Abstract
Seleno-L-methionine (SeMet) can be oxidized to L-methionine selenoxide (MetSeO) by flavin-containing monooxygenase 3 (FMO3) and rat liver microsomes in the presence of NADPH. MetSeO can be reduced by GSH to yield SeMet and GSSG. In the present study, the potential reduction of MetSeO to SeMet by other cellular components and antioxidants was investigated. Besides GSH, other thiols (L-cysteine, or N-acetyl-L-cysteine) and antioxidants (ascorbic acid and methimazole) also reduced MetSeO to SeMet. This reduction is unique to MetSeO since methionine sulfoxide was not reduced to methionine under similar conditions. The MetSeO reduction by thiols was instaneous and much faster than the reduction by ascorbic acid or methimazole. However, only one molar equivalent of ascorbic acid or methimazole was needed to complete the reduction, as opposed to two molar equivalents of thiols. Whereas the disulfides produced by the reactions of MetSeO with thiols are chemically stable, methimazole disulfide readily decomposed at pH 7.4, 37°C to yield methimazole, methimazole-sulfenic acid, methimazole sulfinic acid, methimazole S-sulfonate, 1-methylimidazole and sulfite anion. Collectively, the results demonstrate reduction of MetSeO to SeMet by multiple endogenous thiols, ascorbic acid, and methimazole. Thus, oxidation of SeMet to MetSeO may result in depletion of endogenous thiols and antioxidant molecules. Furthermore, the novel reduction of MetSeO by methimazole provides clear evidence that methimazole should not be used as an alternative FMO substrate when studying FMO-mediated oxidation of SeMet.
Keywords: selenomethionine, methionine selenoxide, methimazole, Vitamin C, GSH oxidation, flavin-containing monooxygenases
1. Introduction
Seleno-L-methionine (SeMet1), a naturally occurring amino acid, has potent growth inhibitory and apoptotic activities against multiple human tumor cell lines, including breast, colon, liver, lung, prostate, skin and lymphoid cells [1–5]. SeMet has also been suggested to have anti-cancer/chemopreventive properties in human clinical studies [6–7]. However, acute or chronic exposures to high concentrations of SeMet have caused toxicity [8–10]. For example, mallards fed diets containing SeMet (10 ppm Se) had lower body weights relative to controls and exhibited teratogenic effects [9], whereas mallard ducklings fed higher levels of SeMet (30 ppm Se) resulted in high mortality, impaired growth in survivors, and exhibited decreased GSH and increased GSSG levels in the liver [10]. Yet the mechanisms of the biological activities of SeMet remain unclear.
SeMet can potentially serve as an antioxidant in biological systems [11]. It can be readily oxidized by peroxynitrite, the reaction product of nitric oxide and superoxide [12–13]. The two-electron oxidation results in formation of L-methionine selenoxide (MetSeO). Recent work in our laboratory demonstrated that SeMet can also be oxidized to MetSeO by recombinant or purified flavin-containing monooxygenase 1 (FMO1) and FMO3 from rats or humans [14–15]. Because SeMet exhibited higher affinity for purified rat liver FMO3 (Km = 0.11–0.31 mM) than purified rat liver FMO1 (Km = 7.8 mM), and because the rat liver microsomal activity was not inhibited by the inclusion of 1-benzylimidazole, superoxide dismutase, or deferoxamine, FMO3 was suggested to be the major enzyme involved in SeMet oxidation in rat liver microsomes. Indeed, because the SeMet Km value for FMO3 was similar or lower than the SeMet Km values for methionine adenosyltransferase (0.6–0.8 mM), methionine γ-lyase (0.51 mM), or glutamine transaminase (0.13 mM), FMO3 was suggested to play an important role in the biological activities of SeMet at low exposure levels [14]. The finding that MetSeO can be recycled back to SeMet by two molecules of GSH, producing GSSG in the process, suggests that GSH may protect against the toxicity of MetSeO [14,16–17].
In the present study, the ability of the endogenous thiols L-cysteine, N-acetyl-L-cysteine and GSH, the FMO substrates methimazole (MIZ, 1-methyl-2-mercaptoimidazole) and L-methionine [18–19], and the antioxidant, ascorbic acid [20] to carry out the nonenzymatic reduction of MetSeO was examined to clarify the reactivity and/or selectivity of MetSeO towards these molecules and allow a better understanding of the mechanisms involved in SeMet-induced biological activities. MIZ was included because of its common use as a competitive inhibitor of FMO-mediated oxidations [19]. Ascorbic acid was previously shown to react with selenoxides under mild acidic conditions to give dehydroascorbic acid [21]. Ascorbic acid depletion has also been reported as a result of product recycling during the selenoxidation of phenyl 2-aminoethyl selenide by dopamine β-monooxygenase [22].
