Abstract
Th2 cytokines induce the release of vascular endothelial growth factor (VEGF) from cultured human airway smooth muscle cells. The objective of this study was to examine the mechanistic basis for IL-4– and IL-13–induced VEGF release and to determine whether genetic differences are responsible for donor-to-donor variability in VEGF release. We measured VEGF mRNA expression by real-time PCR, mRNA stability using actinomycin D, and promoter activity with a VEGF-promoter luciferase reporter construct. We measured IL-4– and IL-13–induced VEGF release in cells from 21 donors by ELISA, genotyped the cells for common single nucleotide polymorphisms in the IL-4Rα (Ile50Val, Ser478Pro, and Gln551Arg) and VEGF (−460T/C, −160C/T, −152G/A, +405C/G and +936 C/T) genes, and stratified the data by IL-4Rα and VEGF genotype. IL-4 and IL-13 increased VEGF release and VEGF mRNA expression. IL-4 also increased mRNA stability but did not affect VEGF promoter activity. There was marked donor-to-donor variability in VEGF release from smooth muscle cells. The presence of Val50, Pro478/Arg551, or the Val50/Pro478/Arg551 IL-4Rα haplotype had little effect on VEGF release. VEGF genotype at +405 or +936 alone had no effect on VEGF release, whereas cells bearing at least one −460C/−152A/+405G VEGF allele had lower release of VEGF in response to IL-13 or IL-4 than cells with other genotypes. Our data suggest that IL-4 and IL-13 mediate their effects on VEGF expression post-transcriptionally and indicate that polymorphisms in the VEGF, but not the IL-4Rα, gene affect VEGF release from smooth muscle cells.
Keywords: asthma, IL-4Rα, polymorphism, TNFα, mRNA stability
Angiogenesis is a feature of asthma (1–3); the number of vessels in the airways correlates with asthma severity (4). Vascular endothelial growth factor (VEGF), a potent regulator of angiogenesis (5), is upregulated within the airways of persons with asthma and has been correlated with decline in FEV1 and with airway hyper-responsiveness (3, 6, 7). The importance of VEGF for asthma is emphasized by observations that VEGF inhibitors and VEGF receptor inhibitors reduce lung inflammation and airway hyper-responsiveness in mice (6, 8). Overexpression of VEGF in the airways enhances Th2-mediated sensitization and inflammation and is sufficient to induce airway hyperresponsiveness (8). Even short periods of increased VEGF expression promote airway remodeling and persistent airway hyperresponsiveness (8). In addition, the human VEGF gene is located on chromosome 6p21.3 (9), a region that has been linked to asthma and atopy (6, 10).
Potential cellular sources of VEGF in the airways include epithelial cells (11), mast cells (12), macrophages (13), and airway smooth muscle cells (13–16). Immunohistochemical studies indicate VEGF expression in airway smooth muscle in human, rat, and mouse airways (13, 17). Moreover, human airway smooth muscle (HASM) cells in culture produce VEGF constitutively and in response to stimulation with a variety of inflammatory mediators, including the Th2 cytokines IL-4 and IL-13 (14–16, 18, 19). VEGF also enhances IL-13 expression in the airways (8), suggesting a positive feedback loop with VEGF enhancing Th2 sensitization and inflammation and IL-13 subsequently enhancing VEGF production. Because of the importance of interactions between VEGF and Th2 cytokines, we sought to examine the mechanism of action of IL-4 and IL-13 on VEGF expression in airway smooth muscle. We measured the effects of Th2 cytokines on VEGF protein and mRNA expression, VEGF promoter activity, and VEGF mRNA stability. We noted substantial variability in the expression of VEGF from donor to donor. Thus, an additional purpose of this study was to examine the hypothesis that genetic factors may modulate IL-4 or IL-13 induced VEGF release.
In HASM cells, IL-4 and IL-13 signal through the IL-4Rα (20, 21). Three relatively common nonsynonymous single-nucleotide polymorphisms (SNP) in the coding region of the IL-4Rα (Ile50Val, Ser478Pro, and Gln551Arg) have been associated with atopy or asthma (10). The Val50/Pro478/Arg551 haplotype is also associated with increased IL-13– and IL-4–induced thymus- and activation-regulated chemokine (TARC) expression (22) and with increased IL-13–induced β-adrenergic desensitization in HASM cells (23).
The VEGF gene is also highly polymorphic (24, 25). Two common SNP in the VEGF gene, −460 T/C in the promoter region and +405 C/G in the 5′-untranslated region (UTR), and other SNP (−160 C/T and −152 G/A) in the VEGF promoter region and in the 3′-UTR (+936C/T) have been associated with diseases with an angiogenic basis and/or with VEGF expression (24, 26, 27). There is strong linkage disequilibrium across the VEGF gene, and these SNP may be markers for other unidentified causative SNP elsewhere in the gene.
