Abstract
Rationale: HIV-infected patients with pulmonary arterial hypertension have histologic manifestations that are indistinguishable from those found in patients with idiopathic pulmonary arterial hypertension. In addition, the role of pleiotropic viral proteins in the development of plexiform lesions in HIV-related pulmonary hypertension (HRPH) has not been explored. Simian immunodeficiency virus (SIV) infection of macaques has been found to closely recapitulate many of the characteristic features of HIV infection, and thus hallmarks of pulmonary arterial hypertension should also be found in this nonhuman primate model of HIV.
Objectives: To determine whether pulmonary arterial lesions were present in archived SIV-infected macaque lung tissues from Johns Hopkins University and two National Primate Research Centers.
Methods: Archived macaque and human lung sections were examined via immunohistochemistry for evidence of complex vascular lesions.
Results: Complex plexiform-like lesions characterized by lumenal obliteration, intimal disruption, medial hypertrophy, thrombosis, and recanalized lumena were found exclusively in animals infected with SHIV-nef (a chimeric viral construct containing the HIV nef gene in an SIV backbone), but not in animals infected with SIV. The mass of cells in the lesions were factor VIII positive, and contained cells positive for muscle-specific and smooth muscle actins. Lung mononuclear cells were positive for HIV Nef, suggesting viral replication. Endothelial cells in both the SHIV-nef macaques and patients with HRPH, but not in patients with idiopathic pulmonary arterial hypertension, were also Nef positive.
Conclusions: The discovery of complex vascular lesions in SHIV-nef– but not SIV-infected animals, and the presence of Nef in the vascular cells of patients with HRPH, suggest that Nef plays a key role in the development of severe pulmonary arterial disease.
Keywords: idiopathic pulmonary arterial hypertension, HIV-1, Nef, pulmonary hypertension, SHIV-nef
The first case of HIV infection associated with severe pulmonary hypertension appeared in 1987 (1) and confirmation by several follow-up studies (2–5) indicated that HIV infection may be causally related to pulmonary hypertension, with histologic lesions that are indistinguishable from those present in idiopathic pulmonary arterial hypertension (IPAH) (6, 7). Although HIV-related pulmonary hypertension (HRPH) is still a rare disease, the incidence is many times higher than that of IPAH in the general population (approximately 1 in 4,000 vs. 2 in 1 million in the United States [N. Gallie, personal communication] or 1 in 200 in the Swiss or French populations [2, 8, 9]). Importantly, there is no evidence of viral particles in the complex pulmonary vascular lesions (3, 10) or bone morphogenetic protein receptor II mutations (11) in HRPH.
The mechanisms underlying HRPH may rely on indirect action of HIV proteins and/or the associated immune dysregulation present in patients with HIV infection. Among proteins of HIV that could participate in the pathogenesis of HRPH, the Tat protein causes increased endothelial cell permeability and injury via production of IL-6, shown to be present in lungs of patients with severe pulmonary hypertension (12). Nef, a 27-kD, N-myristoylated protein first misidentified as a negative regulatory factor for viral replication, is critical for the maintenance of high viral loads during the course of HIV infection in vivo (13, 14), and is an adaptor protein with multiple domains important for the interaction with the cellular signaling machinery. Its proline-rich (PxxP)3 domain interacts with Src homology 3–containing signaling proteins (15, 16), while other Nef domains interact with the endocytotic cellular machinery responsible for the down-regulation of CD4 (17–19).
Nonhuman primate models of HIV-1 infection with either SIV or genetically manipulated pathogenic viruses containing HIV-1 genes recapitulate many of the end-organ pathologies and immune deficiencies seen in humans (20). Only a few investigations using this model for noninfectious lung complications in SIV infection have been done. However, one report by Chalifoux and coworkers described a pulmonary arteriopathy in the lungs of 19 of 85 macaques infected with SIV at the New England National Primate Research Center (NENPRC, Southborough, MA) (21). As HIV modeling in monkeys has significantly evolved with the use of recombinant, chimeric SIV vectors containing potentially pathogenetic HIV genes (SIV/HIV, or SHIV constructs), or SIV with tropism to organs such as brain or lung, we therefore investigated whether SIV-infected macaque lungs displayed the characteristic complex vascular lesions, including the characteristic plexiform lesions, present in HRPH. We further extended the scope of our study to address whether the HIV-1 Nef protein might be detected in the lungs of patients with HRPH and in macaques infected with a chimeric viral construct containing the HIV nef gene in an SIV backbone (SHIV-nef), but not in normal or uninfected IPAH lungs. Our findings indicate that HIV-1 Nef may be involved in the development of vascular lesions found in HRPH. Some of the results of these studies have been previously reported in the form of an abstract (22).
METHODS
Animal Selection
From the NENPRC, we obtained eight animals: two infected with a chimeric SHIV-nef virion (23, 24) and six infected with SIVmac251 or SIVE660 containing the native SIV nef allele. From the California National Primate Research Center (CNPRC; University of California at Davis, Davis, CA) we obtained five macaque lungs: one infected with SIVmac239 and four infected with SHIV-nef chimeric viruses (25). From the Retrovirus Laboratory, Department of Comparative Medicine, Johns Hopkins University (JHU; Baltimore, MD) we obtained 12 lungs from macaques infected with a mixture of lymphocytotropic and monocytotropic SIV strains (26). Table 1 describes the different animals and their known characteristics. No hemodynamic parameters were available from any of the macaque cohorts. All animal procedures conformed to the requirements of the Animal Welfare Act and protocols were approved before implementation by the Institutional Animal Care and Use Committees at the University of California at Davis, JHU, and Harvard University.