2. Materials and Methods
2.1. Reagents
SeMet, L-cysteine, N-acetyl-L-cysteine, N-acetyl-L-methionine, L-methionine, L-methionine sulfoxide, ascorbic acid (vitamin C), MIZ, and both the reduced and oxidized forms of GSH were obtained from Sigma-Aldrich. Additional SeMet and 30% hydrogen peroxide were purchased from Acros Chemical. MetSeO (purity > 95%) was synthesized as previously described [14]. Bis-(1-methylimidazole)-2,2′-disulfide (MIZ-disulfide) was prepared by reacting MIZ in NaHCO3 with I2/KI [23] and its identity was confirmed by electrospray ionization (ESI)-mass spectrometry (MS) carried out at the Mass Spectrometry Facility of the Biotechnology Center at University of Wisconsin - Madison (Madison, WI). An Applied Biosystems 3200 Q Trap LC/MS/MS with direct injection was used. HPLC grade acetonitrile was obtained from EM Science. All other chemicals were of the highest quality commercially available.
2.2. Reduction of MetSeO by potential reductants
MetSeO (1 μmol) and GSH (1, 2, or 5 μmol) were reacted in phosphate buffer (0.1 M KH2PO4, 0.1 M KCl, 5 mM EDTA, pH 7.4) or PBS (137 mM NaCl, 2.7 mM KCl, 5.2 mM NaHPO4, 0.9 mM KH2PO4, pH 7.4) in a total reaction volume of 1 ml at 37°C. Aliquots of the reaction were taken at 5 and 25 min and analyzed by HPLC as described below. Stabilities of SeMet, MetSeO, GSH, and GSSG in the phosphate buffer in the presence or absence of GSH or GSSG were also examined after incubation at 37°C for 75 min.
To determine if endogenous compounds, other than GSH, and antioxidants could serve as a reductant of MetSeO, several compounds including L-cysteine, N-acetyl-L-cysteine, L-methionine, methimazole and ascorbic acid were used. Briefly, MetSeO (1 μmol) was reacted with 1, 2, or 5 μmol antioxidant in phosphate buffer in a total reaction volume of 1 ml at 37°C. In order to characterize the early eluting products in the MetSeO with MIZ reaction, the buffer was changed to PBS in some incubations. Time points were taken initially and then every 25 min thereafter up to 150 min. Samples were filtered through Acrodisc filters and analyzed by HPLC as described below.
In some experiments, L-methionine sulfoxide was used in place of MetSeO to determine if the presence of GSH, MIZ or ascorbic acid at the 1:5 ratio as described above would also result in the reduction of L-methionine sulfoxide. Time points were taken initially and monitored every 30 min until 120 min.
2.3. HPLC analyses of MetSeO reduction samples
HPLC analyses were carried out with UV detection using a Gilson gradient controlled HPLC system equipped with a Gilson 117 UV detector and a Beckman Ultrasphere ODS 5 μm reverse-phase C-18 column (4.6 × 250 mm). Injection volume was 100 μl by a Gilson 234 autoinjector and the wavelength was 220 nm. The mobile phase on pump A was 0.015 M H3PO4 (pH 2.3) and on pump B was 0.015 M H3PO4 (pH 2.3) in 50% acetonitrile/water with a flowrate of 1 ml/min. The gradient used was initially 0% pump B for 7 min. The gradient was then increased to 10%B over 1 min where it was held for 6 min before it returned to 0% B over one min for a total run time of 20 min. The retention times for MetSeO, GSH, SeMet, and GSSG were 2.7, 5.8, 8.4, and 12.8 min, respectively. The retention times of ascorbic acid and MIZ were 4.2 and 12.2 min, respectively. UV/Vis spectra of products in the reaction of MetSeO and MIZ were obtained using chromatographic conditions as described above except a Beckman HPLC system equipped with a Beckman 168 diode array detector was used. Spectra were obtained from 200–500 nm. Standard curves for SeMet, MetSeO, GSH, and GSSG were obtained by plotting the peak areas versus the concentration. Linear regression produced correlation values of >0.99.
2.4. Characterization of products formed by the reaction of MetSeO with MIZ
Because several new peaks were observed in the HPLC chromatogram of the reaction of MetSeO with MIZ, the reaction mixture was analyzed by LC/MS using an Agilent 1100 LC-time of flight (TOF)-ESI/MS. The system was equipped with a Zorbax™ 80Å (50 × 2.1 mm) column (Agilent, Palo Alto, CA) run at a flowrate of 200 μl/min. The mobile phase on pump A was 0.1% formic acid and pump B was acetonitrile containing 0.1% formic acid. The gradient used had an initial composition of 0%B where it was held for 3 min. The %B then increased to 100% over 27 min and then decreased to the initial concentration over 2 min.
2.5. Stability of MIZ S-sulfonate
Attempts to isolate MIZ S-sulfonate by HPLC fractionation from the reaction mixture of MIZ and MetSeO were not successful. Therefore, the stability of MIZ S-sulfonate was characterized in the crude reaction mixture. Aliquots from the reaction mixture (pH 7), before and after adjusting to pH 3 and 5, were incubated at 37°C for 0–120 min and analyzed by HPLC as described above.