To examine the importance of SNP in the IL-4Rα and VEGF genes, we measured IL-4– and IL-13–induced VEGF release in HASM cells from multiple donors and stratified the results by IL-4Rα and VEGF genotype. Our results indicate a substantial decrease in IL-13– or IL-4–induced VEGF release in cells bearing the −460C/−152A/+405G VEGF haplotype but no effect of IL-4Rα genotype.
MATERIALS AND METHODS
Cell Culture
Human tracheas were obtained from lung transplant donors in accordance with procedures approved by the University of Pennsylvania Committee on Studies Involving Human Beings and cultured as previously described (21, 28, 29). Cells from 21 donors in passages 4 to 8 were used in the studies described below. Unless otherwise indicated, cells were studied 2 wk after passage and were serum deprived and supplemented with 5.7 μg/ml insulin and 5 μg/ml transferrin 48 h before use.
ELISA
Cells were treated with IL-4, IL-13, or TNF-α (R&D Systems, Minneapolis, MN) for 24 h. VEGF165 and MCP-1 in cell supernatants were analyzed by ELISA (R&D Systems).
RNA Extraction and Real-Time-PCR
Cells were treated with IL-4 or IL-13 for 6 h. Total RNA was extracted with TRIzol reagent (Invitrogen; Life Technologies, Carlsbad, CA). Reverse transcription and quantitative real-time PCR for VEGF and GAPDH were performed as previously described (19).
Transfection of HASM Cells
Cells were transfected with 2 μg of a 2.4-kb human VEGF promoter-firefly luciferase reporter construct (courtesy of Dr. Richard Jove, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL) using Fugene 6 (Roche, Indianapolis, IN) as previously described (19). Twenty-four hours later, cells were treated with IL-4 for 24 h. A positive control (oncostatin M) was included in each experiment. Luciferase activity was assayed as previously described (19).
Analysis of mRNA Stability
Cells were treated with IL-4 for 6 h. Actinomycin D (Sigma, St. Louis, MO) was added (10 μg/ml), and total mRNA was isolated 0, 1, 2, and 4 h later. Total mRNA was extracted and analyzed for VEGF mRNA by colorimetric quantitation using the Quantikine mRNA system (R&D Systems).
Genotyping
Genomic DNA was extracted from HASM cells using DNA spin columns (Dneasy; Qiagen, Valencia, CA). The methods used for IL-4Rα genotyping have been previously described (22). To genotype loci in the VEGF, genomic DNA was amplified by PCR using three specific primer sets: F1: 5′-CGA GCA GCG TCT TCG AGA-3′ and R1: 5′-CAA GCC TCC GCG ATC CTC-3′ (amplifies a region containing the −460 T/C, −160 C/T, −152 G/A, and −116 G/A SNP); F2: 5′-CAG AGA GAA GTC GAG GAA GA-3′ and R2: 5′-CAA GGG GGA GGG CTC ACG-3′ (amplifies a region containing the +405 C/G SNP); and F3: 5′-AAG GAA GAG GAG ACT CTG CGC-3′ and R3: 5′-TAT GTG GGT GGG TGT GTC TAC AGG-3′ (amplifies a region containing the +936 C/T SNP). PCR products were resolved on 1.5% agarose gel followed by direct sequencing for −460 T/C, −160 C/T, −152 G/A, −116 G/A, and +405C/G. For determination of the +936 C/T SNP, PCR products were digested with NlaIII (New England Biolabs, Beverly, MA) for 2 h at 37°C followed by 20 min at 65°C. Fragments were analyzed on 2% agarose gels stained with ethidium bromide. The +936C allele remained uncut (198 bp), and the +936T allele was cut into two fragments of 122 and 86 bp.
Statistics
VEGF and MCP values were compared by ANOVA using Sigma Stat 3.0 (Jandel Corp., San Raphael, CA). Post hoc t tests were used if ANOVA indicated a significant effect. A P < 0.05 was considered significant.
RESULTS
IL-4 and IL-13 caused a concentration- and time-related increase in VEGF protein release from HASM cells (Figure 1), as reported by others (15). IL-4 induced VEGF release at concentrations as low as 0.3 ng/ml, whereas higher concentrations were required for IL-13 (Figure 1A), which is consistent with the relative efficacy of IL-4 and IL-13 for eotaxin release from HASM cells (20, 28).
Figure 1.
(A) Effects of increasing doses of IL-4 or IL-13 administered for 24 h on VEGF release from HASM cells. Values represent mean ± SE from 4–13 donors. Each donor was studied in duplicate.*P < 0.05, t test, compared with untreated cells. (B) Time course of VEGF protein release after treatment with IL-4 (10 ng/ml) or IL-13 (30 ng/ml). Values represent mean ± SE from three donors. Each donor was studied in duplicate.
