. Nitrated proteins were identified by capillary-liquid chromatography-nanospray tandem mass spectrometry. A total of 23 proteins were identified as being nitrated after I/R and 10 of them were from mitochondria. The nitrated mitochondrial proteins included 4 subunits from the oxidative phosphorylation system (the 24 and the 30 kDa subunits of complex I, the Rieske ISP of complex III, and the α subunit of ATP synthase), five enzymes in the matrix, and voltage-dependent anion channel. In purified complex I treated with ONOO-, 3-NT was identified locating at the residue of Y247 of the 30 kDa subunit and the residues of Y47, Y53 of the 49 kDa subunit. In conclusion, I/R induced protein nitration and mitochondrial proteins were the major targets. Selective nitration of proteins from the oxidative phosphorylation system at the beginning of reperfusion may contribute to the suppression of oxygen consumption.
Keywords: Peroxynitrite, Nitric oxide, Oxidative phosphorylation, Energy Metabolism, Oxygen consumption
1. Introduction
Nitric oxide (NO) has many important physiological roles on cardiac function [1, 2]. However, excess production of NO under disease conditions can be toxic. For example, NO can react with superoxide (O2•-) at a diffusion-controlled rate (k∼1010 M-1s-1) and form peroxynitrite (ONOO-). It is now well established that many of the toxic actions of NO are not directly due to NO itself but are mediated through formation of ONOO- which rapidly decomposes to highly reactive oxidant species [1, 3]. Thereby, the bioavailability and the beneficial effects of NO are lost [4].
It has been demonstrated by direct spin-trapping electron paramagnetic resonance (EPR) measurements that during the early period of reperfusion, amount of NO and O2•- were increased greatly above normal control values [5, 6], which resulted in the formation of ONOO- and protein tyrosine nitration [5]. Experiments with isolated cardiac muscle showed that formation of ONOO- contributed to cardiac dysfunction in hypoxia/reoxygenation [7, 8]. Inhibiting NO production decreased ONOO--mediated tyrosine nitration and attenuated myocardial hyperoxygenation after reperfusion in the post-ischemic heart [9]. Administration of superoxide dismutase (SOD) mimetics reduced the formation of ONOO- and protected heart from I/R injury [10, 11]. Furthermore, ONOO- was reported as a major trigger of cardiomyocyte apoptosis after I/R [11]. Therefore, identifying the target of ONOO- in myocardium is of great importance to the understanding of the mechanisms of I/R injury.
The biological targets of ONOO- include proteins, lipids, and DNA. Protein tyrosine nitration is increasingly recognized as a marker of ONOO--mediated cytotoxicity [12] because tyrosine nitration affects protein structure and function resulting in alterations in the catalytic activity of enzymes [13, 14], cytoskeletal organization [15, 16], and cell signal transduction [17, 18]. Although ONOO- is not the only nitrating species [19, 20], it is generally accepted that elevation of protein tyrosine nitration reflects the actions of ONOO- [21-23]. Protein tyrosine nitration in mitochondria from diabetic mouse heart [24] and rat cardiac proteins [25, 26] has been studied using two dimensional electrophoresis and mass spectrometry. It has been suggested that tyrosine nitration in those processes leads to functional decline of myocardial proteins [24-26]. It has been demonstrated that mitochondrial respiration is impaired by ONOO- in isolated cardiac muscle [7, 8]. Evidence has also shown that protein nitration contributes to the impairment of in vivo mitochondrial respiration in post-ischemic heart [9, 27]. To date, protein nitration caused by myocardial I/R injury has not been characterized at the molecular level.
In this study, we employed a proteomic approach to determine the formation of 3-nitrotyrosine (3-NT) in proteins obtained from mouse heart after I/R injury. We found that the level of tyrosine nitration was higher in the post-ischemic myocardium and mitochondrial proteins were identified to be the major targets of ONOO- formed during myocardial I/R. To gain further insights into the specific sites in the protein tyrosine nitration, myocardial complex I was isolated from mitochondria and subjected to in vitro protein tyrosine nitration with ONOO−. The specific amino acid residues involved in tyrosine nitration were identified by LC/MS/MS.
2. Materials and methods
2.1. Animals and myocardial I/R model
Male C57BL/6 mice obtained from Jackson Laboratory (Bar Harbor, ME) were housed under a 12:12-h light-dark cycle and were provided with water and food ad libitum. All procedures were performed with the approval of the Institutional Animal Care and Use Committee at The Ohio State University, Columbus, Ohio, and conformed to the Guide for the Care and Use of Laboratory Animals (NIH publication No. 86-23, revised 1996).
The in vivo myocardial I/R model was performed by a method described previously [9]. Briefly, mice were anesthetized intraperitoneally with ketamine (55 mg/kg) and xylazine (15 mg/kg). Animals were intubated and ventilated with a mouse mini-ventilator (Harvard Apparatus) at a frequency of 120 breaths/min. Tidal volume was set at 250 μL. Following left intercostal thoracotomy, regional ischemia was induced by ligation of the left anterior descending (LAD) coronary artery with a 7-0 silk suture. After 60 min of ischemia, the occlusion was released and reperfusion was assured for 60 min. Sham operations were performed by encircling the LAD without tightening the suture for sham control group.
2.2. Tissue sample preparation
After 60 min of reperfusion, myocardial tissue from the risk region was excised, rinsed in PBS, and immediately frozen in liquid N2. The tissue was kept at −80°C before use.
2.2.1. Sample preparation for one-dimensional gel electrophoresis (1-DE)
Frozen tissue was thawed and finely minced and homogenized in RIPA buffer (25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) supplemented with protease-inhibitor cocktail (1:40, Sigma). The suspension was then briefly sonicated and centrifuged at 13,000 g for 10 min. The supernatant after centrifugation was collected. Protein concentration was determined with the Lowry method [28]. Tissue homogenate was boiled in the Laemmli sample buffer at 70 °C for 5 min and directly subjected to 1-DE (75 μg of protein/lane). Tissue homogenate was also subjected to immnoprecipitation (IP) with a polyclonal anti-3-NT antibody followed by 1-DE. In addition, to confirm the specificity of the anti-3-NT antibody, samples were reduced with 10 mM sodium dithionite and used as a negative control.
2.2.2. Sample preparation for two-dimensional gel electrophoresis (2-DE)
For 2-DE, myocardial tissue was finely minced in the lysis buffer containing 7 M urea, 2 M thiourea, 4% CHAPS, 0.5% Triton X-100, 20 mM DTT, 0.5% carrier ampholytes, and 1% protease-inhibitor cocktail. The minced tissue was homogenized with a homogenizer (Fisher), briefly sonicated, and then incubated at room temperature for 1 h with constant agitation. The homogenate was centrifuged at 13,000 g for 10 min and the sediment was discarded.
2.3. Immunoprecipitation (IP) with anti-3-NT antibody
IP was performed as described [26] with slight modification. Concentration of the sample prepared in RIPA buffer was adjusted to 8 mg/ml, and 0.5 ml of the solution was incubated with an anti-3-NT polyclonal antibody (1:100; rabbit IgG, Upstate Biotechnology) at 4°C overnight. Then 60 μl protein A agarose beads were added. The mixture was incubated at 4°C for 6 h with constant slow shaking. The beads were collected and exhaustively washed with 20 mM Tris-HCl pH 7.4, 150 mM NaCl and 0.1% Tween 20 (TTBS). The washed pellets were boiled in the Laemmli sample buffer at 70 °C for 5 min to fully dissociate the antigen-antibody complex and then subjected to 1-DE.