3. Results
3.1. Chemical reaction of MetSeO with endogenous thiols
A highly sensitive HPLC method was developed to monitor the chemical reaction of MetSeO with GSH and the resulting products SeMet and GSSG. Control experiments showed that the peak area of MetSeO, SeMet, GSH, or GSSG (5 mM) did not change after individual incubations at pH 7.4 and 37°C for 75 min. Moreover, incubation of MetSeO (2.5 mM) in the presence of GSSG (2.5 mM) or incubation of SeMet (2.5 mM) in the presence of GSH (2.5 mM) did not affect the HPLC peak areas of any of these compounds. Immediately after the addition of MetSeO (1 μmol) and GSH (1, 2 and 5 μmol), two new peaks whose formation was dependent on the presence of both MetSeO and GSH were detected. The retention times of these peaks were identical to those of reference SeMet and GSSG, and the reaction was complete instantaneously (Figure 1A). The results showed formation of 0.5, 1.0 and 1.0 μmol of both SeMet and GSSG at the 1:1, 1:2 and 1:5 SeMet:thiol molar ratios (Figure 1A; data for the 1:5 molar ratio was not shown for clarity of this figure). This demonstrates that the reduction of MetSeO requires two molar equivalents of GSH to be completed.
Figure 1.
Loss of MetSeO and formation of SeMet by endogenous thiols (GSH, A, and L-cysteine, B) and antioxidants (MIZ, C, and ascorbic acid, D) after in vitro incubations of MetSeO with these compounds at different molar ratios at physiological conditions (pH 7.4, 37ºC). Solid lines indicate SeMet values and dashed lines indicate MetSeO values. Filled circles represent experiments conducted at a 1:1 molar ratio, open circles 1:2 molar ratio, and filled triangles 1:5 molar ratio. Values presented are means ± S.D. (n=3).
Similar to GSH, L-cysteine, reduced MetSeO to SeMet (Figure 1B). At the 1:1 molar ratio only half of the MetSeO was converted to SeMet whereas at the 1:2 molar ratio, complete reduction of MetSeO occurred immediately and 1 μmol SeMet was formed in the process. Similar results were also obtained with N-acetyl-L-cysteine (data not shown).
3.2. Reaction of MetSeO with ascorbic acid or MIZ
Ascorbic acid or MIZ initially reduced MetSeO to SeMet at slower rates than the thiol-containing compounds (Figure 1). The reaction with ascorbic acid or methimazole was nearly complete by 25 min at the 1:2 MetSeO:ascorbic acid or MIZ molar ratio. However, compared to GSH that produced 0.5 μmole SeMet from a 1:1 molar ratio of MetSeO:GSH, the final extent of MetSeO reduction by ascorbic acid or MIZ was higher at the 1:1 molar ratio and was similar to the extent of reduction observed at the 1:2 molar ratio (Figure 1C, D).