To determine whether the effects of Th2 cytokines occurred at the transcriptional level, we measured VEGF mRNA expression by real-time PCR. There was constitutive VEGF mRNA expression in HASM cells, and IL-4 caused a concentration-dependent increase in VEGF mRNA expression. A significant increase in VEGF mRNA was observed with IL-4 doses as low as 3 ng/ml, although a trend could be observed even with 1 ng/ml (Figure 2A). IL-13 also increased VEGF mRNA expression (Figure 2A). IL-4 had no effect on VEGF promoter activity even though cells from the same donors treated at the same time with oncostatin M (OSM) showed a significant increase in VEGF promoter activity (Figure 2B) as previously described (19). To determine whether IL-4 modulated VEGF mRNA stability, we measured VEGF mRNA expression in cells treated or untreated with IL-4 for 6 h. Actinomycin D was added to the cells to inhibit transcription, and VEGF mRNA was measured at 0, 1, 2, and 4 h after the addition of actinomycin D. In cells treated with IL-4, VEGF mRNA had a longer half life (273 ± 22 min) than untreated cells (162 ± 39 min) (P < 0.05) (Figure 3).
Figure 2.
(A) Real-time PCR measurements of VEGF mRNA in HASM cells that were left untreated or treated for 6 h with IL-4 (0.3–30 ng/ml) or IL-13 (30 ng/ml). Results are mean ± SE of data from five to nine RNA samples in each case and are normalized relative to GAPDH mRNA. Results are expressed relative to VEGF expression in control cells. *P < 0.05 compared with untreated cells. (B) Luciferase activity in cells transfected with a VEGF promoter luciferase reporter construct. Cells were treated with OSM (20 ng/ml) or IL-4 (10 or 30 ng/ml) for 24 h. Results are mean ± SE of data from 9–21 cell wells in each case and were obtained in cells from three donors. Results are expressed relative to VEGF expression in control cells. *P < 0.05 compared with control cells.
Figure 3.
Effect of IL-4 (30 ng/ml) on VEGF mRNA stability. Cells were incubated with IL-4 for 6 h and treated with actinomycin D (10 μg/ml) at time 0. Results are mean ± SE from four donors.
Great variability in VEGF release was observed among cells from different donors after IL-13 or IL-4 stimulation. To evaluate the role of IL-4Rα genotype in this variability, we assayed VEGF in supernatants of HASM cells from donors chosen for their IL-4Rα genotype and previously stimulated with IL-4 or IL-13 (22). Each cell donor was studied in duplicate on one or two occasions. Although we have previously reported a marked increase in TARC release induced by TNF-α plus IL-4 or IL-13 in cells from donors with at least one Val50/Pro478/Arg551 allele, we observed no effect of IL-4Rα genotype on IL-4– or IL-13–induced VEGF release (Table 1). Two different conventions have been used for numbering amino acids in IL-4Rα, beginning from the start of the signal peptide or from the start of the mature protein. We use the latter method.
TABLE 1.
EFFECT OF IL-4Rα GENOTYPE AT AMINO ACID 50 AND AMINO ACIDS 478/551 ON VEGF PROTEIN RELEASE (ng/ml) FROM UNTREATED HASM CELLS OR CELLS TREATED WITH IL-13 (3 ng/ml), IL-4 (1 ng/ml), OR TNF-α (10 ng/ml)
| Genotype | Wt | He | Mu |
|---|---|---|---|
| Ile50 Val | |||
| Untreated | 0.342 ± 0.06 | 0.477 ± 0.09 | 0.382 ± 0.08 |
| IL-13 | 0.591 ± 0.14 | 1.024 ± 0.31 | 0.653 ± 0.12 |
| IL-4 | 0.808 ± 0.19 | 1.390 ± 0.52 | 1.036 ± 0.21 |
| TNF-α | 0.650 ± 0.13 | 1.308 ± 0.55 | 0.836 ± 0.20 |
| Ser478Pro/Gln551Arg | |||
| Untreated | 0.342 ± 0.04 | 0.443 ± 0.10 | 0.529 ± 0.09 |
| IL-13 | 0.782 ± 0.18 | 0.702 ± 0.13 | 0.664 ± 0.12 |
| IL-4 | 1.151 ± 0.28 | 0.901 ± 0.18 | 1.102 ± 0.23 |
| TNF-α | 0.961 ± 0.27 | 0.746 ± 0.27 | 1.100 ± 0.29 |
Definition of abbreviations: He, heterozygous; Mu, mutant; Wt, wild type. Values are mean ± SE of 5–11 cell donors in each group. Each donor was studied in two to five occasions in duplicate.
Because numerous SNP have also been described in the VEGF gene, we sought to determine whether the observed donor-related differences in VEGF release were dependent on the VEGF genotype of the cells. HASM cells were genotyped for −460T/C, −160C/T, −152G/A SNP in the promoter region; for +405C/G in the 5′-UTR; and for +936C/T in the 3′-UTR of the VEGF gene. Table 2 lists the VEGF genotypes of the 21 cell donors we examined. The VEGF allele frequencies in our bank of 59 HASM cell donors were as follows: −460C 0.52, −160T 0.0, −152A 0.50, +405G 0.61, and +936T 0.14. The frequencies were consistent with the literature (24, 25, 27). For example, others have reported that −460C and 152A are in strong linkage disequilibrium (26, 27). All cell donors we studied who had at least one −460C allele also had at least one −152A allele; these cells were designated as −460C/−152A. Others have also reported linkage disequilibrium between −460C and +405G polymorphisms (24, 27), which was confirmed by our data (see below).
