SUMMARY
Using cryo-electron microscopy, we have solved the structure of a novel icosidodecahedral COPII coat involved in cargo export from the endoplasmic reticulum (ER) co-assembled from purified cargo adaptor Sec23–24 and Sec13–31 lattice forming complexes. The coat structure shows a tetrameric assembly of the Sec23–24 adaptor layer that is positioned beneath the vertices and edges of the Sec13–31 lattice. Fitting the known crystal structures of the COPII proteins into the density map reveals a flexible hinge region stemming from interactions between WD40 β-propeller domains present in Sec13 and Sec31 at the vertices. The structure shows that the hinge region can direct geometric cage expansion to accommodate a wide range of bulky cargo including procollagen and chylomicrons that are sensitive to adaptor function in inherited disease. The COPII coat structure leads us to propose a new mechanism by which cargo drives cage assembly and membrane curvature for budding from the ER.
INTRODUCTION
Vesicular transport through exocytic and endocytic pathways requires multiple classes of coat protein assemblies found in the cytosol including clathrin, COPI and COPII (Bethune et al., 2006; Stagg et al., 2007; Young, 2007). These coat assemblies are composed of cage components that generate a lattice that directs and integrates the overall process of vesicle budding with recruitment of protein cargo linked to adaptor protein complexes (APCs) that are recognized by the cage components (Gurkan et al., 2006; Lee et al., 2004; Miller et al., 2002; Ungewickell and Hinrichsen, 2007).
The cytosolic COPII machinery is responsible for the transport of cargo molecules from the endoplasmic reticulum (ER) to downstream compartments of the secretory pathway and for delivery to the cell surface and endocytic pathways. The Sar1 GTPase (Aridor et al., 2001; Kuge et al., 1994; Yoshihisa et al., 1993) is anchored to the ER membrane in its activated form and recruits the Sec23–24 APC through recognition of the Sec23 subunit (Lee et al., 2004; Schekman and Orci, 1996; Stagg et al., 2007).The Sec24 subunit has a multivalent cargo recruitment platform facing the bilayer that interacts with export-competent cargo molecules to form pre-budding complexes (Aridor et al., 1998) at ER export sites - regions of the ER that are specialized for vesicle budding (Gurkan et al., 2006; Lee et al., 2004). Together, the cargo-Sec23–24 ternary complex interacts in an unknown fashion with Sec13–31, a heterotetrameric cytosolic protein complex, that can self-assemble to form a cage-like oligomeric lattice (Stagg et al., 2006) directing coat assembly along the axis of Sec23–24 containing elongated, tubular ER exit sites in mammalian cells (Aridor et al., 2001). In addition to these basic components that are both necessary and sufficient for formation of vesicles from liposomes in vitro (Matsuoka et al., 1998), a number of additional components are now recognized to facilitate budding, presumably contributing to initiation and/or stability of the assembling cargo-adaptor components and the Sec13–31 cage in vivo (Gurkan et al., 2006; Lee and Miller, 2007). Rare diseases have been directly attributed to defects in COPII assembly. A variety of lipid absorption disorders are associated with mutations in the Sar1 GTPase (Shoulders et al., 2004). Cranio-lenticulo-sutural dysplasia (CLSD), a disease that results from defective collagen export from the ER, is a consequence of a mutation in the Sec23A subunit that leads to defective recruitment of Sec13–31 (Boyadjiev et al., 2006; Fromme et al., 2007).
The structural relationships between cargo, APC components and cage components that allow for the creation of vesicles at ER exit sites have not been elucidated. Similarly, how vesicles of variable size that are capable of accommodating small cargo such as membrane receptors as well as very large cargo molecules such as procollagen or chylomicron particles - exceeding the size of typical COPII vesicle observed in vivo - remains an enigma. To address these questions, we now have solved the molecular structure of the complete COPII coat containing both Sec13–31 and Sec23–24. The structure reveals new principles for the formation of vesicle coats, the organization of the adaptor layer and provides an explanation for possible mechanisms that regulate vesicle size and functionality in response to cargo-adaptor assemblies that have been reported to be dysfunctional in disease
RESULTS
Generation of cage and coat assemblies in the presence of Sec13–31 and Sec23–24
In order to study the structure of COPII particles containing both Sec23–24 and Sec13–31, it is necessary to self-assemble cages and coats in the absence of the lipid bilayer. Previously, we used overnight dialysis to slowly and efficiently promote self-assembly of the Sec13–31 cuboctahedron in vitro (Stagg et al., 2006). In this system, the biologically regulated processes occurring in vivo that drive the assembly of Sec13–31 are mimicked by the substitution of Cl− anions for CH3COO− anions. Because dialysis is not amenable to higher throughput analysis of assembly under different conditions, we developed a new, more rapid approach (see Experimental Procedures). We followed assembly of COPII proteins in solution based on sedimentation in response to a rapid adjustment of the ionic environment of the incubation, referred to as a ‘salt-shift’. In these experiments, large complexes that form can be pelleted at low speed (15,000 × g; referred to as the low speed pellet (LSP)) whereas smaller complexes, such as Sec13–31 cuboctahedrons, can only be recovered by sedimentation at high speed (200,000 × g; referred to as the high speed pellet (HSP)) as described previously (Stagg et al., 2006). When incubations containing only Sec13–31 are salt-shifted, we observe recovery of Sec13–31 in both the LSP and HSP, with roughly 16% of the Sec13–31 assembled into cuboctahedrons that are recovered in the HSP (Fig. 1A, HSP lane). Interestingly, salt-shift of incubations containing only Sec23–24 results in its complete recovery in the LSP (Fig. 1A, HSP lane and Fig. 1B lanes 8–12), suggesting that Sec23–24 can also self-assemble into very large structures (Antonny et al., 2003)
Fig. 1.
(A) Differential centrifugation COPII assembly assay. COPII proteins Sec13–31 (5µg) and Sec23–24 (3µg) were incubated separately in assembly conditions (1M potassium acetate) for one hour on ice. The samples were then spun at 15,000g for 10 minutes to remove large aggregates; different amounts (marked as percentage) of this pellet are shown in panel A for Sec13–31 and Sec23–24 respectively. The supernatant from this spin was then spun at 200,000g for 20 minutes, this pelleted any cuboctahedron cages that are formed and is shown in the lane marked HSP (high speed pellet). (B) 5µl of a 1.7µM Sec13–31 stock was used for all lanes marked with a +, the volume of Sec23–24 stock (1.2µM) are indicated for each lane. The samples were spun for 10 minutes at 15,000 g (to remove large aggregates – see panel A) and then for 20’ at 200,000 g to pellet cuboctahedrons (shown in (panel C)). Only the high speed pellet fractions were run on the gel shown here. (C) shows a negative stained EM micrograph of Sec13–31 cages and (D) shows an electron micrograph for Sec13–31/Sec23–24 cages. Both (C) and (D) are shown at the same magnification, and the scale bars correspond to 2000Å. (E) Three COPII particles assembled with both Sec13–31 and Sec23–24 observed by cryo-EM. The cages are highlighted by red circles. The scale bar corresponds to 500Å. (F) Reference-free class average of largest cages (the right panel in E), show 5-fold symmetry. (G) Proposed Sec13–31 geometries where four edges combine to form a vertex. From left to right, these are the cuboctahedron, small rhombicuboctahedron, icosidodecahedron, and small rhombicosidodecahedron.