3.3. Characterization of additional products from the MetSeO incubation with MIZ
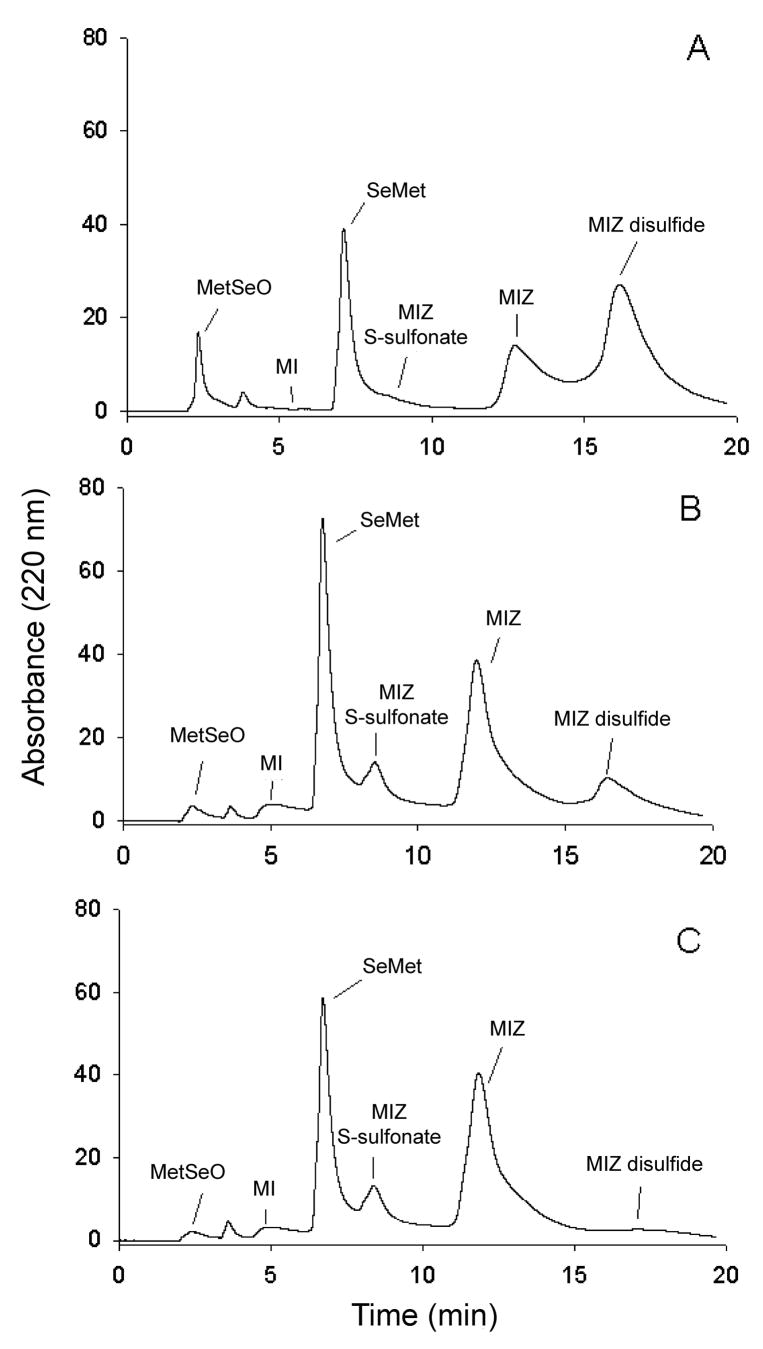
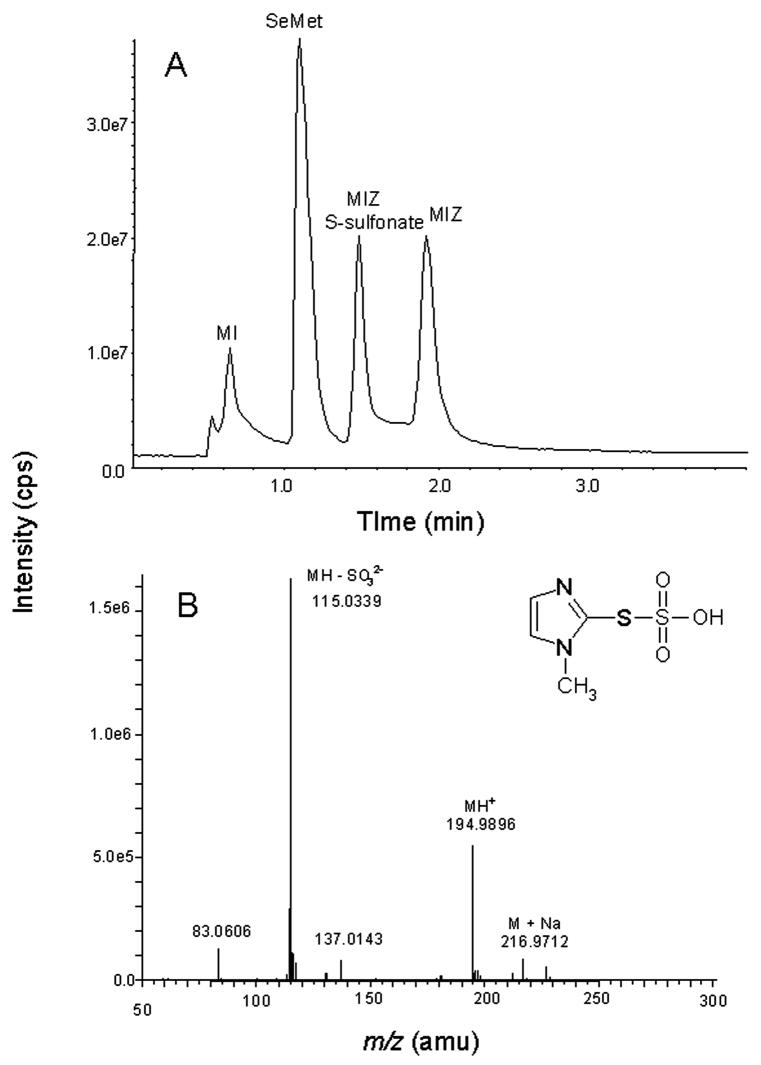
In addition to SeMet, three additional peaks were observed in the HPLC chromatograms of the reaction of MetSeO and MIZ (Figure 2). These peaks were identified as MIZ-disulfide (retention time 15.8 min; Figure 2), MIZ S-sulfonate (retention time 8.5 min) and 1-methylimidazole (retention time 3.6 min) based on coelution with reference compounds and/or MS characterization. MIZ-disulfide was synthesized as previously described [23] and characterized by ESI/MS. The MH+ detected was m/z 227 which is the same as reported in Freeman et al. [24]. The MS/MS of m/z 227 resulted in m/z 114 which corresponds to MIZ (data not shown). The UV/Vis spectra of MIZ-disulfide and MIZ showed a λmax of 253 nm and 252 nm, respectively. However, only the spectrum of generated MIZ-disulfide had a broad peak from 280–320 nm similar to that of the MIZ-disulfide produced by the reaction of MIZ with hypochlorous acid [25]. In the reaction of MetSeO with MIZ, the MIZ-disulfide peak was detectable immediately but then decreased over time until only a trace could be detected at 150 min (Figure 2). Another new peak in the chromatogram that eluted at 8.5 min (Figure 2) was detected initially in only trace amounts and increased until 50 min where it remained stable until 150 min. This peak was identified as the S-sulfonate of MIZ by LC/MS (retention time 1.48 min; Figure 3A) of the reaction mixture. The peak at m/z 194.9 corresponds to MH+ (Figure 3B). The small peak at 216.9 is M+Na. The major fragment at m/z 115.0 corresponds to the loss of SO32−. The Na salt of this anion was also detected at m/z 137.0. The UV/Vis spectrum showed that the λmax of MIZ S-sulfonate was 245 nm. The MIZ peak increased until 50 min rather than decreased as expected, indicating that it was being recycled after initial reaction with MetSeO. 1-Methylimidazole (MI) was also identified in the reaction (Figure 2). The area of this peak also increased until 50 min where it remained relatively stable in the reaction mixture. In the LC/MS run, this peak (MI) was also identified at a retention time of 0.64 min (Figure 3A) and produced an m/z 83.1 that corresponds to MH+.