2.4. One- and two-dimensional gel electrophoresis
For 1-DE, samples were loaded onto pre-cast 4-20% Tris-glycine polyacrylamide gradient gels (Invitrogen) and run at room temperature for 2 h at 120 V. For 2-DE, samples (300 μg of protein) were focused on 11 cm, pH 3-10 isoelectric focusing (IEF) strips and separated on precast Criterion 8–16% second-dimension gels (Bio-Rad). The obtained gels were then subjected for either staining with colloidal Coomassie blue (Pierce) or transferring to membranes.
2.5. Western blot analysis
Proteins were electrophoretically transferred to nitrocellulose membranes for 1-DE. To verify equal amount of sample loaded, nitrocellulose membranes containing transferred proteins were reversibly stained in 0.5% (w/v) Ponceau S (in 1% acetic acid) for 5 min, followed by destaining in distilled water for 3 min [29]. Proteins separated by 2-DE were electrophoretically transferred to 0.45 μm polyvinylidene difluoride (PVDF) membranes. After blocking with 5% dry milk in TTBS for 1 h at room temperature, the membranes were probed with an anti-3-NT monoclonal antibody (1:1000; clone 1A6, mouse IgG, Upstate Biotechnology) at 4 °C overnight. After incubation, the membranes were washed with TTBS and exposed to the peroxidase-linked species-specific anti-mouse IgG (1:2000; GE healthcare) for 1 h at room temperature. The membranes were washed again with TTBS. The 3-NT-positive proteins were visualized using chemiluminescence (ECL) Western blotting detection reagents (Amersham Biosciences). Densitometric analysis of the immunoblots was performed using an Alphaimager 3300 system (Alpha Innotech, San Leandro, CA).
2.6. Preparation and in vitro protein tyrosine nitration of mitochondrial complex I
Bovine heart mitochondrial complex I was prepared as described [30]. Submitochondrial particles (SMP) were prepared as reported and used as the starting material [31]. The SMP was suspended in 50 mM Tris-HCl (pH 8.0), 0.66 M sucrose and 1 mM histidine (TSH), and then subjected to KCl (72 g/l) fractionation in the presence of deoxycholate (0.3 mg/mg protein). The resulting supernatant was mixed with an appropriate amount of cold water to precipitate trace amounts of cytochrome c oxidase, and then dialyzed against 10 mM Tris-HCl (pH 8.0) containing 1 mM EDTA for 6 h with one change of buffer. The dialysate was subjected to centrifugation at 96,000 g for 75 min. The pellet containing complexes I, II, and III was homogenized in TSH buffer, and then subjected to repeated ammonium acetate fractionation in the presence of deoxycholate (0.5 mg/mg protein). Complex I was finally resolved (39% saturation of ammonium sulfate) and separated using ammonium sulfate precipitation (35.9% saturation) in the presence of potassium cholate (0.4 mg/mg protein).
The purified complex I was nitrated by adding 100 or 200 μM ONOO- into 1 mg protein/ml complex I in 50 mM phosphate buffer (pH 7.4) containing 0.1% Lauryl-β-D-Maltoside. The mixture was then incubated at room temperature for 1 h, treated with 9% of ethanol at 40 °C for 10 min, and then centrifuged at 75,600 g for 30 min. The ONOO--treated complex I (40 μg/lane) was subjected to 1-DE on 4-12% Bis-Tris polyacrylamide gradient gel. Western blot analysis was performed with the anti-3-NT polyclonal antibody (1:3000; Upstate Biotechnology). To confirm the specificity of the antibody, nitrated complex I was reduced with 10 mM sodium dithionite. Equal amount of protein was loaded and processed in parallel.
2.7. Capillary-liquid chromatography-nanospray tandem mass spectrometry (Nano-LC/MS/MS)
2.7.1 In-gel digestion
Coomassie blue-stained gel spots of interest were excised from the gel. The obtained gels were digested with sequencing grade trypsin (Promega, Madison, WI), chymotrypsin (Roche Diagnostics, Indianapolis, IN), or a combination of trypsin and chymotrypsin using the Montage In-Gel Digestion Kit from Millipore (Bedford, MA). The manufacturer-recommended protocol was followed. Briefly, the spots of interest were trimmed as closely as possible to minimize background contaminations. After being washed twice in 50% methanol/5% acetic acid for several hours, the gel spots were dried with acetonitrile and reconstituted with dithiothreitol (DTT) solution (5 μg/μl) to reduce the cysteines. Iodoacetamide solution (15 μg/μl) was then added to alkylate the cysteines. The gel was washed again with cycles of acetonitrile and 100 mM ammonium bicarbonate. The gel was then dried using a SpeedVac concentrator system. A 50 μl aliquot of trypsin (20 ng/μl), chymotrypsin (25 ng/μl) or a combination of trypsin and chymotrypsin in 50 mM ammonium bicarbonate buffer was added to the dehydrated gel. The gel was set on ice for 10 min for rehydration before the addition of another 20 μl of 50 mM ammonium bicarbonate buffer. The mixture was then incubated at room temperature overnight. The peptides were extracted with 50% acetonitrile and 5% formic acid several times and pooled together. The extracted pools were concentrated in the concentrator system to ∼25 μl.
2.7.2 Mass spectrometry
Nano-LC/MS/MS was performed on a Thermo Finnigan LTQ mass spectrometer equipped with a nanospray source operated in positive ion mode. The LC system was an UltiMate™ Plus system from LC-Packings A Dionex Co (Sunnyvale, CA) with a Famos autosampler and Switchos column switcher. Solvent A was 50 mM acetic acid and solvent B was acetonitrile. Sample (5 μl) was first injected onto the trapping column (LC-Packings A Dionex Co), and washed with 50 mM acetic acid. The injector port was switched to inject and the peptides were eluted off the trap onto the column. A 5 cm 75 μM ID ProteoPep II C18 column (New Objective, Inc. Woburn, MA) packed directly in the nanospray tip was used for chromatographic separations. Peptides were eluted directly off the column into the LTQ system using a gradient of 2-80% solvent B over 30 min, with a flow rate of 300 nl/min. The total run time was 58 minutes. The scan sequence of the mass spectrometer was programmed for a full scan and MS/MS scans of the 10 most abundant peaks in the spectrum. Dynamic exclusion was used to exclude multiple MS/MS of the same peptide after detecting it three times. Sequence information from the MS/MS data was processed using the Mascot 2.0 active perl script with standard data processing parameters. Data was searched with the MASCOT 2.0 (Matrix Science, Boston, MA). The mass accuracy of the precursor ions was set to 1.8 Da to accommodate accidental selection of the 13C ion and the fragment mass accuracy was set to 0.8 Da. Number of missed cleavages permitted in the search was 2 for both tryptic and chymotryptic digestions. An individual peptide was considered as a good match if it produced a probability-based MOWSE score greater than 20 (P<0.05). Cysteine, Carbamidomethylation, methionine oxidation and tyrosine nitration were considered as variable modifications. The cut-off score for significant protein identification was set at 45 and the 3-NT-containing peptides were all manually verified.