Using this simple approach for self-assembly, we looked at the sedimentation behavior of Sec13–31 and Sec23–24 when co-incubated in solution. Mixing increasing amounts of Sec23–24 with a fixed amount of Sec13–31 followed by salt-shift revealed two things. First, when sub-stoichiometric amounts of Sec23–24 was added to Sec13–31 prior to salt shift, Sec23–24 appeared in the HSP containing the cuboctahedron (Fig. 1B, lanes 2–4). This suggests that Sec23–24 can be incorporated into assembling Sec13–31 cuboctahedron-sized structures (Fig. 1B, compare lanes 8–10 with lanes 2–4). Second, the addition of increasing amounts of Sec23–24 resulted in a progressive decrease in the amount of Sec13–31 recovered in the HSP (Fig. 1B, lanes 2–6) and its complete recovery in the LSP (not shown). This demonstrates that when Sec23–24 is stoichiometric or greater, Sec13–31 and Sec23–24 co-assemble into structures larger than cuboctahedrons under the same conditions that favor the formation of cuboctahedrons in the absence of Sec23–24. To rule out the possibility that the very large Sec23–24 assemblies that form under these conditions non-specifically bring down Sec13–31 cuboctahedrons, we salt shifted Sec13–31 and Sec23–24 separately and then mixed them together. Under these conditions, Sec13–31 is recovered in the HSP (Fig. 1B, compare lane 7 with lane 5), indicating that preformed Sec23–24 assemblies do not non-specifically bind preformed Sec13–31 cuboctahedrons.
Taken together, the above experiments demonstrate that Sec13–31 and Sec23–24 can co-assemble into very large complexes that pellet at low speeds (15,000g). To investigate the morphological nature of the larger assemblies formed from Sec13–31 and Sec23–24, we performed negative stain EM analysis. Unlike the cuboctahedral particles we observed with Sec13–31 alone that can only be recovered in the HSP (Fig. 1C), the structures generated by incubation of limiting Sec13–31 and excess Sec23–24 contained an abundant number of coat-like particles that appeared considerably larger than cuboctahedrons (Fig. 1D). These results suggest that defined salt conditions can promote in solution the rapid formation of Sec13–31 and Sec23–24 mixed composition assemblies that may correspond to the complete coat structures that drive vesicle budding in vivo.
Single particle reconstructions of Sec13–31/Sec23–24 structures
We assembled in vitro COPII cage-like structures from purified full-length human Sec13–31 and Sec23–24 using dialysis into optimized acetate buffer conditions to improve the overall yield of mixed composition particles and characterized these assembled structures using cryo-EM and single particle analysis (Antonny et al., 2003; Stagg et al., 2006). In these particle preparations, we observed at least three discrete sizes of assemblies with diameters ranging from 600 Å to 1000 Å (Fig. 1E). The diameter of the smallest structures corresponds to the cuboctahedral geometry observed previously for the 30 Å resolution structure built from Sec13–31 alone (Stagg et al., 2006). The diameter of the intermediate-sized structures was ~700 Å, and in contrast to the cuboctahedral particles, which had square or hexagonal shapes, many appeared to have pentagonal shapes. The largest and by far the most abundant particles in the Sec13–31/Sec23–24 assembly conditions were ~1000 Å in diameter. These large particles had a very low signal-to-noise ratio that made them refractory to alignment and classification that is required for single particle analysis. To overcome this limitation, we collected a larger dataset of ~12,000 of the large particles using moderate dose (30e-/Å2) and high defocus (10–12 µm under focus at 200keV) (Supplemental Fig. S1A). When these particles were subjected to reference-free alignment and classification, this resulted in coherent class averages, some of which had 5-fold symmetry (Fig. 1F, Supplemental Fig. S1B). Based on the geometry of the cage generated by self-assembly of Sec13–31 alone, there are only two geometries that will form cages with a diameter of ~1000 Å: these are the icosidodecahedron, which has icosahedral symmetry, and the small rhombicuboctahedron, which has octahedral symmetry (Fig. 1G). Since icosahedral symmetry has a five-fold symmetry axis and octahedral does not, we predicted that the new, large structures would be icosidodecahedrons.
Three dimensional (3-D) model of the Sec13–31 cage formed in the presence of Sec23–24
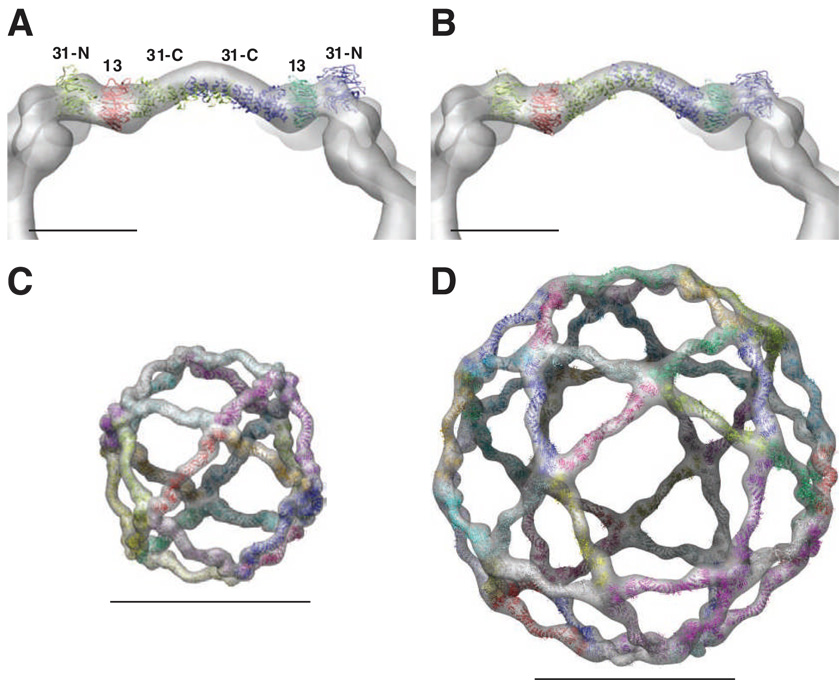
We performed a single particle reconstruction to generate a 3-D model of the large cage structure. To minimize model bias, we took the best 5-fold reference-free class average, aligned it to the 5-fold axis of an icosahedron and backprojected it with icosahedral symmetry to arrive at an initial model. We then refined this structure against the ~12,000 particle data set to convergence. The final reconstruction has a resolution of 43 Å and forms an outer cage structure that corresponds to an icosidodecahedron (Fig. 2A, Supplemental Fig. S1C). Although the resolution is relatively low given the icosahedral symmetry (likely reflecting the relatively high dose and high-defocus that was required to solve the structure), we observed two strong similarities between the new reconstruction and the cuboctahedral structure. First, the structure of the icosidodecahedral edge, which consists of a Sec13–31 heterotetramer, has identical contours to that of the cuboctahedral structure (Fig. 2B). The lengths of the edges are the same in both structures (about 300 Å), they both have characteristic lobes at the ends of the edges that are connected by a continuous curving area of density, and the two ends appear to be related by a 180° rotation indicating that the edges have two-fold symmetry (Fig. 2B). Second, the asymmetric units, which are the Sec13–31 heterotetramers (Stagg et al., 2006), are off-center with respect to the polyhedral edge (Fig. 2A, B). In other words, one end is closer to the center of its corresponding vertex than that of the other end. We refer to these as the ‘plus’ and ‘minus’ ends of the edge (see below). The fact that the cuboctahedron and icosidodecahedron structures were solved completely independently and yet share many similarities provides a high level of confidence in the reconstruction.