TABLE 2.
VEGF GENOTYPE OF HASM CELL DONORS STUDIED
| Position +405
|
|||
|---|---|---|---|
| +405CC | +405CG | +405GG | |
| Position −460/−152 | |||
| −460TT/−152GG | 6 | 4 | 1* |
| −460TC/−152GA | 0 | 5 | 0 |
| −460CC/−152AA | 0 | 0 | 4* |
Definition of abbreviations: HASM, human airway smooth muscle; VEGF, vascular endothelial growth factor.
One of these donors was heterozygous at −152.
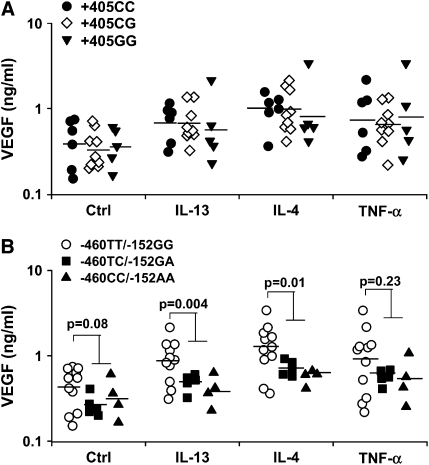
Cells were first stratified according to their genotype for +405C/G (Figure 4A) without regard for the other SNP. ANOVA indicated no effect of +405C/G genotype on baseline or IL-13–, IL-4–, or TNF-α–induced VEGF release. Cells were stratified according to their genotype at −460T/C and −152G/A (Figure 4B). ANOVA indicated a significant difference among cells from donors with −460TT/−152GG, −460TC/−152GA, and −460CC/−152AA genotypes. Because cells from donors heterozygous or homozygous for the −460C/−152A allele had similar behavior, these two genotypes were grouped together and compared with −460TT/−152GG cells. The presence of at least one −460C/−152A allele significantly decreased VEGF release induced by IL-13 (3 ng/ml) (P = 0.004) or IL-4 (1 ng/ml) (P = 0.012) (Figure 4B). Similar results were obtained with higher doses of IL-4 (10 ng/ml) or IL-13 (30 ng/ml) (data not shown). Presence of the −460C/−152A allele also tended to decrease basal and TNF-α–stimulated expression of VEGF by HASM cells, although the differences were not significant (P = 0.08 and P = 0.229, respectively). The −460/−152/+405 haplotype was then analyzed (Figure 5). In our sample, there were no cells wild-type at +405 that had at least one −460C/−152A allele. Thus, only three −460/−152/+405 haplotypes were observed. ANOVA indicated a significant difference among haplotypes after treatment with IL-13 or IL-4. Cells with at least one +405G allele that were wild type at −460/−152 had similar VEGF release to cells that were wild type at +405 and −460/−152. Therefore, cells with these two haplotypes were grouped together and compared with cells with at least one −460C/−152A and at least one +405G allele. The presence of at least one −460C/−152A and at least one +405G allele led to significantly lower IL-4– and IL-13–induced VEGF release than cells with the other haplotypes (Figure 5). Although the same trend was observed in untreated cells and in cells treated with TNF-α, the difference was not significant. We considered the possibility that the genotype effect might be the result of some unperceived bias in the status of the cells or of some unmeasured common genetic variation in another aspect of the IL-4/IL-13 signaling pathway. We reasoned that such effects would likely affect not just changes in VEGF release induced by IL-4 or IL-13, but also other effects of these cytokines. Therefore, we measured MCP-1 in the same cell supernatants because IL-4 and IL-13 have also been shown to induce MCP-1 release (18). MCP-1 release was measured in untreated cells and in cells treated with IL-4, IL-13, or TNF-α. Results were stratified according to −460/−152/+405 haplotype. IL-13, IL-4, and TNF-α each increased MCP-1 release, but ANOVA did not show any difference in MCP-1 release among −460/−152/+405 haplotypes (data not shown), indicating that the effect of VEGF genotype was specific to VEGF.
Figure 4.
Effect of +405 genotype (A) and of −460/−152 genotype (B) on VEGF release under basal conditions (Ctrl) and after treatment with IL-13 (3 ng/ml), IL-4 (1 ng/ml), or TNF-α (10 ng/ml). Each data point represents the mean value obtained for one cell donor. Each donor was studied on one or two occasions in duplicate. The presence of at least one −460C/−152A allele resulted in decreased IL-4– and IL-13–induced VEGF release. Similar results were obtained at higher concentrations of IL-4 and IL-13 (data not shown). Horizontal bars indicate geometric mean.