3. Results
3.1 1-DE and Western blot analysis
Previous results of immunohistochemical staining have shown that ONOO- is formed in postischemic myocardium resulting in nitration of proteins within cardiac myocytes [5, 9]. To directly compare the levels of nitrated proteins between the sham control and the I/R hearts, 1-DE and Western blotting were performed. The amount of protein from Heart homogenate loaded in each lane of the gel was 75 μg. Ponceau S staining of the membrane confirmed equal loading of the protein samples (Fig. 1B). After destaining, the same membrane was subjected to Western blot analysis with the monoclonal anti-NT antibody. The results clearly demonstrated that a Western blot band at the position of about 25 kDa was more 3-NT-immunopositive in the I/R hearts than that in the sham control (Fig. 1A). Proteins can be enriched by IP with appropriate antibodies. To reveal low abundant nitrated proteins in the heart tissue, IP was performed with a polyclonal anti-3-NT antibody (rabbit IgG). After IP, Western blotting with the monoclonal anti-3-NT antibody (mouse IgG) and the species-specific second antibody showed that except a Western blot band at the position of about 25 kDa (a), there were 3 additional 3-NT-immunoreactive bands at positions of about 50 (b), 52 (c), and 54 (d) kDa respectively (Fig. 1C). As indicated by densitometric analysis (Fig. 1D), the total protein nitration in the whole lane was significantly increased to 158.5±3.68% of that of sham control after I/R injury (P<0.05; n=6). For the 4 individual Western blot bands, protein nitration was significantly increased to 144.6±2.85%, 154.0±8.37%, 199.5±13.28%, and 379.0±34.69% of that of sham control. These results clearly demonstrated that there was some intrinsic nitration in some proteins in the normal heart, and the intrinsic protein nitration was significantly enhanced after I/R injury. The different magnitude of enhancement suggests that protein nitration in vivo is specific and selective. It is worth noting that our results are consistent with the marked increase in 3-nitrotyrosine formation with several major bands located at 45-50 and 25-30 kDa following I/R in an in vivo rat myocardial I/R model [11].
Fig. 1.

Western blot analysis of tyrosine nitration in cardiac proteins separated by one-dimensional gel electrophoresis. A: Western blot analysis of proteins (75 μg of protein/lane) from heart tissue homogenate of 3 sham and 3 I/R mice with the monoclonal anti-3NT antibody. Lanes 1-6 showed that 3-NT was increased in heart tissue after I/R. Proteins in lanes 8-9 were reduced with 10 mM sodium dithionite (DTN) before loading. B: Ponceau S staining of cardiac proteins in the nitrocellulose membrane indicated equal protein loading. C: representative Western blots of 3-NT in immunoprecipitated proteins from heart homogenate. Heart homogenate (4 mg of protein) was incubated with an anti-3-NT polyclonal antibody and then with protein A agarose beads. After separation by 1-DE, proteins in the membrane were probed with the monoclonal anti-3NT antibody. D: densitometric analysis of the immunoblots of immuoprecipitated proteins showed that protein nitration was significantly increased after I/R injury. (*P<0.05; n=6). M represents a molecular weight marker. Other experimental details are described in Materials and Methods
3.2 2-DE and Western blot analysis
2-DE provides another dimension to separate proteins according to their respective isoelectric points (pI). To identify the nitrated proteins, tissue homogenates (300 μg of protein) from mouse heart were focused on IEF strips and separated on second-dimension gels. After separation, the gels were subjected to colloidal Coomassie blue staining or Western blot analysis with the monoclonal anti-3-NT antibody. Colloidal Coomassie blue staining of the gels indicated similar levels of protein expression in sham and I/R hearts (data not shown). Western blot analysis demonstrated that there was some intrinsic nitration in normally perfused sham heart (Fig. 2A). Protein nitration was increased prominently in I/R heart (Fig. 2B). These nitrated proteins included one group of proteins with molecular weight about 25 kDa and pI value in the range of 6-8, and another group of proteins with molecular weight about 50-55 kDa and pI value of 8-9, which was consistent with results of 1-DE and Western blotting (Fig. 1A). A total of 10 immunopositive spots were excised and subjected to mass spectrometry analysis (Fig. 2C). The same experiment with 2-DE was repeated for 4 times (n=4) and similar results were obtained.
Fig. 2.

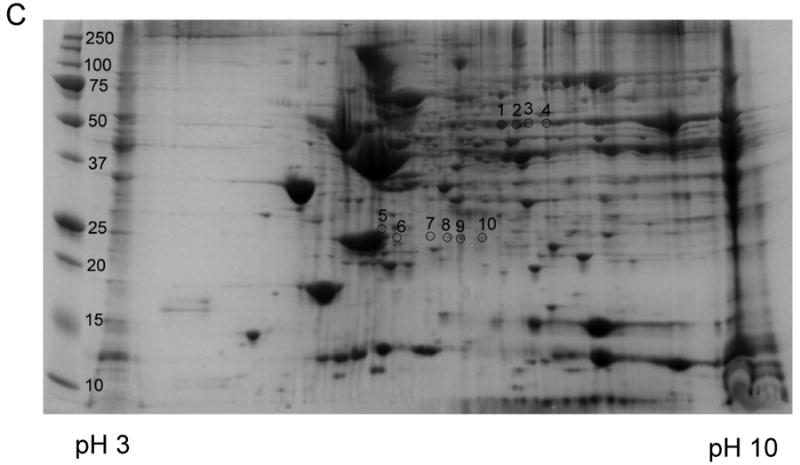
Representative results of four independent experiments for the Western blot analysis of protein nitration in cardiac proteins separated by two-dimensional gel electrophoresis. Heart tissue was homogenized and cardiac proteins were extracted into 2-DE lysis buffer. A load of 300 μg protein was applied to each gel. The PI range of the first dimension IEF was 3-10. See Materials and Methods for other details. Western blots of 3-NT in proteins from A: sham heart and B: I/R heart. C: colloidal Coomassie blue-stained 2-DE gel of I/R heart and the gel spots selected for mass spectrometry analysis. The blots in B were matched to the gel spots in C with the Bio-Rad gel imaging system and the corresponding gel spots were excised. M represents a molecular weight marker. The data again showed that protein nitration was increased in the I/R heart tissue.
3.3 Protein identification with mass spectrometry
To identify the nitrated proteins in the I/R hearts, the excised spots from the 2D gel were digested with trypsin (20 ng/μl). The tryptic peptides were then subjected to Nano-LC/MS/MS analysis. More than one protein was identified in most of the spots, indicating overlap of proteins with similar pI and molecular weight. Meanwhile, some of the identical proteins were found in several different spots due to degradation or modification that can alter the protein pI value and molecular weight [26]. A total of 23 proteins were identified from the 10 spots as summarized in Table 1. The identified proteins included 10 mitochondrial proteins. Among them are 4 polypeptides from the oxidative phosphorylation system: Ndufv2 protein (the 24 kDa subunit of complex I), Ndufs3 protein (the 30 kDa subunit of complex I), Uqcrfs1 protein (the Rieske ISP of complex III), and Atp5a1 protein (the α subunit of ATP synthase). In addition, five enzymes in mitochondrial matrix involved in energy metabolism as well as Vdac1 protein were also identified to be nitrated. The 13 non-mitochondrial proteins include proteins from cytoplasm, plasma membrane, as well as extracellular region. The peptide sequences detected from all the proteins were shown in supplementary Table 1.
Table 1.