Fig. 2. Structure of the COPII icosidodecahedron.
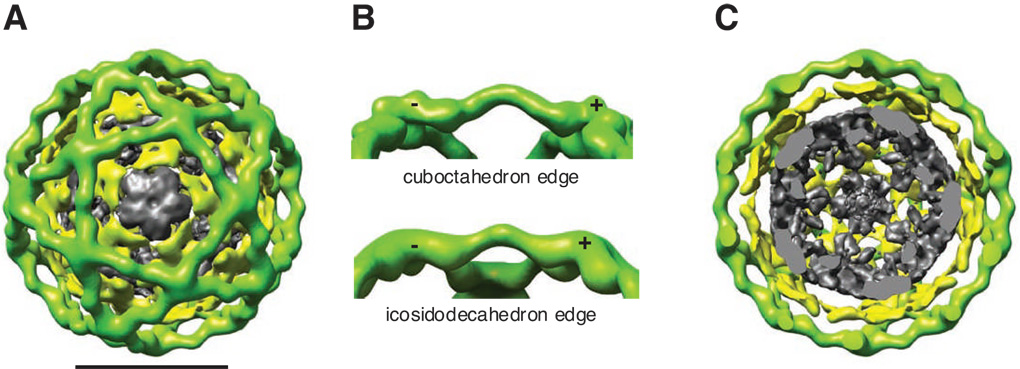
(A) Single particle reconstruction of the icosidodecahedral cages colored by radius from gray to green. Three layers of EM density are observed corresponding to Sec13–31 (green), Sec23–24 (yellow), and nonspecifically bound proteins (gray). The scale bar corresponds to 500Å. (B) Comparison of the edges of the cuboctahedral cage (top) and icosidodecahedral cage (bottom) shows that their contours are identical. They both show a polarity to the edges so that the ends of the Sec13–31 edges that are closer to the vertex are labeled with a plus (+) and those farther from the vertex are labeled with a minus (−). (C) Slice through the middle of the reconstruction highlighting the three unique layers of density.
The new icosidodecahedron cage has 60 edges and 32 faces of which 20 are triangles and 12 are pentagons (Fig. 2A, Supplemental Movie S2). As with the Sec13–31 cuboctahedron, the defining feature is not the geometry of the faces, but the fact that the vertices are formed by the intersection of four edges. Thus, consistent with the LSP sedimentation properties observed during assembly of Sec13–31 in the presence of Sec23–24 following salt-shift, we conclude that the larger particles observed in negative-stain EM (Fig. 1D) correspond to the considerably larger COPII particles.
The icosidodecahedron is a multi-layered assembly
In contrast to the cuboctahedron generated with Sec13–31 alone (Stagg et al., 2006), the icosidodecahedron structure appears to have three layers of density (Fig. 2A, C, Supplemental Fig. S3). There is an outer layer that corresponds to the Sec13–31 cage (Fig. 2A, C, green) followed by a second or middle layer (Fig. 2A, C, yellow), and an innermost layer that is distributed through the center of the central cavity and lacks regular structure (Fig. 2A, C, gray). Given that only two components are in the mixture, Sec13–31 and Sec23–24, we interpret the second layer to be Sec23–24 (see below) and the disordered innermost layer likely consists of unassembled Sec13–31 and/or Sec23–24 complexes that are captured during self-assembly (Supplemental Fig. S3C). The membrane proximal surface of Sec23–24 is highly positively charged (Bi et al., 2002) and may interact in solution non-specifically with unassembled coat components. The space inside of the Sec23–24 layer is large enough to accommodate a vesicle of about 67 nm in diameter, in line with the sizes observed in vivo (Matsuoka et al., 1998). We now refer to the multilayered structure that is self-assembled in a solution that contains both Sec13–31 and Sec23–24 as the COPII coat with Sec13–31 forming the surrounding COPII cage.
Modeling the atomic structure of the COPII cage
Crystal structures have recently been solved of the C-terminal domain of yeast Sec31 in complex with Sec13 and of the N-terminal domain of Sec31 in complex with Sec13 (Fath et al., 2007). Combining these two structures together results in a model for the yeast Sec13–31 heterotetramer that is composed of two Sec13–31 monomers that dimerize through the α-solenoid domains of Sec31 (Fath et al., 2007). The overall topology of the yeast heterotetramer structure agrees very well with that of the edge of the cryo-EM structure of the human Sec13–31 cuboctahedron (Fath et al., 2007; Stagg et al., 2006) (Fig. 3A). We found a similar result for the icosidodecahedron containing human Sec13–31 and Sec23–24, suggesting that generation of a larger cage structure in the presence of Sec23–24 does not alter the basic structural organization and dimensions of the Sec13–31 edge. The major difference in the crystal structure of the yeast Sec13–31 (Fath et al., 2007) and the cryo-EM maps of the human COPII cage (Stagg et al., 2006) and the icosidodecahedron COPII coat (described herein) is observed at the middle of the Sec31 dimerization interface (Fig. 3A). In both the cuboctahedron and icosidodecahedron cryo-EM structures, the dimerization region in the center of the heterotetramer creates an approximately 135° angle in the edge (Fig. 2A, B). However, in the crystal structure of yeast Sec13–31 (Fath et al., 2007) this angle is approximately 165° (Fig. 3A). This may reflect crystal packing (Fath et al., 2007), be due to differences in the yeast and human Sec31 sequences and/or reflect the absence of nearly half of the Sec31 sequence in the yeast crystal structure (Fath et al., 2007) but that is present in both cryo-EM structures generated from full-length human components. Furthermore, it could reflect a conformational change that occurs during assembly of the COPII cage revealed by the cryo-EM structure (Fig. 3C, D).
Fig. 3. Atomic models for different COPII cages.
(A) Atomic model for the Sec13–31 heterotetramer from Fath et al. (colored ribbons) shown relative to the Sec13–31 cuboctahedron cryo-EM structure (grey). (B) Model for the Sec13–31 heterotetramer fit into the cryo-EM density of a 22 Å resolution cuboctahedron cage. The curve in the center of the edge was modeled by normal modes flexible fitting (coloring as in (A)). (C) Atomic model for the Sec13–31 cuboctahedron (colored ribbons) fit into the cryo-EM density (transparent grey). (D) Atomic model for the icosidodecahedron fit into the cryo-EM density (coloring as in (C)). The scale bars in (A) and (B) correspond to 100 Å while the scale bars in (C) and (D) correspond to 500 Å.
The angle in the human heterotetramer was modeled using normal modes flexible fitting (Tama et al., 2004) of the yeast crystal structure into an isolated edge from a new, higher-resolution (22 Å) cuboctahedron reconstruction (Fig. 3B, Supplemental Movie S4, see Supplemental Methods). The modeled heterotetramer was then fit as a rigid body into the cuboctahedron and icosidodecahedron structures. The modeled Sec13–31 atomic structure fits well into the cryo-EM density of both the cuboctahedron and icosidodecahedron (Fig. 3C, D), providing detailed molecular descriptions of both the COPII cage and, now, the COPII coat.