TABLE 1.
ANIMALS WITH PULMONARY VASCULAR ARTERIOPATHY FROM NEW ENGLAND NATIONAL PRIMATE RESEARCH CENTER, CALIFORNIA NATIONAL PRIMATE RESEARCH CENTER, AND JOHNS HOPKINS UNIVERSITY
| Animal | Viral Strain* | Survival | Pulmonary Vascular Lesions | Lung Pathologies | |||
|---|---|---|---|---|---|---|---|
| New England National Primate Research Center | |||||||
| NENPRC1 | SIVE660 | 1 yr, 3 mo | None | Hemorrhagic necrotizing bronchopneumonia, pulmonary alveolar proteinosis | |||
| NENPRC2 | SIVmac251, BCG, SEB | 1 yr, 3 mo | None | Pulmonary edema, thromboemboli | |||
| NENPRC3 | SIVmac251 | 1 yr, 3 mo | None | Hyperplasia of BALT | |||
| NENPRC4 | SIVmac251 | 1 yr, 4 mo | None | Organizing thromboemboli, hyperplasia of BALT, hemosiderin | |||
| NENPRC5 | SIVmac251 | 3 yr, 4 mo | None | Nonnecrotizing vasculitis (predominantly lymphocytic) | |||
| NENPRC6 | SIVmac251 | 1 yr | None | Nonnecrotizing vasculitis (predominantly lymphocytic), pulmonary edema, fibrin exudates | |||
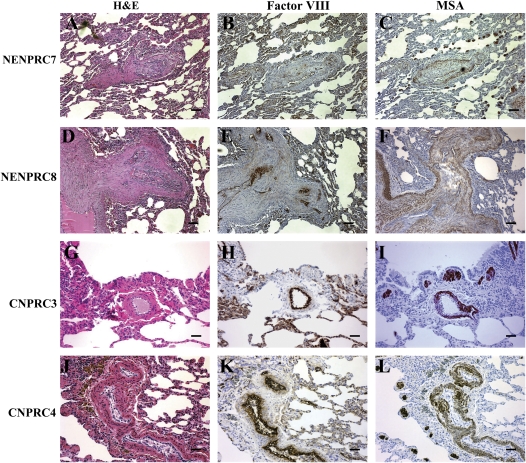
| NENPRC7 | SHIV-nef1486D95 | 1 yr, 9 mo | EC proliferation, medial hypertrophy, plexiform-like lesions | Nonnecrotizing vasculitis, (predominantly lymphocytic), focal thromboemboli | |||
| NENPRC8 | SHIV-nef1486D95 | N/A | EC proliferation, medial hypertrophy, intimal hyperplasia, plexiform-like lesions | Hyperplasia of BALT | |||
| California National Primate Research Center | |||||||
| CNPRC1 | SIVmac239 | 1 yr | None | Alveolar proteinosis (Pneumocystis carinii), type II pneumocyte hyperplasia, multinucleated giant cells, neutrophilic infiltration | |||
| CNPRC2 | SHIV-nefSF33 | 2 yr, 3 mo | Mild vascular remodeling of medium to large vessels | Intraepithelial infiltrates of neutrophils and lymphocytes | |||
| CNPRC3 | SHIV-nefSF33A | 2 yr, 3 mo | Significant vascular remodeling, abnormal EC layers in the vasculature | Multifocal lymphoid hyperplasia, mild invading mononuclear cells (neutrophilic and lymphocytic) | |||
| CNPRC4 | SHIV-nefSF33A | 1 yr, 6 mo | Significant vascular remodeling, abnormal EC layers in the vasculature | Subpleural alveolar proteinosis (P. carinii), bronchopneumonia, Cryptosporidium | |||
| CNPRC5 | SHIV-nefSF33A | 3 mo | Pulmonary arteries normal, unorganized architecture | Alveolar proteinosis (P. carinii), neutrophilic and lymphocytic alveolitis, septal damage | |||
| Johns Hopkins University | |||||||
| JHU1–JHU12 | SIV/ΔB670 and SIV/17E-Fr | N/A | None | Perivascular lymphocytic infiltrates, interstitial pneumonitis, septal damage with airspace enlargement | |||
Definition of abbreviations: BALT = bronchial-associated lymphoid tissue; BCG = Mycobacterium bovis bacillus calmette-gaérin; EC = endothelial cells; N/A = not available; SEB = staphylococcal enterotoxin B superantigen.
Subscript refers to the viral strain.
Human Tissues
Archived, paraffin-embedded lung tissue sections were obtained from two patients with IPAH, two patients with HRPH, and one patient with no clinical or histologic evidence of IPAH. These tissues were used for immunohistochemistry of the HIV-1 Nef protein. Patient characteristics have already been described by Cool and coworkers (6, 27).
Tissue and Histologic Examination
All tissues were obtained at necropsy, and were fixed and embedded in paraffin. Serial sections were prepared and shipped to the University of Colorado at Denver and Health Sciences Center (Denver, CO) for hematoxylin and eosin (H&E) staining and immunohistochemistry as indicated. The sections were masked concerning the identity of the experimental groups and examined independently by two pulmonary pathologists (C.D.C. and R.M.T.) for the presence of pulmonary vascular lesions as recommended by Pietra and coworkers (28). A total of 10 and 12 serial sections from a single lung block were obtained from the NENPRC and CNPRC, respectively. From JHU, we received one H&E-stained section for each animal (n = 12). Representative sections were performed on the basis of random selection by the respective institutions. Therefore, we had to limit our analyses to the archived material already collected for other studies. Nevertheless, we are confident that the random nature of the archival process allowed us an analysis of representative samples from many different areas of the lung.