Proteins identified in the 3-NT immunoreactive spots in Fig 2C with Nano-LC/MS/MS
| Spot No. |
Protein | Gene abbreviation |
GenInfo Accession No. |
Mass (kDa) | pI | Matched Peptides |
Sequence Coverage |
Score | Location |
|---|---|---|---|---|---|---|---|---|---|
| 1 | dihydrolipoamide dehydrogenase | Dld | 2078522 | 54.18 | 7.97 | 26 | 25% | 509 | Mitochondrion |
| propionyl-CoA carboxylase beta | PccB | 13507244 | 58.36 | 7.18 | 2 | 7% | 64 | Mitochondrion | |
| chain pyruvate kinase M2 | Pkm2 | 1098063 | 57.82 | 7.58 | 4 | 7% | 56 | Cytoplasm | |
| protocadherin gamma B6 | Pcdhgb6 | 22164120 | 16.5 | 4.55 | 2 | 5% | 58 | Plasma membrane | |
| Etl4 protein | Etl4 | 20073019 | 53.78 | 9.23 | 4 | 2% | 50 | Cytoplasm, nucleus | |
| Psmd1 protein | Psmd1 | 27370667 | 90.88 | 5.68 | 3 | 6% | 48 | Cytoplasm | |
|
| |||||||||
| 2 | ATP synthase, H+ transporting, mitochondrial F1 complex, alpha subunit, isoform 1 | Atp5a1 | 15928789 | 59.72 | 9.22 | 28 | 35% | 765 | Mitochondrion |
| 3-oxoacid CoA transferase 1 | Oxct1 | 13097345 | 55.95 | 8.73 | 24 | 28% | 564 | Mitochondrion | |
| dihydrolipoamide dehydrogenase | Dld | 2078522 | 54.18 | 7.97 | 8 | 11% | 202 | Mitochondrion | |
| nicotinamide phosphoribosyltransferase | Nampt | 10946948 | 55.35 | 6.69 | 2 | 6% | 149 | Cytoplasm | |
| pyruvate kinase M2 | Pkm2 | 1098063 | 57.82 | 7.58 | 3 | 4% | 71 | Cytoplasm | |
| aldehyde dehydrogenase family 6, subfamily A1 | Aldh6a1 | 23271115 | 57.92 | 8.25 | 1 | 3% | 59 | Mitochondrion | |
| glutamate dehydrogenase 1 | Glud1 | 30931187 | 61.3 | 8.05 | 2 | 3% | 59 | Mitochondrion | |
|
| |||||||||
| 3 | 3-oxoacid CoA transferase 1 | Oxct1 | 13097345 | 55.95 | 8.73 | 21 | 28% | 532 | Mitochondrion |
| ATP synthase, H+ transporting, mitochondrial F1 complex, alpha subunit, isoform 1 | Atp5a1 | 15928789 | 59.72 | 9.22 | 10 | 16% | 384 | Mitochondrion | |
| aldehyde dehydrogenase family 6, subfamily A1 | Aldh6a1 | 23271115 | 57.92 | 8.25 | 8 | 12% | 193 | Mitochondrion | |
| dihydrolipoamide dehydrogenase | Dld | 2078522 | 54.18 | 7.97 | 5 | 9% | 167 | Mitochondrion | |
| glutamate dehydrogenase 1 | Glud1 | 30931187 | 61.3 | 8.05 | 3 | 7% | 118 | Mitochondrion | |
|
| |||||||||
| 4 | ATP synthase, H+ transporting, mitochondrial F1 complex, alpha subunit, isoform 1 | Atp5a1 | 15928789 | 59.72 | 9.22 | 27 | 28% | 707 | Mitochondrion |
| 3-oxoacid CoA transferase 1 | Oxct1 | 13097345 | 55.95 | 8.73 | 16 | 26% | 432 | Mitochondrion | |
| UDP-glucose pyrophosphorylase 2 | Ugp2 | 56202395 | 56.94 | 7.18 | 4 | 12% | 242 | Cytoplasm | |
| aldehyde dehydrogenase family 6, subfamily A1 | Aldh6a1 | 23271115 | 57.92 | 8.25 | 8 | 10% | 205 | Mitochondrion | |
| dihydrolipoamide dehydrogenase | Dld | 2078522 | 54.18 | 7.97 | 2 | 5% | 82 | Mitochondrion | |
|
| |||||||||
| 5 | myosin, light polypeptide 3 | Myl3 | 38173999 | 22.41 | 5.03 | 10 | 23% | 263 | Cytoskeleton |
| heat shock protein HSP27 | Hspb1 | 424145 | 21.96 | 6.45 | 1 | 8% | 67 | Cytoplasm, Nucleus | |
| apolipoprotein A-I binding protein | Apoa1bp | 37231544 | 30.95 | 7.59 | 1 | 6% | 62 | Extracellullar Region | |
|
| |||||||||
| 6 | NADH dehydrogenase (ubiquinone) flavoprotein 2 | Ndufv2 | 21410924 | 27.27 | 7.00 | 10 | 30% | 283 | Mitochondrion |
| NADH dehydrogenase (ubiquinone) Fe-S protein 3 | Ndufs3 | 20071222 | 30.19 | 6.40 | 6 | 18% | 268 | Mitochondrion | |
| RAB11B, member RAS oncogene family | Rab11b | 55391484 | 24.47 | 5.64 | 7 | 28% | 252 | Cytoplasm | |
| myosin, light polypeptide 3 | Myl3 | 38173999 | 22.41 | 5.03 | 1 | 6% | 64 | Cytoplasm | |
|
| |||||||||
| 7 | nonselenium glutathione peroxidase | Prdx6 | 2072655 | 24.86 | 5.71 | 2 | 8% | 89 | Cytoplasm |
|
| |||||||||
| 8 | heat shock protein HSP27 | Hspb1 | 424145 | 21.96 | 6.45 | 11 | 44% | 334 | Cytoplasm, Nucleus |
| triosephosphate isomerase | Tpi | 1864018 | 22.49 | 5.62 | 2 | 14% | 116 | Cytoplasm | |
|
| |||||||||
| 9 | nonselenium glutathione peroxidase | Prdx6 | 2072655 | 24.86 | 5.71 | 15 | 50% | 473 | Cytoplasm |
| heat shock protein HSP27 | Hspb1 | 424145 | 21.96 | 6.45 | 6 | 39% | 259 | Cytoplasm, Nucleus | |
| triosephosphate isomerase | Tpi | 1864018 | 22.49 | 5.62 | 2 | 14% | 137 | Cytoplasm | |
| carnitine transporter 2 | Slc22a16 | 74484339 | 73.89 | 8.06 | 5 | 8% | 48 | Plasma Membrane | |
|
| |||||||||
| 10 | nonselenium glutathione peroxidase | Prdx6 | 2072655 | 24.86 | 5.71 | 6 | 27% | 241 | Cytoplasm |
| voltage-dependent anion-selective channel protein 1 | Vdac1 | 10720404 | 32.33 | 8.55 | 5 | 18% | 229 | Mitochondrion | |
| ubiquinol-cytochrome c reductase, Rieske iron-sulfur polypeptide 1 | Uqcrfs1 | 18044191 | 29.35 | 8.91 | 6 | 15% | 171 | Mitochondrion | |
| triosephosphate isomerase | Tpi | 1864018 | 22.49 | 5.62 | 4 | 12% | 86 | Cytoplasm | |
Spot numbers refer to the numbers shown in Fig. 2C. The 9th column shows the MOWSE score obtained with Mascot software.