Modeling the atomic structure of the COPII coat adaptor layer
While Sec13–31 forms an outer shell with icosidodecahedral geometry, we reasoned that the middle layer was Sec23–24. The mass of the middle layer corresponds to approximately 120 Sec23–24 molecules. Thus, there are two Sec23–24 dimers per edge. When viewed down the two-fold axis of symmetry of the coat (i.e., directly below a vertex), one observes a four-lobed structure with two-fold symmetry (Fig. 4A–D). The crystal structure of Sec23–24 has a bowtie shape that resembles each of these lobes (Bi et al., 2002; Matsuoka et al., 2001), and we can dock four Sec23–24 dimers into this four-lobed structure (Fig. 4B). The position of the 4-lobed Sec23–24 structure in relationship to the Sec13–31 edge is more clearly seen in a surface rendering of the Sec23–24 (Bi et al., 2002) and Sec13–31 (Stagg et al., 2006) crystal structures in the context of the icosidodecahedron coat (Fig. 4C, D). This demonstrates that Sec23–24 occupies two different positions relative to Sec13–31. One Sec23–24 in the asymmetric unit extends along the Sec13–31 edge, while the other extends out into the middle of the triangular face. While the 43 Å resolution of the reconstruction does not permit us to unambiguously determine the relative orientations of the four Sec23–24 dimers (Fig. 4E, F), the new COPII coat structure demonstrates that Sec23–24 can form a regular complex consisting of four dimers now referred to as the Sec23–24 tetramer cluster (Sec23–24TC) that is oriented directly under each vertex.
Fig. 4. Organization of Sec23–24 in the icosidodecahedral cage.
(A) View down the two-fold axis of symmetry. (B) Four lobes of density are observed just beneath the vertices. Two Sec23–24 crystal structures (ribbons) fit well in the asymmetric unit, though an unambiguous fit could not be determined. (C) Reduced representation (surface rendering) view of the crystal structures of Sec13–31 and Sec23–24 that have been fit into the icosidodecahedron cryo-EM density in the same orientation as (A). Dark green lobes labeled with plus and minuses are Sec31 WD40 β-propeller domains. The light green lobes adjacent to Sec31 WD40 domains are Sec13 WD40 β-propeller containing structures. (D) The same view as (C) but with the Sec13–31 vertex made transparent. This shows that the crystal structure of Sec23–24 has the same general shape and features as the EM density (B). (E) Two possible parallel orientations for Sec23–24. Red triangles correspond to Sec23 and orange triangles correspond to Sec24. (F) Two possible antiparallel orientations for Sec23–24. Coloring is the same as in (E). The scale bars in (A – D) correspond to 500 Å.
The middle layer Sec23–24 layer is disconnected from the outer Sec13–31 at moderate contour levels in our structure, though at low contour levels connections can be seen (Supplemental Fig S3D). There are two likely reasons for the lack of observable density between the two layers. One possibility is that the connections between the layers are heterogeneous and thus are averaged out – the portion of Sec31 known to interact with Sec23 is in a proline-rich and likely unstructured region of the protein. Another possibility is that the resolution of the reconstruction is simply too low to resolve the slender connections between the layers.
Expanding the size of COPII vesicles to accommodate large cargo
The COPII cuboctahedron cage (Stagg et al., 2006) and icosidodecahedron coat structures we have now generated share properties that illustrate the structural basis for vesicle assembly. First, it is now apparent that four edges combine to form a vertex. Second, as we observed for the edge in the cuboctahedron (Stagg et al., 2006), Sec13–31 heterotetramers in the icosidodecahedron define geometric edges which are assembled in an off-center fashion where one end (the ‘plus’ end) lies closer to a vertex than the other end (the ‘minus’ end), thereby conferring polarity to the edge (Fig. 2, Fig. 5A, B). Thus, asymmetry is a key organizational feature of edge assembly in COPII cages and contributes significantly to cage architecture.
Fig. 5. Angles influencing the structure of the COPII cage.
(A) The cuboctahedral cage with α and β angles labeled. Sec13–31 is off-center with respect to the geometric edge, giving the structure polarity. As in figure 2, the ends of the Sec13–31 edges containing the Sec31 WD40 β-propeller motifs that are closer to the vertex are labeled with a plus (+) and those farther from the vertex are labeled with a minus (−). α is the angle between the plus end of an edge and the adjacent edge in the clockwise direction. Likewise, β is the angle between the minus end of an edge and the adjacent edge in the clockwise direction. (B) A diagram of the different Sec13–31 edges linked at the vertex to form the fixed α and variable β angles in relationship to the underlying Sec23–24 tetramer cluster observed in the structure (Fig. 5). It should be noted that because the faces derived from the α and β angles do not lie in the same plane, their sum does not equal 360° until a planar conformation is achieved (i.e. β angle of 120°). A possible hinge interface for the tetramer cluster is indicated by a dashed line. (C) Reference-free class average of pentagonal-shaped intermediate-sized particles shown in Fig. 2B, middle panel. The scale bar corresponds to 500 Å. (D) Model of the D5 coat based on class average in C.
A third and new property of COPII assembly emerges as a consequence of solving the structure of the Sec13–31/Sec23–24 icosidodecahedron and provides a mechanism for cage expansion. The observed discrepancy between the angles at the Sec31 dimerization interface in the yeast crystal structure and our cryo-EM reconstructions (Fig. 3A) led Fath et al. to the propose that flexibility in the middle of the heterotetramer facilitates cuboctahedral cage expansion (Fath et al., 2007). Such a mechanism, while possible, would only result in the expansion of the 600 Å cuboctahedron (with a 135° angle in the middle of the edge and a length of 300 Å) to a maximum diameter of 650 Å (when the heterotetramer is straightened to 180° angle giving it a length of 325 Å) (see Supplemental Movie S4).
In contrast to the middle of the edge functioning as a potential pivot for cage expansion, our structure of the COPII coat containing both Sec13–31 and Sec23–24 provides a geometric solution to cage expansion that is driven not by the straightening of the Sec13–31 heterotetramers, but by the angles formed between the heterotetramers at the vertex elements. Here, we define α and β as angles between adjacent edges lying clockwise from a plus or a minus end, respectively (Fig. 5A, B). In comparing the cuboctahedron with the icosidodecahedron, we observe that two of the four angles that arise from the intersection of four edges at a vertex are invariant (Fig. 5A, B). The α angles are 60° for every vertex, resulting in triangular faces in each structure. Moreover, the fact that α is 60° regardless of the presence of Sec23–24 implies that α is predetermined by the Sec13–31 heterotetramers at the vertices. If COPII cage geometries are limited by evolutionary constraints that restrict Sec13–31 oligomerization to an α angle of 60°, then the predicted structures with α angles different from 60°, such as the small rhombicuboctahedron and small rhombicosidodecahedron (Fig. 1G) are not possible.