Immunohistochemistry
Identification of vascular cells.
Sections were immunostained at the Histology Laboratory (University of Colorado at Denver and Health Sciences Center) with a HexES automated apparatus (Ventana Medical Systems, Tucson, AZ), using the following antibodies: rabbit polyclonal anti-human von Willebrand factor (factor VIII; A0082; Dako, Carpinteria, CA), mouse monoclonal anti–α-smooth muscle actin (α-SMA; A5228; Sigma-Aldrich, St. Louis, MO), and mouse monoclonal anti–muscle specific actin (MSA; C34931; Enzo Life Sciences, Inc., Farmingdale, NY). Immunodetection was performed with the Ventana avidin–biotin peroxidase system with anti-mouse or anti-rabbit IgG. Both positive and negative control tissues were provided with each antibody.
Detection of HIV-1 Nef.
A mouse monoclonal antibody to amino acids 153–158 of HIV-1 Nef (Advanced Biotechnologies, Inc., Columbia, MD) was used to stain sections with a Zymed AEC (aminoethyl carbazole) immunohistochemical staining kit (Invitrogen, Carlsbad, CA) according to the manufacturer's protocols (for further details, see the online supplement). Negative controls for the immunohistochemistry were performed using secondary antibody alone.
Statistics
A Fisher's exact test of a 4 × 4 matrix was used to analyze the observations, with p < 0.05 considered significant.
RESULTS
Pulmonary Pathologies in SIV- and SHIV-nef–infected Macaques
We examined a total of 24 different monkey lungs from two separate primate centers based in New England (NENPRC), California (CNPRC), and Johns Hopkins University (JHU), with a total of 18 monkeys infected with SIV and six infected with SHIV-nef (Table 1). The SIV strains contained the simian nef allele whereas the SHIV-nef strains were chimeric viruses with HIV nef from various HIV isolates cloned in place of the native SIV nef sequence. Figure E1 of the online supplement shows a diagrammatic depiction of the genomic structures of parental and chimeric SIV strains.
From the NENPRC, we obtained eight animals: two infected with a chimeric SHIV-nef virion that contained the SIVmac251 backbone and the HIV nef gene, and six infected with SIVmac251 or SIVE660 containing the native SIV nef allele. We found that the two SHIV-nef animals (designated NENPRC7 and NENPRC8 in Table 1) showed pulmonary vessels with medial smooth muscle cell hypertrophy and lumenal obstruction with endothelial cells (Figures 1B and 1C). The lesions were histologically similar to the plexiform lesions seen in patients with HRPH. One animal infected with SIVmac251 (Figure 1A and Table 1; designated as NENPRC1), showed significant perivascular cellular infiltration, but little disruption of the intimal or medial layers of the pulmonary vasculature. Furthermore, none of the animals infected with parental SIV strains (SIVmac251 or SIVE660, NENPRC1–NENPRC6 in Table 1) had any evidence of endothelial layer disruption or smooth muscle cell proliferation. Several of the prominent histopathologic features in these lungs included pulmonary edema, organizing thromboemboli, nonnecrotizing perivascular lymphocytic infiltrates, neutrophilic infiltration, and hyperplasia of bronchial-associated lymphoid tissue (Table 1). In four animals, alveolar proteinosis from Pneumocystis carinii was also evident, suggesting severe immunodeficiency with opportunistic infections.
Figure 1.
Pulmonary vasculature and plexiform lesions in macaques infected with simian immunodeficiency virus (SIV) or SHIV-nef (a chimeric viral construct containing the HIV nef gene in an SIV backbone). Paraffin-embedded sections from the lungs of macaques infected with SIVmac251-NENPRC1 (A), SHIV-nef1486D95-NENPRC7 (B), SHIV-nef1486D95-NENPRC8 (C), SIVmac239-CNPRC1 (D), SHIV-nefSF33A-CNPRC3 (E), or SHIV-nefSF33A-CNPRC4 (F) were stained with hematoxylin and eosin (H&E) and examined for vascular pathologies by two independent pathologists. Arrows indicate vessels with lymphocytic infiltration (SIV) and plexiform-like lesions (SHIV-nef). Scale bars: 100 μm.
No measurements of pulmonary artery pressure or cardiac output were taken in the NENPRC early study. On the other hand, examination of echocardiographic data from monkeys infected with the parental SIV strains showed no evidence of right ventricular changes in these monkeys (i.e., no echocardiographic evidence of right ventricular hypertrophy in approximately 30 SIV-infected animals examined [R.P.S., unpublished data]), despite evidence of widespread myocarditis and left ventricular changes.
We then compared the findings in the studies from the NENPRC with those in five additional monkey lungs obtained from the CNPRC (animals designated as CNPRC1–CNPRC5 in Table 1). Animal CNPRC1 had been infected with SIVmac239 and animal CNPRC2 had been infected with a chimeric SHIV-nef containing the HIV-1 nefSF33 allele in the SIVmac239 backbone. CNPRC1 had no involvement of the pulmonary vasculature but showed neutrophilic and lymphocytic infiltration of the perivascular space (Figure 1D). One SHIV-nef–infected monkey (CNPRC2) showed mild vascular remodeling (see Figure E2). Another animal infected with SHIV-nefSF33 (tissues unavailable) died of immunodeficiency, but its SHIV-nef virus was isolated, recovered, and used to infect three additional macaques (CNPRC3, CNPRC4, and CNPRC5 in Table 1). The nef allele, which had acquired four amino acid mutations during passage in vivo, was designated as Nef SF33A (A, for adapted) (25). Two animals (CNPRC3 and CNPRC4; Figures 1E and 1F, respectively) showed abnormal vascular lesions with medial hypertrophy and a disrupted, cuboidal endothelial cell layer. Pentachrome staining corroborated both medial and intimal hypertrophy of the pulmonary arteries (see Figure E3). CNPRC5 died of severe wasting and opportunistic infections 3 mo postinfection, with lung samples showing extensive inflammation, but no vascular remodeling (see Figure E2). As with animals in the NENPRC cohort, no evidence of cardiac abnormalities was found in young animals infected with parental SIVmac251 at the CNPRC (A.F.T., unpublished data).