In order to confirm the identified proteins separated by 2-DE, we used the polyclonal antibody against 3-NT to perform IP with the I/R heart homogenate. Proteins after IP were subjected to 1-DE, followed by colloidal Coomassie blue staining (supplementary Fig. 1). Two relatively clear spots were excised. The proteins were identified as Slc25a4 protein in spot 11, and Oxct1 protein and Atp5a1 protein in spot 12 (supplementary Table 2). The heavy chain of the antibody against 3-NT was also identified in spot 12. Among them, Oxct1 protein and Atp5a1 protein were also identified with 2-DE and Western blotting, which confirmed the reliability of this method. Slc25a4 protein was not observed in the 2-DE experiments. This may be due to the very basic pI value (9.76) that affects its separation and identification with 2-DE [26]. The peptide sequences detected from these proteins were shown in supplementary Table 3.
3.4 In vitro protein nitration of mitochondrial complex I
It has been reported that even with all the tissue from a whole rat heart, it is difficult to obtain satisfactory MS/MS data for 3-NT-containing peptides [26]. In order to identify the sites of tyrosine nitration in the amino acid sequences and provide direct evidence for protein nitration, we also have tried to determine the nitrated sites in the identified proteins from the in vivo mouse heart homogenate. However, due to all the potential reasons (discussed in section 4.3) we were unable to identify the modified sites in those nitrated proteins obtained from the myocardial tissue subjected to in vivo I/R. Therefore, we used purified complex I subjected to in vitro protein nitration with ONOO- as a model for identification of those possible nitrated sites.
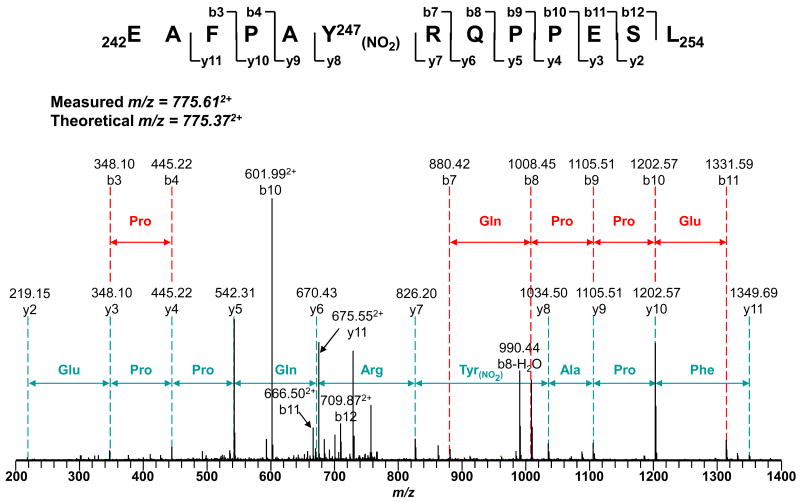
In vitro protein nitration of mitochondrial complex I was induced by treating the isolated complex with 100 μM or 200 μM ONOO-. The reaction mixture was subjected to 1-DE and protein nitration was confirmed with immunoblotting with the polyclonal antibody against 3-NT (Fig. 3). The intensity of the Western blot signal was enhanced with higher (200 μM) ONOO- concentration (Fig. 3B). The detected Western signal was subsequently diminished after reduction of nitrated complex I with sodium dithionite, confirming the specificity of the antibody. Not all the subunits separated by 1-DE were immunopositive and the intensity of different Western blot bands also varied, implying that proteins were selectively nitrated. Immunopositive spots with the molecular weight of about 27 (spot I), 30 (spot II), and 50 kDa (spot III) were excised (Fig. 3A) and subjected to in-gel digestion with trypsin, chymotrypsin, or a combination of trypsin and chymotrypsin, followed by Nano-LC/MS/MS analysis. The identified subunits are shown in Table 2 and their corresponding peptide sequences detected were shown in the supplementary Table 4. Several subunits from complex I were identified with great sequence coverage. The overall coverage of the amino acid sequence was 75% for the 24 kDa subunit (Ndufv2 protein, identified in spot I), 85% for the 30 kDa subunit (Ndufs3 protein, from spot II), 83% for the 49 kDa subunit (Ndufs2 protein, from spot III) and 91% for the 51 kDa subunit (Ndufv1 protein, from spot III), respectively. Compared with unmodified peptides, nitration causes a mass shift of +45 Da on 3-NT-containing peptides. This specific mass shift was observed on both the 30 kDa and the 49 kDa subunits, revealing the presence of nitrated peptides in both proteins. Further MS/MS data suggested that 3-NT was located to the residue of Y247 (242EAFPAY(NO2)RQPPESL254) of the 30 kDa subunit (Fig. 4 and supplementary Table 5). Additional tyrosine nitration was found in the 49 kDa subunit at the residues of Y47 (36QWQPDVEWAEQY(NO2)47, Fig. 5A and supplementary Table 6) and Y53 (48GGAVM(OX)Y(NO2)PTK56, Fig. 5B and supplementary Table 7). The MS/MS results clearly showed that the polypeptides in mitochondrial complex I were nitrated by ONOO- in a site-specific manner. As shown in the online Fig. 2, Y193, a 30kDa subunit in the spectrum of chymotryptic peptide 191TDY193GFEGHPH200 was observed as unmodified indicating the specificity of the process.
Fig. 3.
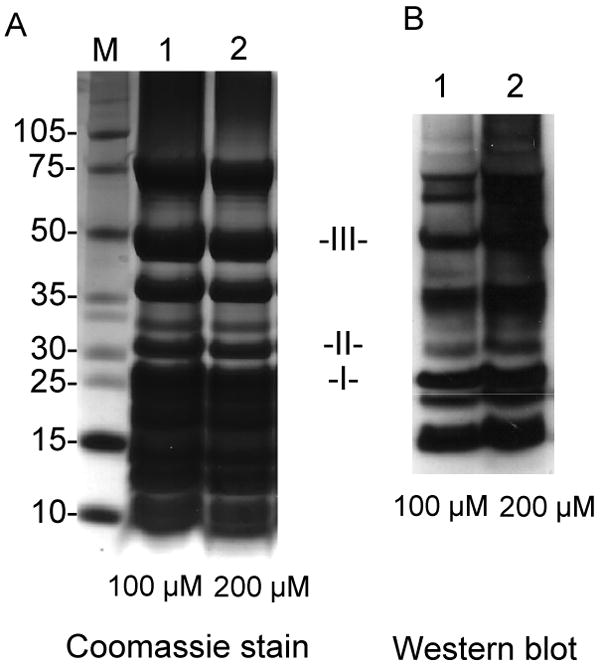
Nitration of purified mitochondrial complex I in vitro. Complex I was treated with 100 or 200 μM ONOO-. The nitrated complex I (40 μg of protein/lane) was subjected to 1-DE on 4-12% Bis-Tris polyacrylamide gradient gel and Western blotting was performed with the anti-3-NT polyclonal antibody (1:3000). The Immunopositive spots at the positions of about 27, 30 and 50 kDa were excised and subjected to LC/MS/MS analysis. A: SDS-PAGE: lane 1, complex I treated with 100 μM ONOO-; lane 2, complex I treated with 200 μM ONOO-. M represents a molecular weight marker. B: Western blot analysis using an anti-3NT antibody: lane 1, complex I treated with 100 μM ONOO-; lane 2, complex I treated with 200 μM ONOO-. These data demonstrated that the subunits in complex I were selectively nitrated by ONOO-.
Table 2.