Whereas α is a fixed angle, the β angle is variable. β is 90° in the square face found in the cuboctahedron (Fig. 1G)) or 108° in the pentagonal face found in the icosidodecahedron (Fig. 1G)), suggesting that the β angle is adjustable and can determine the size of coats during cage assembly. To provide additional support for the observed variable β and the fixed α angles, we performed single particle analysis of the intermediate-size particles observed in the cryo-EM images of COPII coat preparations with both Sec13–31 and Sec23–24 (Fig. 1E, middle panel). Though there were not enough particles for a full single particle reconstruction, reference-free class averages (Fig. 5C) in combination with modeling indicate a structure with D5 symmetry and the same design principles as the cuboctahedron and icosidodecahedron in that four edges combine to make the vertices, thus we call this structure the D5 coat (Fig. 5D). Notably, α in the D5 coat is 60° and β varies between 90° and 108°. These observations agree well with what is observed in the both the cuboctahedron and icosidodecahedron structures. Thus, three independent structures confirm the observation that α is fixed and β is varied in different cage and coat assemblies.
Molecular organization of the COPII vertex
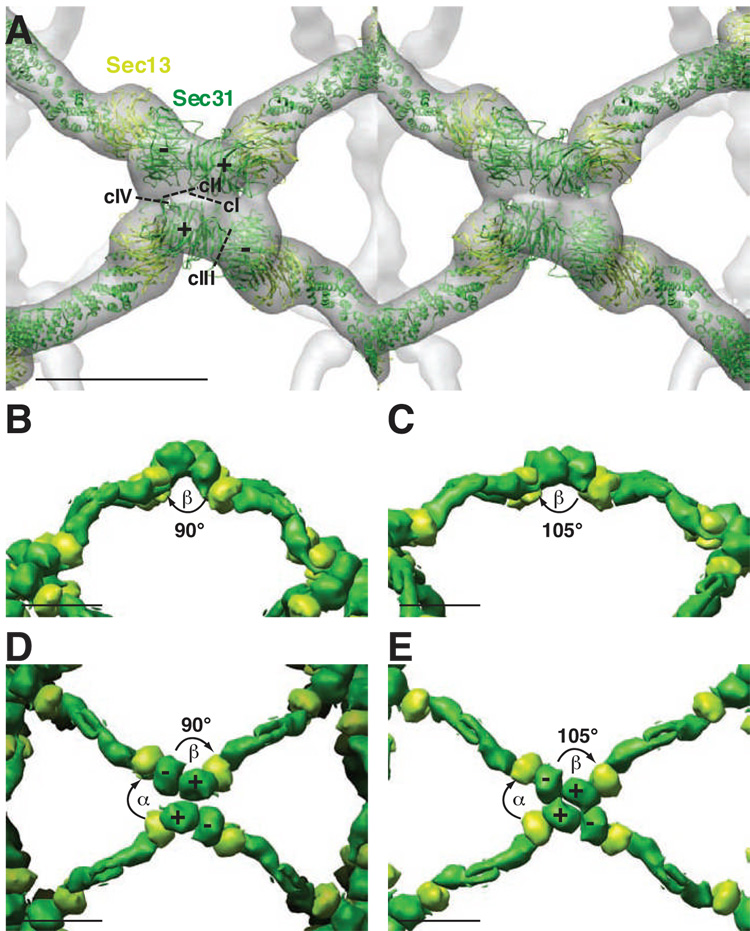
What are the molecular interactions that determine the α and β angles? Fath et al. identified three unique contacts at the vertices of our 30 Å cuboctahedron cage (Fath et al., 2007; Stagg et al., 2006). Contact I (cI) occurs between the WD40 domains (Sec31 WD40 β-propeller domain shown in dark green) at the plus ends of two Sec31 heterotetramers that are 180° apart from each other at a vertex. Contacts II and III (cII and cIII) occur between the WD40 domain of a plus end of Sec31 and the WD40 domain at the minus end of the neighboring Sec31 in the clockwise and counterclockwise directions, respectively (Fig. 6A). Based on a new 22 Å resolution reconstruction of the Sec13–31 cuboctahedron (Fig. 3C), we can now identify a fourth contact (cIV) (Fig. 6A) between the WD40 domain of Sec31 at a minus end and WD40 domain of Sec13 (shown in light green) at the plus end of the neighboring edge in the counterclockwise direction (Fig. 6A). Given these observations, it appears that α is determined by the cII and cIV interactions involving both Sec13 and Sec31. Because α is always 60°, these interactions would remain unchanged with different β angles (Fig 6A, D, E) and now suggests a role for Sec13 as a determinant of the invariant α angles that contribute to cage design.
Fig. 6. Mechanism for the expansion of the COPII cage from the cuboctahedron to the icosidodecahedron.
(A) Stereo view of the vertex of the cuboctahedron with the atomic model of Sec13–31 fit into the cryo-EM density (transparent grey). Sec13 WD40 β-propeller domain is represented by light green ribbons while Sec31 is represented by dark green ribbons with the WD40 β-propeller domain labeled with pluses and minuses. The putative contacts mediating the formation of the vertex are indicated by cI-cIV. These contacts all appear to be facilitated through the WD40 β-propeller containing domains. Contacts I and III appear to rotate relative to each other when transitioning from a cuboctahedron to an icosidodecahedron (B–E). (B) Reduced representation (surface rendering) depiction of the side view of the Sec13–31 cuboctahedron vertex. Colors are the same as (A). α and β angles are indicated. (C) Side view of the Sec13–31 icosidodecahedron vertex. (D) Top view of the cuboctahedron vertex. (E) Top view of the icosidodecahedron vertex. See Supplemental Movie S5 for animation of changes in the vertex hinge region during transition from the cuboctahedron to the icosidodecahedron. The scale bars in (A – E) correspond to 100 Å.
What is the mechanism that facilitates the formation of different β angles? Contacts cI and cIII are observed to change significantly between the cuboctahedron and icosidodecahedron cage reconstructions (Fig. 6B–E, Supplemental Movie S5). This change facilitates the expansion of the cage from the cuboctahedron to the icosidodecahedron. For contact cI, the faces of the two WD40 domains that comprise the contact rotate against each other (Fig. 6B–E). For cIII, the face of the WD40 at the minus end of Sec31 rotates against the edge of the WD40 domain at the plus end of the adjacent Sec31 (Fig. 6B–E). Thus, the Sec31 WD40 domain could be considered a structurally flexible domain that can accommodate different cage architectures through variation of the β angle to accommodate cargo recruitment.
DISCUSSION
Our previous structure of the cuboctahedron COPII cage assembled from Sec13–31 alone (Stagg et al., 2006) and now our new reconstruction of the icosidodecahedron COPII coat assembled in the presence of both Sec23–24 and Sec13–31 provide key insights into a number of fundamental molecular, biochemical and biophysical principles underlying vesicle budding from the ER at exit sites.