Figure 2.
Typical HPLC chromatogram of the reaction of MetSeO with MIZ with UV detection at 220 nm after 0 min (A), 50 min (B), and 150 min (C) reaction time. MI = 1-methylimidazole.
Figure 3.
A) LC/MS chromatogram of the reaction mixture of MetSeO and MIZ at pH 7.4, 37ºC. The identities of peaks I-IV, were confirmed by MS analyses as 1-methylimidazole, SeMet, MIZ-S-sulfonate and MIZ, respectively. The mass spectrum of the MIZ S-sulfonate peak is shown (B) as to our knowledge, the mass spectrum of this compound has not been previously reported.
3.4. Stability of MIZ S-sulfonate at 37°C and different pHs
MIZ S-sulfonate was stable at pH 7.0 for at least 2 h, whereas it was almost totally decomposed by 60 min at pH 5.0 (data not shown). The only other peak in the chromatogram whose area changed was MIZ and it increased, demonstrating that MIZ S-sulfonate breaks down into MIZ; similar results were observed at pH 3.0 (data not shown).
3.5. Reaction of L-methionine and N-acetyl-L-methionine with MetSeO under physiological conditions
The ability of L-methionine or N-acetyl-L-methionine to reduce MetSeO at pH 7.4, 37°C over time (up to 150 min) was also examined. However, little to no reduction (< 5%) was observed with these compounds, even at a molar ratio as high as 1:5 (MetSeO:reductant; data not shown).
3.6. Reaction of L-methionine sulfoxide with GSH, ascorbic acid or MIZ
To determine if the reaction of GSH, ascorbic acid or MIZ with MetSeO was unique to MetSeO, the structural analog, L-methionine sulfoxide was incubated under similar conditions as carried out with MetSeO using GSH, ascorbic acid, and MIZ. However, no change in the concentration of L-methionine sulfoxide or formation of L-methionine was observed with any of these compounds (data not shown).
4. Discussion
Similar to GSH, L-cysteine and N-acetyl-L-cysteine reduced MetSeO to SeMet at similar stoichiometry (2:1 thiol:MetSeO). These results suggest multiple endogenous thiols could be depleted secondary to SeMet exposure. SeMet has been shown to cause growth inhibition in several human tumor cell lines at concentrations ranging from 40-130 μM [1]. Although mammalian cells typically have much higher GSH concentrations (0.1-10 mM) [26], repeated recycling of SeMet between reduced and oxidized forms can cause depletion of GSH and formation of GSSG and protein mixed disulfides.
Ascorbic acid, an antioxidant that does not contain a thiol moiety, has also been shown to reduce MetSeO to SeMet. The stoichiometry observed for the latter reaction (1:1) is similar to that observed for the reduction of phenyl 2-aminoethyl selenoxide by ascorbic acid [22]. Ascorbic acid protects cells by reducing reactive oxygen and nitrogen species to stable molecules. It also acts as a cofactor in the biosynthesis of catecholamine, carnitine and collagen [27]. Vitamin C is typically found in human plasma at concentrations ranging from 50–70 μmol/L [28] and can accumulate 50-fold in human tissues compared to plasma [29]. Similar to GSH, repeated recycling of SeMet could result in depletion of ascorbic acid. Thus, oxidative metabolism of SeMet to MetSeO and reduction of MetSeO back to SeMet can lead to depletion of endogenous thiols and antioxidants, such as ascorbic acid, leading to cellular oxidative stress and injury.