Identification of nitrated sites in subunits from ONOO--treated Complex I with Nano-LC/MS/MS
| Spot No. | Subunit Identified | Gene abbreviation | GenInfo Accession No. | Mass (kDa) | Overall Sequence Coverage | Nitrated site identified |
|---|---|---|---|---|---|---|
| I | NADH dehydrogenase [ubiquinone] flavoprotein 2, mitochondrial precursor (NADH-ubiquinone oxidoreductase 24 kDa subunit) | Ndufv2 | 128865 | 27.58 | 70% | none |
| II | NADH dehydrogenase [ubiquinone] iron-sulfur protein 3, mitochondrial precursor (NADH-ubiquinone oxidoreductase 30 kDa subunit) | Ndufs3 | 128860 | 30.44 | 85% | Y246 |
| III | NADH dehydrogenase [ubiquinone] iron-sulfur protein 2, mitochondrial precursor (NADH-ubiquinone oxidoreductase 49 kDa subunit) | Ndufs2 | 116242673 | 52.92 | 83% | Y46 and Y52 |
| NADH dehydrogenase [ubiquinone] flavoprotein 1, mitochondrial precursor (NADH-ubiquinone oxidoreductase 51 kDa subunit) | Ndufv1 | 548387 | 51.30 | 91% | none |
Spot numbers refer to the numbers shown in Fig. 3A.
Fig. 4.
Tandem mass spectrum of the doubly protonated molecular ions of the nitrated peptide 242EAFPAYRQPPESL254 of the 30 kDa subunit of complex I. The protein was digested with 20 ng/μl trypsin and then subjected to Nano-LC/MS/MS analysis. The modified peptide was manually verified. The sequence-specific ions are labeled as y and b ions on the spectra. The peptide contained 3-NT at the residue of Y247.
Fig. 5.
Tandem mass spectra of the doubly protonated molecular ions of the nitrated peptides of 49 kDa subunit of complex I. The protein was digested with trypsin/chymotrypsin and then subjected to Nano-LC/MS/MS analysis. The modified peptides were manually verified. The sequence-specific ions are labeled as y and b ions on the spectra. A: the nitrated peptide 36QWQPDVEWAEQY47 contained 3-NT at the residue of Y47. B: the nitrated peptide 48GGAVMYPTK56 contained 3-NT at the residue of Y53.
4. Discussion
4.1 Nitration of proteins after I/R
It has been demonstrated that mitochondrion constitutes a primary locus for the formation and reactions of peroxynitrite, which contributes to the biological and pathological effects of both NO and ONOO- [32, 33]. Specifically, protein tyrosine nitration has been detected in the mitochondria of endothelial cells after I/R [52]. Small amounts of ONOO- may also diffuse across the mitochondrial membranes and react with non-mitochondrial proteins [33]. During reperfusion, decreased availability of reduced co-factors of the mitochondrial respiratory chain will increase mitochondrial O2-• generation [34]. O2-• can react with endothelium-derived NO [9] and results in ONOO- formation. ONOO--mediated protein nitration contributes to the suppression of mitochondrial respiration during reperfusion [9, 27]. Amino acid residues located proximally to the specific site of OONO- formation have a higher probability of being nitrated [35]. However, it is difficult to determine the precise stoichiometry of nitration due to limitations of currently available approaches to characterize the nitrated proteins in vivo, which unexceptionally relied on the visualization of nitrated proteins by Western blotting followed by mass spectrometry to identify specific site of modification. Ideally, the method requires full sequence coverage of protein, which is rarely observed in the case of tissue samples [36]. In addition, denitration [37-39] and increased turnover of nitrated proteins [40] may also add difficulties to the detection of nitrated sites and the precise stoichiometry of nitration.
Our results have demonstrated that protein nitration is greatly increased after I/R and nearly half of the nitrated proteins are from mitochondria. Four polypeptides are from the oxidative phosphorylation system in the mitochondrial inner membrane, the 24 and 30 kDa subunit of complex I, the Rieske ISP of complex III, and the α subunit of ATP synthase. These subunits are vital for maintaining the normal function of electron transport and H+ translocation in mitochondria. The 24 kDa subunit of complex I hosts the 2Fe-2S cluster (N1a center) which can temporarily store electron from flavosemiquinone and thus minimize ROS production during turnover of complex I [41]. The 30 kDa subunit appears to play an important role in the stabilization of complex I [41]. The Rieske ISP accepts the first electron from ubiquinone, which is the rate limiting reaction of complex III [42]. ATP synthase couples the energy of electrochemical H+ gradient established by the respiratory chain to the formation of ATP [43].
Nitration of proteins from the oxidative phosphorylation system has also been found during other in vivo processes of oxidative stress in heart. For instance, the 24 kDa subunit of complex I was shown to be 3-NT-immunopositive in diabetic mouse heart [24]. During the process of aging, increased levels of nitration were observed in subunits from complex I, complex III, complex IV and ATP synthase [26]. Tyrosine nitration of mitochondrial proteins results in mitochondrial dysfunction through the impairment of catalysis and protein-protein interaction [24, 44]. The activities of complexes I, II, IV and V are significantly inhibited in ONOO--treated mitochondria [13]. Previous work in our laboratory has shown that a marked hyperoxygenation state was detected after reperfusion due to suppression of mitochondrial respiration by NO and its derivative, ONOO-. The activity of complex I is significantly decreased after reperfusion [9]. In the present study, we have found that subunits from complex I, complex III and ATP synthase are nitrated after I/R, suggesting that nitration of proteins from oxidative phosphorylation system may contribute to the suppression of mitochondrial oxygen consumption upon reperfusion.
The nitrated proteins identified also include proteins with the functions involved in the physiological processes of energy metabolism and signaling transduction. However, nitration of these proteins after I/R and the consequential impact on myocardial oxygen consumption and other cardiac functions need to be further studied.
4.2. Localization of nitrated mitochondrial proteins
In the intact and tightly coupled mitochondria, the pH value is within the range of 7.5-7.8 in the matrix and 6.9-7.0 in the intermembrane space. Nitration of proteins occurs largely in the matrix side since ONOO- is more stable in high pH [45]. Among the 10 nitrated mitochondrial proteins, a total of 5 proteins are located in the matrix. In addition, the 24 kDa and the 30 kDa subunits of complex I are located in the hydrophilic peripheral arm protruding into the matrix [41]. The α subunit of ATP synthase is also exposed to the matrix [43]. Proteins in the matrix or subunits protruding into the matrix are more likely to be nitrated after I/R. The Rieske ISP of complex III, which is very close to one of the major sites of O2-• production [46], and VDAC, which may act as a channel to release O2-• into the cytosol [47]. Nitration of these two proteins may have an impact on the cellular physiology mediated by the redox signal from mitochondria.
4.3. Nitration of complex I
When mapping protein post-translational modifications with mass spectrometry, a frequent obstacle is the low sensitivity of the current proteomic approach [48]. Only a limited number of 3-NT-containing peptide sequences have been observed to date [26, 49]. For example, of all the 48 proteins identified, only 1 protein was shown to have a nitrated site in aged heart tissue [26]. The possible reasons include: 1) limited sample load, low solubility of proteins, poor separation of proteins with very low and high molecular weight and pI, and low abundance of some nitrated proteins in tissue [36, 38, 50]; 2) low recovery of proteins from in-gel digestion; differential tryptic digestion and elution efficiencies of nitrated and non-nitrated proteins; 3) dynamic protein tyrosine denitration [37-39] and increased turnover of nitrated protein [40] in vivo.