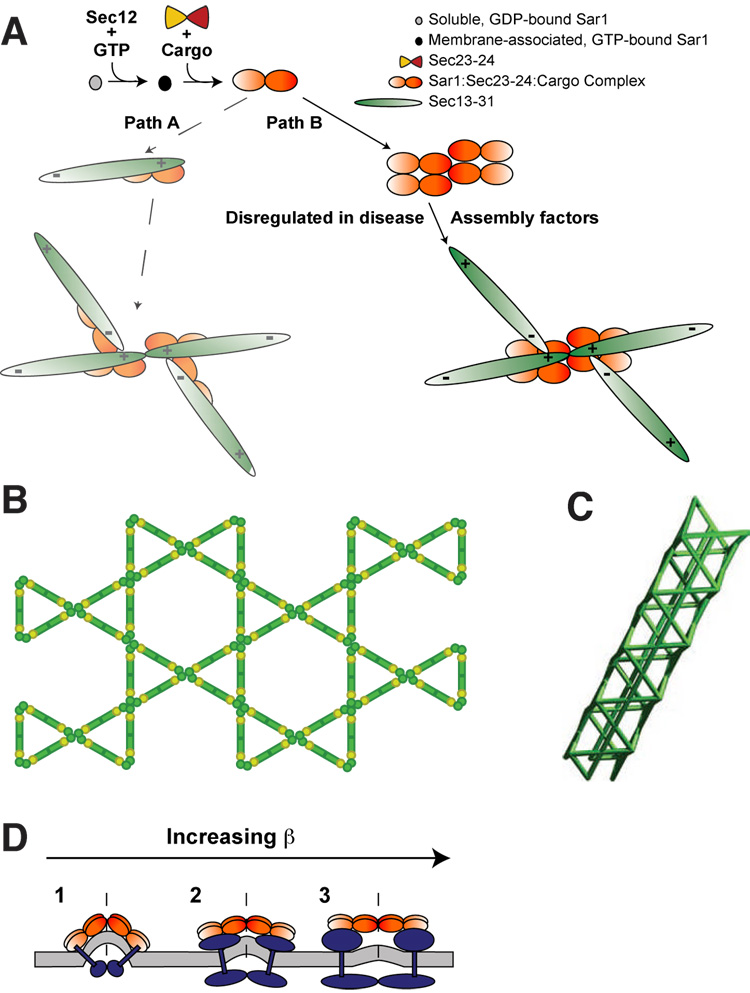
We expected that we would observe a symmetric structural relationship between Sec23–24 and Sec13–31 in the assembled COPII coat. Surprisingly, in our reconstruction, we observe a tetramer cluster where two of the four Sec23–24 dimers extend out along two opposing Sec13–31 edges while the other two dimers extend out into the middle of the triangular faces. A conventional model of coat assembly suggested the possibility that each Sar1/Sec23–24/cargo ternary pre-budding complex (Aridor et al., 1999; Supek et al., 2002) will recruit a Sec13–31 heterotetramer that will subsequently assemble a vertex (Fig. 7A, path A). We now consider this unlikely given the asymmetric organization of Sec13–31 with respect to the Sec23–24TC. Instead, our structure suggests that Sec23–24 must first form an oligomer, perhaps coalescing first as a minimal Sec23–24TC to define a site for Sec13–31 recruitment. While other permutations are possible, such a pathway based on assembly of Sec23–24 tetramer clusters could establish the polarity and geometry of the observed vertices as well as facilitate regulation by cargo (see below) (Fig. 7A, path B). This possibility is consistent with our salt-shift experiments in which Sec23–24 forms higher oligomeric species recovered in the LSP even in the absence of Sec13–31 (WEB, unpublished). Oligomeric organization of Sec23–24 could be enhanced by other components of the COPII coat such as Sec16 (Connerly et al., 2005; Supek et al., 2002). Furthermore, the location of the Sec23–24 tetramer clusters is consistent with the proposal that the TRAPPI complex, which integrates tether function in homotypic fusion of COPII vesicles with coat assembly through Sec23 (Cai et al., 2007), could bridge the two adaptor layers. Here, the closest approach between the Sec23–24 layers of two directly adjacent COPII coats is ~140Å, which is less than the 180Å length of the TRAPPI complex.
Fig. 7. Models for COPII coat formation and expanded COPII structures.
(A) A scheme of sequential COPII protein recruitment to the ER membrane. In path A, Sar1 activation results in recruitment of Sec23–24 (which binds protein cargo) and, according to current models, Sec13–31. This sequence predicts the congruous Sec23–24 arrangement shown at the end of path A, a result that is not observed in the COPII coat structure. Path B shows how Sec23-24 oligomerization prior to and independent of Sec13-31 recruitment could result in the observed arrangement of Sec23–24 in the COPII coat structure and define the physical properties of the vertex. The possible role of assembly factors in combination with Sec23–24 in facilitating tetramer cluster organization at exit sites is indicated. (B) Ạ possiblẹ planạr structurẹ of̣ Sec13–31 if̣ β can form angles up to 120°. Sec13 is represented as light green circles and Sec31 is represented in dark green. (C) A hypothetical tubular structure of Sec13–31. (D) A side view of the Sec23–24 tetramer cluster showing the effect of increasing cargo size on the hinge region (dashed lines). Increasing cargo size would result in Sec23–24 tetramer cluster configurations that would increase the resultant β angle generated by Sec13–31 recruitment.
COPII cages are required to transport a wide variety of differently sized cargo in the cell. The cuboctahedron, D5 coat, and icosidodecahedron structures that we have solved increase in diameter from 600Å to 1000Å and reveal new structural rules for COPII cage assembly. The formation of a vertex depends on two angles: α and β. α is invariant for all the COPII structures we have observed thus far. β non the other hand, varies between 90° and 108°, and this is what allows COPII cages to form with different diameters. The COPII structures observed thus far should be capable of recruiting a wide range of molecules of size classes including single and multi-membrane spanning proteins. If COPII cages are to incorporate cargo larger than can be accommodated by the 1000 Å coat, β must be capable of assuming angles even greater than 108°. One possibility is that 1000 Å is the largest coat that can be formed. If this is true, then large cargo such as procollagen and chylomicrons (up to 3,000 Å or 10,000 Å respectively) would have to be transported by some other means, perhaps a non-canonical COPII vesicle that lacks Sec13–31 (Siddiqi et al., 2003). However, this largely is inconsistent with biochemical studies (Shoulders et al., 2004) and genetic studies in which a defect in Sec23A prevents the recruitment and assembly of the Sec13–31 cage (Boyadjiev et al., 2006). If, on the other hand, β is capable of forming angles greater than 108°, a myriad of structures becomes possible. One intriguing possibility is if β can form up to a 120° angle, then Sec13–31 can assemble to form planar structures (Fig. 7B). This opens the possibility for COPII components to form 2-D sheets on the ER membrane, much like what has been observed for clathrin at the plasma membrane (Larkin et al., 1986). Intriguingly, we also note that with a β angle of 120°, COPII coats could also form tubular structures (Fig. 7C) that could be used for transporting elongated cargo like procollagen, which is 500 Å in diameter and can be as long as 3000 Å.
In addition to capture of large molecules such as procollagen, a largely planar Sec13–31 structure could readily assemble a cage/coat around large chylomicron particles to facilitate export from the ER. This interpretation is highly consistent with the need for Sar1B for export of chylomicron particles (Shoulders et al., 2004). Because Sec31 interacts with Sec23 along a surface that involves Sar1 (Bi et al., 2007), it is ultimately the combination a cargo/cargo receptor and the ternary Sar1/Sec23–24TC that may provide a catalyst for Sec13–31 self-assembly, dynamically accelerating cage assembly. The Sec23–24TC in our COPII coat reconstruction is well positioned to control vesicle size in response to cargo by also acting as a hinge that bends along the two-fold interface of the tetramer cluster - in other words, functioning as a lever to push apart opposing Sec13–31 edges in response to bound cargo and thereby control β and cage diameter (Fig. 7D).