MIZ is a thioureylene antithyroid drug that is also used as an alternative FMO substrate to inhibit FMO-mediated reactions. Because SeMet is a substrate for multiple FMOs, it was of interest to examine if MIZ could also reduce MetSeO under physiological conditions (pH 7.4. 37°C) to yield SeMet. The results of the reaction of MetSeO with MIZ were unexpected since MIZ (pKa = 12) [30] was previously shown not to react with 5,5′-dithiobis(2-nitrobenzoate) whereas GSH (pKa = 9.2) [31–32] readily reacted with this compound [33]. MIZ has been previously shown to have antioxidant properties and protected against nephrotoxicity mediated by gentamicin, cephaloridine, cisplatin, S-(1,2-dichlorovinyl)-L-cysteine, or 2-bromohydroquinone [34–35]. In the present study, MIZ readily reduced MetSeO back to SeMet, suggesting MIZ should not be used as an alternate substrate when studying FMO-mediated oxidation of SeMet.
Similar to the reaction of MetSeO with GSH, the disulfide of MIZ was detected in the reaction mix. However, unlike GSSG which is chemically stable and requires GSH reductase and NADPH to be recycled back to GSH [16–17], MIZ-disulfide was unstable and quickly decomposed to MIZ and MIZ S-sulfonate (Figures 3, 4). The instability of MIZ disulfide which is consistent with its chemical structure (imidazoyl disulfide) has previously been reported [23, 25]. A possible mechanism for MIZ S-sulfonate formation involves the hydrolysis of MIZ disulfide to yield MIZ, MIZ-sulfenic acid, MIZ sulfinic acid, MI, and sulfite anion followed by reaction of the sulfite anion with MIZ-disulfide (Figure 4). MIZ S-sulfonate was stable at pH 7, 37°C (at least 150 min) but at pH 3 or pH 5, 37°C, it decomposed to yield MIZ over 60 min (data not shown). Small amounts of 1-methylimidazole, a known metabolite of MIZ in rat liver microsomes [36] were detected in incubations of MetSeO and MIZ for 25–150 min. However, if the reaction mixture was allowed to remain at 4°C overnight, the amount of detectable 1-methylimidazole increased. The exact mechanism for the formation of MI was not investigated, but as indicated above may involve the decomposition of MIZ sulfenic and sulfinic acids formed by the hydrolysis of MIZ-disulfide [24].
Figure 4.
Structures of MIZ and related compounds formed in its reaction with MetSeO and possible mechanisms of MIZ disulfide and MIZ S-sulfonate formation and/or breakdown. MI = 1-methylimidazole; MIZ-S-S-MIZ = MIZ disulfide.
Methionine sulfoxide, the sulfur analog of MetSeO, was also tested in the presence of GSH, Vitamin C or MIZ to determine if it could also be reduced to Met in a similar manner. Unlike MetSeO, methionine sulfoxide was completely stable under the conditions tested. Methionine sulfoxide has been previously shown to be stable in the presence of GSH [16]. This demonstrates that the reaction with thiols, Vitamin C or MIZ is unique to the selenoxide. Methionine sulfoxide is also known to be reduced to methionine by methionine sulfoxide reductases. Although MetSeO may potentially serve as a substrate for methionine sulfoxide reductases, the high reactivity of MetSeO towards endogenous thiols and the high concentration of these thiols in mammalian cells suggest a preference for non-enzymatic reduction of MetSeO.
In summary, the present study shows that several endogenous thiols and antioxidants are capable of reducing MetSeO, an oxidation product of SeMet, to recycle it back to SeMet. Thus, depletion of the cellular protectors, GSH and ascorbic acid, can occur after exposure to high levels of SeMet. Depletion of GSH and subsequent formation of GSSG and protein mixed disulfides (Figure 5) could cause changes in gene expression, and lead to oxidative stress, alterations of cellular function and/or cytotoxicity [37]. Finally, the FMO alternative substrate, MIZ is capable of reducing MetSeO to SeMet, thus, MIZ should not be used as an inhibitor to study FMO metabolism of selenium-containing compounds.
Figure 5.
Oxidative metabolism of SeMet by FMO3 and the reduction of MetSeO by GSH, MIZ, or ascorbic acid to regenerate SeMet.
Acknowledgments
A preliminary report of the data described in this manuscript was presented at the Experimental Biology meeting (2007) held in Washington, D.C. This work was supported by Grant DK 044295 from the National Institute of Diabetes, Digestive and Kidney Diseases, National Institutes of Health.