To further confirm that SIV alone is not associated with complex pulmonary vascular lesions, we examined an additional 12 monkeys infected with an immunosuppressive swarm SIV-ΔB670 and a macrophage-tropic molecular clone SIV/17E-Fr mixture (JHU1–JHU12; Table 1) from the Retrovirus Laboratory, Department of Comparative Medicine, Johns Hopkins University. These macaques developed a rapid and lethal immunodeficiency within 3 mo of infection (26). Although significant inflammatory lung involvement with inflammation and alveolar simplification was observed, no complex vascular lesions were detected (see Figure E4).
Combining the results from the three geographically separate primate centers, four of the six SHIV-nef–infected animals were found to have complex vascular lesions, and one additional animal had evidence of less prominent pulmonary vascular remodeling. Thus, five of the six SHIV-nef–infected animals (83%) with remodeling were chronically infected (more than 12 mo), whereas none of the animals infected with the parental SIV strains containing the SIV nef allele (n = 18) developed complex pulmonary vascular lesions. Taken together, there is a statistically significant association between SHIV-nef infection in macaques and the presence of pulmonary vascular lesions (Fisher's exact test, p < 0.0001).
Characterization of Pulmonary Vascular Lesions in SHIV-nef–infected Animals
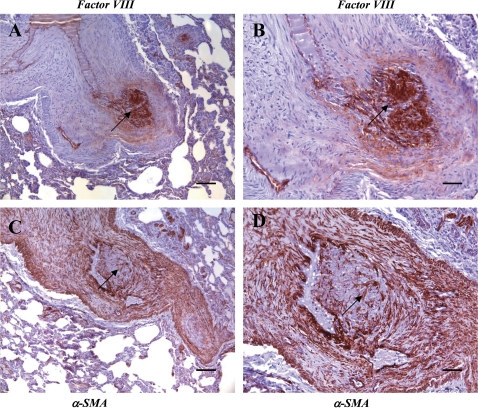
To further characterize the lesions, we immunostained the sections with the endothelial cell marker factor VIII, and the smooth muscle markers muscle-specific actin (MSA) and α-smooth muscle actin (Figures 2 and 3). Animals NENPRC7 and NENPRC8 showed marked medial hypertrophy along with a mass of endothelial cells at a site of bifurcation (Figures 2D–2F). The pattern of factor VIII staining (Figures 2B, 2E, 2H, and 2K) highlighted the endothelial cell layer and intimal thickening, and the muscle-specific actin staining pattern (Figures 2C, 2F, 2I, and 2L) indicated medial hypertrophy. In the SHIV-nef animals CNPRC3 and CNPRC4 (Figure 2, rows 3 and 4), the pattern of factor VIII staining highlighted the disrupted, cuboidal endothelial cells, and the muscle-specific actin staining indicated medial hypertrophy. In animal NENPRC7, in a vessel at a bifurcation point, the lesion showed a core of factor VIII–positive endothelial cells obliterating the lumen (Figures 3A and 3B). Furthermore, at higher magnification, the smooth muscle actin–positive cells (Figures 3C and 3D) can be seen as interdigitating strings surrounded by an endothelial cell core.
Figure 2.
Characterization of vascular cells in the lesions of SHIV-nef macaques from the New England National Primate Research Center (NENPRC) and the California National Primate Research Center (CNPRC) cohorts. Paraffin-embedded serial sections from animals NENPRC7 (A–C) and NENPRC8 (D–F), and from animals CNPRC3 (G–I) and CNPRC4 (J–L), were stained with H&E (A, D, G, and J), factor VIII (B, E, H, and K), and mouse monoclonal anti–muscle specific actin (MSA; C, F, I, and L) for visualization of endothelial cells and smooth muscle cells in the plexiform lesions. Scale bars: 50 μm.
Figure 3.
Identification of endothelial and smooth muscle cell markers in a lesion from an SHIV-nef animal (NENPRC7). Endothelial cells stained with factor VIII (A and B) and smooth muscle cells stained with α-smooth muscle actin (α-SMA; C and D) in lesions from the SHIV-nef animal NENPRC7. Arrows indicate regions of mixed endothelial and smooth muscle cell staining in the lesion. (B) and (D) are magnifications of the lesion in (A) and (C), respectively. Scale bars: (A and C) 50 μm; (B and D) 100 μm.