Our combined evidence emerging from the new COPII icosidodecahedron coat structure suggests that the purpose of Sec13–31 may not only be to stabilize spatial interactions between Sec23–24TCs in the surface of the ER, but to promote membrane curvature and fission (Aridor et al., 2001; Fromme et al., 2007). Given the apparent geometric flexibility of the COPII coat structures, how is membrane curvature generated? Because Sec13–31 is capable of assembling into multiple geometries, it is unlikely that the cage element directly controls membrane curvature. Although Sar1-GTP can induce membrane curvature (Bielli et al., 2005; Lee et al., 2005) and the structure of Sec23–24 reveals a slightly concave surface associated with its membrane-oriented face (Bi et al., 2002), the Sec23-24TCs in the COPII coat structure have little if any contact between one another (Fig. 4B). Thus, the Sar1/Sec23–24TC/cargo prebudding complexes may generate local areas of curvature, but these are likely insufficient to induce the coherent, global curvature required to form a vesicle. We propose that it is the assembly of Sec13–31 that collects and organizes these local areas of curvature, resulting in their coalescence into a more global perturbation of the bilayer that ultimately forms a vesicle and dictates the timing of the fission event (Stagg et al., 2007). Furthermore, the length of the Sec13–31 heterotetramer may dictate the minimal separation required to promote coherence of multiple local regions of curvature defined by the Sec23–24TCs. Such a model is consistent with the COPI pathway where Arf1 and the Arf1 GTPase activating protein functioning as an adaptor are proposed to generate and sense curvature to regulate COPI vesicle budding in response to COPI coat assembly (Antonny et al., 2005).
The structure of the complete, multi-layered COPII coat reveals new fundamental principles that define the interactions between APCs and cage components that can generate budding structures of variable architecture resulting in a wide range of coat geometries. Key to COPII coat assembly is the newly identified role of the Sec23–24TCs that are asymmetrically organized directly beneath the Sec13–31 vertices. We suggest that the unprecedented structure of a vesicle coat containing an adaptor layer observed herein generated by self-assembly of cytosolic components in solution may provide a model for the assembly of adaptors in other coat structures such as COPI and clathrin. As such, the COPII coat structure now provides a basis for investigation of the interactive roles of cargo, APCs and edge assembly in the general operation of the exocytic and endocytic pathways.
EXPERIMENTAL PROCEDURES
Preparation of COPII coats
Human Sec23A, Sec24C, Sec13R and Sec31L1 (Genbank accession NM_006364.2, NM_004922.2, NM_183352 and NM_014933 respectively) were expressed in baculovirus and purified as described (Stagg et al., 2006). COPII coats were assembled by incubating equimolar ratios of purified human Sec13–31 and Sec23–24 on ice for 30 minutes followed by dialysis against 50 mM MES pH 6.8, 700 mM KOAc, 1 mM MgOAc, 1 mM DTT overnight. Analytical ‘salt shift’ experiments were carried out by adding KOAc (final) directly to Sec13–31 and/or Sec23–24 and incubating on ice for 30 minutes. These mixtures were subjected to a low speed spin (15,000g) and the supernatant subsequently to a high speed spin (200,000g). Pellets from each of these spins were analyzed by SDS-PAGE and coomassie staining.
Electron microscopy
Stained COPII coats and cages were prepared with 1% PTA.
COPII coats were prepared for cryo-EM analysis by preserving the samples in vitreous ice (Stagg et al., 2006). Four µl of the COPII coat sample was placed on a Quantifoil R2/2 400 mesh holey carbon grid that had been plasma cleaned for 30s using a Fischione model 1020 plasma cleaner (Fischione Instruments, Inc). The sample was vitrified using an FEI Vitrobot (FEI Company).
Two data sets were collected using an FEI F20 transmission electron microscope that was equipped with a Gatan Ultrascan 4k × 4k pixel CCD camera. The microscope was operated at 200keV. For the first data set, 474 exposure pairs were collected at a nominal magnification of 50,000×, dose of 20 e−/Å2, and defocus ranging from 1.0 µm to 3.5 µm under focus. This resulted in a 738 particle dataset, but the particles were refractory to single particle analysis because the signal to noise ratio (SNR) was very low. A second dataset was collected in order to increase the SNR,. For this dataset, 757 exposures were collected at a nominal magnification of 29,000×, dose of 30 e−/Å2, and defocus ranging from 10.0 µm to 12.0 µm under focus. Data was acquired using the Leginon automated electron microscopy package (Suloway et al., 2005) for both datasets.
Single particle reconstruction
Particles were manually selected using boxer from the EMAN package (Ludtke et al., 1999). The contrast transfer function (CTF) was estimated using the ACE software package (Mallick et al., 2005). Phases were corrected for individual particles based on the defocus reported from ACE for the particular micrograph from which the particle was picked.
12,120 large particles from the second data set were subjected to reference-free alignment and classification using the Spider package (Frank et al., 1996). The class average with the best five-fold symmetry was used to make an initial 3D model by aligning the average to the icosahedral five-fold symmetry axis and backprojecting it with icosahedral symmetry. This structure was then refined against the 12,120 particles. The refinement was performed using the standard projection matching scheme in EMAN. Icosahedral symmetry was applied, and ~25% of particles were excluded during the course of the refinement. Since the inside layer of the icosidodecahedral structure was disordered, this part of the density map was masked out during the refinement. The resolution of the final reconstruction was 43 Å as measured by the FSC0.5 criterion (Supplemental Fig. S6A). A refinement was also performed with the Sec23–34 middle layer masked out leaving only the outer Sec13–31 layer. The resulting structure still showed the characteristic Sec23–24 density indicating that it is consistently bound to Sec13–31, but the resolution of the reconstruction was lower (data not shown).
The mass was estimated for the middle layer of the COPII coat using the volume utility in EMAN. Briefly, this program calculates the volume occupied for a given density value and multiplies that by the average density for proteins. We estimated the mass of the middle layer at the same contour level used to visualize the reconstruction in Fig. 2 (2.3 sigma).
Supplementary Material
ACKNOWLEDGEMENTS
Work presented here was conducted at the National Resource for Automated Molecular Microscopy which is supported by the National Institutes of Health (NIH) though the National Center for Research Resources’ P41 program (RR17573) with additional NIH support to W.E.B. (GM42336) and S.S. (GM073509). Paul LaPointe, Abbas Razvi and Cemal Gurkan were supported by Fellowships form the Cystic Fibrosis Foundation Consortium.