Figure 5.
Effect of the −460/−152/+405 haplotype on VEGF release from untreated HASM cells (Ctrl) and from cells treated with IL-13 (3 ng/ml), IL-4 (1 ng/ml), or TNF-α (10 ng/ml). Each data point represents the mean value obtained for one cell donor. Each donor was studied on one or two occasions in duplicate. The presence of at least one −460C/−152A/+405G allele resulted in decreased IL-4– and IL-13–induced VEGF release. Similar results were obtained at higher concentrations of IL-4 or IL-13. Horizontal bars indicate geometric mean.
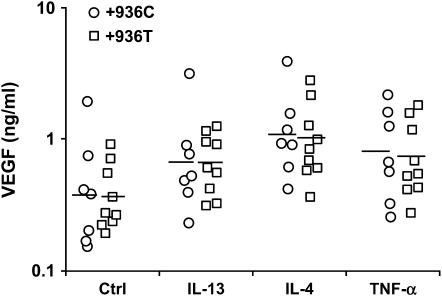
The 3′-UTR of the VEGF gene participates in post-transcriptional regulation of VEGF expression by stabilization of its mRNA (30), and the +936 C/T mutation in the 3′-UTR has been associated with VEGF plasma levels (25). To examine the importance of +936C/T SNP at the 3′-UTR of the VEGF gene on IL-13–, IL-4–, and TNF-α–induced VEGF release, HASM cells were stratified according to +936 C/T genotype. The +936 C/T genotype had no impact on VEGF release (Figure 6), but because we only had heterozygotes in our population, we cannot rule out the possibility that homozygosity for +936T could have an impact.
Figure 6.
Effect of the presence of at least one +936T allele on VEGF release from untreated HASM cells (Ctrl) and from cells treated with IL-13 (3 ng/ml), IL-4 (1 ng/ml), or TNF-α (10 ng/ml). Each data point represents the mean value obtained for one cell donor. Each donor was studied on one or two occasions in duplicate. Horizontal bars indicate geometric mean.
DISCUSSION
Our data indicate that the ability of Th2 cytokines to induce VEGF from HASM cells is likely mediated at the level of VEGF mRNA stability. To our knowledge, this is the first report of an effect of Th2 cytokines on VEGF mRNA stability in any cell type. Our data also indicate that VEGF genotype modulates the ability of IL-4 and IL-13 to induce VEGF release from HASM cells. This is the first report of a role for VEGF genotype in these events in any cell type.
Our data confirm reports of others that IL-13 and IL-4, Th2 cytokines that play an important role in asthma, induce VEGF release from HASM cells (15, 18). We also report that IL-4 and IL-13 increased IL-4 mRNA expression (Figure 2A), an effect not previously reported in any cell type. Although IL-4 increased VEGF mRNA, IL-4 did not alter VEGF promoter activity (Figure 2). The absence of any effect at the promoter level could be the result of the reporter construct used. Although the construct contained 2.4 kb of the VEGF promoter and was activated by another stimulus (OSM), we cannot rule out the possibility that IL-4 activates elements upstream of this or exerts its effects on intronic elements in the VEGF gene. On the other hand, IL-4 increased VEGF mRNA stability (Figure 3), suggesting that effects of IL-4 on VEGF mRNA are post-transcriptional rather than transcriptional. The VEGF mRNA 3′-UTR, like many labile mRNAs, contains AU-rich elements that are associated with rapid mRNA turnover (30, 31). Consequently, increases in VEGF mRNA stability would be expected to exert profound effects on the expression of this gene. Indeed, hypoxia, one of the most potent stimuli for VEGF expression, increases VEGF release in part through stabilization of VEGF mRNA (32, 33). Previous reports have demonstrated the presence of binding sites for hypoxia- or stress-inducible proteins in the VEGF mRNA 3′-UTR (30), but the molecular mechanisms underlying VEGF mRNA stability remain poorly understood.
Our data indicate that the VEGF genotype of the cells substantially affected IL-4− and IL-13–induced VEGF release. In particular, we found a decrease in IL-13– and IL-4–induced VEGF release in the presence of the −460C/−152A/+405G haplotype (Figure 5), whereas the genotype of the cells at +405 considered independently of −460/−152 did not have an impact (Figure 4A). This effect was not a function of some unperceived bias in the status of the cells or of some unmeasured common genetic variation in some component of the IL-4/IL-13 signaling pathway because when cells were analyzed for another IL-4/IL-13–induced effect (MCP-1 release) and the results stratified according to their VEGF promoter genotype, no effect was observed.