Presence of Nef in the Lungs of HIV-1–infected Patients and SHIV-nef–infected Macaques
The presence of plexiform lesions in the pulmonary vasculature of SHIV-nef but not in the SIV-infected animals suggested a role for HIV-1 Nef in the pathogenesis of HRPH. As the presence of Nef in the lungs of patients with HRPH has not been reported previously, we examined paraffin-embedded lung sections from a normal human lung, two patients with IPAH, two patients with HRPH, and two SHIV-nef–infected macaques (CNPRC3 and CNPRC4) for the presence of the HIV-1 Nef protein. Figure 4 (first column) shows some representative lung sections stained with a monoclonal HIV-1 Nef antibody, whereas a separate section and region of the same subject in the remaining panels show dual-immunofluorescence stains for factor VIII and Nef. We detected no Nef staining in an uninfected control (Figure 4A; and see Figure E5A) or in patients with IPAH (Figure 4E; and see Figure E5B), illustrating the specificity of the antibody and the absence of any molecular mimicry (29). On the other hand, the lungs from patients with HRPH showed distinct positive staining in alveolar mononuclear cells (most likely lymphocytes and macrophages) as well as in endothelial cells lining the vessels (see Figures E5C and E6). The occluding cells and some lumenal cells found in the complex lesion in the patient with HRPH also stained positive for Nef (Figure 4I), and Nef-positive cells were observed clustering at sites adjacent to the vessel walls. In addition, Nef-positive cells were observed in lymphoid-associated tissues (see Figure E6), suggesting a potential source for the viral protein.
Figure 4.
Colocalization of HIV-1 Nef in pulmonary arterial endothelial cells in human patients with HIV-related pulmonary hypertension (HRPH), but not in normal humans or in patients with idiopathic pulmonary arterial hypertension (IPAH). Paraffin-embedded lung sections from normal human lung (A–D), human IPAH lung with plexiform lesions (E–H), and human HRPH lung (I–L) were stained with a monoclonal antibody to HIV-1 Nef and counterstained with methyl green (A, E, and I). Separate sections from the same patients were dual-stained with a rabbit polyclonal antibody to factor VIII and detected with an Alexa Fluor 488–conjugated goat anti-rabbit secondary antibody (B, F, and J) and a mouse monoclonal antibody to HIV-1 Nef and detected with a goat anti-mouse antibody, using Vector red (C, G, and K). The factor VIII and Nef images were digitally merged (D, H, and L) to demonstrate colocalization of Nef in endothelial cells. Human lungs from normal subjects and patients with IPAH lacked staining in either infiltrating mononuclear cells, plexiform lesions, or the unaffected vasculature. The lung from a patient with HRPH showed distinct positive staining in the occluding cells of a plexiform lesion (I, arrows) as well as localization to the endothelial cell layer (K and L, arrows) in an uninvolved vessel from the same section. N = normal patient; I = patient with IPAH; H = patient with HRPH; L = lumen.
Figures 4 and 5 show immunofluorescence staining for factor VIII and Nef in human and macaque lungs, respectively. The pattern of staining in the merged images clearly indicates that Nef colocalizes with endothelial cells. This pattern in the SHIV-nef macaque lungs recapitulated that found in the human lungs, with staining in several endothelial cells within the vasculature as well as in perivascular sites (Figure 5I). We also found factor VIII-negative, Nef-positive medial and parenchymal cells. Interestingly, some adventitial cells in HRPH stained positive for both factor VIII and Nef (see Figure E6). As expected, lymphoid tissue showed staining for Nef, but no factor VIII positivity (Figure 5). These findings provide additional evidence that HIV-1 Nef may be a critical player in the development of complex pulmonary vascular lesions.
Figure 5.
Colocalization of HIV-1 Nef in pulmonary arterial endothelial cells in SHIV-nef–infected macaques, but not in SIV-infected macaques. Paraffin-embedded lung sections from SIV-infected macaque lung (A–D), SHIV-nefSF33A-CNPRC3 lung (E–H), and SHIV-nefSF33A-CNPRC4 lung (I–L) were stained with a monoclonal antibody to HIV-1 Nef and counterstained with methyl green (A, E, and I). Separate sections from the same animals were dual-stained with a rabbit polyclonal antibody to factor VIII and detected with an Alexa Fluor 488–conjugated goat anti-rabbit secondary antibody (B, F, and J) and a mouse monoclonal antibody to HIV-1 Nef and detected with a goat anti-mouse antibody, using Vector red (C, G, and K). The factor VIII and Nef images were digitally merged (D, H, and L) to demonstrate colocalization in vascular cells. SIV-infected lungs showed no evidence of Nef staining. The staining pattern in the SHIV-nef macaque lungs mimicks the findings in human tissues, with staining in the endothelial cell layer (E, G, and H, arrows) as well as in perivascular sites with massive lymphocytic infiltration (I, K, and L, arrows). S = SIV; SH = SHIV-nef.
DISCUSSION
The main findings of our study are that complex vascular structures resembling plexiform lesions of IPAH are found in the lungs of macaques infected with a chimeric virus with the SIV backbone and the HIV nef gene. None of the monkeys infected with the parental SIV nef showed pulmonary vascular lesions, suggesting that there are functional differences between HIV and SIV nef. These differences may result in a protein that can act either alone or in conjunction with other viral or cellular factors in promoting alterations of pulmonary arteries characteristic of severe pulmonary hypertension. These findings were somewhat unanticipated, because HIV nef can complement nef deletions in SIV (24, 25), suggesting that at least in terms of immunodeficiency alleles from both lentiviruses are functionally equivalent. We found that Nef was present in endothelial and in factor VIII–negative cells, including the infected mononuclear cells. To our knowledge, this is the first time that any group has identified plexiform lesions in macaque lungs infected with a lentivirus.
Although all the animals in cohorts from the New England and California National Primate Research Centers have abnormal endothelial cell morphology, the characteristics of the vasculopathies are different. Vascular lesions in the NENPRC SHIV-nef1486D95 cohort more closely resemble the plexiform lesions seen in HRPH patients with medial smooth muscle cell hypertrophy and multiple lumenal obstructions containing factor VIII-positive endothelial cells. Interestingly, lesions in the SHIV-nefSF33A animals from the CNPRC cohort were dominated by medial hypertrophy and a disrupted, cuboidal endothelial cell layer. The different pathologies in the two cohorts may be related to the stage of plexiform lesion formation in SHIV-nef animals, the rate of progression of lesion development, or the specific nef isolates in the chimeras. Without prospective studies using both of the constructs in cohorts infected in parallel, we are unable to provide direct evidence for the underlying mechanistic reasons for the differences. Nevertheless, the appearance of a disrupted endothelial cell layer containing the HIV-1 Nef protein suggests a role for Nef in the development of the complex severe pulmonary vascular lesions.