Footnotes
Abbreviations: FMOs, flavin-containing monooxygenases; SeMet, seleno-L-methionine; MetSeO, L-methionine selenoxide; MIZ, methimazole; MI, 1-methylimidazole.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Redman C, Scott JA, Baines AT, Basye JL, Clark LC, Calley C, Roe D, Payne CM, Nelson MA. Inhibitory effect of selenomethionine on the growth of three selected human tumor cell lines. Cancer Lett. 1998;125:103–10. doi: 10.1016/s0304-3835(97)00497-7. [DOI] [PubMed] [Google Scholar]
- 2.Goulet A-C, Chigbrow M, Frisk P, Nelson MA. Selenomethionine induces sustained ERK phosphorylation leading to cell-cycle arrest in human colon cancer cells. Carcinogenesis. 2005;26:109–17. doi: 10.1093/carcin/bgh306. [DOI] [PubMed] [Google Scholar]
- 3.Miki K, Xu M, Gupta A, Ba Y, Tan Y, Al-Refaie W, Bouvet M, Makuuchi M, Moossa AR, Hoffman RM. Methionase cancer gene therapy with selenomethionine as suicide prodrug substrate. Cancer Res. 2001;61:6805–10. [PubMed] [Google Scholar]
- 4.Zhao R, Domann FE, Zhong W. Apoptosis induced by selenomethionine and methioninase is superoxide mediated and p53 dependent in human prostate cancer cells. Mol Cancer Ther. 2006;5:3275–84. doi: 10.1158/1535-7163.MCT-06-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kajander EO, Harvima RJ, Eloranta TO, Martikainen H, Kantola M, Kärenlampi SO, Åkerman K. Metabolism, cellular actions, and cytotoxicity of selenomethionine in cultured cells. Biol Trace Elem Res. 1991;28:39–45. doi: 10.1007/BF02990463. [DOI] [PubMed] [Google Scholar]
- 6.Clark LC, Combs GF, Turnbull WB, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL, Park HK, Sanders BB, Smith CL, Taylor JR The Nutrition Prevention of Cancer Study Group. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: a randomized controlled trial. J Am Med Assoc. 1996;276:957–1963. [PubMed] [Google Scholar]
- 7.Klein EA, Lippman SM, Thompson IM, Goodman PJ, Albanes D, Taylor PR, Coltman C. The selenium and vitamin E cancer prevention trial. World J Urology. 2003;21:21–7. doi: 10.1007/s00345-002-0314-z. [DOI] [PubMed] [Google Scholar]
- 8.Schrauzer GN. Selenomethionine: a review of its nutritional significance, metabolism and toxicity. J Nutr. 2000;130:1653–6. doi: 10.1093/jn/130.7.1653. [DOI] [PubMed] [Google Scholar]
- 9.Heinz GH, Hoffman DJ, Krynitsky AJ, Weller DMG. Reproduction in mallards fed selenium. Environ Toxicol Chem. 1987;6:423–33. [Google Scholar]
- 10.Hoffman DJ, Heinz GH, LeCaptain LJ, Eisemann JD, Pendleton GW. Toxicity and oxidative stress of different forms of organic selenium and dietary protein in mallard ducklings. Arch Environ Contam Toxicol. 1996;31:120–7. doi: 10.1007/BF00203916. [DOI] [PubMed] [Google Scholar]
- 11.Walter R, Roy J. Selenomethionine, a potential catalytic antioxidant in biological systems. J Org Chem. 1971;36:2561–3. doi: 10.1021/jo00816a045. [DOI] [PubMed] [Google Scholar]
- 12.Roussyn I, Briviba K, Masumoto H, Sies H. Selenium-containing compounds protect DNA from single-strand breaks caused by peroxynitrite. Arch Biochem Biophys. 1996;330:216–8. doi: 10.1006/abbi.1996.0245. [DOI] [PubMed] [Google Scholar]
- 13.Padmaja S, Squadrito GL, Lemercier J-N, Cueto R, Pryor WA. Rapid oxidation of DL-selenomethionine by peroxynitrite. Free Radic Biol Med. 1996;21:317–22. doi: 10.1016/0891-5849(96)00132-3. [DOI] [PubMed] [Google Scholar]
- 14.Krause RJ, Glocke SC, Sicuri AR, Ripp SL, Elfarra AA. Oxidative metabolism of seleno-L-methionine to L-methionine selenoxide by flavin-containing monooxygenases. Chem Res Toxicol. 2006;12:1643–9. doi: 10.1021/tx0601915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novick RM, Elfarra AA. Purification and characterization of flavin-containing monooxygenase isoform 3 from rat kidney microsomes. Drug Metab Dispos. 2008 doi: 10.1124/dmd.108.021436. epub Sept. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assmann A, Briviba K, Sies H. Reduction of methionine selenoxide to selenomethionine by glutathione. Arch Biochem Biophys. 1998;349:201–3. doi: 10.1006/abbi.1997.0462. [DOI] [PubMed] [Google Scholar]