The −460 T/C and +405 C/G SNP have been implicated in a number of diseases with an angiogenic basis, although the results have not been consistent (24, 26, 27). For example, +405C has been associated with diabetic retinopathy, psoriasis, and higher serum VEGF levels (26). In contrast, others have reported that +405G allele is associated with higher VEGF protein production by peripheral blood mononuclear cells stimulated with lipopolysaccharide (24). One possible explanation for this apparent discrepancy in the impact of +405C/G is that there are cell-type– or stimulus-dependent effects of VEGF SNP. Alternatively, linkage disequilibrium may account for these apparently disparate observations. For example, our data indicate that the presence of the −460C/−152A allele decreases the ability of HASM cells to release VEGF in response to IL-4 or IL-13. −460C always occurred with +405G, and cells with at least one –460C/−152A/+405G allele also had reduced VEGF expression (Figure 5), whereas we could not observe any effect of +405C/G SNP alone.
Our results indicate no effect of IL-4 on VEGF promoter activity (Figure 2). Rather, IL-4 seemed to act by increasing VEGF mRNA stability. Therefore, we do not think that the impact of the −460C/−152A allele (Figure 5) on IL-4– and IL-13–induced VEGF release was related to effects on VEGF promoter activity. It is more likely that the reason for the decrease in IL-4– and IL-13–induced VEGF release from cells bearing the −460C/−152A/+405G haplotype is linkage disequilibrium with some other SNP in the 3′-UTR, a region important for stabilization of VEGF mRNA (30, 32). Strong linkage disequilibrium has been reported across the VEGF gene (24, 26, 27) and was confirmed in this group of HASM cell donors. Because the +936T allele in the 3′UTR has been associated with reduced VEGF plasma levels (25, 34), we examined its impact on IL-4– and IL-13–induced release in these HASM cells. Our results indicated no impact of the presence of the +936T allele in these cells, although because we had only heterozygous cells in our population, we cannot rule out the possibility that this SNP has the capacity to affect VEGF release. It is more likely that the reduction in VEGF in cells bearing at least one −460C/−152A/+405G allele is the result of linkage disequilibrium between this haplotype and another undescribed mutation elsewhere in the VEGF gene.
Although the presence of the −460C/−152A/+405G haplotype accounted for some of the variability in IL-13– and IL-4–induced VEGF release (Figures 4 and 5), substantial variability in VEGF release remained even in cells that were −460TT/−152GG (Figure 4B, open symbols). In HASM cells, IL-4 and IL-13 act through the type II IL-4 receptor, a dimer consisting of the IL-4Rα and the IL-13RαI (20, 21). We considered the possibility that SNP in the IL-4Rα gene accounted for some of this variability but found no evidence to that effect. We were somewhat surprised by these results because we have previously demonstrated that the Val50/Pro478/Arg551 haplotype of the IL-4Rα is associated with increased IL-13– and IL-4–induced TARC expression (22) and increased IL-13–induced β-adrenergic desensitization in HASM cells (23). This difference is likely related to differences in the regulation of VEGF, TARC, and β-adrenergic responsiveness by Th2 cytokines. It is possible that genetic variation in STAT-6 or in other components of the IL-4/IL-13 signaling pathway account for some this remaining variability or that there are other unidentified SNP in the VEGF gene that contribute to this variability. There may also be other sources of variability, including heterogeneity in VEGF release among cells within a single culture.
The importance of VEGF for asthma is underscored by the observations that inhibiting VEGF reduces lung inflammation and airway hyper-responsiveness in mice and that overexpression of VEGF enhances Th2 inflammation and induces airway hyper-responsiveness (6, 8). Given the ability of IL-4 and IL-13 to induce VEGF and the important role played by Th2 cytokines in the asthmatic airway, it is likely that IL-4 and IL-13 contribute to the increased VEGF expression observed in asthma. Although the magnitude of the change in VEGF release induced by Th2 cytokines may seem small (2- to 3-fold; see Figure 1), it is similar to that reported by Wen and colleagues (15) and similar to that induced by the potent proinflammatory cytokine, TNF-α (Figure 4). Data with another potent proinflammatory cytokine, IL-1β, indicate comparable levels of induction of VEGF (15, 19) in HASM cells. Even hypoxia, one of the most potent stimuli for VEGF expression, induces only a 5-fold increase in VEGF release by lung epithelial cells (11).
Although it is likely that IL-14 and IL-13 contribute to VEGF release in the airways of patients with asthma, we do not know to what extent VEGF released from airway smooth muscle is important. However, the basal VEGF release from HASM cells found in this study is comparable to the basal release of VEGF from lung epithelial cells (11). Moreover, VEGF release from HASM cells has the capacity for autocrine effects because VEGF alters the expression of extracellular matrix proteins by HASM cells (16).
In summary, this study indicates that IL-4 and IL-13 induce VEGF release in airway smooth muscle cells likely at the level of VEGF mRNA stability. In addition, the ability of HASM cells to release VEGF when stimulated with IL-4 or IL-13 is strongly dependent on VEGF haplotype, but it is not affected by IL-4Rα genotype. VEGF is a potent angiogenesis factor released in the asthmatic airway. Our data suggest that VEGF haplotype can have an impact on airway remodeling in asthma.