Weesner and Kaplan (30) found elevated pulmonary artery pressures in a subset of SIV-infected stumptailed macaques (Macaca arctoides) and only moderate medial thickening of the pulmonary arteries. Chalifoux and coworkers (21) described an arteriopathy characterized by intimal and medial thickening in 19 of 85 macaques infected with SIV at the NENPRC. The medial thickening was seen only in large and medium-sized pulmonary arteries, and was reported to be the result of extracellular matrix deposition. Thirty-seven percent of the macaques with arteriopathy contained lumena with thrombi in various stages of organization and recanalization. In fact, after exhaustive examination, the authors did not find evidence of plexogenic lesions of small pulmonary arterioles and mention that the endothelial layer was always intact in these small arterioles. Infection of C57BL/6 mice with the LP-BM5 retroviral mixture, a murine model of immunodeficiency, leads to a diffuse interstitial pneumonitis similar to AIDS-associated interstitial pneumonitis, characterized by lymphocytic and macrophage infiltration in a setting of immunodeficiency (31). Although these mice develop right ventricular hypertrophy and show an increase in the percentage of muscularized pulmonary arteries with increased perivascular collagen deposition, they are remarkable for not developing plexiform lesions or intimal fibrosis of the pulmonary arteries (32). Transgenic mice expressing the entire HIV-1–coding sequence under the control of the CD4 promoter develop a severe AIDS-like disease and a lymphoid interstitial pneumonitis as well (33). These studies highlight the relevance of our identification of HIV-1 Nef as a potential contributor to advanced pulmonary vascular remodeling and plexiform lesions in HIV-associated pulmonary hypertension. The present study, in combination with the observation that lung tissue samples from many patients with pulmonary hypertension were positive for human herpesvirus type 8 (27), suggests a central role for viral gene products and their influence on endothelial cell biology.
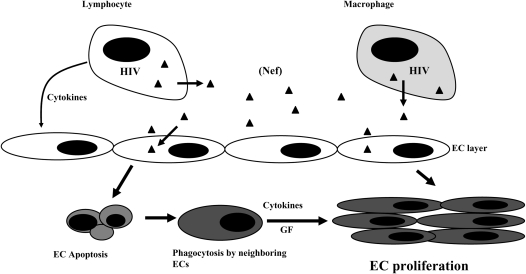
How could Nef exert its effects on endothelial cells? HIV does not appear to infect endothelial cells directly, but extracellular Nef has been found in HIV-infected patients at a level of approximately 10 ng/ml (34). We also found Nef-positive cells in medial layers as well in the parenchyma (Figures 4K and 4L, arrows; and see Figure E6), and the cell morphology suggests that these may be smooth muscle and epithelial cells, respectively. As with endothelial cells, productive infection of these non-CD4+ cells has not been described. Acheampong and coworkers (35) demonstrated that either exogenous or intracellular Nef induced apoptosis of human brain microvascular endothelial cells. In lymphocytes, Nef dose- and time-dependently induced apoptosis; however, infected cells were protected from death through down-regulation of class I major histocompatibility complex (36). Because Nef has been shown to enter lymphocytes via the chemokine receptor CXCR4 (37, 38) which is expressed on endothelial cells in the lung vasculature, active infection is not a prerequisite for HIV-induced pulmonary vascular injury. Furthermore, HIV-infected alveolar macrophages and lymphocytes may act as Nef reservoirs and increase angiogenic factors such as platelet-derived growth factor-α (39) to induce endothelial and smooth muscle cell proliferation in the lung vasculature. Thus, the viral replication–independent effects of Nef may directly affect uninfected cells or indirectly activate alveolar macrophages to induce a proangiogenic response. On the basis of all the known properties of Nef, our own studies with cultured endothelial cells, and the presence of Nef in HRPH and SHIV-nef lungs, we propose the model shown in Figure 6. Infected lymphocytes or macrophages can either secrete Nef into the extracellular compartment or transfer Nef to surrounding cells via cell-to-cell contact (40). Nef may then induce changes in the uninfected cells that may include apoptosis and growth factor release, proliferation and/or transdifferentiation, and formation of obliterative vascular lesions. In this model, an initial insult leads to subsequent selection of an apoptosis-resistant angioproliferative phenotype (41, 42). Whether this effect is mediated by the direct action of Nef on the target cells or indirectly through angiogenic growth factors remains to be determined.
Figure 6.
Hypothetical relationship between HIV-1 Nef and the development of severe angioproliferative pulmonary hypertension. The accessory HIV-1 protein Nef (solid triangles) is expressed early during infection in HIV-infected resident alveolar macrophages and circulating lymphocytes, providing proximate sources for viral proteins for surrounding cells and the circulation. The uptake of Nef into vascular endothelial cells (ECs), in conjunction with immune insufficiency, leads to endothelial cell dysfunction and apoptosis. The engulfment of apoptotic cells by surrounding endothelial cells leads to an apoptosis-resistant population with increased secretion of cytokines and growth factors (GFs). The uncontrolled endothelial cell proliferation leads to complex vascular remodeling, plexiform lesions, and HRPH.