We have tried to map 3-NT-containing peptides from the in vivo heart tissue after I/R. In order to increase the starting material, immunoprecipitation was also performed with a polyclonal antibody against 3-NT. Our colleagues recently demonstrated that after immunoprecipitation of tissue homogenate with an antibody against the 70 kDa flavin protein of mitochondrial, Western blotting showed that protein tyrosine nitration of the 70 kDa flavin protein was increased in the post-ischemic heart tissue, however, no 3-NT sites were able to be identified with LC/MS/MS [1].
As the in-gel digestion leads to low recovery of proteins, we have also tried a higher resolution approach, protein separation by solution isoelectric focusing followed by in-solution digestion and LC/MS/MS. It was reported that this approach could overcome the limitations of traditional 2-DE method [10], and more nitrated proteins were detected in cardiac tissue of old rat. In our study, many proteins was identified in each fraction, most of which with low sequence coverage. However, we couldn't detect any nitrated sites with this method either from the in vivo tissue homogenate. Therefore, we used in vitro nitrated complex I as a model system to confirm the nitration of proteins from the oxidative phosphorylation system and to identify the targeting sites of nitration.
To help understanding the molecular mechanism of protein nitration, isolated complex I was subjected to in vivo protein tyrosine nitration to facilitate mapping of specific tyrosyl residue(s) involved in nitration. Complex I, which catalyzes electron transfer from NADH to ubiquinone, is one of the two entry points to the oxidative phosphorylation system. Complex I has been identified to be a major site of oxidative damage in mitochondria after myocardial I/R [9, 51, 52]. Specific tyrosyl residues detected include the residue Y247 of the 30 kDa subunit and the residues Y47, Y53 of the 49 kDa subunit, which suggests the specificity of the anti-3-NT antibody and the selectivity of OONO--mediated tyrosine nitration. Specifically, the 30 kDa subunit, which is nitrated in vivo after I/R, has a nitrated site identified in the ONOO--treated complex I, indicating that this protein is prone to nitration under both in vivo and in vitro conditions.
In summary, there is a significant increase in protein tyrosine nitration in the myocardium after I/R and mitochondria are major targets. Protein subunits from the oxidative phosphorylation system such as the 24 kDa and the 30 kDa subunits of complex I are identified as being selectively nitrated. Nitration of tyrosine in ONOO--treated complex I is selective and site-specific. The nitration of proteins from the oxidative phosphorylation system may contribute to the suppression of oxygen consumption on mitochondrial respiratory chain.
Supplementary Material
Acknowledgments
This work was supported by NIH HL081630 and AHA GRT962974 (GH), HL083237(YRC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2003;138:532–43. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hare JM, Colucci WS. Role of nitric oxide in the regulation of myocardial function. Prog Cardiovasc Dis. 1995;38:155–66. doi: 10.1016/s0033-0620(05)80004-0. [DOI] [PubMed] [Google Scholar]
- 3.Valdez LB, Alvarez S, Arnaiz SL, Schopfer F, Carreras MC, Poderoso JJ, Boveris A. Reactions of peroxynitrite in the mitochondrial matrix. Free Radic Biol Med. 2000;29:349–56. doi: 10.1016/s0891-5849(00)00301-4. [DOI] [PubMed] [Google Scholar]
- 4.Peluffo G, Radi R. Biochemistry of protein tyrosine nitration in cardiovascular pathology. Cardiovasc Res. 2007;75:291–302. doi: 10.1016/j.cardiores.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. Evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem. 1996;271:29223–30. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- 6.Zweier JL, Kuppusamy P, Williams R, Rayburn BK, Smith D, Weisfeldt ML, Flaherty JT. Measurement and characterization of postischemic free radical generation in the isolated perfused heart. J Biol Chem. 1989;264:18890–5. [PubMed] [Google Scholar]
- 7.Xie YW, Kaminski PM, Wolin MS. Inhibition of rat cardiac muscle contraction and mitochondrial respiration by endogenous peroxynitrite formation during posthypoxic reoxygenation. Circ Res. 1998;82:891–7. doi: 10.1161/01.res.82.8.891. [DOI] [PubMed] [Google Scholar]
- 8.Xie YW, Wolin MS. Role of nitric oxide and its interaction with superoxide in the suppression of cardiac muscle mitochondrial respiration. Involvement in response to hypoxia/reoxygenation. Circulation. 1996;94:2580–6. doi: 10.1161/01.cir.94.10.2580. [DOI] [PubMed] [Google Scholar]
- 9.Zhao X, He G, Chen YR, Pandian RP, Kuppusamy P, Zweier JL. Endothelium-derived nitric oxide regulates postischemic myocardial oxygenation and oxygen consumption by modulation of mitochondrial electron transport. Circulation. 2005;111:2966–72. doi: 10.1161/CIRCULATIONAHA.104.527226. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, Liu B, Zweier JL, He G. Formation of hydrogen peroxide and reduction of peroxynitrite via dismutation of superoxide at reperfusion enhances myocardial blood flow and oxygen consumption in postischemic mouse heart. J Pharmacol Exp Ther. 2008;327:402–10. doi: 10.1124/jpet.108.142372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levrand S, Vannay-Bouchiche C, Pesse B, Pacher P, Feihl F, Waeber B, Liaudet L. Peroxynitrite is a major trigger of cardiomyocyte apoptosis in vitro and in vivo. Free Radic Biol Med. 2006;41:886–95. doi: 10.1016/j.freeradbiomed.2006.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray J, Taylor SW, Zhang B, Ghosh SS, Capaldi RA. Oxidative damage to mitochondrial complex I due to peroxynitrite: identification of reactive tyrosines by mass spectrometry. J Biol Chem. 2003;278:37223–30. doi: 10.1074/jbc.M305694200. [DOI] [PubMed] [Google Scholar]
- 14.MacMillan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry. 1998;37:1613–22. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- 15.Aslan M, Ryan TM, Townes TM, Coward L, Kirk MC, Barnes S, Alexander CB, Rosenfeld SS, Freeman BA. Nitric oxide-dependent generation of reactive species in sickle cell disease. Actin tyrosine induces defective cytoskeletal polymerization. J Biol Chem. 2003;278:4194–204. doi: 10.1074/jbc.M208916200. [DOI] [PubMed] [Google Scholar]
- 16.Eiserich JP, Estevez AG, Bamberg TV, Ye YZ, Chumley PH, Beckman JS, Freeman BA. Microtubule dysfunction by posttranslational nitrotyrosination of alpha-tubulin: a nitric oxide-dependent mechanism of cellular injury. Proc Natl Acad Sci USA. 1999;96:6365–70. doi: 10.1073/pnas.96.11.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takakura K, Beckman JS, MacMillan-Crow LA, Crow JP. Rapid and irreversible inactivation of protein tyrosine phosphatases PTP1B, CD45, and LAR by peroxynitrite. Arch Biochem Biophys. 1999;369:197–207. doi: 10.1006/abbi.1999.1374. [DOI] [PubMed] [Google Scholar]
- 18.Yakovlev VA, Barani IJ, Rabender CS, Black SM, Leach JK, Graves PR, Kellogg GE, Mikkelsen RB. Tyrosine nitration of IkappaBalpha: a novel mechanism for NF-kappaB activation. Biochemistry. 2007;46:11671–83. doi: 10.1021/bi701107z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oury TD, Tatro L, Ghio AJ, Piantadosi CA. Nitration of tyrosine by hydrogen peroxide and nitrite. Free Radic Res. 1995;23:537–47. doi: 10.3109/10715769509065275. [DOI] [PubMed] [Google Scholar]