Acknowledgments
The authors thank Dr. Eric Silverman for critiquing this manuscript and Dr. James Butler for assistance with statistical analysis.
This study was supported by HL67664, HL33009, and ES00002.
Conflict of Interest Statement: D.S.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.A.P. received $5,000/yr from GlaxoSmithKline (GSK) from 2002–2005 and $2,000/yr from Merck from 2002–2005 for consultant services. He has served on the advisory boards of GSK from 2002–2005 at $4,000/yr, Merck from 2002–2004 at $2,000/yr, and Sepracor from 2002–2005 at $3,000/yr. He has received lecture fees from GSK of $12,000/yr from 2002–2005 and from Merck of $10,000/yr from 2002–2005. He has received industry-sponsored grants from GSK of $1.1 million/yr from 2002–2003 and from Merck of $50,000 from 2002–2003. S.A.S. received $2,500 in consulting fees from GlaxoSmithKline in 2004.
Originally Published in Press as DOI: 10.1165/rcmb.2005-0147OC on October 6, 2005
References
- 1.Carroll NG, Cooke C, James AL. Bronchial blood vessel dimensions in asthma. Am J Respir Crit Care Med 1997;155:689–695. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med 1997;156:229–233. [DOI] [PubMed] [Google Scholar]
- 3.Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J Allergy Clin Immunol 2001;107:1034–1038. [DOI] [PubMed] [Google Scholar]
- 4.Salvato G. Quantitative and morphological analysis of the vascular bed in bronchial biopsy specimens from asthmatic and non-asthmatic subjects. Thorax 2001;56:902–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989;246:1306–1309. [DOI] [PubMed] [Google Scholar]
- 6.Lee YC, Kwak YG, Song CH. Contribution of vascular endothelial growth factor to airway hyperresponsiveness and inflammation in a murine model of toluene diisocyanate-induced asthma. J Immunol 2002;168:3595–3600. [DOI] [PubMed] [Google Scholar]
- 7.Asai K, Kanazawa H, Otani K, Shiraishi S, Hirata K, Yoshikawa J. Imbalance between vascular endothelial growth factor and endostatin levels in induced sputum from asthmatic subjects. J Allergy Clin Immunol 2002;110:571–575. [DOI] [PubMed] [Google Scholar]
- 8.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med 2004;10:1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincenti V, Cassano C, Rocchi M, Persico G. Assignment of the vascular endothelial growth factor gene to human chromosome 6p21.3. Circulation 1996;93:1493–1495. [DOI] [PubMed] [Google Scholar]
- 10.Ober C, Tsalenko A, Parry R, Cox NJ. A second-generation genomewide screen for asthma-susceptibility alleles in a founder population. Am J Hum Genet 2000;67:1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boussat S, Eddahibi S, Coste A, Fataccioli V, Gouge M, Housset B, Adnot S, Maitre B. Expression and regulation of vascular endothelial growth factor in human pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol 2000;279:L371–L378. [DOI] [PubMed] [Google Scholar]
- 12.Boesiger J, Tsai M, Maurer M, Yamaguchi M, Brown LF, Claffey KP, Dvorak HF, Galli SJ. Mast cells can secrete vascular permeability factor/ vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of fc epsilon receptor I expression. J Exp Med 1998;188:1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corne J, Chupp G, Lee CG, Homer RJ, Zhu Z, Chen Q, Ma B, Du Y, Roux F, McArdle J, et al. IL-13 stimulates vascular endothelial cell growth factor and protects against hyperoxic acute lung injury. J Clin Invest 2000;106:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knox AJ, Corbett L, Stocks J, Holland E, Zhu YM, Pang L. Human airway smooth muscle cells secrete vascular endothelial growth factor: up-regulation by bradykinin via a protein kinase C and prostanoid-dependent mechanism. FASEB J 2001;15:2480–2488. [DOI] [PubMed] [Google Scholar]
- 15.Wen FQ, Liu X, Manda W, Terasaki Y, Kobayashi T, Abe S, Fang Q, Ertl R, Manouilova L, Rennard SI. TH2 cytokine-enhanced and TGF-beta-enhanced vascular endothelial growth factor production by cultured human airway smooth muscle cells is attenuated by IFN-gamma and corticosteroids. J Allergy Clin Immunol 2003;111:1307–1318. [DOI] [PubMed] [Google Scholar]
- 16.Kazi AS, Lotfi S, Goncharova EA, Tliba O, Amrani Y, Krymskaya VP, Lazaar AL. Vascular endothelial growth factor-induced secretion of fibronectin is ERK dependent. Am J Physiol Lung Cell Mol Physiol 2004;286:L539–L545. [DOI] [PubMed] [Google Scholar]