In summary, we describe the associations between HIV-1 Nef and complex pulmonary vascular lesions in SHIV-nef–infected monkeys, which recapitulate the alterations seen in HRPH and IPAH. Our findings should lead to future investigations of the monkey model to elucidate the pathogenesis of these vascular lesions, and molecular examination of how the Nef protein might alter pulmonary vascular cells.
Supplementary Material
Acknowledgments
The authors thank M. Christine Zink, Joseph L. Mankowski, and Janice E. Clements of the Johns Hopkins University School of Medicine for providing archived macaque lung tissues, and Dr. Douglas Everett for assistance with the statistical analyses.
Supported by NHLBI R01 HL59785 (supplement to S.C.F.), RR00169 California National Primate Research Center (CNPRC), RR00168 New England National Primate Research Center (NENPRC), and NHLBI RO1HL66554 (R.M.T.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200601-005OC on May 25, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the manuscript.
References
- 1.Kim KK, Factor SM. Membranoproliferative glomerulonephritis and plexogenic pulmonary arteriopathy in a homosexual man with acquired immunodeficiency syndrome. Hum Pathol 1987;18:1293–1296. [DOI] [PubMed] [Google Scholar]
- 2.Speich R, Jenni R, Opravil M, Pfab M, Russi EW. Primary pulmonary hypertension in HIV infection. Chest 1991;100:1268–1271. [DOI] [PubMed] [Google Scholar]
- 3.Mette SA, Palevsky HI, Pietra GG, Williams TM, Bruder E, Prestipino AJ, Patrick AM, Wirth JA. Primary pulmonary hypertension in association with human immunodeficiency virus infection: a possible viral etiology for some forms of hypertensive pulmonary arteriopathy. Am Rev Respir Dis 1992;145:1196–1200. [DOI] [PubMed] [Google Scholar]
- 4.Pellicelli AM, Palmieri F, D'Ambrosio C, Rianda A, Boumis E, Girardi E, Antonucci G, D'Amato C, Borgia MC. Role of human immunodeficiency virus in primary pulmonary hypertension: case reports. Angiology 1998;49:1005–1011. [DOI] [PubMed] [Google Scholar]
- 5.Sitbon O, Gressin V, Speich R, Macdonald PS, Opravil M, Cooper DA, Fourme T, Humbert M, Delfraissy JF, Simonneau G. Bosentan for the treatment of human immunodeficiency virus–associated pulmonary arterial hypertension. Am J Respir Crit Care Med 2004;170:1212–1217. [DOI] [PubMed] [Google Scholar]
- 6.Cool CD, Kennedy D, Voelkel NF, Tuder RM. Pathogenesis and evolution of plexiform lesions in pulmonary hypertension associated with scleroderma and human immunodeficiency virus infection. Hum Pathol 1997;28:434–442. [DOI] [PubMed] [Google Scholar]
- 7.Newman JH. Pulmonary hypertension. Am J Respir Crit Care Med 2005;172:1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Opravil M, Pechere M, Speich R, Joller-Jemelka HI, Jenni R, Russi EW, Hirschel B, Luthy R. HIV-associated primary pulmonary hypertension: a case control study. Swiss HIV Cohort Study. Am J Respir Crit Care Med 1997;155:990–995. [DOI] [PubMed] [Google Scholar]
- 9.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173:1023–1030. [DOI] [PubMed] [Google Scholar]
- 10.Humbert M, Monti G, Fartoukh M, Magnan A, Brenot F, Rain B, Capron F, Galanaud P, Duroux P, Simonneau G, et al. Platelet-derived growth factor expression in primary pulmonary hypertension: comparison of HIV seropositive and HIV seronegative patients. Eur Respir J 1998;11:554–559. [PubMed] [Google Scholar]
- 11.Nunes H, Humbert M, Sitbon O, Morse JH, Deng Z, Knowles JA, Le Gall C, Parent F, Garcia G, Herve P, et al. Prognostic factors for survival in human immunodeficiency virus–associated pulmonary arterial hypertension. Am J Respir Crit Care Med 2003;167:1433–1439. [DOI] [PubMed] [Google Scholar]
- 12.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995;151:1628–1631. [DOI] [PubMed] [Google Scholar]
- 13.Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med 1995;332:228–232. [DOI] [PubMed] [Google Scholar]
- 14.Kestler HW III, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 1991;65:651–662. [DOI] [PubMed] [Google Scholar]
- 15.Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J 1995;14:484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng H, Hoxie JP, Parks WP. The conserved core of human immunodeficiency virus type 1 Nef is essential for association with Lck and for enhanced viral replication in T-lymphocytes. Virology 1999;264:5–15. [DOI] [PubMed] [Google Scholar]
- 17.Grzesiek S, Stahl SJ, Wingfield PT, Bax A. The CD4 determinant for downregulation by HIV-1 Nef directly binds to Nef: mapping of the Nef binding surface by NMR. Biochemistry (Mosc) 1996;35:10256–10261. [DOI] [PubMed] [Google Scholar]
- 18.Bresnahan PA, Yonemoto W, Ferrell S, Williams-Herman D, Geleziunas R, Greene WC. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr Biol 1998;8:1235–1238. [DOI] [PubMed] [Google Scholar]
- 19.Stoddart CA, Geleziunas R, Ferrell S, Linquist-Stepps V, Moreno ME, Bare C, Xu W, Yonemoto W, Bresnahan PA, McCune JM, et al. Human immunodeficiency virus type 1 Nef-mediated downregulation of CD4 correlates with Nef enhancement of viral pathogenesis. J Virol 2003;77:2124–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Rompay KK. Antiretroviral drug studies in nonhuman primates: a valid animal model for innovative drug efficacy and pathogenesis experiments. AIDS Rev 2005;7:67–83. [PubMed] [Google Scholar]