- 20.Ko J, Yan J, Zhu L, Qi Y. Subtilisin QK, a fibrinolytic enzyme, inhibits the exogenous nitrite and hydrogen peroxide induced protein nitration, in vitro and in vivo. J Biochem Mol Biol. 2005;38:577–83. doi: 10.5483/bmbrep.2005.38.5.577. [DOI] [PubMed] [Google Scholar]
- 21.Crow JP, Ischiropoulos H. Detection and quantitation of nitrotyrosine residues in proteins: in vivo marker of peroxynitrite. Methods Enzymol. 1996;269:185–94. doi: 10.1016/s0076-6879(96)69020-x. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn DM, Sakowski SA, Sadidi M, Geddes TJ. Nitrotyrosine as a marker for peroxynitrite-induced neurotoxicity: the beginning or the end of the end of dopamine neurons? J Neurochem. 2004;89:529–36. doi: 10.1111/j.1471-4159.2004.02346.x. [DOI] [PubMed] [Google Scholar]
- 23.MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci USA. 1996;93:11853–8. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turko IV, Li L, Aulak KS, Stuehr DJ, Chang JY, Murad F. Protein tyrosine nitration in the mitochondria from diabetic mouse heart. Implications to dysfunctional mitochondria in diabetes. J Biol Chem. 2003;278:33972–7. doi: 10.1074/jbc.M303734200. [DOI] [PubMed] [Google Scholar]
- 25.Hong SJ, Gokulrangan G, Schoneich C. Proteomic analysis of age dependent nitration of rat cardiac proteins by solution isoelectric focusing coupled to nanoHPLC tandem mass spectrometry. Exp Gerontol. 2007;42:639–51. doi: 10.1016/j.exger.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanski J, Behring A, Pelling J, Schoneich C. Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: effects of biological aging. Am J Physiol Heart Circ Physiol. 2005;288:H371–81. doi: 10.1152/ajpheart.01030.2003. [DOI] [PubMed] [Google Scholar]
- 27.Zhu X, Liu B, Zhou S, Chen YR, Deng Y, Zweier JL, He G. Ischemic preconditioning prevents in vivo hyperoxygenation in postischemic myocardium with preservation of mitochondrial oxygen consumption. Am J Physiol Heart Circ Physiol. 2007;293:H1442–50. doi: 10.1152/ajpheart.00256.2007. [DOI] [PubMed] [Google Scholar]
- 28.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 29.Salinovich O, Montelaro RC. Reversible staining and peptide mapping of proteins transferred to nitrocellulose after separation by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1986;156:341–7. doi: 10.1016/0003-2697(86)90263-0. [DOI] [PubMed] [Google Scholar]
- 30.Chen CL, Zhang L, Yeh A, Chen CA, Green-Church KB, Zweier JL, Chen YR. Site-specific S-glutathiolation of mitochondrial NADH ubiquinone reductase. Biochemistry. 2007;46:5754–65. doi: 10.1021/bi602580c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vinogradov AD, King TE. The Keilin-Hartree heart muscle preparation. Methods Enzymol. 1979;55:118–27. doi: 10.1016/0076-6879(79)55017-4. [DOI] [PubMed] [Google Scholar]
- 32.Radi R, Rodriguez M, Castro L, Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 33.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451–64. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 34.Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res. 2008;77:334–43. doi: 10.1093/cvr/cvm005. [DOI] [PubMed] [Google Scholar]
- 35.Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun. 2003;305:776–83. doi: 10.1016/s0006-291x(03)00814-3. [DOI] [PubMed] [Google Scholar]
- 36.Schoneich C, Sharov VS. Mass spectrometry of protein modifications by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;41:1507–20. doi: 10.1016/j.freeradbiomed.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Aulak KS, Koeck T, Crabb JW, Stuehr DJ. Dynamics of protein nitration in cells and mitochondria. Am J Physiol Heart Circ Physiol. 2004;286:H30–8. doi: 10.1152/ajpheart.00743.2003. [DOI] [PubMed] [Google Scholar]
- 38.Koeck T, Fu X, Hazen SL, Crabb JW, Stuehr DJ, Aulak KS. Rapid and selective oxygen-regulated protein tyrosine denitration and nitration in mitochondria. J Biol Chem. 2004;279:27257–62. doi: 10.1074/jbc.M401586200. [DOI] [PubMed] [Google Scholar]
- 39.Koeck T, Stuehr DJ, Aulak KS. Mitochondria and regulated tyrosine nitration. Biochem Soc Trans. 2005;33:1399–403. doi: 10.1042/BST0331399. [DOI] [PubMed] [Google Scholar]
- 40.Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in mammalian cells. Faseb J. 1997;11:526–34. [PubMed] [Google Scholar]
- 41.Sazanov LA, Hinchliffe P. Structure of the hydrophilic domain of respiratory complex I from Thermus thermophilus. Science. 2006;311:1430–6. doi: 10.1126/science.1123809. [DOI] [PubMed] [Google Scholar]
- 42.Crofts AR. Proton-coupled electron transfer at the Qo-site of the bc1 complex controls the rate of ubihydroquinone oxidation. Biochim Biophys Acta. 2004;1655:77–92. doi: 10.1016/j.bbabio.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Leyva JA, Bianchet MA, Amzel LM. Understanding ATP synthesis: structure and mechanism of the F1-ATPase (Review) Mol Membr Biol. 2003;20:27–33. doi: 10.1080/0968768031000066532. [DOI] [PubMed] [Google Scholar]
- 44.Chen CL, Chen J, Rawale S, Varadharaj S, Kaumaya PP, Zweier JL, Chen YR. Protein tyrosine nitration of the flavin subunit is associated with oxidative modification of mitochondrial complex II in the post-ischemic myocardium. J Biol Chem. 2008;283:27991–8003. doi: 10.1074/jbc.M802691200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghafourifar P, Colton CA. Compartmentalized nitrosation and nitration in mitochondria. Antioxid Redox Signal. 2003;5:349–54. doi: 10.1089/152308603322110913. [DOI] [PubMed] [Google Scholar]
- 46.Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B, Link TA, Ramaswamy S, Jap BK. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- 47.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–63. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 48.Witze ES, Old WM, Resing KA, Ahn NG. Mapping protein post-translational modifications with mass spectrometry. Nat Methods. 2007;4:798–806. doi: 10.1038/nmeth1100. [DOI] [PubMed] [Google Scholar]
- 49.Zhan X, Desiderio DM. The human pituitary nitroproteome: detection of nitrotyrosyl-proteins with two-dimensional Western blotting, and amino acid sequence determination with mass spectrometry. Biochem Biophys Res Commun. 2004;325:1180–6. doi: 10.1016/j.bbrc.2004.10.169. [DOI] [PubMed] [Google Scholar]
- 50.Irie Y, Saeki M, Kamisaki Y, Martin E, Murad F. Histone H1.2 is a substrate for denitrase, an activity that reduces nitrotyrosine immunoreactivity in proteins. Proc Natl Acad Sci USA. 2003;100:5634–9. doi: 10.1073/pnas.1131756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Federici A, Ruggiero FM. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ Res. 2004;94:53–9. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- 52.Hardy L, Clark JB, Darley-Usmar VM, Smith DR, Stone D. Reoxygenation-dependent decrease in mitochondrial NADH:CoQ reductase (Complex I) activity in the hypoxic/reoxygenated rat heart. Biochem J. 1991;274(Pt 1):133–7. doi: 10.1042/bj2740133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.