REFERENCES
- Antonny B, Bigay J, Casella JF, Drin G, Mesmin B, Gounon P. Membrane curvature and the control of GTP hydrolysis in Arf1 during COPI vesicle formation. Biochem Soc Trans. 2005;33:619–622. doi: 10.1042/BST0330619. [DOI] [PubMed] [Google Scholar]
- Antonny B, Gounon P, Schekman R, Orci L. Self-assembly of minimal COPII cages. EMBO Rep. 2003;4:419–424. doi: 10.1038/sj.embor.embor812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Bannykh SI, Rowe T, Balch WE. Cargo can modulate COPII vesicle formation from the endoplasmic reticulum. J Biol Chem. 1999;274:4389–4399. doi: 10.1074/jbc.274.7.4389. [DOI] [PubMed] [Google Scholar]
- Aridor M, Fish KN, Bannykh S, Weissman J, Roberts TH, Lippincott-Schwartz J, Balch WE. The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J Cell Biol. 2001;152:213–229. doi: 10.1083/jcb.152.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Weissman J, Bannykh S, Nuoffer C, Balch WE. Cargo selection by the COPII budding machinery during export from the ER. J Cell Biol. 1998;141:61–70. doi: 10.1083/jcb.141.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethune J, Wieland F, Moelleken J. COPI-mediated transport. J Membr Biol. 2006;211:65–79. doi: 10.1007/s00232-006-0859-7. [DOI] [PubMed] [Google Scholar]
- Bi X, Corpina RA, Goldberg J. Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature. 2002;419:271–277. doi: 10.1038/nature01040. [DOI] [PubMed] [Google Scholar]
- Bi X, Mancias JD, Goldberg J. Insights into COPII coat nucleation from the structure of Sec23.Sar1 complexed with the active fragment of Sec31. Dev Cell. 2007;13:635–645. doi: 10.1016/j.devcel.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielli A, Haney CJ, Gabreski G, Watkins SC, Bannykh SI, Aridor M. Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J Cell Biol. 2005;171:919–924. doi: 10.1083/jcb.200509095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyadjiev SA, Fromme JC, Ben J, Chong SS, Nauta C, Hur DJ, Zhang G, Hamamoto S, Schekman R, Ravazzola M, et al. Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat Genet. 2006;38:1192–1197. doi: 10.1038/ng1876. [DOI] [PubMed] [Google Scholar]
- Cai H, Yu S, Menon S, Cai Y, Lazarova D, Fu C, Reinisch K, Hay JC, Ferro-Novick S. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007;445:941–944. doi: 10.1038/nature05527. [DOI] [PubMed] [Google Scholar]
- Connerly PL, Esaki M, Montegna EA, Strongin DE, Levi S, Soderholm J, Glick BS. Sec16 is a determinant of transitional ER organization. Curr Biol. 2005;15:1439–1447. doi: 10.1016/j.cub.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Fath S, Mancias JD, Bi X, Goldberg J. Structure and organization of coat proteins in the COPII cage. Cell. 2007;129:1325–1336. doi: 10.1016/j.cell.2007.05.036. [DOI] [PubMed] [Google Scholar]
- Frank J, Radermacher M, Penczek P, Zhu J, Li Y, Ladjadj M, Leith A. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J Struct Biol. 1996;116:190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- Fromme JC, Ravazzola M, Hamamoto S, Al-Balwi M, Eyaid W, Boyadjiev SA, Cosson P, Schekman R, Orci L. The genetic basis of a craniofacial disease provides insight into COPII coat assembly. Dev Cell. 2007;13:623–634. doi: 10.1016/j.devcel.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurkan C, Stagg SM, Lapointe P, Balch WE. The COPII cage: unifying principles of vesicle coat assembly. Nat Rev Mol Cell Biol. 2006;7:727–738. doi: 10.1038/nrm2025. [DOI] [PubMed] [Google Scholar]
- Kuge O, Dascher C, Orci L, Rowe T, Amherdt M, Plutner H, Ravazzola M, Tanigawa G, Rothman JE, Balch WE. Sar1 promotes vesicle budding from the endoplasmic reticulum but not Golgi compartments. J Cell Biol. 1994;125:51–65. doi: 10.1083/jcb.125.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JM, Donzell WC, Anderson RG. Potassium-dependent assembly of coated pits: new coated pits form as planar clathrin lattices. J Cell Biol. 1986;103:2619–2627. doi: 10.1083/jcb.103.6.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Miller EA. Molecular mechanisms of COPII vesicle formation. Semin Cell Dev Biol. 2007;18:424–434. doi: 10.1016/j.semcdb.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Lee MC, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- Lee MC, Orci L, Hamamoto S, Futai E, Ravazzola M, Schekman R. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 2005;122:605–617. doi: 10.1016/j.cell.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Ludtke SJ, Baldwin PR, Chiu W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J Struct Biol. 1999;128:82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- Mallick SP, Carragher B, Potter CS, Kriegman DJ. ACE: automated CTF estimation. Ultramicroscopy. 2005;104:8–29. doi: 10.1016/j.ultramic.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998;93:263–275. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Schekman R, Orci L, Heuser JE. Surface structure of the COPII-coated vesicle. Proc Natl Acad Sci U S A. 2001;98:13705–13709. doi: 10.1073/pnas.241522198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E, Antonny B, Hamamoto S, Schekman R. Cargo selection into COPII vesicles is driven by the Sec24p subunit. EMBO J. 2002;21:6105–6113. doi: 10.1093/emboj/cdf605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Shoulders CC, Stephens DJ, Jones B. The intracellular transport of chylomicrons requires the small GTPase, Sar1b. Curr Opin Lipidol. 2004;15:191–197. doi: 10.1097/00041433-200404000-00012. [DOI] [PubMed] [Google Scholar]
- Siddiqi SA, Gorelick FS, Mahan JT, Mansbach CM., 2nd COPII proteins are required for Golgi fusion but not for endoplasmic reticulum budding of the pre-chylomicron transport vesicle. J Cell Sci. 2003;116:415–427. doi: 10.1242/jcs.00215. [DOI] [PubMed] [Google Scholar]
- Stagg SM, Gurkan C, Fowler DM, LaPointe P, Foss TR, Potter CS, Carragher B, Balch WE. Structure of the Sec13/31 COPII coat cage. Nature. 2006;439:234–238. doi: 10.1038/nature04339. [DOI] [PubMed] [Google Scholar]
- Stagg SM, LaPointe P, Balch WE. Structural design of cage and coat scaffolds that direct membrane traffic. Curr Opin Struct Biol. 2007;17:221–228. doi: 10.1016/j.sbi.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Suloway C, Pulokas J, Fellmann D, Cheng A, Guerra F, Quispe J, Stagg S, Potter CS, Carragher B. Automated molecular microscopy: the new Leginon system. J Struct Biol. 2005;151:41–60. doi: 10.1016/j.jsb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Supek F, Madden DT, Hamamoto S, Orci L, Schekman R. Sec16p potentiates the action of COPII proteins to bud transport vesicles. J Cell Biol. 2002;158:1029–1038. doi: 10.1083/jcb.200207053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tama F, Miyashita O, Brooks CL., 3rd Normal mode based flexible fitting of high-resolution structure into low-resolution experimental data from cryo-EM. J Struct Biol. 2004;147:315–326. doi: 10.1016/j.jsb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Ungewickell EJ, Hinrichsen L. Endocytosis: clathrin-mediated membrane budding. Curr Opin Cell Biol. 2007;19:417–425. doi: 10.1016/j.ceb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Yoshihisa T, Barlowe C, Schekman R. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science. 1993;259:1466–1468. doi: 10.1126/science.8451644. [DOI] [PubMed] [Google Scholar]
- Young A. Structural insights into the clathrin coat. Semin Cell Dev Biol. 2007;18:448–458. doi: 10.1016/j.semcdb.2007.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.