- 17.Fehrenbach H, Kasper M, Haase M, Schuh D, Muller M. Differential immunolocalization of VEGF in rat and human adult lung, and in experimental rat lung fibrosis: light, fluorescence, and electron microscopy. Anat Rec 1999;254:61–73. [DOI] [PubMed] [Google Scholar]
- 18.Faffe DS, Flynt L, Mellema M, Moore PE, Silverman ES, Subramaniam V, Jones MR, Mizgerd JP, Whitehead T, Imrich A, et al. Oncostatin M causes eotaxin-1 release from airway smooth muscle: synergy with IL-4 and IL-13. J Allergy Clin Immunol 2005;115:514–520. [DOI] [PubMed] [Google Scholar]
- 19.Faffe DS, Flynt L, Mellema M, Whitehead TR, Bourgeois K, Panettieri RA Jr, Silverman ES, Shore SA. Oncostatin M causes VEGF release from human airway smooth muscle: synergy with IL-1beta. Am J Physiol Lung Cell Mol Physiol 2005;288:L1040–L1048. [DOI] [PubMed] [Google Scholar]
- 20.Hirst SJ, Hallsworth MP, Peng Q, Lee TH. Selective induction of eotaxin release by interleukin-13 or interleukin-4 in human airway smooth muscle cells is synergistic with interleukin-1beta and is mediated by the interleukin-4 receptor alpha-chain. Am J Respir Crit Care Med 2002;165:1161–1171. [DOI] [PubMed] [Google Scholar]
- 21.Laporte JC, Moore PE, Baraldo S, Jouvin MH, Church TL, Schwartzman IN, Panettieri RA Jr, Kinet JP, Shore SA. Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am J Respir Crit Care Med 2001;164:141–148. [DOI] [PubMed] [Google Scholar]
- 22.Faffe DS, Whitehead T, Moore PE, Baraldo S, Flynt L, Bourgeois K, Panettieri RA, Shore SA. IL-13 and IL-4 promote TARC release in human airway smooth muscle cells: role of IL-4 receptor genotype. Am J Physiol Lung Cell Mol Physiol 2003;285:L907–L914. [DOI] [PubMed] [Google Scholar]
- 23.Shore SA, Moore PE. Regulation of beta-adrenergic responses in airway smooth muscle. Respir Physiol Neurobiol 2003;137:179–195. [DOI] [PubMed] [Google Scholar]
- 24.Watson CJ, Webb NJ, Bottomley MJ, Brenchley PE. Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: correlation with variation in VEGF protein production. Cytokine 2000;12:1232–1235. [DOI] [PubMed] [Google Scholar]
- 25.Renner W, Kotschan S, Hoffmann C, Obermayer-Pietsch B, Pilger E. A common 936 C/T mutation in the gene for vascular endothelial growth factor is associated with vascular endothelial growth factor plasma levels. J Vasc Res 2000;37:443–448. [DOI] [PubMed] [Google Scholar]
- 26.Awata T, Inoue K, Kurihara S, Ohkubo T, Watanabe M, Inukai K, Inoue I, Katayama S. A common polymorphism in the 5′-untranslated region of the VEGF gene is associated with diabetic retinopathy in type 2 diabetes. Diabetes 2002;51:1635–1639. [DOI] [PubMed] [Google Scholar]
- 27.Stevens A, Soden J, Brenchley PE, Ralph S, Ray DW. Haplotype analysis of the polymorphic human vascular endothelial growth factor gene promoter. Cancer Res 2003;63:812–816. [PubMed] [Google Scholar]
- 28.Moore PE, Church TL, Chism DD, Panettieri RA Jr, Shore SA. IL-13 and IL-4 cause eotaxin release in human airway smooth muscle cells: a role for ERK. Am J Physiol Lung Cell Mol Physiol 2002;282:L847–L853. [DOI] [PubMed] [Google Scholar]
- 29.Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol 1989;256:C329–C335. [DOI] [PubMed] [Google Scholar]
- 30.Claffey KP, Shih SC, Mullen A, Dziennis S, Cusick JL, Abrams KR, Lee SW, Detmar M. Identification of a human VPF/VEGF 3′ untranslated region mediating hypoxia-induced mRNA stability. Mol Biol Cell 1998;9:469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross J. mRNA stability in mammalian cells. Microbiol Rev 1995;59:423–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein I, Neeman M, Shweiki D, Itin A, Keshet E. Stabilization of vascular endothelial growth factor mRNA by hypoxia and hypoglycemia and coregulation with other ischemia-induced genes. Mol Cell Biol 1995;15:5363–5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy NS, Chung S, Furneaux H, Levy AP. Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J Biol Chem 1998;273:6417–6423. [DOI] [PubMed] [Google Scholar]
- 34.Krippl P, Langsenlehner U, Renner W, Yazdani-Biuki B, Wolf G, Wascher TC, Paulweber B, Haas J, Samonigg H. A common 936 C/T gene polymorphism of vascular endothelial growth factor is associated with decreased breast cancer risk. Int J Cancer 2003;106:468–471. [DOI] [PubMed] [Google Scholar]