- 17.Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57:145–55. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elfarra AA, Krause RJ. Potential roles of flavin-containing monooxygenases in sulfoxidation reactions of L-methionine, N-acetyl-L-methionine and peptides containing L-methionine. Biochim Biophys Acta. 2005;1703:183–9. doi: 10.1016/j.bbapap.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Krause RJ, Ripp SL, Sausen PJ, Overby LH, Philpot RM, Elfarra AA. Characterization of the methionine S-oxidase activity of rat liver and kidney microsomes: Immunochemical and kinetic evidence for FMO3 being the major catalyst. Arch Biochem Biophys. 1996;333:109–16. doi: 10.1006/abbi.1996.0370. [DOI] [PubMed] [Google Scholar]
- 20.Rumsey SC, Daruwala R, Al-Hasani H, Zarnowski MJ, Simpson IA, Levine M. (2000) Dehydroascorbic acid transport by GLUT4 in Xenopus oocytes and isolated rat adipocytes. J Biol Chem. 2000;275:28246–53. doi: 10.1074/jbc.M000988200. [DOI] [PubMed] [Google Scholar]
- 21.Balenovic K, Bregant N, Perina I. Oxidations with selenoxides; III1. A convenient method for the preparation of adrenochrome. Synthesis. 1973;3:172. [Google Scholar]
- 22.May SW, Herman HH, Roberts SF, Ciccarello MC. Ascorbate depletion as a consequence of product recycling during dopamine β-monooxygenase catalyzed selenoxidation. Biochem. 1987;26:1626–33. doi: 10.1021/bi00380a021. [DOI] [PubMed] [Google Scholar]
- 23.Taurog A, Dorris ML, Guziec FS., Jr Metabolism of 35S- and 14C-labeled 1-methyl-2-mercaptoimidazole in vitro and in vivo. Endocrinology. 1989;124:30–9. doi: 10.1210/endo-124-1-30. [DOI] [PubMed] [Google Scholar]
- 24.Freeman F, Keindl MC, Po HN, Brinkman E, Masse JA. Electrochemical synthesis of imidazolyl disulfides. Synthesis. 1989;9:714–5. [Google Scholar]
- 25.Nakamura M, Shishido N, Akutsu H. Reactions of 1-methyl-2-mercaptoimidazole with hypochlorous acid and superoxide. Jpn J Infect Dis. 2004;57:S34–5. [PubMed] [Google Scholar]
- 26.Jornot L, Junod AF. Variable glutathione levels and expression of antioxidant enzymes in human endothelial cells. Am J Physiol Lung Cell Mol Physiol. 1993;264:L482–489. doi: 10.1152/ajplung.1993.264.5.L482. [DOI] [PubMed] [Google Scholar]
- 27.Wilson JX. The physiological role of dehydroascorbic acid. FEBS Lett. 2002;527:5–9. doi: 10.1016/s0014-5793(02)03167-8. [DOI] [PubMed] [Google Scholar]
- 28.Catley L, Anderson KC. Velcade and vitamin C: too much of a good thing? Clin Cancer Res. 2006;12:3–4. doi: 10.1158/1078-0432.CCR-05-2272. [DOI] [PubMed] [Google Scholar]
- 29.Welch RW, Wang Y, Crossman A, Jr, Park JB, Kirk KL, Levine M. Accumulation of vitamin C (ascorbate) and its oxidized metabolite dehydroascorbic acid occurs by separate mechanisms. J Biol Chem. 1995;270:12584–92. doi: 10.1074/jbc.270.21.12584. [DOI] [PubMed] [Google Scholar]
- 30.Bailey PJ, Lorono-Gonzales D, McCormack C, Millican F, Parsons S, Pfeifer R, Pinho PP, Rudolphi F, Sanchez Perucha A. Reaction of azole heterocycles with tris(dimethylamino)borane, a new method for the construction of tripodal borate-centred ligands. Chem Eur J. 2006;12:5293–300. doi: 10.1002/chem.200501323. [DOI] [PubMed] [Google Scholar]
- 31.Jemth P, Mannervik B. Kinetic characterization of recombinant human glutathione transferase T1-1, a polymorphic detoxication enzyme. Arch Biochem Biophys. 1997;348:247–54. doi: 10.1006/abbi.1997.0357. [DOI] [PubMed] [Google Scholar]
- 32.Jung G, Breitmaier E, Voelter W. Dissoziationsgleichgewichte von glutathion: eine fourier-transform-13C-NMR spektroskopische untersuchhung der pH-abhängigkeit der ladungsverteilung. Eur J Biochem. 1972;24:438–45. doi: 10.1111/j.1432-1033.1972.tb19704.x. [DOI] [PubMed] [Google Scholar]
- 33.Dixit A, Roche TE. Spectrophotometric assay of the flavin-containing monooxygenase and changes in its activity in female mouse liver with nutritional and diurnal conditions. Arch Biochem Biophys. 1984;233:50–63. doi: 10.1016/0003-9861(84)90600-3. [DOI] [PubMed] [Google Scholar]
- 34.Sausen PJ, Elfarra AA, Cooley AJ. Methimazole protection of rats against chemically induced kidney damage in vivo. J Pharmacol Exper Therap. 1992;260:393–401. [PubMed] [Google Scholar]
- 35.Elfarra AA, Duescher RJ, Sausen PJ, O’Hara TM, Cooley AJ. Methimazole protection of rats against gentamicin-induced nephrotoxicity. Can J Physiol Pharmacol. 1994;72:1238–44. doi: 10.1139/y94-176. [DOI] [PubMed] [Google Scholar]
- 36.Lee PW, Neal RA. Metabolism of methimazole by rat liver cytochrome P-450-containing monoxygenases. Drug Metab Dispos. 1978;6:591–600. [PubMed] [Google Scholar]
- 37.Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–88. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]