- 21.Chalifoux LV, Simon MA, Pauley DR, MacKey JJ, Wyand MS, Ringler DJ. Arteriopathy in macaques infected with simian immunodeficiency virus. Lab Invest 1992;67:338–349. [PubMed] [Google Scholar]
- 22.Marecki J, Cool C, Voelkel N, Luciw P, Flores S. Evidence for vascular remodeling in the lungs of macaques infected with simian immunodeficiency virus/HIV NEF recombinant virus [abstract]. Chest 2005;128:621S–622S. [DOI] [PubMed] [Google Scholar]
- 23.Alexander L, Du Z, Howe AY, Czajak S, Desrosiers RC. Induction of AIDS in rhesus monkeys by a recombinant simian immunodeficiency virus expressing nef of human immunodeficiency virus type 1. J Virol 1999;73:5814–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander L, Weiskopf E, Greenough TC, Gaddis NC, Auerbach MR, Malim MH, O'Brien SJ, Walker BD, Sullivan JL, Desrosiers RC. Unusual polymorphisms in human immunodeficiency virus type 1 associated with nonprogressive infection. J Virol 2000;74:4361–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandell CP, Reyes RA, Cho K, Sawai ET, Fang AL, Schmidt KA, Luciw PA. SIV/HIV nef recombinant virus (SHIVnef) produces simian AIDS in rhesus macaques. Virology 1999;265:235–251. [DOI] [PubMed] [Google Scholar]
- 26.Babas T, Vieler E, Hauer DA, Adams RJ, Tarwater PM, Fox K, Clements JE, Zink MC. Pathogenesis of SIV pneumonia: selective replication of viral genotypes in the lung. Virology 2001;287:371–381. [DOI] [PubMed] [Google Scholar]
- 27.Cool CD, Rai PR, Yeager ME, Hernandez-Saavedra D, Serls AE, Bull TM, Geraci MW, Brown KK, Routes JM, Tuder RM, et al. Expression of human herpesvirus 8 in primary pulmonary hypertension. N Engl J Med 2003;349:1113–1122. [DOI] [PubMed] [Google Scholar]
- 28.Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder RM. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol 2004;43:25S–32S. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Nardi MA, Karpatkin S. Role of molecular mimickry to HIV-1 peptides in HIV-1–related immunologic thrombocytopenia. Blood 2005;106:572–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weesner KM, Kaplan K. Hemodynamic and echocardiographic evaluation of the stumptailed macaque: a potential nonhuman primate model for pulmonary vascular disease. J Med Primatol 1987;16:185–202. [PubMed] [Google Scholar]
- 31.Cohen DA, Fitzpatrick EA, Hartsfield C, Gillespie MN, Avdiushko M, Kaplan AM. Pulmonary lymphoid cell activation and cytokine expression in murine AIDS-associated interstitial pneumonitis. Am J Respir Cell Mol Biol 1997;16:153–161. [DOI] [PubMed] [Google Scholar]
- 32.Gillespie MN, Hartsfield CL, O'Connor WN, Cohen DA. Pulmonary hypertension in a murine model of the acquired immunodeficiency syndrome. Am J Respir Crit Care Med 1994;150:194–199. [DOI] [PubMed] [Google Scholar]
- 33.Hanna Z, Kay DG, Cool M, Jothy S, Rebai N, Jolicoeur P. Transgenic mice expressing human immunodeficiency virus type 1 in immune cells develop a severe AIDS-like disease. J Virol 1998;72:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujii Y, Otake K, Tashiro M, Adachi A. Soluble Nef antigen of HIV-1 is cytotoxic for human CD4+ T cells. FEBS Lett 1996;393:93–96. [DOI] [PubMed] [Google Scholar]
- 35.Acheampong EA, Parveen Z, Muthoga LW, Kalayeh M, Mukhtar M, Pomerantz RJ. Human immunodeficiency virus type 1 Nef potently induces apoptosis in primary human brain microvascular endothelial cells via the activation of caspases. J Virol 2005;79:4257–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 1998;391:397–401. [DOI] [PubMed] [Google Scholar]
- 37.James CO, Huang MB, Khan M, Garcia-Barrio M, Powell MD, Bond VC. Extracellular Nef protein targets CD4+ T cells for apoptosis by interacting with CXCR4 surface receptors. J Virol 2004;78:3099–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang MB, Jin LL, James CO, Khan M, Powell MD, Bond VC. Characterization of Nef–CXCR4 interactions important for apoptosis induction. J Virol 2004;78:11084–11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graziani A, Galimi F, Medico E, Cottone E, Gramaglia D, Boccaccio C, Comoglio PM. The HIV-1 Nef protein interferes with phosphatidylinositol 3-kinase activation 1. J Biol Chem 1996;271:6590–6593. [DOI] [PubMed] [Google Scholar]
- 40.Qiao X, He B, Chiu A, Knowles DM, Chadburn A, Cerutti A. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat Immunol 2006;7:302–310. [DOI] [PubMed] [Google Scholar]
- 41.Golpon HA, Fadok VA, Taraseviciene-Stewart L, Scerbavicius R, Sauer C, Welte T, Henson PM, Voelkel NF. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J 2004;18:1716–1718. [DOI] [PubMed] [Google Scholar]
- 42.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Vascular endothelial growth factor receptor blockade by SU5416 combined with pulsatile shear stress causes apoptosis and subsequent proliferation of apoptosis-resistant endothelial cells. Chest 2005;128:610S–611S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.